Impacts of Cropping Systems on Glyphosate and Aminomethylphosphonic Acid Contents and Microbial Community in Field Crop Soils in Quebec (Canada)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sites selection and Description
2.2. Soil Sampling
2.3. Analysis of Glyphosate and AMPA Contents
2.4. Soil Physico-Chemical Properties Analysis
2.5. DNA Extraction, Sequencing and Quantitative PCR of Bacterial and Fungal DNA
2.6. Downstream Data Analysis and Statistical Analysis
3. Results
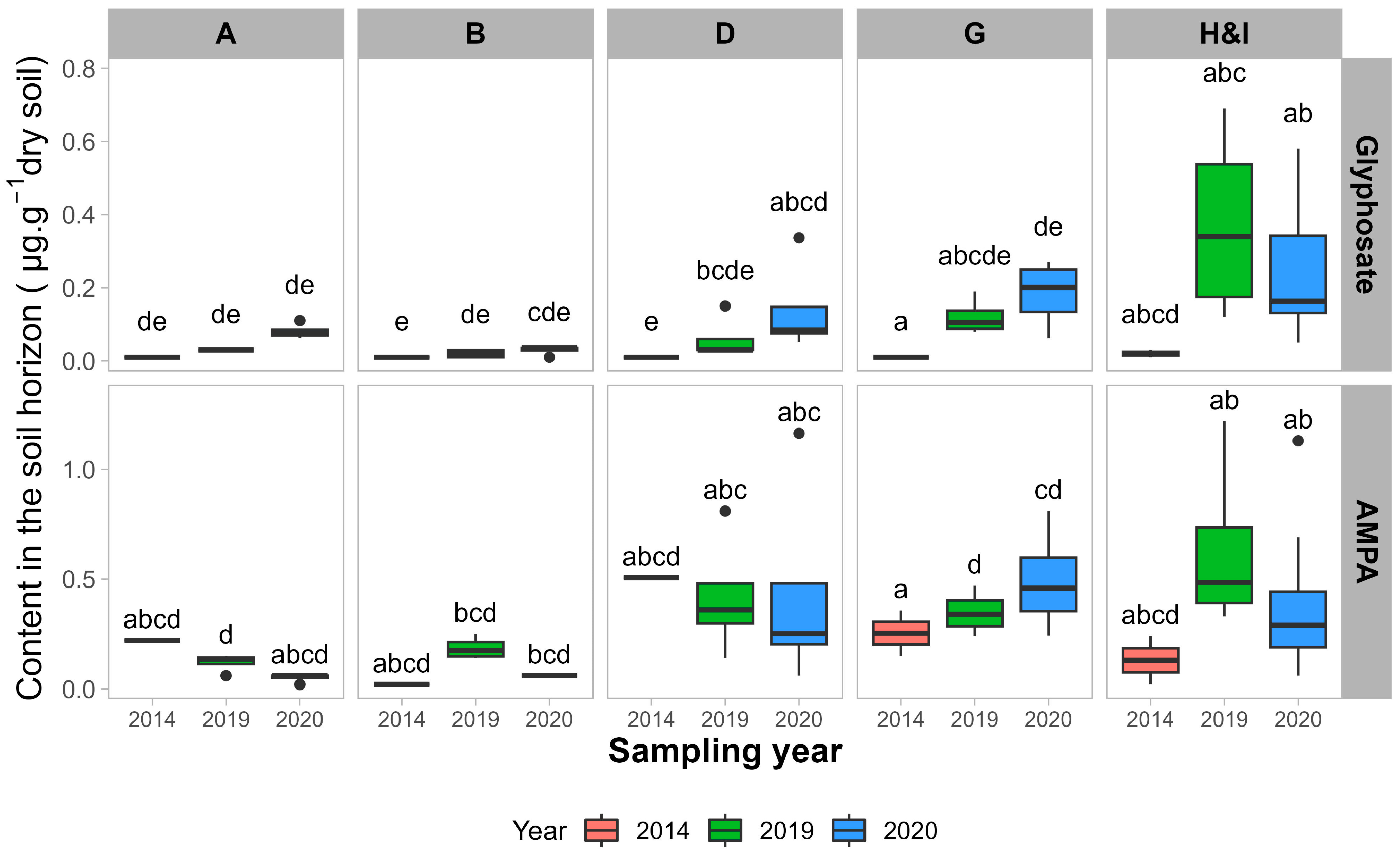
3.1. Glyphosate and AMPA Soil Contents
3.1.1. Impacts of Sampling Year and Sampling Depth
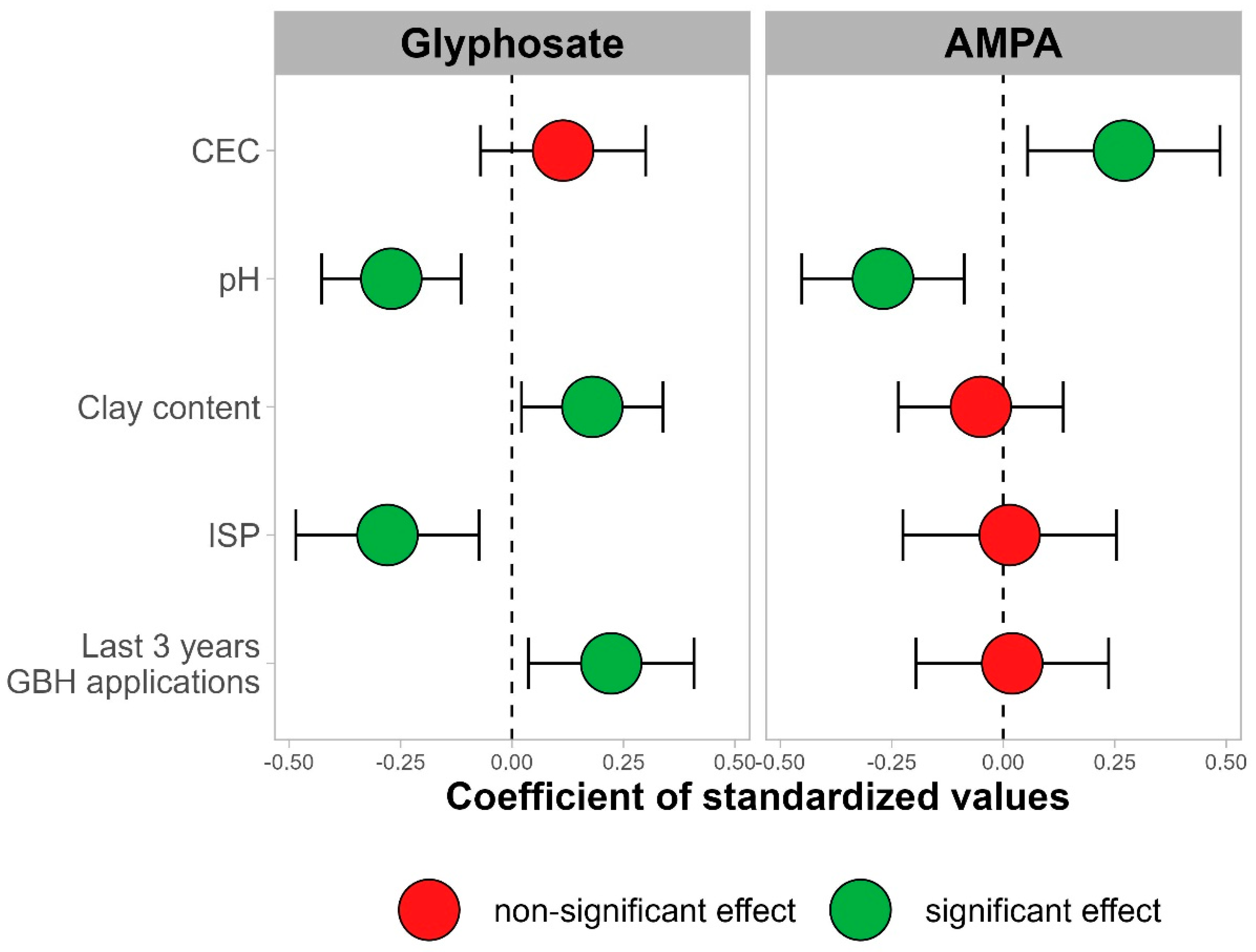
3.1.2. Impacts of Management Practices and Soil Properties
3.2. Soil Microbial Community
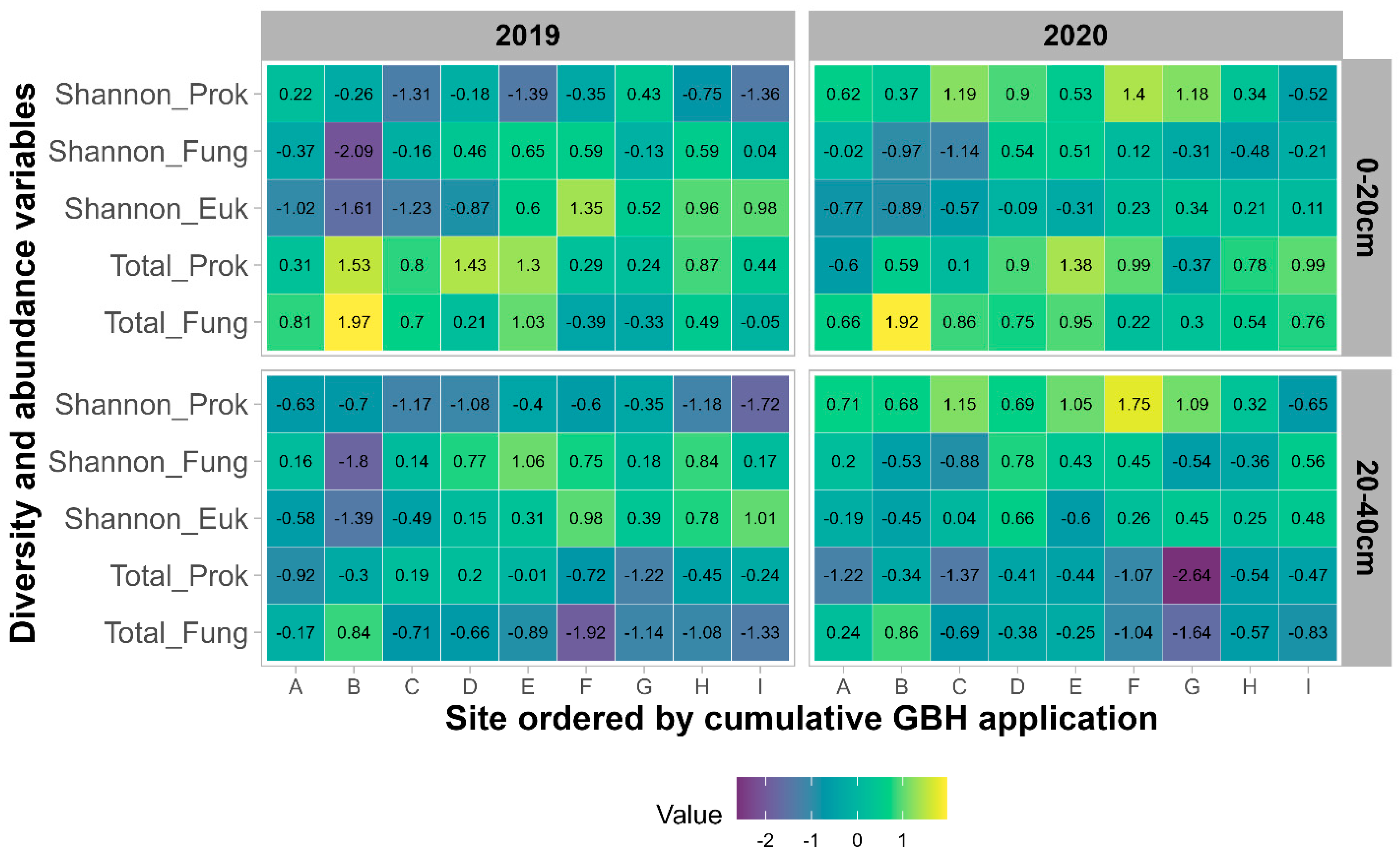
3.2.1. Alpha-Diversity and Abundance
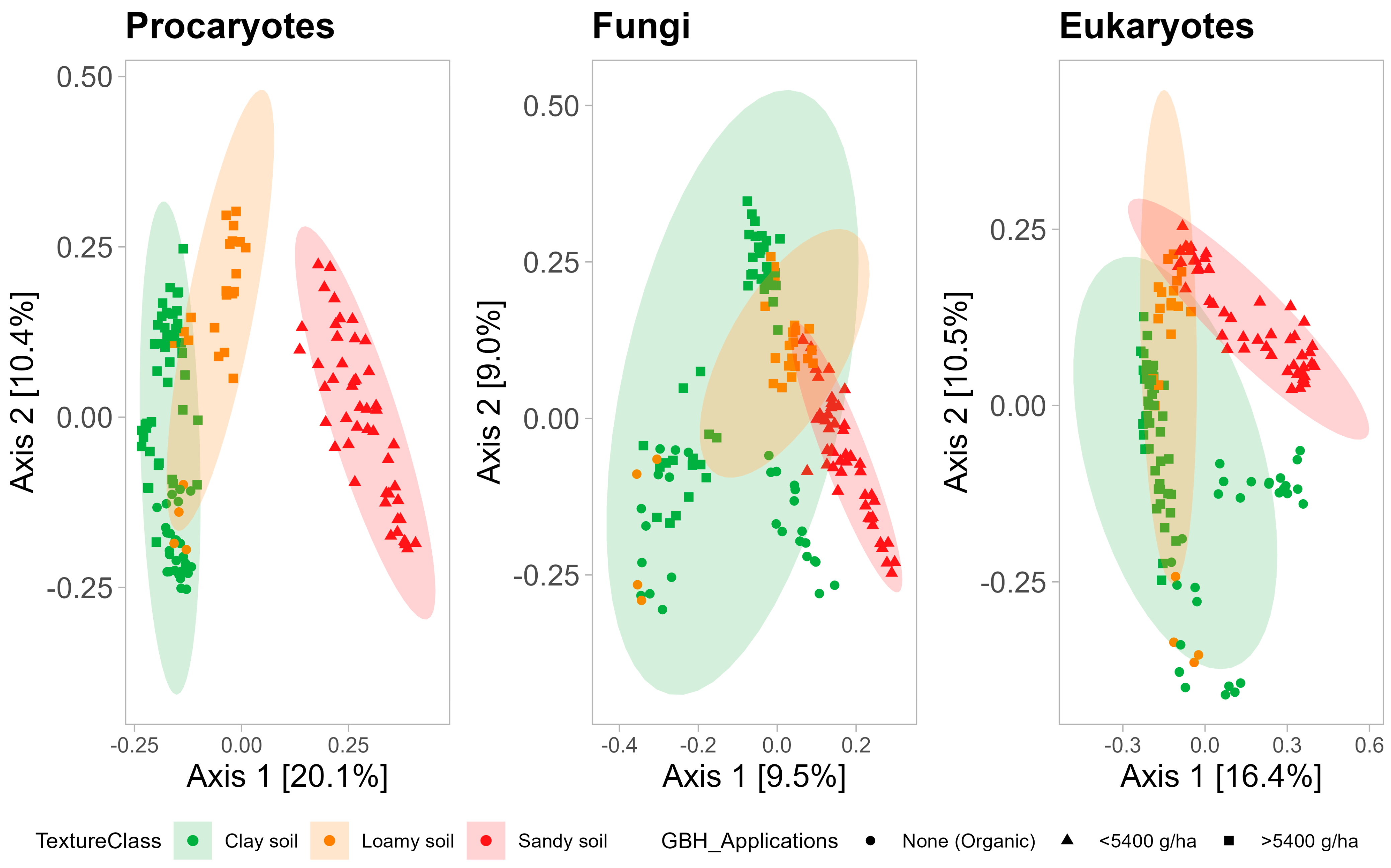
3.2.2. Beta-Diversity
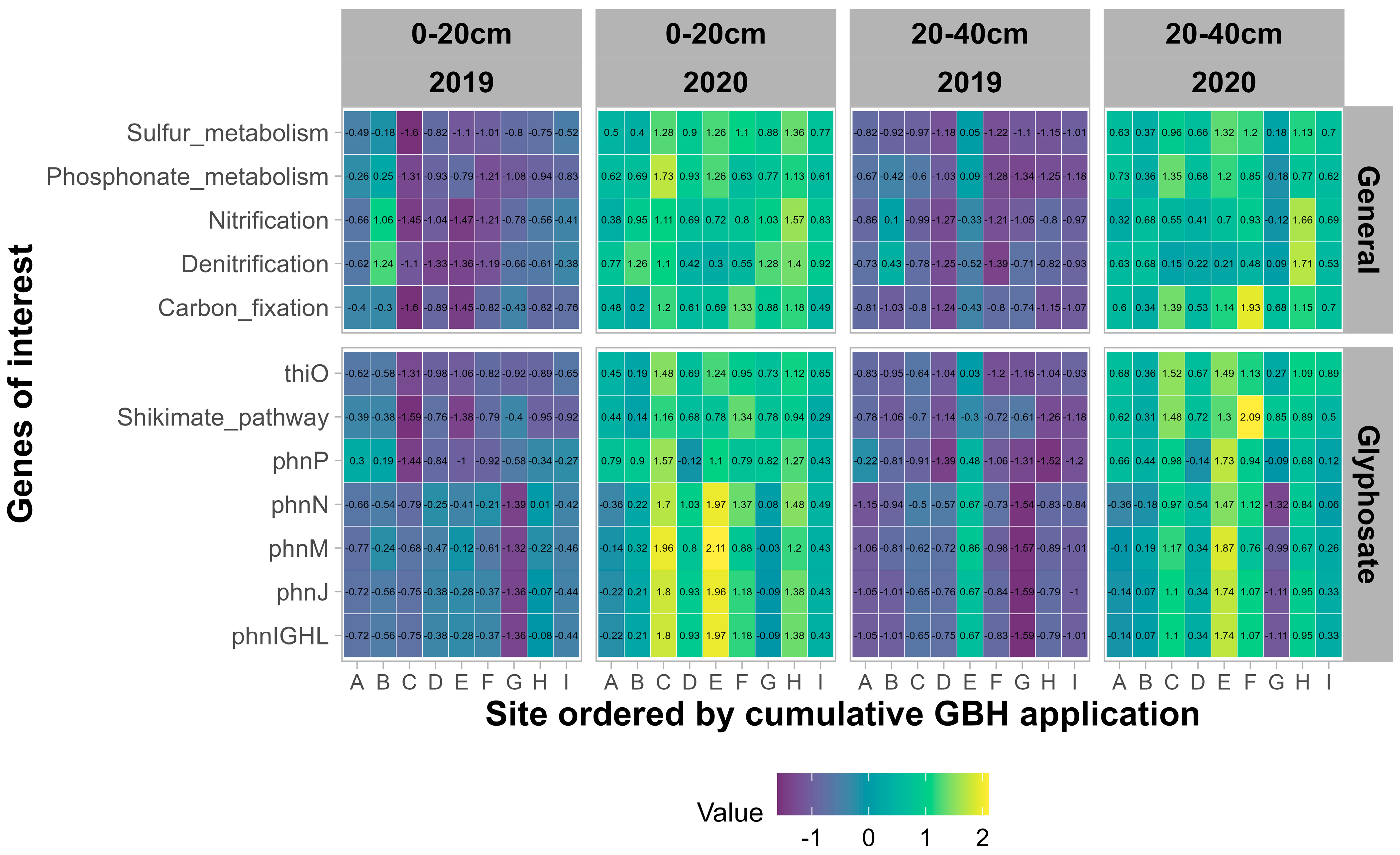
3.2.3. Functional Potential
4. Discussion
4.1. Pseudo-Persistence of Glyphosate and AMPA in Soils
4.2. Impacts of Soil Properties and Cropping Systems on Glyphosate and AMPA Contents
4.3. Impacts of Cropping Systems and Soil Properties on Soil Microbial Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novotny, E. Glyphosate, Roundup and the Failures of Regulatory Assessment. Toxics 2022, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Szekacs, A.; Darvas, B. Forty Years with Glyphosate. Herbic.-Prop. Synth. Control Weeds 2012, 14, 247–284. [Google Scholar]
- Ministère de l’Environnement, de la Lutte Contre les Changements Climatiques. Bilan des Ventes de Pesticides au Québec—Année 2021; Bibliothèque et Archives Nationales du Québec: Montréal, QC, Canada, 2023. [Google Scholar]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its Environmental Persistence and Impact on Crop Health and Nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Carretta, L.; Cardinali, A.; Onofri, A.; Masin, R.; Zanin, G. Dynamics of Glyphosate and Aminomethylphosphonic Acid in Soil Under Conventional and Conservation Tillage. Int. J. Environ. Res. 2021, 15, 1037–1055. [Google Scholar] [CrossRef]
- Motharasan, M.; Yunus; Shukor, Y.; Yasid, N.; Wan Johari, W.L.; Johari, W.; Aqlima, S. Environmental Fate and Degradation of Glyphosate in Soil. Pertanika J. Sch. Res. Rev. 2018, 4, 102–116. [Google Scholar]
- Dollinger, J.; Dagès, C.; Voltz, M. Glyphosate sorption to soils and sediments predicted by pedotransfer functions. Environ. Chem. Lett. 2015, 13, 293–307. [Google Scholar] [CrossRef]
