Reduced Translocation Confers Paraquat Resistance in Plantago lanceolata
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Collection
P. lanceolata Selection
2.2. Dose-Response Trials
2.3. Quantum Efficiency of Open Photosystem II
2.4. Paraquat Transport
2.5. Absorption and Translocation of [14C]-Paraquat
2.6. Paraquat Metabolism
2.7. Phosphor Imaging
2.8. Data Collection and Statistical Analysis
3. Results
3.1. Survival and Biomass (%)
3.2. Quantum Efficiency of Open Photosystem II
3.3. Paraquat Transport
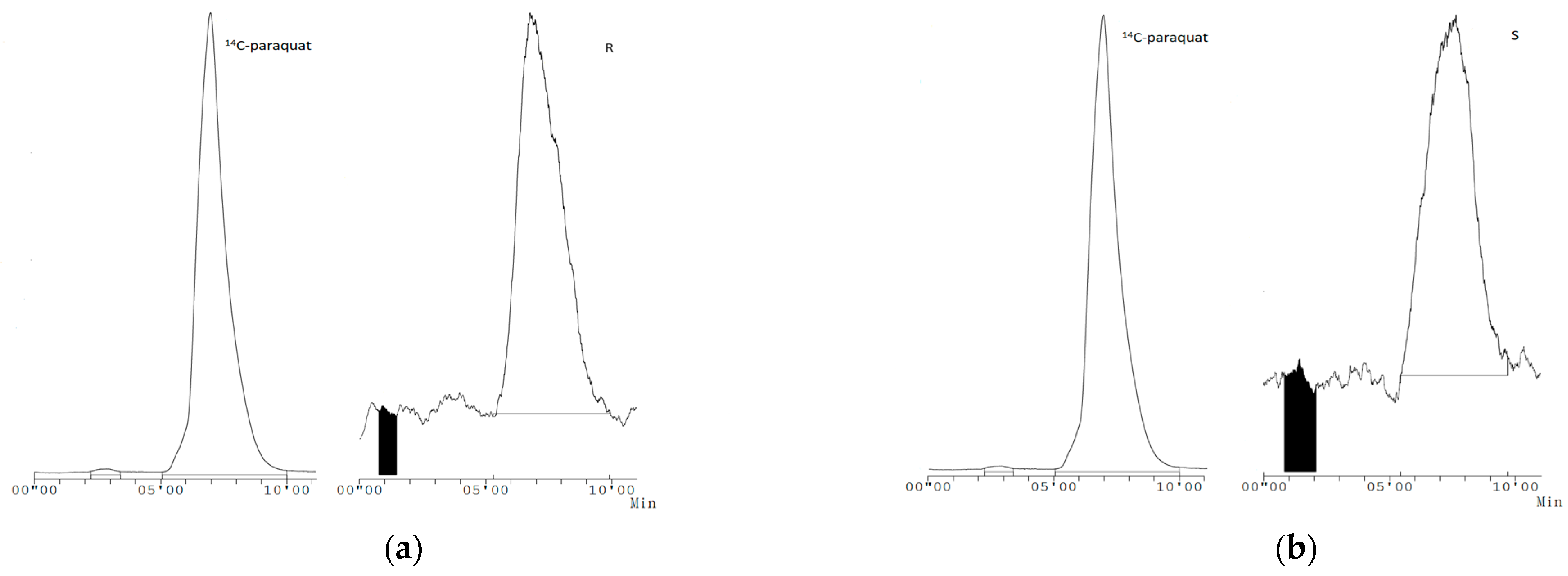
3.4. Leaf Uptake and Translocation of [14C]-Labelled Paraquat
3.5. Paraquat Metabolism
3.6. Phosphor Imaging
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazish, T.; Huang, Y.J.; Zhang, J.; Xia, J.Q.; Alfatih, A.; Luo, C.; Cai, X.T.; Xi, J.; Xu, P.; Xiang, C.B. Understanding paraquat resistance mechanisms in Arabidopsis thaliana to facilitate developing paraquat-resistant crops. Plant Commun. 2022, 25, 100321. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.Q.; Nazish, T.; Javaid, A.; Ali, M.; Liu, Q.Q.; Wang, L.; Zhang, Z.Y.; Zhang, Z.S.; Huang, Y.J.; Wu, J.; et al. A gain-of-function mutation of the MATE family transporter DTX6 confers paraquat resistance in Arabidopsis. Mol. Plant 2021, 14, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Huang, Y.P.; Xia, J.Q.; Fu, Z.P.; Chen, Y.F.; Huang, Y.P.; Ma, A.; Hou, W.T.; Chen, Y.X.; Qi, X.; et al. AtPQT11, a P450 enzyme, detoxifies paraquat via N-demethylation. J. Genet. Genom. 2022, 49, 1169–1173. [Google Scholar] [CrossRef]
