The Impact of Combining Robinia pseudoacacia Leaves and Corn Straw on Soil Carbon Content and Corn Yield in Loess Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Sample Collection
2.4. Measurements and Metrics
2.4.1. Soil Physical Properties
2.4.2. Chemical Properties and Soil Respiration
2.4.3. Soil Carbon-Degrading Enzyme Activity and Soil Respiration
2.5. Statistical Analysis
2.5.1. Soil Carbon Emission
2.5.2. Data Analysis
3. Results
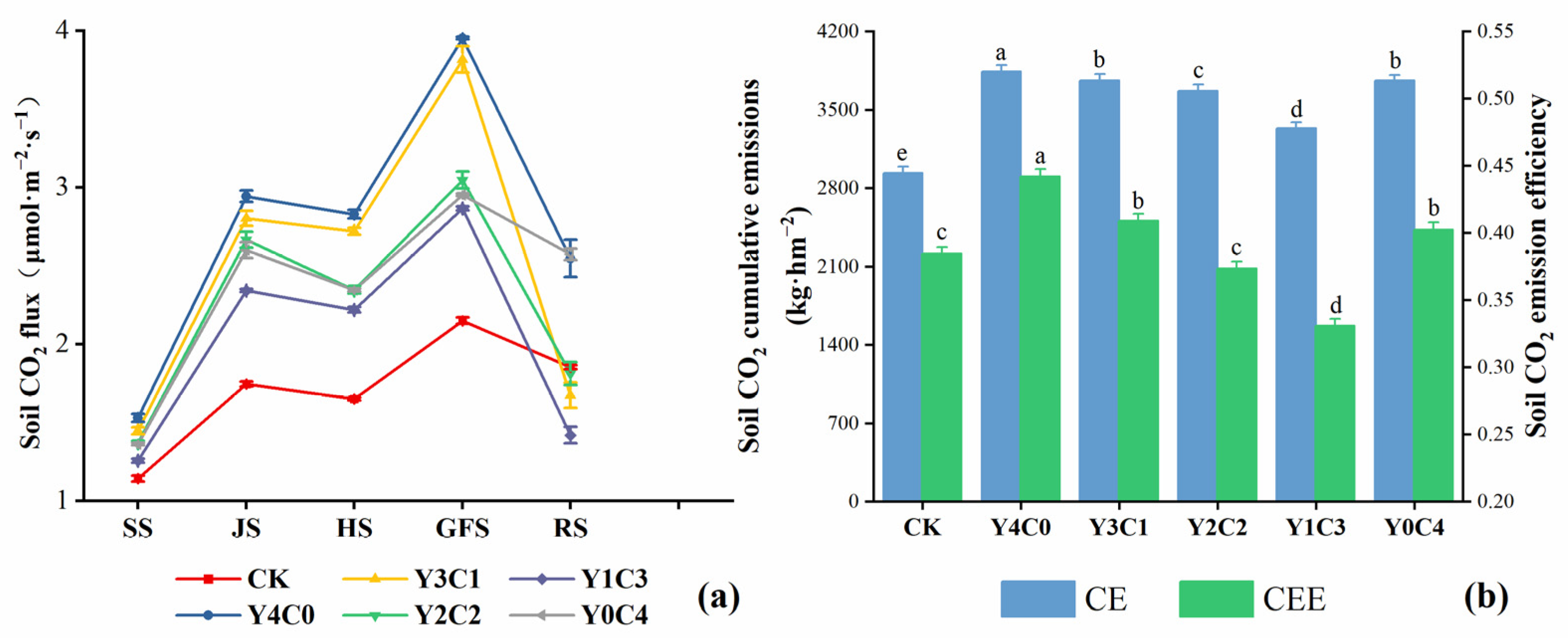
3.1. Characteristics of Carbon Emissions
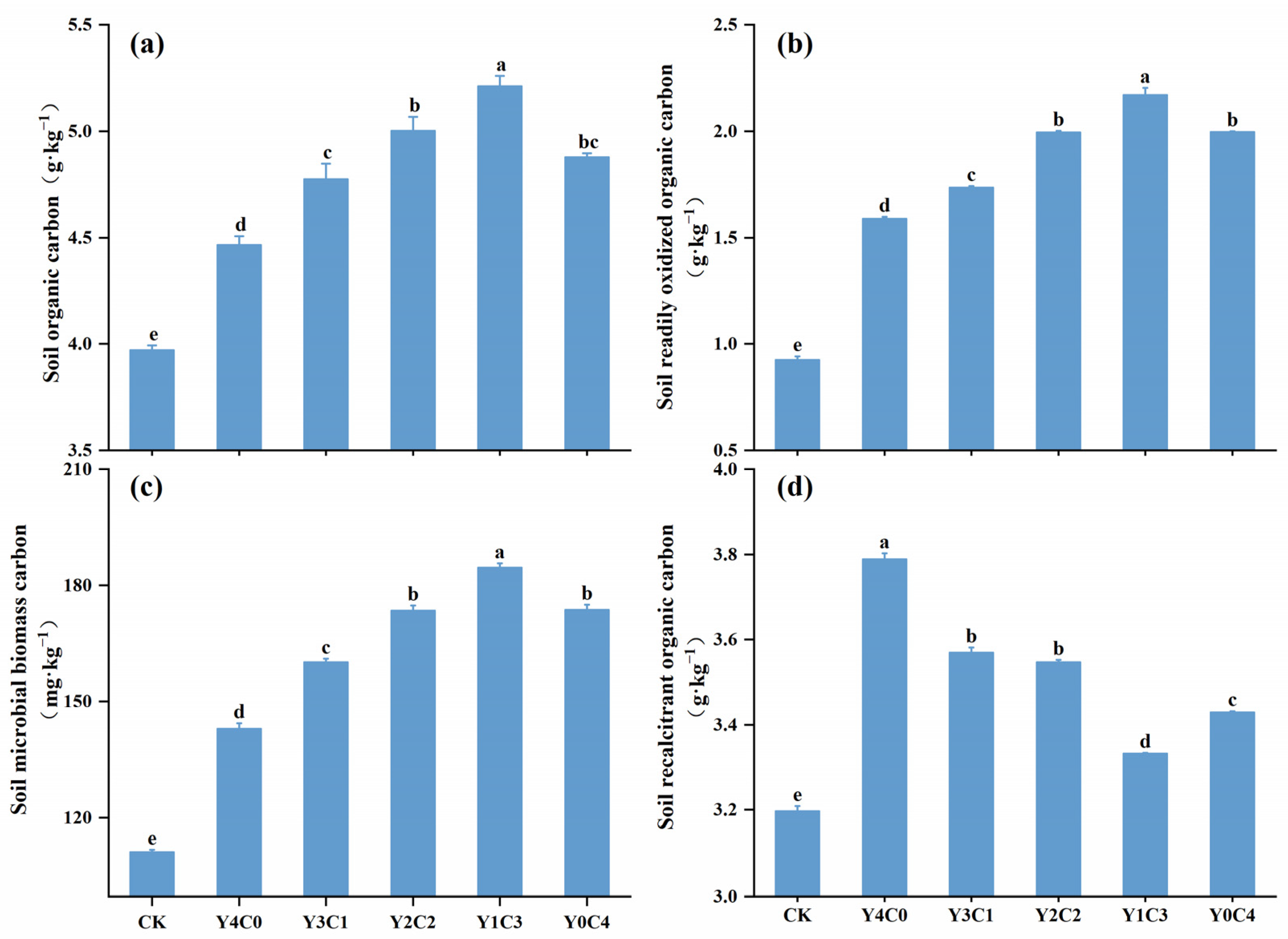
3.2. Characteristics of the Soil Carbon Pool
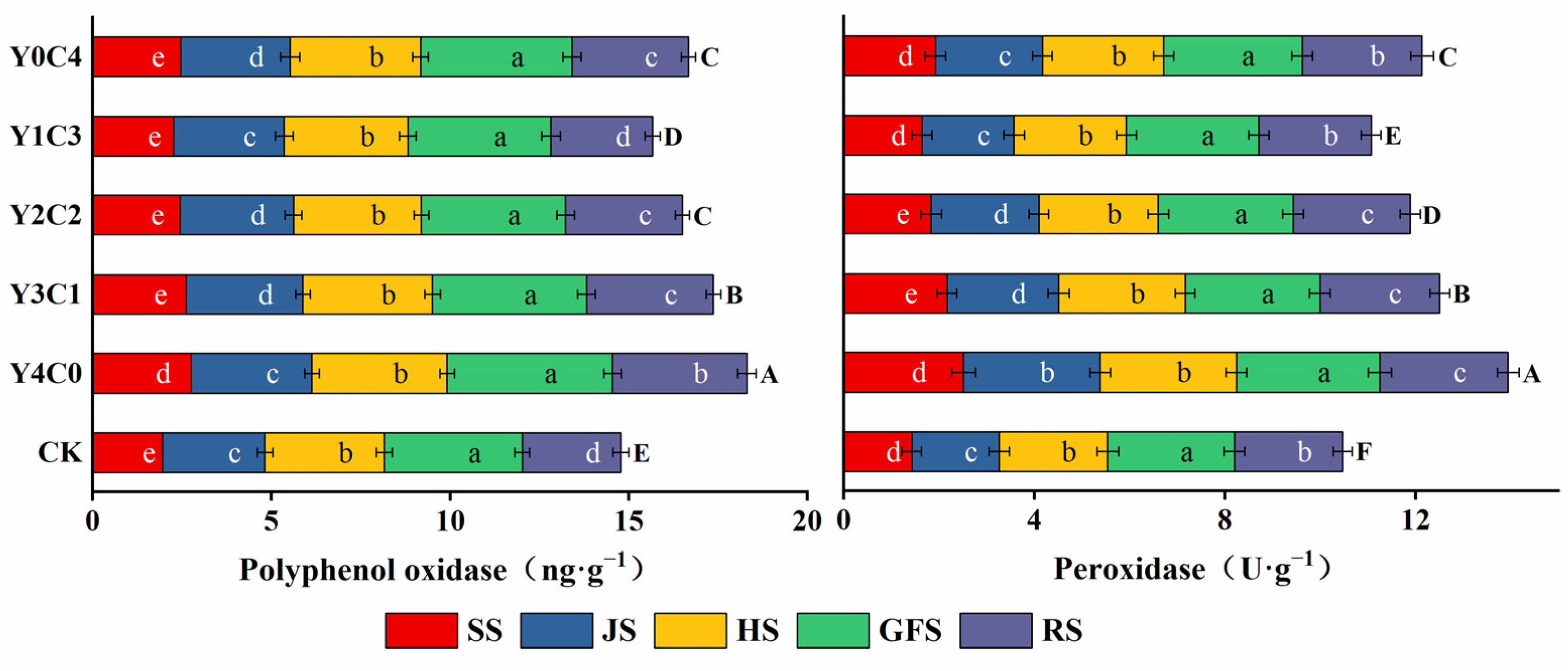
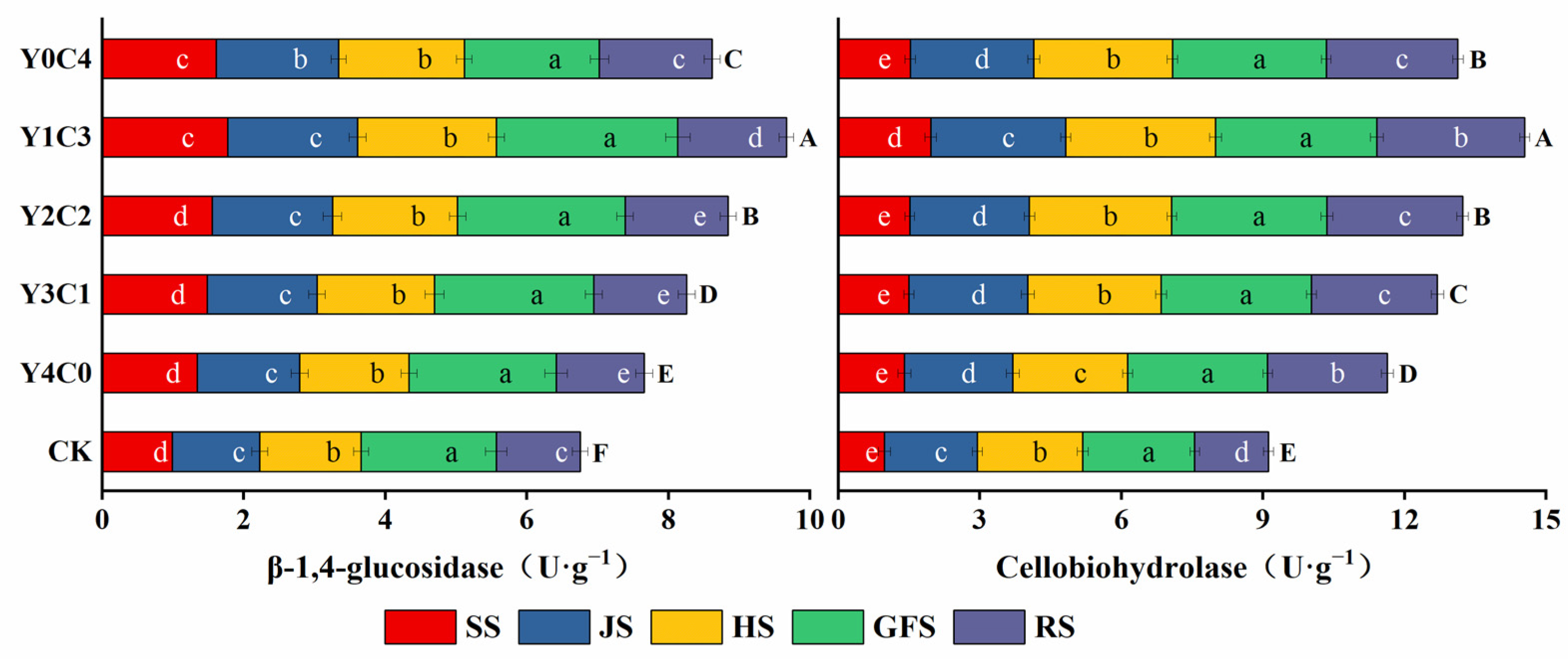
3.3. Characteristics of Soil Carbon-Degrading Enzyme Activity
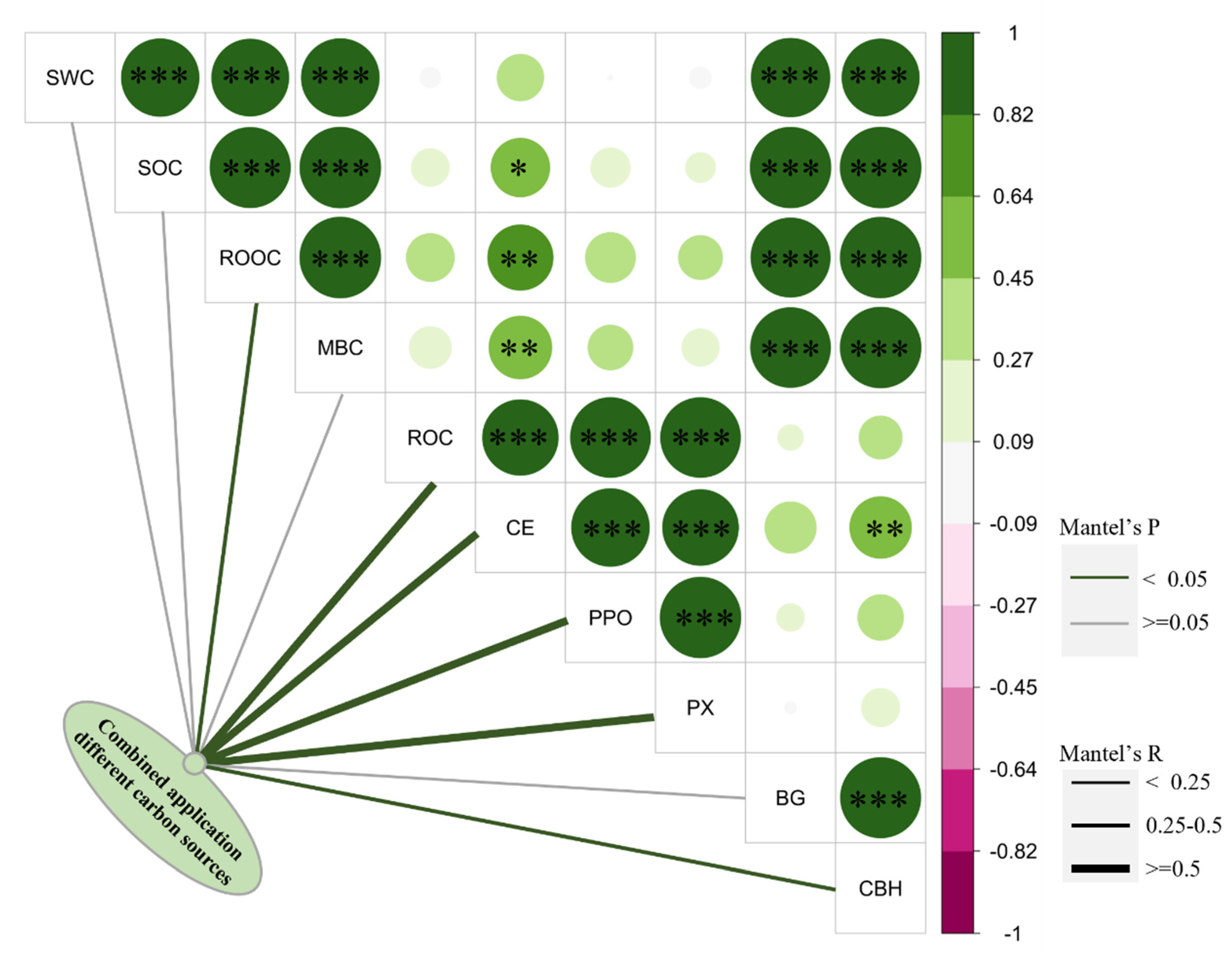
3.4. The Relationship between Soil Carbon-Degrading Enzymes and Soil Carbon Pool
4. Discussion
4.1. Response of Soil Carbon-Degrading Enzymes to Combined Application of Different Carbon Sources
4.2. Relationship between Soil Carbon-Degrading Enzymes and Soil Carbon Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Li, X.S.; Liang, Z.Y.; Li, Y.N.; Zhu, Y.H.; Tian, X.H.; Shi, J.L.; Wei, G.H. Short-term effects of combined organic amendments on soil organic carbon sequestration in a rain-fed winter wheat system. Agron. J. 2021, 113, 2150–2164. [Google Scholar] [CrossRef]