- De Gerónimo, E.; Aparicio, V.C.; Costa, J.L. Glyphosate sorption to soils of Argentina. Estimation of affinity coefficient by pedotransfer function. Geoderma 2018, 322, 140–148. [Google Scholar] [CrossRef]
- Ascolani Yael, J.; Fuhr, J.D.; Bocan, G.A.; Daza Millone, A.; Tognalli, N.; dos Santos Afonso, M.; Martiarena, M.L. Abiotic Degradation of Glyphosate into Aminomethylphosphonic Acid in the Presence of Metals. J. Agric. Food Chem. 2014, 62, 9651–9656. [Google Scholar] [CrossRef]
- Papagiannaki, D.; Medana, C.; Binetti, R.; Calza, P.; Roslev, P. Effect of UV-A, UV-B and UV-C irradiation of glyphosate on photolysis and mitigation of aquatic toxicity. Sci. Rep. 2020, 10, 20247. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide Glyphosate: Toxicity and Microbial Degradation. Int. J. Environ. Res. Public Health 2020, 17, 7519. [Google Scholar] [CrossRef]
- Carretta, L.; Masin, R.; Zanin, G. Review of studies analysing glyphosate and aminomethylphosphonic acid (AMPA) occurrence in groundwater. Environ. Rev. 2021, 30, 88–109. [Google Scholar] [CrossRef]
- Tang, F.H.M.; Jeffries, T.C.; Vervoort, R.W.; Conoley, C.; Coleman, N.V.; Maggi, F. Microcosm experiments and kinetic modeling of glyphosate biodegradation in soils and sediments. Sci. Total Environ. 2019, 658, 105–115. [Google Scholar] [CrossRef]
- Wimmer, B.; Langarica-Fuentes, A.; Schwarz, E.; Kleindienst, S.; Huhn, C.; Pagel, H. Mechanistic modeling indicates rapid glyphosate dissipation and sorption-driven persistence of its metabolite AMPA in soil. J. Environ. Qual. 2023, 52, 393–405. [Google Scholar] [CrossRef]
- Blake, R.; Pallett, K. The Environmental Fate and Ecotoxicity of Glyphosate. Outlooks Pest Manag. 2018, 29, 266–269. [Google Scholar] [CrossRef]
- von Mérey, G.; Manson, P.S.; Mehrsheikh, A.; Sutton, P.; Levine, S.L. Glyphosate and aminomethylphosphonic acid chronic risk assessment for soil biota. Environ. Toxicol. Chem. 2016, 35, 2742–2752. [Google Scholar] [CrossRef]
- Kim, Y.J.; Nitin, N.; Kim, K.B. Negligible Toxicokinetic Differences of Glyphosate by Different Vehicles in Rats. Toxics 2023, 11, 67. [Google Scholar] [CrossRef]
- Kassam, A.; Friedrich, T.; Derpsch, R. Global spread of Conservation Agriculture. Int. J. Environ. Stud. 2019, 76, 29–51. [Google Scholar] [CrossRef]
- FAO. Top 5 Need-to-Knows about Conservation Agriculture. Available online: http://www.fao.org/zhc/detail-events/en/c/238478/ (accessed on 27 February 2024).
- Bilodeau, L.; Marcoux, A.; Miville, D.; Fréchette, I.; Samson, V. Désherbage du Maïs de Grandes Cultures. 2020. Available online: https://www.agrireseau.net/documents/Document_100450.pdf (accessed on 20 February 2024).