- Luo, Q.; Chen, S.; Zhu, J.; Ye, L.; Hall, N.D.; Basak, S.; McElroy, J.S.; Chen, Y. Overexpression of EiKCS confers paraquat-resistance in rice (Oryza sativa L.) by promoting the polyamine pathway. Pest Manag. Sci. 2022, 78, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, M.; Koetz, E.; Wu, H.; Shephard, A. Paraquat resistance and hormetic response observed in Conyza sumatrensis (Retz.) E. Walker (tall fleabane) in Australian cotton cropping systems. Phytoparasitica 2022, 50, 269–279. [Google Scholar] [CrossRef]
- Hawkes, T.R. Mechanisms of resistance to paraquat in plants. Pest Manag. Sci. 2014, 70, 1316–1323. [Google Scholar] [CrossRef]
- Heap, I. The International Survey of Herbicide-Resistant Weeds. 2024. Available online: www.weedscience.org (accessed on 1 January 2024).
- Ghanizadeh, H.; Harrington, K.C. Non-target site mechanisms of resistance to herbicides. Crit. Rev. Plant Sci. 2017, 36, 24–34. [Google Scholar] [CrossRef]
- Hart, J.J.; DiTomaso, J.M.; Linscott, D.L.; Kochian, L.V. Transport interactions between paraquat and polyamines in roots of intact maize seedlings. Plant Physiol. 1992, 99, 1400–1405. [Google Scholar] [CrossRef]
- Xi, J.; Xu, P.; Xiang, C.B. Loss of AtPDR11, a plasma membrane localized ABC transporter, confers paraquat tolerance in Arabidopsis thaliana. Plant J. 2012, 69, 782–791. [Google Scholar] [CrossRef]
- Matshidze, M.M.; Ndou, V. Herbicide resistance cases in South Africa: A review of the current state of knowledge. S. Afr. J. Sci. 2023, 119, 11–12. [Google Scholar] [CrossRef]
- Matshidze, M.M.; Ndou, V. A bibliometric analysis of herbicide resistance in Africa. Sci. Afr. 2023, 22, e01899. [Google Scholar] [CrossRef]
- Sharma, N.; Koul, P.; Koul, A.K. Pollination biology of some species of genus Plantago L. Bot. J. Linn. Soc. 1993, 111, 129–138. [Google Scholar] [CrossRef]