- Lu, J.S.; Zhang, W.; Li, Y.; Liu, S.T.; Khan, A.; Yan, S.C.; Hu, T.T.; Xiong, Y.C. Effects of reduced tillage with stubble remaining and nitrogen application on soil aggregation, soil organic carbon and grain yield in maize-wheat rotation system. Eur. J. Agron. 2023, 149, 126920. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, W.W.; Martinez-Murillo, J.F.; Pereira, P. Loess Plateau: From degradation to restoration. Sci. Total. Environ. 2020, 738, 140206. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Xin, Z.B.; Qin, Y.B.; Xiao, Y.L. Impact of ecological vegetation construction on the landscape pattern of a Loess Plateau Watershed. Acta Ecol. Sin. 2013, 33, 6277–6286. [Google Scholar]
- Rouifed, S.; Handa, I.T.; David, J.F.; Hattenschwiler, S. The importance of biotic factors in predicting global change effects on decomposition of temperate forest leaf litter. Oecologia 2010, 163, 247–256. [Google Scholar] [CrossRef]
- Caruso, T.; De Vries, F.T.; Bardgett, R.D.; Lehmann, J. Soil organic carbon dynamics matching ecological equilibrium theory. Ecol. Evol. 2018, 8, 11169–11178. [Google Scholar] [CrossRef]
