Allelopathic Activity of a Novel Compound and Two Known Sesquiterpene from Croton oblongifolius Roxb.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction
2.3. Growth Bioassay
2.4. Separation of the Active Substances
2.5. Measurement of NMR Spectral Data and Chemical Shifts of Identified Compounds
2.6. Bioassays of the Isolated Compounds
2.7. Statistical Analysis
3. Results
3.1. Growth-Suppressive Activity of C. oblongifolius
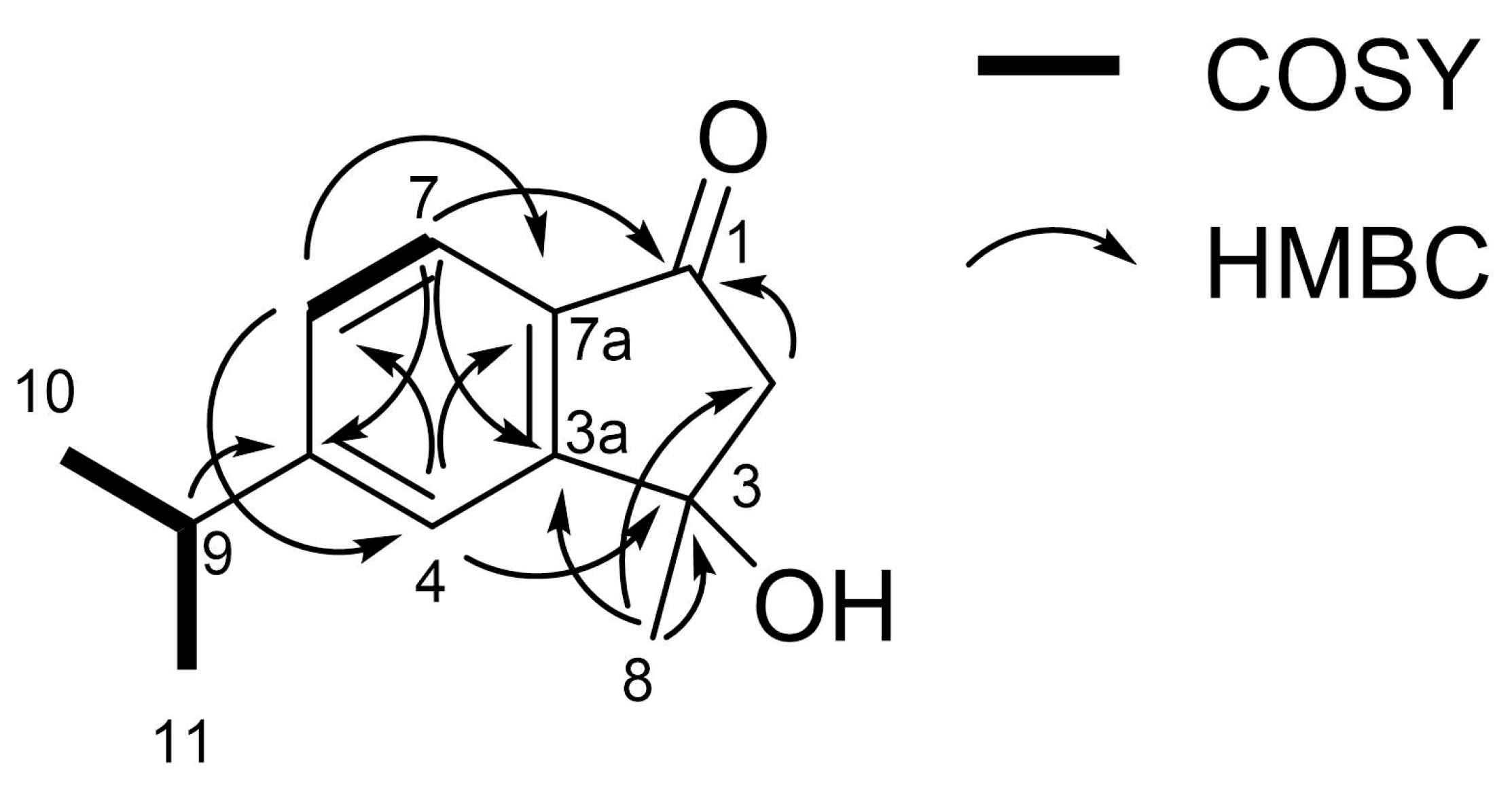
3.2. Characterization of the Allelopathic Compounds
3.3. Bioactivity of Compounds 1, 2, and 3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haq, S.M.; Lone, F.A.; Kumar, M.; Calixto, E.S.; Waheed, M.; Casini, R.; Mahmoud, E.A.; Elansary, H.O. Phenology and diversity of weeds in the agriculture and horticulture cropping systems of Indian Western Himalayas: Understanding implications for agro-ecosystems. Plants 2023, 12, 1222. [Google Scholar] [CrossRef]
- Monteiro, A.; Santos, S. Sustainable Approach to Weed Management: The Role of Precision Weed Management. Agronomy 2022, 12, 118. [Google Scholar] [CrossRef]
- Saric-Krsmanovic, M.; Umiljendic, J.G.; Radivojevi, L.; Santric, L.; Potocnik, I.; Durovic-Pejcev, R. Bio-herbicidal effects of five essential oils on germination and early seedling growth of velvetleaf (Abutilon theophrasti Medik.). J. Environ. Sci. Health B 2019, 54, 247–254. [Google Scholar] [CrossRef]
- Harker, K.N.; O’Donovan, J.T. Recent weed control, weed management, and integrated weed management. Weed Technol. 2013, 27, 1–11. [Google Scholar] [CrossRef]
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The challenge of herbicide resistance around the world: A current summary. Pest Manage. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: https://www.weedscience.org (accessed on 21 January 2024).
- Adhikari, S.P.; Yang, H.; Kim, H. Learning semantic graphics using convolutional encoder–decoder network for autonomous weeding in paddy. Front. Plant Sci. 2019, 10, 1404. [Google Scholar] [CrossRef]