- Yu, Q.; Cairns, A.; Powles, S. Glyphosate, paraquat and ACCase multiple herbicide resistance in a Lolium rigidum biotype. Planta 2007, 225, 499–513. [Google Scholar] [CrossRef]
- Heap, I. Identification and documentation of herbicide resistance. Phytoprotection 1994, 75, 85–90. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- Brunharo, C.A.C.G.; Hanson, B.D. Vacuolar sequestration of paraquat is involved in the resistance mechanism in Lolium perenne L. spp. multiflorum. Front. Plant Sci. 2017, 8, 1485. [Google Scholar] [CrossRef]
- Eksteen, F.H. Resistance of Ryegrass (Lolium spp.) to Paraquat and Glyphosate in the Western Cape. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2007. [Google Scholar]
- Meyer, C.; Norsworthy, J.; Kruger, G. Antagonism in mixtures of glufosinate + glyphosate and glufosinate + clethodim on grasses. Weed Technol. 2021, 35, 12–21. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.; Gerhard, D. Dose–response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Tehranchian, P.; Nandula, V.; Jugulam, M.; Putta, K.; Jasieniuk, M. Multiple resistance to glyphosate, paraquat and ACCase-inhibiting herbicides in Italian ryegrass populations from California: Confirmation and mechanisms of resistance. Pest Manag. Sci. 2018, 74, 868–877. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; Pereira, V.G.C.; Albrecht, A.J.P.; Rubin, R.S.; Adegas, F.S.; Albrecht, L.P. Paraquat resistance of sumatran fleabane (Conyza sumatrensis). Planta Daninha 2019, 37, e019183264. [Google Scholar] [CrossRef]