- Duan, F.Y.; Peng, P.; Yang, K.P.; Shu, Y.H.; Wang, J.W. Straw return of maize and soybean enhance soil biological nitrogen fixation by altering the N-cycling microbial community. Appl. Soil Ecol. 2023, 192, 105094. [Google Scholar] [CrossRef]
- Guinet, M.; Adeux, G.; Cordeau, S.; Courson, E.; Nandillon, R.; Zhang, Y.Y.; Munier-Jolain, N. Fostering temporal crop di-versification to reduce pesticide use. Nat. Commun. 2023, 14, 7416. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhang, Y.H.; Sun, Y.G.; Zhang, Q.; Liu, P.Z.; Wang, X.L.; Li, J.; Wang, R. No-tillage with straw mulching improved grain yield by reducing soil water evaporation in the fallow period: A 12-year study on the Loess Plateau. Soil Tillage Res. 2022, 224, 105504. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.W.; Xu, L.; Nadeem, M.Y.; Li, W.W.; Jiang, Y.; Ding, Y.F.; Liu, Z.H.; Li, G.H. Long-term fertilizer postponing promotes soil organic carbon sequestration in paddy soils by accelerating lignin degradation and increasing microbial necromass. Soil Biol. Biochem. 2022, 175, 108839. [Google Scholar] [CrossRef]
- Kan, Z.R.; Zhou, J.J.; Li, F.M.; Sheteiwy, M.S.; Qi, J.Y.; Chen, C.Q.; Yang, H.S. Straw incorporation interacting with earthworms mitigate N2O emissions from upland soil in a rice-wheat rotation system. Sci. Total Environ. 2023, 859, 160338. [Google Scholar] [CrossRef]