- Kostina-Bednarz, M.; Płonka, J.; Barchanska, H. Allelopathy as a source of bioherbicides: Challenges and prospects for sustainable agriculture. Rev. Environ. Sci. Biotechnol. 2023, 22, 471–504. [Google Scholar] [CrossRef]
- Farooq, M.; Jabran, K.; Cheema, Z.A.; Wahid, A.; Siddique, K.H.M. The role of allelopathy in agricultural pest management. Pest Manag. Sci. 2011, 67, 493–506. [Google Scholar] [CrossRef]
- Hossen, K.; Das, K.R.; Asato, Y.; Teruya, T.; Kato-Noguchi, H. Allelopathic activity and characterization of allelopathic substances from Elaeocarpus floribundus Blume leaves for the development of bioherbicides. Agronomy 2022, 12, 57. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An eco-friendly tool for sustainable weed management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- El-Darier, S.M.; Abdelaziz, H.A.; ZeinEl-Dien, M.H. Effect of soil type on the allelotoxic activity of Medicago sativa L. residues in Vicia faba L. agroecosystems. J. Taibah Univ. Sci. 2014, 8, 84–89. [Google Scholar] [CrossRef][Green Version]
- Jabran, K. Sorghum allelopathy for weed control. In Manipulation of Allelopathic Crops for Weed Control, 1st ed.; SpringerBriefs in Plant Science; Springer: Cham, Switzerland, 2017; pp. 65–75. [Google Scholar]
- Czarnota, M.A.; Paul, R.N.; Dayan, F.E.; Nimbal, C.I.; Weston, L.A. Mode of action, localization of production, chemical nature, and activity of sorgoleone: A potent PSII inhibitor in Sorghum spp. root exudates. Weed Technol. 2001, 15, 813–825. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E.; Romagni, J.G.; Rimando, A.M. Natural products as sources of herbicides: Current status and future trends. Weed Res. 2000, 40, 99–111. [Google Scholar] [CrossRef]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdow, A. Allelochemicals as Bioherbicides—Present and Perspectives. In Herbicides—Current Research and Case Studies in Use; Price, A.J., Kelton, J.A., Eds.; InTech: Rijeka, Croatia, 2013; pp. 517–542. [Google Scholar]