- Kumar, V.; Mahajan, G.; Dahiya, S.; Chauhan, B.S. Challenges and Opportunities for Weed Management in No-Till Farming Systems. In No-Till Farming Systems for Sustainable Agriculture: Challenges and Opportunities; Dang, Y.P., Dalal, R.C., Menzies, N.W., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 107–125. [Google Scholar]
- Giroux, I. Présence de Pesticides dans l’Eau au Québec: Portrait et Tendances dans les Zones de Maïs et de Soya—2018 à 2020; Ministère de l’Environnement et de la Lutte Contre les Changements Climatiques, Direction de la Qualité des Milieux Aquatiques: Québec, QC, Canada, 2022. [Google Scholar]
- Maccario, S.; Lucotte, M.; Moingt, M.; Samson-Brais, É.; Smedbol, É.; Labrecque, M. Impact of Soil Characteristics and Weed Management Practices on Glyphosate and AMPA Persistence in Field Crops Soils from the St. Lawrence Lowlands (Quebec, Canada). Agronomy 2022, 12, 992. [Google Scholar] [CrossRef]
- Nkongolo, K.K.; Narendrula-Kotha, R. Advances in monitoring soil microbial community dynamic and function. J. Appl. Genet. 2020, 61, 249–263. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Mills, D.A. Improved selection of internal transcribed spacer-specific primers enables quantitative, ultra-high-throughput profiling of fungal communities. Appl. Environ. Microbiol. 2013, 79, 2519–2526. [Google Scholar] [CrossRef]
- Comeau, A.M.; Li, W.K.W.; Tremblay, J.-É.; Carmack, E.C.; Lovejoy, C. Arctic Ocean Microbial Community Structure before and after the 2007 Record Sea Ice Minimum. PLoS ONE 2011, 6, e27492. [Google Scholar] [CrossRef]
- Emerson, J.B.; Thomas, B.C.; Alvarez, W.; Banfield, J.F. Metagenomic analysis of a high carbon dioxide subsurface microbial community populated by chemolithoautotrophs and bacteria and archaea from candidate phyla. Environ. Microbiol. 2016, 18, 1686–1703. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; González-López, J.; Vallejo, A.; Bedmar, E.J. Effect of urease and nitrification inhibitors on ammonia volatilization and abundance of N-cycling genes in an agricultural soil. J. Plant Nutr. Soil Sci. 2020, 183, 99–109. [Google Scholar] [CrossRef]
- Morales, M.E.; Allegrini, M.; Basualdo, J.; Villamil, M.B.; Zabaloy, M.C. Primer design to assess bacterial degradation of glyphosate and other phosphonates. J. Microbiol. Methods 2020, 169, 105814. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Kristiansson, E.; Ryberg, M.; Hallenberg, N.; Larsson, K.H. Intraspecific ITS variability in the kingdom fungi as expressed in the international sequence databases and its implications for molecular species identification. Evol. Bioinform. Online 2008, 4, 193–201. [Google Scholar] [CrossRef]