- Erenstein, O. Crop residue mulching in tropical and semi-tropical countries: An evaluation of residue availability and other technological implications. Soil Tillage Res. 2002, 67, 115–133. [Google Scholar] [CrossRef]
- Shang, W.H.; Razavi, B.S.; Yao, S.H.; Hao, C.K.; Kuzyakov, Y.; Blagodatskaya, E.; Tian, J. Contrasting mechanisms of nutrient mobilization in rhizosphere hotspots driven by straw and biochar amendment. Soil Biol. Biochem. 2023, 187, 109212. [Google Scholar] [CrossRef]
- Su, Y.; Yu, M.; Xi, H.; Lv, J.L.; Ma, Z.H.; Kou, C.L.; Shen, A.L. Soil microbial community shifts with long-term of different straw return in a wheat-corn rotation system. Sci. Rep. 2020, 10, 6360. [Google Scholar] [CrossRef]
- Kozjek, K.; Manoharan, L.; Urich, T.; Ahrén, D.; Hedlund, K. Microbial gene activity in straw residue amendments reveals carbon sequestration mechanisms in agricultural soils. Soil Biol. Biochem. 2023, 179, 108994. [Google Scholar] [CrossRef]
- Lee, J. Effect of application methods of organic fertilizer on growth, soil chemical properties and microbial densities in organic bulb onion production. Sci. Hortic. 2010, 124, 299–305. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.Q.; Li, J.W.; Zhou, X.H.; Cao, J.J.; Wang, R.W.; Wang, Y.Q.; Shelton, S.; Jin, Z.; Walker, L.M.; et al. Costimulation of soil glycosidase activity and soil respiration by nitrogen addition. Glob. Chang. Biol. 2017, 23, 1328–1337. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- O’Brien, P.L.; Sauer, T.J.; Archontoulis, S.; Karlen, D.L.; Laird, D. Corn stover harvest reduces soil CO2 fluxes but increases overall C losses. GCB Bioenergy 2020, 12, 894–909. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, P.; Wang, K.; Ding, R.X.; Yang, B.P.; Nie, J.F.; Jia, Z.K.; Han, Q.F. Effects of Wheat Straw Incorporation on the Availability of Soil Nutrients and Enzyme Activities in Semiarid Areas. PLoS ONE 2015, 10, 120994. [Google Scholar] [CrossRef]
- Eberwein, J.R.; Oikawa, P.Y.; Allsman, L.A.; Jenerette, G.D. Carbon availability regulates soil respiration response to nitrogen and temperature. Soil Biol. Biochem. 2015, 88, 158–164. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Zhou, X.H.; Zhang, B.C.; Lu, M.; Luo, Y.Q.; Liu, L.L.; Li, B. Different responses of soil respiration and its components to nitrogen addition among biomes: A meta-analysis. Glob. Chang. Biol. 2014, 20, 2332–2343. [Google Scholar] [CrossRef]