- Dayan, F.; Romagni, J.; Tellez, M.; Rimando, A.; Duke, S. Managing weeds with natural products. Pest. Outlook 1999, 5, 185–188. [Google Scholar]
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef]
- Anaya, A.L. Allelopathic organisms and molecules: Promising bioregulators for the control of plant diseases, weeds, and other pests. In Allelochemicals: Biological Control of Plant Pathogens and Diseases; Inderjit, K.G., Muker, J.I., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 2, pp. 31–78. [Google Scholar]
- Huang, Y.; Xie, F.J.; Cao, X.; Li, M.Y. Research progress in biosynthesis and regulation of plant terpenoids. Biotechnol. Biotechnol. Equip. 2021, 35, 1799–1808. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Fujii, Y.; Parvez, S.S.; Parvez, M.M.; Ohmae, Y.; Iida, O. Screening of 239 medicinal plant species for allelopathic activity using the sandwich method. Weed Biol. Manag. 2003, 3, 233–241. [Google Scholar] [CrossRef]
- Mirmostafaee, S.; Azizi, M.; Fujii, Y. Study of allelopathic interaction of essential oils from medicinal and aromatic plants on seed germination and seedling growth of lettuce. Agronomy 2020, 10, 163. [Google Scholar] [CrossRef]
- Kyaw, E.H.; Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathic potential of Oldenlandia corymbosa and identification of its allelopathic substances. Plant Biosyst. 2024, 1–10. [Google Scholar] [CrossRef]
- Gupta, P. Allelopathic effect of extracts of medicinal plants on mung bean in vivo conditions. Curr. Agri. Res. 2016, 4, 120–124. [Google Scholar] [CrossRef]
- El-Darier, S.M.; Metwally, A.K. Allelopathic interaction between a medicinal plant; Achillea santolina L. and two associated soil algae species. Catrina—Int. J. Environ. Sci. 2020, 20, 1–8. [Google Scholar]