- Kepler, R.M.; Epp Schmidt, D.J.; Yarwood, S.A.; Cavigelli, M.A.; Reddy, K.N.; Duke, S.O.; Bradley, C.A.; Williams, M.M.; Buyer, J.S.; Maul, J.E. Soil Microbial Communities in Diverse Agroecosystems Exposed to the Herbicide Glyphosate. Appl. Environ. Microbiol. 2020, 86, e01744-19. [Google Scholar] [CrossRef]
- Dennis, P.G.; Kukulies, T.; Forstner, C.; Orton, T.G.; Pattison, A.B. The effects of glyphosate, glufosinate, paraquat and paraquat-diquat on soil microbial activity and bacterial, archaeal and nematode diversity. Sci. Rep. 2018, 8, 2119. [Google Scholar] [CrossRef]
- Weaver, M.A.; Krutz, L.J.; Zablotowicz, R.M.; Reddy, K.N. Effects of glyphosate on soil microbial communities and its mineralization in a Mississippi soil. Pest Manag. Sci. 2007, 63, 388–393. [Google Scholar] [CrossRef]
- Guijarro, K.H.; Aparicio, V.; De Gerónimo, E.; Castellote, M.; Figuerola, E.L.; Costa, J.L.; Erijman, L. Soil microbial communities and glyphosate decay in soils with different herbicide application history. Sci. Total Environ. 2018, 634, 974–982. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Larney, F.J.; Blackshaw, R.E.; Kanashiro, D.A.; Pearson, D.C.; Petri, R.M. Pyrosequencing reveals profiles of soil bacterial communities after 12 years of conservation management on irrigated crop rotations. Appl. Soil Ecol. 2017, 121, 65–73. [Google Scholar] [CrossRef]
- Schmidt, R.; Gravuer, K.; Bossange, A.V.; Mitchell, J.; Scow, K. Long-term use of cover crops and no-till shift soil microbial community life strategies in agricultural soil. PLoS ONE 2018, 13, e0192953. [Google Scholar] [CrossRef]
- Giusti, B.; Hogue, R.; Jeanne, T.; Lucotte, M. Impacts of winter wheat and cover crops on soil microbial diversity in a corn–soybean no-till cropping system in Quebec (Canada). Agrosyst. Geosci. Environ. 2023, 6, e20349. [Google Scholar] [CrossRef]
- Samson-Brais, É.; Lucotte, M.; Moingt, M.; Tremblay, G.; Paquet, S. Glyphosate and aminomethylphosphonic acid contents in field crops soils under various weed management practices. Agrosyst. Geosci. Environ. 2022, 5, e20273. [Google Scholar] [CrossRef]
- Alferness, P.L.; Iwata, Y. Determination of Glyphosate and (Aminomethyl)phosphonic Acid in Soil, Plant and Animal Matrixes, and Water by Capillary Gas Chromatography with Mass-Selective Detection. J. Agric. Food Chem. 1994, 42, 2751–2759. [Google Scholar] [CrossRef]