- Diao, S.P.; Gao, R.P.; Gao, Y.; Ren, Y.F.; Zhao, P.Y.; Yuan, W.; Gao, X.F. Effects of Straw Returning on Soil Hydrothermal and Yield of Maize in Loess Plateau of Inner Mongolia. Crops 2019, 6, 83–89. [Google Scholar]
- Ren, C.J.; Chen, J.; Lu, X.J.; Doughty, R.; Zhao, F.Z.; Zhong, Z.K.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Responses of soil total microbial biomass and community compositions to rainfall reductions. Soil Biol. Biochem. 2018, 116, 4–10. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis; Part 2. Agronomy Monographs 9; Page, A.L., Ed.; ASA and SSSA: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-Total. In Methods of Soil Analysis; Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Blair, G.; Lefroy, R.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Rovira, P.; Vallejo, V.R. Examination of thermal and acid hydrolysis procedures in the characterization of soil organic matter. Commun. Soil Sci. Plant Anal. 2000, 31, 81–100. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long-term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Xu, Z.W.; Yu, G.R.; Zhang, X.Y.; He, N.P.; Wang, Q.F.; Wang, S.Z.; Wang, R.L.; Zhao, N.; Jia, Y.L.; Wang, C.Y. Soil enzyme activity and stoichiometry in forest ecosystems along the NorthSouth Transect in eastern China (NSTEC). Soil Biol. Biochem. 2017, 104, 152–163. [Google Scholar] [CrossRef]