- Palma-Bautista, C.; Vázquez-García, J.G.; Domínguez-Valenzuela, J.A.; Ferreira Mendes, K.; Alcantara De la Cruz, R.; Torra, J.; De Prado, R. Non-target-site resistance mechanisms endow multiple herbicide resistance to five mechanisms of action in Conyza bonariensis. J. Agric. Food Chem. 2021, 69, 14792–14801. [Google Scholar] [CrossRef] [PubMed]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef] [PubMed]
- Jalaludin, A.; Yu, Q.; Powles, S.B. Multiple resistance across glufosinate, glyphosate, paraquat and ACCase-inhibiting herbicides in an Eleusine indica population. Weed Res. 2015, 55, 82–89. [Google Scholar] [CrossRef]
- Yu, Q.; Cairns, A.; Powles, S. Paraquat resistance in a population of Lolium rigidum. Funct. Plant Biol. 2004, 31, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Abdallah, I.; Han, H.; Owen, M.; Powles, S. Distinct non-target site mechanisms endow resistance to glyphosate, ACCase and ALS-inhibiting herbicides in multiple herbicide-resistant Lolium rigidum. Planta 2009, 230, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moreno, P.T.; Bastida, F.; De Prado, R. Evidence, mechanism and alternative chemical seedbank-level control of glyphosate resistance of a rigid ryegrass (Lolium rigidum) biotype from southern Spain. Front. Plant Sci. 2017, 8, 450. [Google Scholar] [CrossRef]
- Ismail, B.S.; Chuah, T.S.; Khatijah, H.H. Metabolism, uptake and translocation of 14C-paraquat in resistant and susceptible biotypes of Crassocephalum crepidioides (Benth.) S. Moore. Weed Biol. Manag. 2001, 1, 176–181. [Google Scholar] [CrossRef]
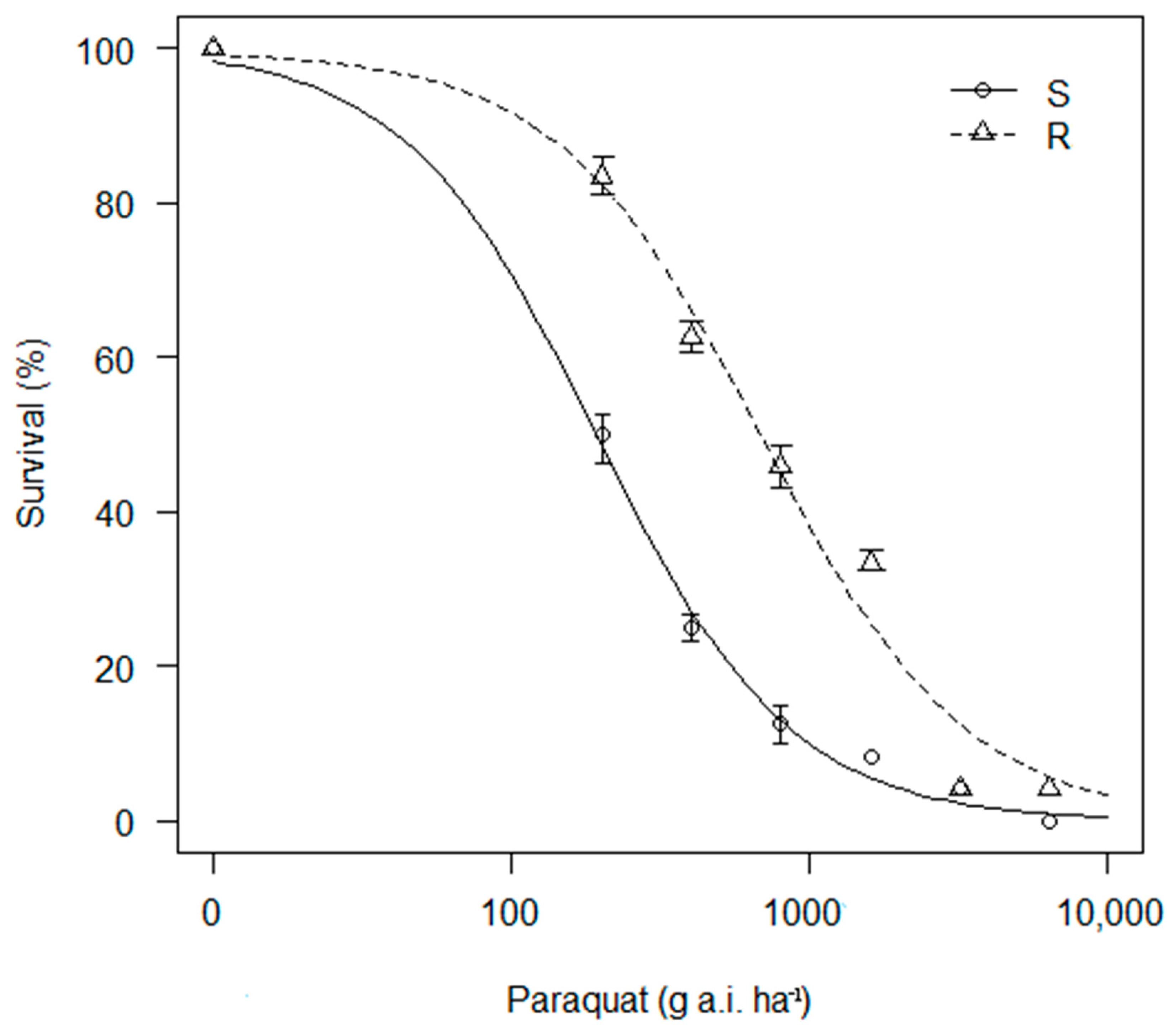
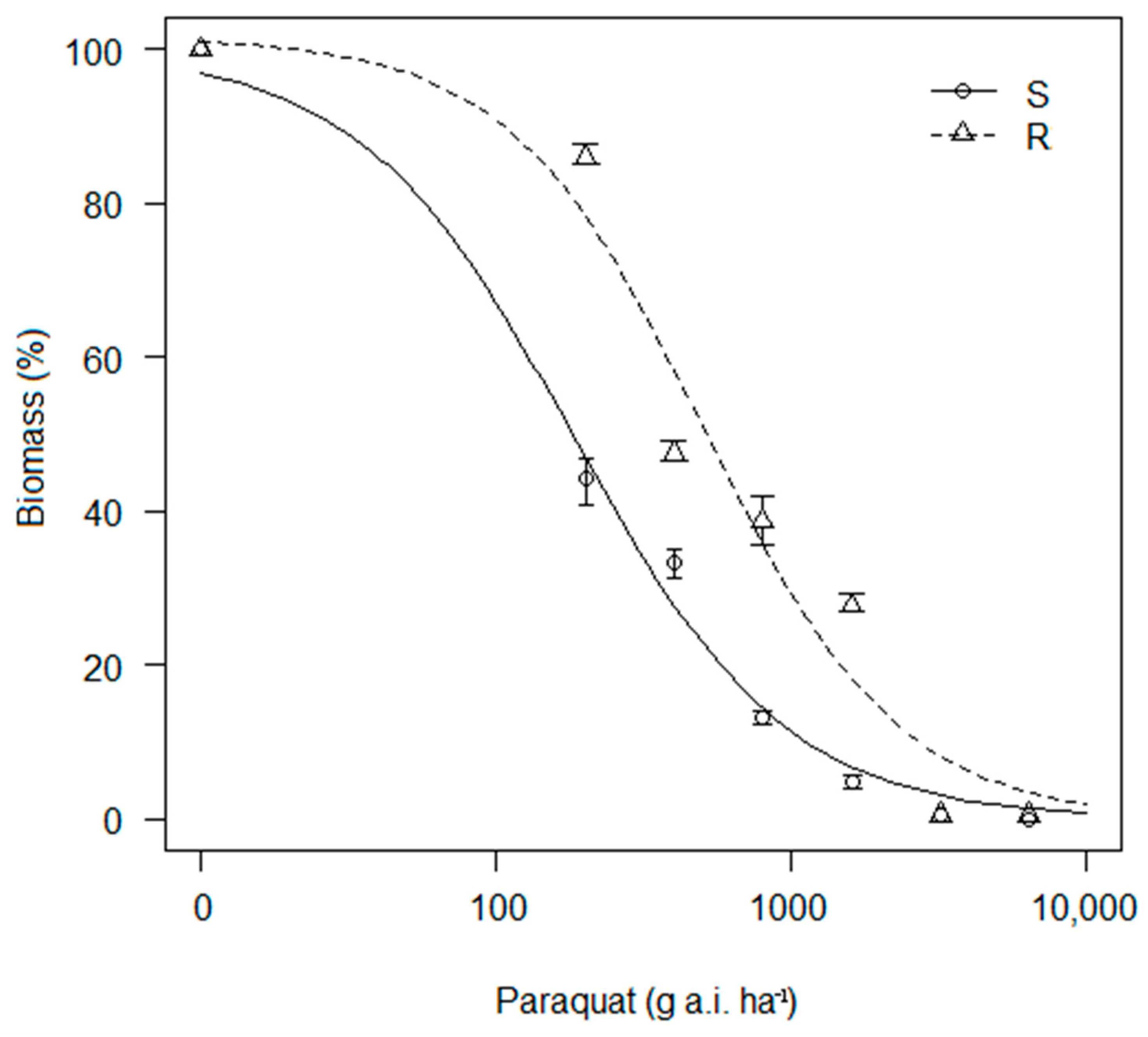


| Biotype | D | B | p Value | LD50 | p Value | R/S Ratio |