- Conseil des Productions Végétales du Québec. Méthode MA-2; Ministère de l’Agriculture, des Pêcheries et de l’Alimentation (Québec): Québec, QC, Canada, 1993. [Google Scholar]
- Centre d’Expertise en Analyse Environnementale du Québec. Détermination du pH: Méthode Électrométrique; Ministère de l’Environnement, de la Lutte contre les changements climatiques, de la Faune et des Parcs: Québec, QC, Canada, 2014; Volume 3. [Google Scholar]
- Shoemaker, H.E.; McLean, E.O.; Pratt, P.F. Buffer Methods for Determining Lime Requirement of Soils With Appreciable Amounts of Extractable Aluminum. Soil Sci. Soc. Am. J. 1961, 25, 274–277. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Culman, S.; Mann, M.; Brown, C. Calculating Cation Exchange Capacity, Base Saturation, and Calcium Saturation. 2019. Available online: https://ohioline.osu.edu/factsheet/anr-81 (accessed on 19 February 2024).
- CRAAQ. Fertilization Reference Guide, 2nd ed.; CRAAQ: Québec, QC, Canada, 2010. (In French) [Google Scholar]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef]
- Jeanne, T.; Parent, S.-É.; Hogue, R. Using a soil bacterial species balance index to estimate potato crop productivity. PLoS ONE 2019, 14, e0214089. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef]
- Overbeek, W.; Jeanne, T.; Hogue, R.; Smith, D.L. Effects of Microbial Consortia, Applied as Fertilizer Coating, on Soil and Rhizosphere Microbial Communities and Potato Yield. Front. Agron. 2021, 3, 714700. [Google Scholar] [CrossRef]
- Jeanne, T.; D’Astous-Pagé, J.; Hogue, R. Spatial, temporal and technical variability in the diversity of prokaryotes and fungi in agricultural soils. Front. Soil Sci. 2022, 2, 945888. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Ferrer, L.; Foerster, H.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016, 44, D471–D480. [Google Scholar] [CrossRef]
- Hernández Guijarro, K.; De Gerónimo, E.; Erijman, L. Glyphosate Biodegradation Potential in Soil Based on Glycine Oxidase Gene (thiO) from Bradyrhizobium. Curr. Microbiol. 2021, 78, 1991–2000. [Google Scholar] [CrossRef]
- Hove-Jensen, B.; Zechel, D.L.; Jochimsen, B. Utilization of Glyphosate as Phosphate Source: Biochemistry and Genetics of Bacterial Carbon-Phosphorus Lyase. Microbiol. Mol. Biol. Rev. 2014, 78, 176–197. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- McMurdie, P.J.; Holmes, S. Waste Not, Want Not: Why Rarefying Microbiome Data Is Inadmissible. PLoS Comput. Biol. 2014, 10, e1003531. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Vegan: Community Ecology Package. 2019. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 14 February 2024).