- Kato-Noguchi, H. Isolation and identification of allelochemicals and their activities and functions. J. Pestic. Sci. 2024, 49, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Salatino, M.L.F.; Negri, G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef]
- Aye, M.M.; Aung, H.T.; Sein, M.M.; Armijos, C. A review on the phytochemistry, medicinal properties and pharmacological activities of 15 selected Myanmar medicinal plants. Molecules 2019, 24, 293. [Google Scholar] [CrossRef] [PubMed]
- Ngamrojnavanich, N.; Sirimongkon, S.; Roengsumran, S.; Petsom, A.; Kamimura, H. Inhibition of Na+, K+-ATPase activity by (−)-ent-kaar-16-en-19-oic acid and its derivatives. Planta Medica 2003, 69, 555–556. [Google Scholar]
- Singh, M.; Pal, M.; Sharma, R.P. Biological activity of the labdane diterpenes. Planta Medica 1999, 65, 2–8. [Google Scholar] [CrossRef]
- Sommit, D.; Petsom, A.; Ishikawa, J.; Roengsumran, S. Cytotoxic activity of natural labdanes and their semi-synthetic modified derivatives from Croton oblongifolius. Planta Medica 2003, 69, 167–170. [Google Scholar] [CrossRef]
- Ahmed, B.; Alam, T.; Varshney, M.; Khan, S.A. Hepatoprotective activity of two plants belonging to the Apiaceae and the Euphorbiaceae family. J. Ethnopharmacol. 2002, 79, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Wijesekera, K. A bioactive diterpene; Nasimalum A from Croton oblongifolius Roxb. Prayog. Ras. 2017, 1, 41–44. [Google Scholar]
- Athikomkulchai, S.; Tadtong, S.; Ruangrungsi, N.; Hongratanaworakit, T. Chemical composition of the essential oils from Croton oblongifolius and its antibacterial activity against Propionibacterium acnes. Nat. Prod. Commun. 2015, 10, 1459–1460. [Google Scholar] [CrossRef]
- Youngsa-ad, W.; Ngamrojanavanich, N.; Mahidol, C.; Ruchirawat, S.; Prawat, H.; Kittakoop, P. Diterpenoids from the roots of Croton oblongifolius. Planta Medica 2007, 73, 1491–1494. [Google Scholar] [CrossRef]