|---|---|---|---|---|---|---|
| R | 99.54 (9.80) | 1.26 (0.31) | *** | 688.99 (191.24) | *** | 3.59 |
| S | 100.14 (10.04) | 1.33 (0.56) | 191.81 (62.62) |
| Biotype | D | B | p Value | LD50 | p Value | R/S Ratio |
|---|---|---|---|---|---|---|
| R | 101.46 (9.08) | 1.31 (0.29) | *** | 505.36 (119.87) | *** | 2.78 |
| S | 99.75 (9.48) | 1.20 (0.41) | 181.61 (62.65) |
| Applied [14C]-Paraquat (%) | ||||
|---|---|---|---|---|
| Biotype | Untreated Leaves | Roots | Treated Leaves | Leaf Wash |
| R | 18.03 ± 6.12 | 7.86 ± 0.10 | 62.13 ± 9.37 | 7.79 ± 0.11 |
| S | 36.04 ± 9.69 | 33.17 ± 7.42 | 10.71 ± 2.65 | 15.17 ± 7.27 |
| p value | NS | ** | *** | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndou, V.; Kotze, D.; Marjanovic-Painter, B.; Phiri, E.E.; Pieterse, P.J.; Sonopo, M.S. Reduced Translocation Confers Paraquat Resistance in Plantago lanceolata. Agronomy 2024, 14, 977. https://doi.org/10.3390/agronomy14050977
Ndou V, Kotze D, Marjanovic-Painter B, Phiri EE, Pieterse PJ, Sonopo MS. Reduced Translocation Confers Paraquat Resistance in Plantago lanceolata. Agronomy. 2024; 14(5):977. https://doi.org/10.3390/agronomy14050977
Chicago/Turabian StyleNdou, Vhuthu, Deon Kotze, Biljana Marjanovic-Painter, Ethel E. Phiri, Petrus J. Pieterse, and Molahlehi S. Sonopo. 2024. "Reduced Translocation Confers Paraquat Resistance in Plantago lanceolata" Agronomy 14, no. 5: 977. https://doi.org/10.3390/agronomy14050977
APA StyleNdou, V., Kotze, D., Marjanovic-Painter, B., Phiri, E. E., Pieterse, P. J., & Sonopo, M. S. (2024). Reduced Translocation Confers Paraquat Resistance in Plantago lanceolata. Agronomy, 14(5), 977. https://doi.org/10.3390/agronomy14050977