- Daughton Christian, G. Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. I. Rationale for and avenues toward a green pharmacy. Environ. Health Perspect. 2003, 111, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Primost, J.; Marino, D.; Aparicio, V.; Costa, J.; Carriquiriborde, P. Glyphosate and AMPA, “pseudo-persistent” pollutants under real-world agricultural management practices in the Mesopotamic Pampas agroecosystem, Argentina. Environ. Pollut. 2017, 229, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Saikkonen, K.; Helander, M.; Tian, Y.; Yang, B.; Engström, M.T.; Salminen, J.-P.; Muola, A. Legacy of agrochemicals in the circular food economy: Glyphosate-based herbicides introduced via manure fertilizer affect the yield and biochemistry of perennial crop plants during the following year. Chemosphere 2022, 308, 136366. [Google Scholar] [CrossRef] [PubMed]
- Giard, F.; Lucotte, M.; Moingt, M.; Gaspar, A. Glyphosate and aminomethyphosphonic (AMPA) contents in Brazilian field crops soils. Agron. Sci. Biotechnol. 2022, 8, 1–18. [Google Scholar] [CrossRef]
- Silva, V.; Montanarella, L.; Jones, A.; Fernández-Ugalde, O.; Mol, H.G.J.; Ritsema, C.J.; Geissen, V. Distribution of glyphosate and aminomethylphosphonic acid (AMPA) in agricultural topsoils of the European Union. Sci. Total Environ. 2018, 621, 1352–1359. [Google Scholar] [CrossRef]
- Mamy, L.; Barriuso, E.; Gabrielle, B. Glyphosate fate in soils when arriving in plant residues. Chemosphere 2016, 154, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Mencaroni, M.; Cardinali, A.; Costa, L.; Morari, F.; Salandin, P.; Zanin, G.; Dal Ferro, N. Glyphosate and AMPA have low mobility through different soil profiles of the prosecco wine production area: A monitoring study in north-eastern Italy. Front. Environ. Sci. 2022, 10, 971931. [Google Scholar] [CrossRef]
- Vereecken, H. Mobility and leaching of glyphosate: A review. Pest Manag. Sci. 2005, 61, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Cassigneul, A.; Benoit, P.; Bergheaud, V.; Dumeny, V.; Etiévant, V.; Goubard, Y.; Maylin, A.; Justes, E.; Alletto, L. Fate of glyphosate and degradates in cover crop residues and underlying soil: A laboratory study. Sci. Total Environ. 2016, 545–546, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Iqbal, A.; Lafolie, F.; Recous, S.; Benoit, P.; Garnier, P. Mulch of plant residues at the soil surface impact the leaching and persistence of pesticides: A modelling study from soil columns. J. Contam. Hydrol. 2018, 214, 54–64. [Google Scholar] [CrossRef]
- Aslam, S.; Jing, Y.; Nowak, K.M. Fate of glyphosate and its degradation products AMPA, glycine and sarcosine in an agricultural soil: Implications for environmental risk assessment. J. Hazard. Mater. 2023, 447, 130847. [Google Scholar] [CrossRef] [PubMed]
- Samson-Brais, É.; Lucotte, M.; Moingt, M.; Tremblay, G.; Paquet, S. Impact of weed management practices on soil biological activity in corn and soybean field crops in Québec (Canada). Can. J. Soil Sci. 2020, 101, 12–21. [Google Scholar] [CrossRef]
- Lane, M.; Lorenz, N.; Saxena, J.; Ramsier, C.; Dick, R. The effect of glyphosate on soil microbial activity, microbial community structure, and soil potassium. Pedobiologia 2012, 55, 335–342. [Google Scholar] [CrossRef]
- McConnell, J.S.; Hossner, L.R. pH-Dependent adsorption isotherms of glyphosate. J. Agric. Food Chem. 1985, 33, 1075–1078. [Google Scholar] [CrossRef]
- de Jonge, H.; Wollesen de Jonge, L. Influence of pH and solution composition on the sorption of glyphosate and prochloraz to a sandy loam soil. Chemosphere 1999, 39, 753–763. [Google Scholar] [CrossRef]
- Weber, J.B.; Wilkerson, G.G.; Reinhardt, C.F. Calculating pesticide sorption coefficients (Kd) using selected soil properties. Chemosphere 2004, 55, 157–166. [Google Scholar] [CrossRef]
- Kumari, K.G.I.D.; Moldrup, P.; Paradelo, M.; Elsgaard, L.; de Jonge, L.W. Soil Properties Control Glyphosate Sorption in Soils Amended with Birch Wood Biochar. Water Air Soil Pollut. 2016, 227, 174. [Google Scholar] [CrossRef]
- De Gerónimo, E.; Aparicio, V.C. Changes in soil pH and addition of inorganic phosphate affect glyphosate adsorption in agricultural soil. Eur. J. Soil Sci. 2022, 73, e13188. [Google Scholar] [CrossRef]