- Roengsumran, S.; Petsom, A.; Sommit, D.; Vilaivan, T. Labdane diterpenoids from Croton oblongifolius. Phytochemistry 1999, 50, 449–453. [Google Scholar] [CrossRef]
- Aiyar, V.N.; Seshadri, T.R. Components of Croton oblongifolius—III constitution of oblongifolic acid. Tetrahedron 1970, 26, 5275–5279. [Google Scholar] [CrossRef]
- Moh, S.M.; Tojo, S.; Teruya, T.; Kato-Noguchi, H. Allelopathic activity of a novel compound, two known sesquiterpenes, and a C13 nor-isopenoid from the leave of Croton oblongifolius Roxb. for weed control. Plants 2023, 12, 3384. [Google Scholar] [CrossRef]
- Itokawa, H.; Morita, H.; Osawa, K.; Watanabe, K.; Iitaka, Y. Novel guaiane- and secoguaiane-type sesquiterpenes from Alpinia japonica (THUNB.) MIQ. Chem. Pharm. Bull. 1987, 35, 2849–2859. [Google Scholar] [CrossRef][Green Version]
- Cheng, A.X.; Lou, Y.G.; Mao, Y.B.; Lu, S.; Wang, L.J.; Chen, X.Y. Plant terpenoids: Biosynthesis and ecological functions. J. Integr. Plant Biol. 2007, 49, 179–186. [Google Scholar] [CrossRef]
- Ervin, G.N.; Wetzel, R.G. Allelochemical autotoxicity in the emergent wetland macrophyte juncus effusus (juncaceae). Am. J. Bot. 2000, 87, 853–860. [Google Scholar] [CrossRef]
- Ashour, M.; Wink, M.; Gershenzon, J. Biochemistry of terpenoids: Monoterpenese, sequiterpenes and diterpenes. Annu. Plant Rev. 2010, 40, 258–303. [Google Scholar]
- Nicol, R.W.; Yousef, L.; Traquair, J.A.; Bernards, M.A. Ginsenosides stimulate the growth of soilborne pathogens of American ginseng. Phytochemistry 2003, 64, 257–264. [Google Scholar] [CrossRef]
- Bari, I.N.; Kato-Noguchi, H.; Iwasaki, A.; Suenaga, K. Allelopathic potency and an active substance from Anredera cordifolia (Tenore) Steenis. Plants 2019, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalik, A.M.; Alshahrani, T.S.; Alqarawi, A.A.; Ahmed, E.M. Allelopathic potential of Nicotiana glauca aqueous extract on seed germination and seedlings of Acacia gerrardii. Diversity 2024, 16, 26. [Google Scholar] [CrossRef]
- Vashishth, D.S.; Bachheti, A.; Bachheti, R.K.; Husen, A. Allelopathic effect of Callistemon viminalis’s leaves extract on weeds, soil features, and growth performance of wheat and chickpea plants. J. Plant Interact. 2023, 18, 2248172. [Google Scholar] [CrossRef]
- Moh, S.M.; Tojo, S.; Teruya, T.; Kato-Noguchi, H. Allelopathy and identification of five allelochemicals in the leaves of the aromatic medicinal tree Aegle marmelos (L.) Correa. Plants 2024, 13, 559. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, L.; Masoni, A.; Pampan, S.; Arduini, I. Allelopathic effects of rye, brown mustard and hairy vetch on redroot pigweed, common lambsquarter and knotweed. Allelopathy J. 2007, 19, 249–256. [Google Scholar]
- Ladhari, A.; Omezzine, F.; Dellagreca, M.; Zarrelli, A.; Haouala, R. Phytotoxic activity of Capparis spinosa L. and its discovered active compounds. Allelopathy J. 2013, 32, 175–190. [Google Scholar]
- Frescura, V.D.; Kuhn, A.W.; Laughinghouse IV, H.D.; Nicoloso, F.T.; Lopes, S.J.; Tedesco, S.B. Evaluation of the allelopathic, genotoxic, and antiproliferative effect of the medicinal species Psychotria brachypoda and Psychotria birotula (Rubiaceae) on the germination and cell division of Eruca sativa (Brassicaceae). Caryologia 2013, 66, 138–144. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Ueno, A.; Ujiie, K.; Sato, S. Structures of sesquiterpenes from Curcuma aromatica SALISB. Chem. Pharm. Bull. 1987, 35, 53–59. [Google Scholar] [CrossRef]
- Itokawa, H.; Morita, H.; Kobayashi, T.; Watanabe, K.; Takase, A.; Iitaka, Y. Two new sesquiterpenoids (alpinolide and hanamyol) from Alpinia Japonica (Thunb.) Miq. Chem. Lett. 1984, 13, 1687–1690. [Google Scholar] [CrossRef]
- Gajger, T.I.; Dar, S.A. Plant allelochemicals as sources of insecticides. Insects 2021, 12, 189. [Google Scholar] [CrossRef]