- Sidoli, P.; Baran, N.; Angulo-Jaramillo, R. Glyphosate and AMPA adsorption in soils: Laboratory experiments and pedotransfer rules. Environ. Sci. Pollut. Res. 2016, 23, 5733–5742. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, D.; Liang, S.; Dang, P.; Qin, X.; Liao, Y.; Siddique, K.H.M. Effect of no-tillage on soil bacterial and fungal community diversity: A meta-analysis. Soil Tillage Res. 2020, 204, 104721. [Google Scholar] [CrossRef]
- Sun, R.; Li, W.; Dong, W.; Tian, Y.; Hu, C.; Liu, B. Tillage Changes Vertical Distribution of Soil Bacterial and Fungal Communities. Front. Microbiol. 2018, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Li, H.; Li, M. Effect of No Tillage System on Soil Fungal Community Structure of Cropland in Mollisol: A Case Study. Front. Microbiol. 2022, 13, 847691. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Lu, X.; Liao, Y. Effect of Tillage Treatment on the Diversity of Soil Arbuscular Mycorrhizal Fungal and Soil Aggregate-Associated Carbon Content. Front. Microbiol. 2018, 9, 410634. [Google Scholar] [CrossRef]
- Liu, W.; Ma, K.; Wang, X.; Wang, Z.; Negrete-Yankelevich, S. Effects of no-tillage and biologically-based organic fertilizer on soil arbuscular mycorrhizal fungal communities in winter wheat field. Appl. Soil Ecol. 2022, 178, 104564. [Google Scholar] [CrossRef]
- Zhang, W.-w.; Wang, C.; Xue, R.; Wang, L.-j. Effects of salinity on the soil microbial community and soil fertility. J. Integr. Agric. 2019, 18, 1360–1368. [Google Scholar] [CrossRef]
- Rath, K.M.; Rousk, J. Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: A review. Soil Biol. Biochem. 2015, 81, 108–123. [Google Scholar] [CrossRef]
- Obayomi, O.; Seyoum, M.M.; Ghazaryan, L.; Tebbe, C.C.; Murase, J.; Bernstein, N.; Gillor, O. Soil texture and properties rather than irrigation water type shape the diversity and composition of soil microbial communities. Appl. Soil Ecol. 2021, 161, 103834. [Google Scholar] [CrossRef]
- Xia, Q.; Rufty, T.; Shi, W. Soil microbial diversity and composition: Links to soil texture and associated properties. Soil Biol. Biochem. 2020, 149, 107953. [Google Scholar] [CrossRef]
- Mann, C.; Lynch, D.; Fillmore, S.; Mills, A. Relationships between field management, soil health, and microbial community composition. Appl. Soil Ecol. 2019, 144, 12–21. [Google Scholar] [CrossRef]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.-J. The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]
- Guo, Z.; Wan, S.; Hua, K.; Yin, Y.; Chu, H.; Wang, D.; Guo, X. Fertilization regime has a greater effect on soil microbial community structure than crop rotation and growth stage in an agroecosystem. Appl. Soil Ecol. 2020, 149, 103510. [Google Scholar] [CrossRef]
- Kracmarova, M.; Uhlik, O.; Strejcek, M.; Szakova, J.; Cerny, J.; Balik, J.; Tlustos, P.; Kohout, P.; Demnerova, K.; Stiborova, H. Soil microbial communities following 20 years of fertilization and crop rotation practices in the Czech Republic. Environ. Microbiome 2022, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Town, J.R.; Dumonceaux, T.; Tidemann, B.; Helgason, B.L. Crop rotation significantly influences the composition of soil, rhizosphere, and root microbiota in canola (Brassica napus L.). Environ. Microbiome 2023, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.M.; Powell, J.R.; Gulden, R.H.; Dunfield, K.E.; Peter Pauls, K.; Swanton, C.J.; Klironomos, J.N.; Antunes, P.M.; Koch, A.M.; Trevors, J.T. Separating the effect of crop from herbicide on soil microbial communities in glyphosate-resistant corn. Pedobiologia 2009, 52, 253–262. [Google Scholar] [CrossRef]
- Daniel, C.S.; Yin, C.; Hulbert, S.; Burke, I.; Paulitz, T. Impacts of Repeated Glyphosate Use on Wheat-Associated Bacteria Are Small and Depend on Glyphosate Use History. Appl. Environ. Microbiol. 2017, 83, e01354-17. [Google Scholar] [CrossRef]

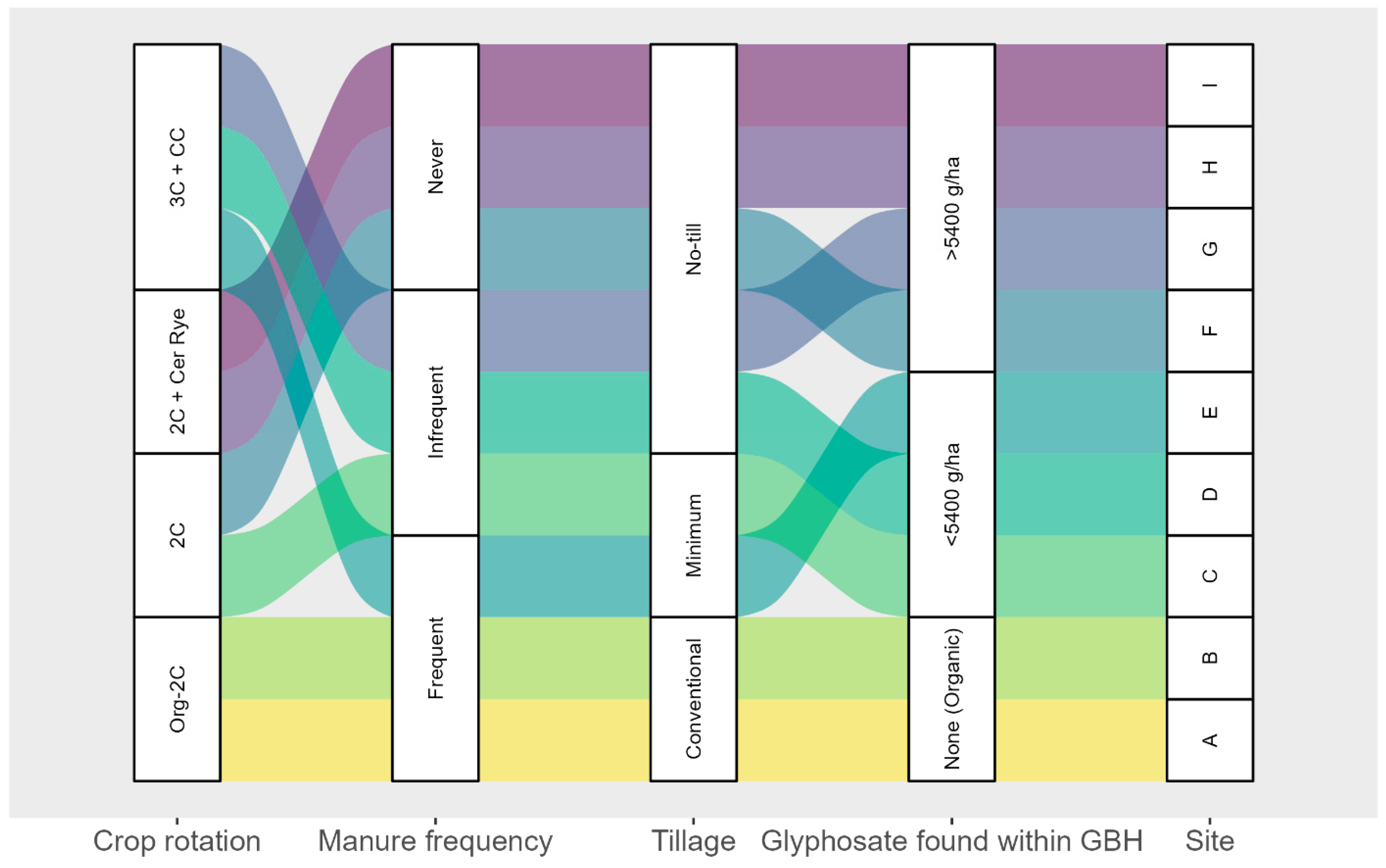
| Sites | GBH Group: Cumulative Applications 2017–2020 (g·ha−1) | GBH 2019 Application (g·ha−1) | Cumulative GBH Applications 2017–2019 (g·ha−1) | GBH 2020 Application (g·ha−1) | Cumulative GBH Applications 2018–2020 (g·ha−1) |
|---|---|---|---|---|---|
| A | None | 0 | 0 | 0 | 0 |
| B | None | 0 | 0 | 0 | 0 |
| C | <5400 | 432 | 793.8 | 1350 | 2144 |
| D | <5400 | 378 | 1998 | 1836 | 2916 |
| E | <5400 | 999 | 2673 | 2160 | 3159 |
| F | >5400 | 1080 | 4590 | 810 | 2430 |