- Sharma, A.; Bajpai, V.K.; Shukla, S. Sesquiterpenes and cytotoxicity. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3515–3550. [Google Scholar]
- Rasul, A.; Bao, R.; Malhi, M. Induction of apoptosis by costunolide in bladder cancer cells is mediated through ROS generation and mitochondrial dysfunction. Molecules 2013, 18, 1418–1433. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; Cai, Y.; Phillipson, J.D. Studies on the anti-tumour, anti-bacterial, and wound-healing properties of dragon’s blood. Planta Medica 1994, 60, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Lorigooini, Z.; Jamshidi-Kia, F.; Dodman, S. Chapter 8—Analysis of sesquiterpenes and sesquiterpenoids. In Recent Advances in Natural Products Analysis; Sanches, S.A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 289–312. [Google Scholar]
- Liu, Z.; Zhang, N.; Ma, X.; Zhang, T.; Li, X.; Tian, G.; Feng, Y.; An, T. Sesquiterpenes from Ambrosia artemisiifolia and their allelopathy. Front. Plant Sci. 2022, 13, 996498. [Google Scholar] [CrossRef] [PubMed]
- Anese, S.; Jatoba, L.J.; Grisi, P.U.; Gualtieri, S.C.J.; Santos, M.F.C.; Berlinck, R.G.S. Bioherbicidal activity of drimane sesquiterpenes from Drimys brasiliensis Miers roots. Ind. Crop. Prod. 2015, 74, 28–35. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Martin, V.J.J.; Pitera, D.J.; Withers, S.T.; Newman, J.D.; Keasling, J.D. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol. 2003, 21, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.A.; Galino, J.C.G.; Molinillo, J.M.G.; Castellano, D. Dehydrozaluzanin C: A potent plant growth regulator with potential use as a natural herbicide template. Phytochem. 2000, 54, 165–171. [Google Scholar] [CrossRef]
- DellaGreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Temussi, F.; Zarrelli, A. New dimeric phenanthrenoids from the rhizomes of Juncus acutus. Structure determination and antialgal activity. Tetrahedron 2003, 59, 2317–2324. [Google Scholar] [CrossRef]