| G | >5400 | 1950 | 5010 | 1500 | 6510 |
| H | >5400 | 2700 | 7182 | 2430 | 6912 |
| I | >5400 | 2700 | 8100 | 2430 | 7830 |
| Sites | Sand % | Silt % | Clay % | OM % | pH | ISP (%) | CEC (meq/100 g) | Na (ppm) |
|---|---|---|---|---|---|---|---|---|
| A | 3.50 ± 2.67 | 50.25 ± 2.31 ab | 46.25 ± 2.31 | 4.72 ± 0.45 ab | 6.71 ± 0.11 a | 4.10 ± 0.94 c | 18.94 ± 1.24 a | 23.74 ± 3.92 a |
| B | 36.25 ± 9.08 | 20.75 ± 4.3 ab | 43.00 ± 6.8 | 4.68 ± 0.58 ab | 6.50 ± 0.16 ab | 6.46 ± 2.48 c | 15.63 ± 1.84 b | 26.28 ± 9.97 a |
| C | 69.75 ± 6.73 | 18.50 ± 4.69 b | 11.75 ± 2.05 | 3.45 ± 0.62 cd | 6.19 ± 0.19 bc | 20.62 ± 3.69 a | 8.99 ± 1.30 d | 3.96 ± 0.9 c |
| D | 63.00 ± 6.96 | 23.75 ± 4.56 ab | 13.25 ± 3.58 | 5.02 ± 0.73 a | 6.50 ± 0.35 ab | 14.39 ± 6.04 b | 10.13 ± 1.57 d | 4.37 ± 1.02 c |
| E | 57.75 ± 2.87 | 29.75 ± 3.73 a | 12.50 ± 0.93 | 4.81 ± 0.31 a | 5.99 ± 0.33 c | 5.30 ± 1.49 c | 12.19 ± 0.90 c | 9.20 ± 2.48 bc |
| F | 42.25 ± 6.61 | 38.50 ± 5.26 ab | 19.25 ± 2.19 | 3.96 ± 0.28 bc | 6.75 ± 0.44 a | 7.48 ± 2.63 c | 9.67 ± 1.50 d | 4.39 ± 0.63 c |
| G | 3.25 ± 2.19 | 45.50 ± 0.53 a | 51.25 ± 2.06 | 4.30 ± 0.22 ab | 6.87 ± 0.24 a | 5.04 ± 1.46 c | 18.12 ± 1.39 a | 14.44 ± 2.22 b |
| H | 33.75 ± 9.77 | 36.25 ± 4.56 ab | 36.50 ± 3.42 | 4.58 ± 0.51 ab | 6.23 ± 0.24 bc | 4.90 ± 1.00 c | 14.75 ± 1.61 bc | 11.45 ± 1.63 b |
| I | 40.25 ± 5.63 | 28.25 ± 3.81 ab | 31.50 ± 4.04 | 2.97 ± 0.26 d | 6.53 ± 0.13 ab | 7.60 ± 2.73 c | 9.58 ± 0.69 d | 10.08 ± 1.28 bc |
| p-value | 0.313 | 0.01 | 0.162 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Cumulative GBH Applications 2017–2020 | Soil Profile | Glyphosate (µg·g−1 Dry Soil) | AMPA (µg·g−1 Dry Soil) | ||||
|---|---|---|---|---|---|---|---|
| Average | Max | Detection % | Average | Max | Detection % | ||
| None | 0–20 cm | 0.04 ± 0.03 | 0.11 | 81 | 0.10 ± 0.06 | 0.25 | 94 |
| 20–40 cm | 0.03 ± 0.02 | 0.06 | 50 | 0.05 ± 0.02 | 0.06 | 69 | |
| <5400 g·ha−1 | 0–20 cm | 0.14 ± 0.13 | 0.58 | 100 | 0.45 ± 0.26 | 1.16 | 100 |
| 20–40 cm | 0.06 ± 0.03 | 0.13 | 83 | 0.20 ± 0.21 | 0.73 | 79 | |
| >5400 g·ha−1 | 0–20 cm | 0.23 ± 0.18 | 0.69 | 100 | 0.45 ± 0.27 | 1.22 | 97 |
| 20–40 cm | 0.23 ± 0.18 | 0.72 | 97 | 0.15 ± 0.12 | 0.69 | 88 | |
| Type of Variable | Variable | d.f. | Prokaryotic Community | Fungal Community | Eukaryotic Community | |||
|---|---|---|---|---|---|---|---|---|
| Pseudo-F Value | p-Value | Pseudo-F Value | p-Value | Pseudo-F Value | p-Value | |||
| Environmental variables | Organic matter | 1 | 0.882 | 0.574 | 1.11 | 0.262 | 0.98 | 0.408 |
| Ca | 1 | 1.20 | 0.211 | 1.65 | 0.023 | 1.87 | 0.023 | |
| Na | 1 | 2.32 | 0.012 | 1.75 | 0.009 | 2.05 | 0.012 | |
| Clay content | 1 | 2.08 | 0.015 | 2.09 | 0.003 | 2.05 | 0.013 | |
| Sand content | 1 | 1.78 | 0.033 | 1.79 | 0.009 | 1.68 | 0.032 | |
| Management variables | Cumulative GBH application | 1 | 0.853 | 0.585 | 1.18 | 0.198 | 0.878 | 0.55 |
| Crop rotation | 3 | 1.41 | 0.045 | 1.46 | 0.004 | 1.69 | 0.005 | |
| Manure inputs | 2 | 2.05 | 0.005 | 2.06 | 0.001 | 2.04 | 0.002 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Overbeek, W.; Lucotte, M.; D’Astous-Pagé, J.; Jeanne, T.; Pin, C.; Moingt, M.; Hogue, R. Impacts of Cropping Systems on Glyphosate and Aminomethylphosphonic Acid Contents and Microbial Community in Field Crop Soils in Quebec (Canada). Agronomy 2024, 14, 686. https://doi.org/10.3390/agronomy14040686
Overbeek W, Lucotte M, D’Astous-Pagé J, Jeanne T, Pin C, Moingt M, Hogue R. Impacts of Cropping Systems on Glyphosate and Aminomethylphosphonic Acid Contents and Microbial Community in Field Crop Soils in Quebec (Canada). Agronomy. 2024; 14(4):686. https://doi.org/10.3390/agronomy14040686
Chicago/Turabian StyleOverbeek, William, Marc Lucotte, Joël D’Astous-Pagé, Thomas Jeanne, Clara Pin, Matthieu Moingt, and Richard Hogue. 2024. "Impacts of Cropping Systems on Glyphosate and Aminomethylphosphonic Acid Contents and Microbial Community in Field Crop Soils in Quebec (Canada)" Agronomy 14, no. 4: 686. https://doi.org/10.3390/agronomy14040686
APA StyleOverbeek, W., Lucotte, M., D’Astous-Pagé, J., Jeanne, T., Pin, C., Moingt, M., & Hogue, R. (2024). Impacts of Cropping Systems on Glyphosate and Aminomethylphosphonic Acid Contents and Microbial Community in Field Crop Soils in Quebec (Canada). Agronomy, 14(4), 686. https://doi.org/10.3390/agronomy14040686