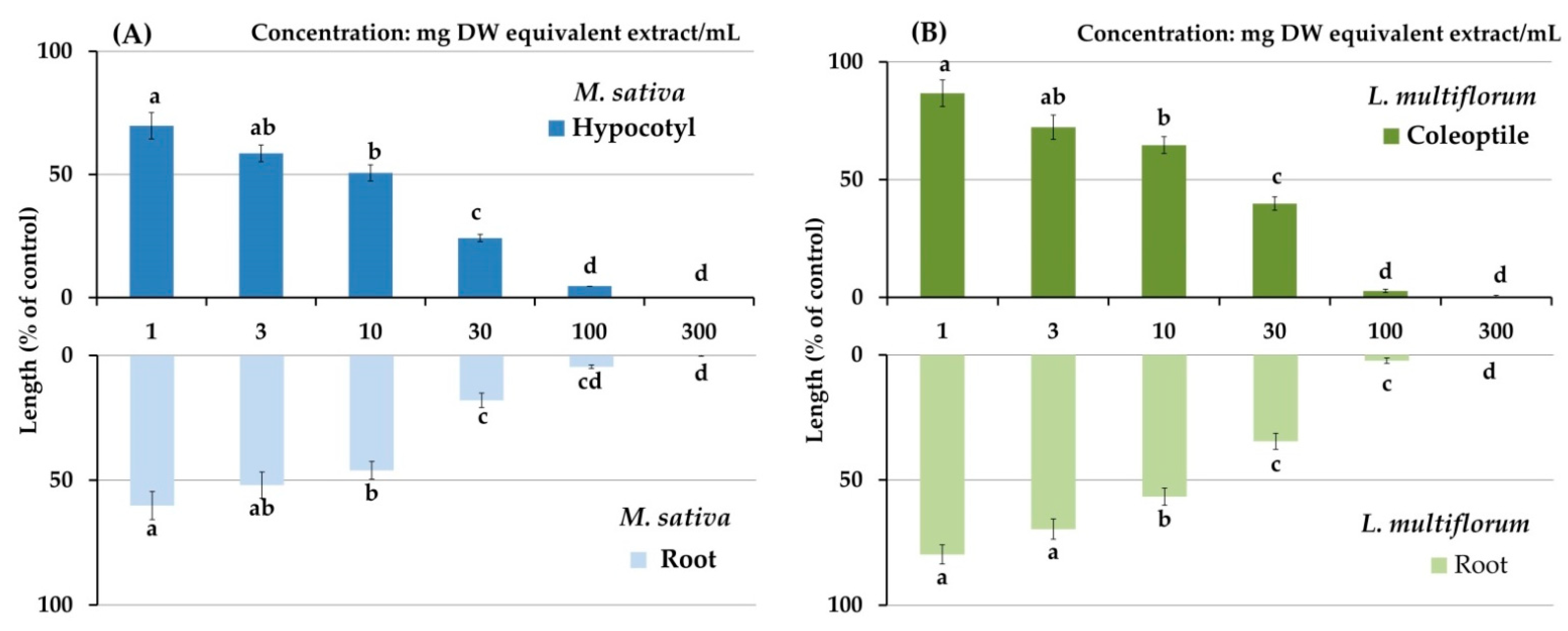
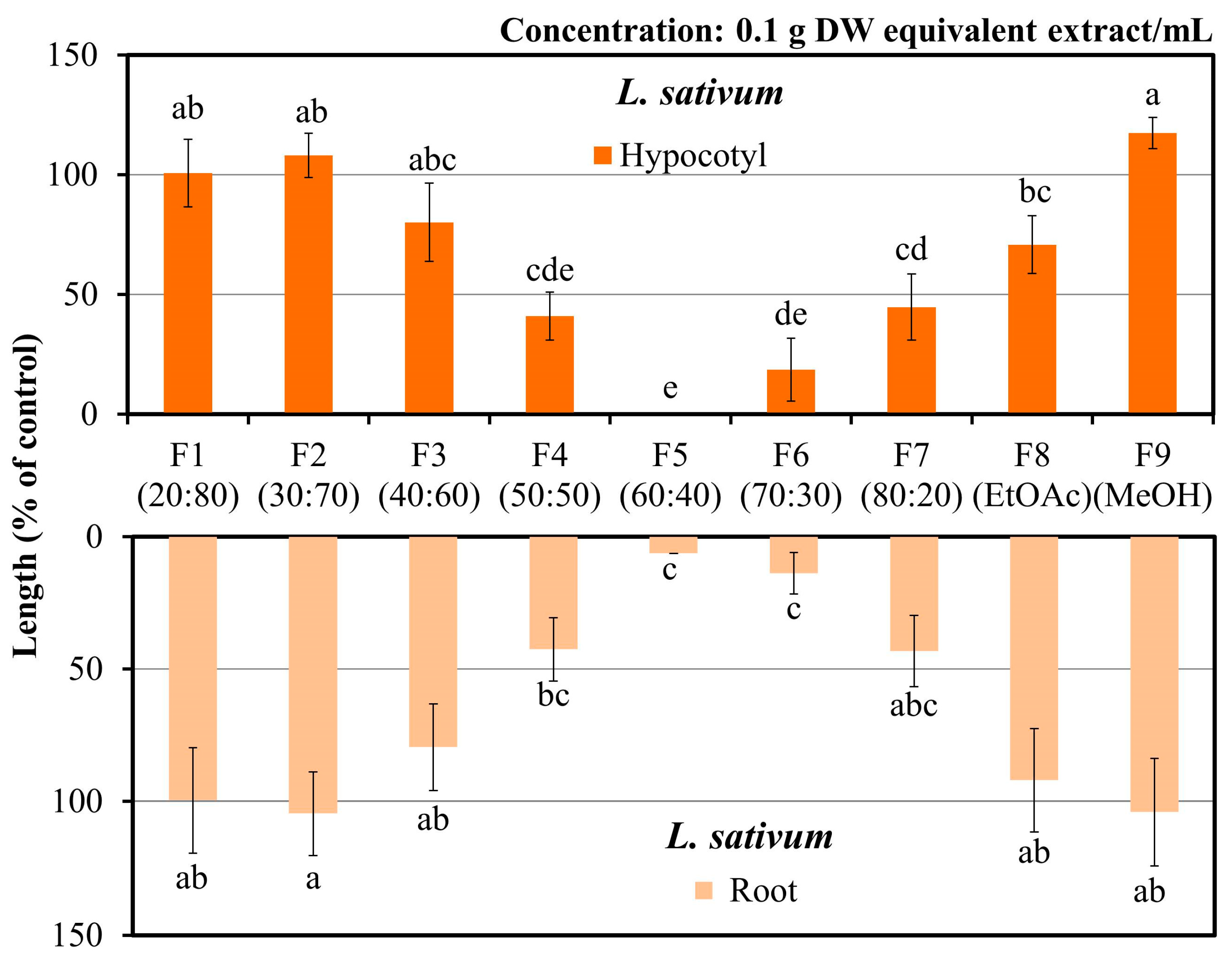
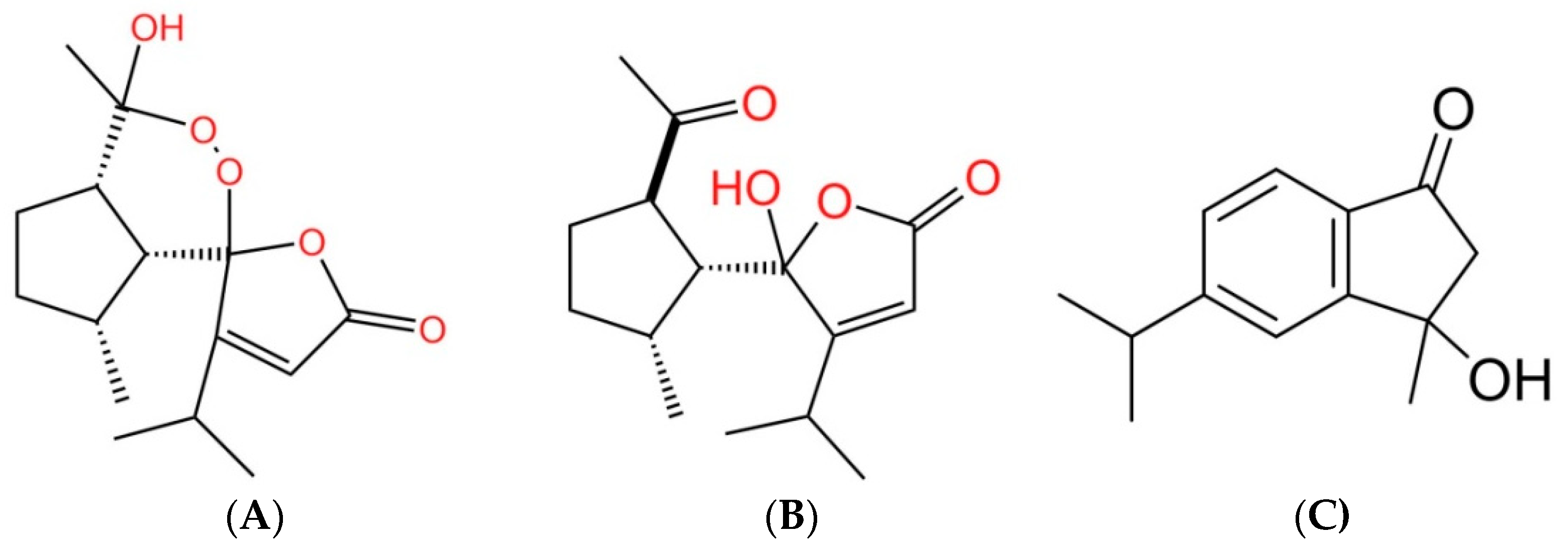
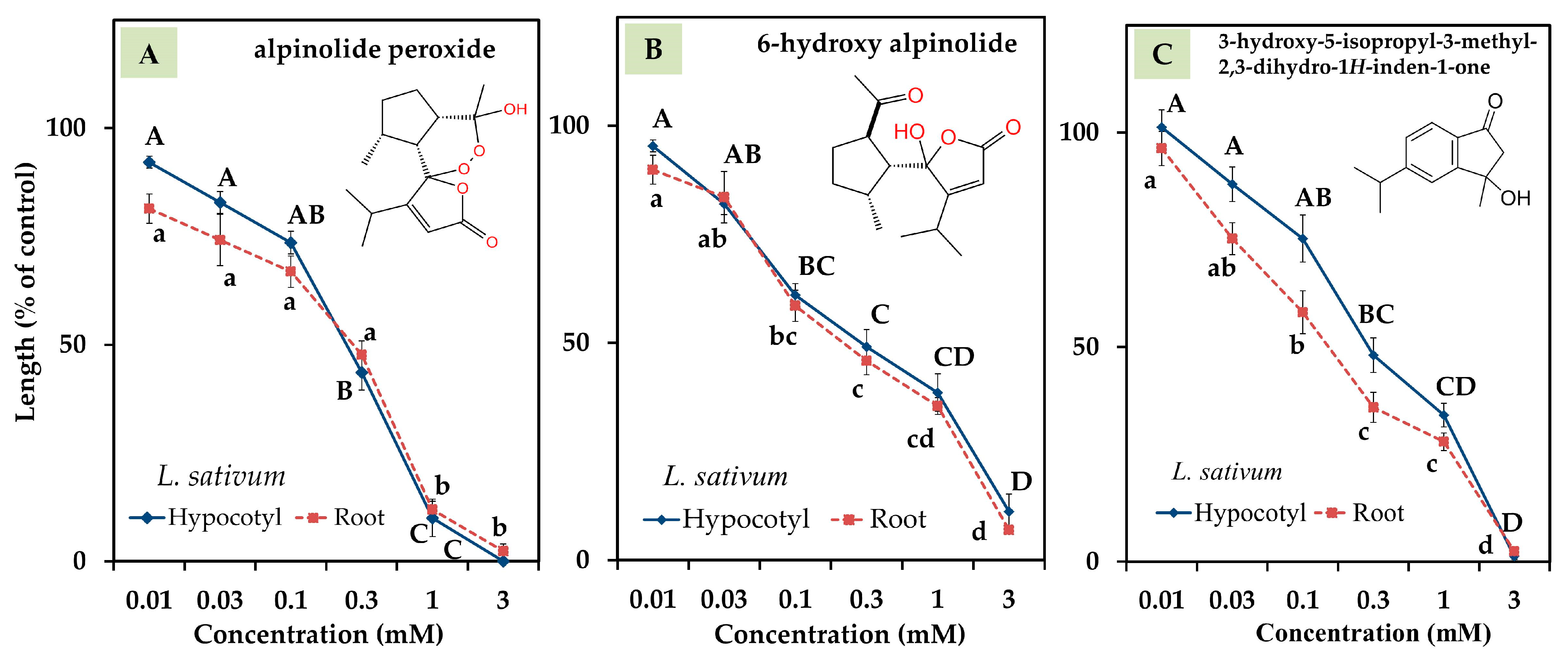
| Plant Species | IC50 (mg DW Equivalent C. oblongifolius Extract/mL) | ||
|---|---|---|---|
| Hypocotyl/Coleoptile | Root | ||
| Dicot | M. sativa | 5.44 | 3.23 |
| Monocot | L. multiflorum | 13.85 | 9.87 |
| Position | δH Mult (J in Hz) a | δC b |
|---|---|---|
| 1 | 202.6 | |
| 2 | 2.89, s | 54.2 |
| 3 | 74.5 | |
| 3a | 159.1 | |
| 4 | 7.52, d (1.4) | 121.1 |
| 5 | 157.8 | |
| 6 | 7.36, dd (8.0, 1.4) | 128.3 |
| 7 | 7.65, d (8.0) | 123.3 |
| 7a | 133.6 | |
| 8 | 1.72, s | 29.3 |
| 9 | 3.04, m | 34.9 |
| 10/11 | 1.31, d (6.9) | 23.8 |
| Identified Compound | IC50 (mM) | |
|---|---|---|
| L. sativum | ||
| Hypocotyl | Root | |
| alpinolide peroxide | 0.21 | 0.17 |
| 6-hydroxy alpinolide | 0.26 | 0.23 |
| 3-hydroxy-5-isopropyl-3-methyl-2,3-dihydro-1H-inden-1-one | 0.34 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moh, S.M.; Tojo, S.; Teruya, T.; Kato-Noguchi, H. Allelopathic Activity of a Novel Compound and Two Known Sesquiterpene from Croton oblongifolius Roxb. Agronomy 2024, 14, 695. https://doi.org/10.3390/agronomy14040695
Moh SM, Tojo S, Teruya T, Kato-Noguchi H. Allelopathic Activity of a Novel Compound and Two Known Sesquiterpene from Croton oblongifolius Roxb. Agronomy. 2024; 14(4):695. https://doi.org/10.3390/agronomy14040695
Chicago/Turabian StyleMoh, Seinn Moh, Shunya Tojo, Toshiaki Teruya, and Hisashi Kato-Noguchi. 2024. "Allelopathic Activity of a Novel Compound and Two Known Sesquiterpene from Croton oblongifolius Roxb." Agronomy 14, no. 4: 695. https://doi.org/10.3390/agronomy14040695
APA StyleMoh, S. M., Tojo, S., Teruya, T., & Kato-Noguchi, H. (2024). Allelopathic Activity of a Novel Compound and Two Known Sesquiterpene from Croton oblongifolius Roxb. Agronomy, 14(4), 695. https://doi.org/10.3390/agronomy14040695