- Deforest, J.L.; Moorhead, D.L. Effects of elevated pH and phosphorus fertilizer on soil C, N and P enzyme stoichiometry in an acidic mixed mesophytic deciduous forest. Soil Biol. Biochem. 2020, 150, 107993. [Google Scholar] [CrossRef]
- Chi, Y.B.; Yang, P.L.; Ren, S.M.; Ma, N.; Yang, J.; Xu, Y. Effects of fertilizer types and water quality on carbon dioxide emissions from soil in wheat-maize rotations. Sci. Total Environ. 2020, 698, 134010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Tian, H.Q.; Shi, H.; Zhang, J.F.; Wang, X.K.; Pan, S.F.; Yang, J. Increased greenhouse gas emission intensity of major croplands in China: Implications for food security and climate change mitigation. Glob. Chang. Biol. 2020, 26, 6116–6133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Zhang, Y.H.; Li, C.; Xu, R.S.; Pei, Z.R.; Li, F.C.; Wu, Y.H.; Chen, F.; Liang, Y.R.; Li, Z.H.; et al. Linking soil organic carbon dynamics to microbial community and enzyme activities in degraded soil remediation by reductive soil disinfestation. Appl. Soil Ecol. 2023, 189, 104931. [Google Scholar] [CrossRef]
- Xu, H.W.; Qu, Q.; Li, G.W.; Liu, G.B.; Geissen, V.; Ritsema, C.J.; Xue, S. Impact of nitrogen addition on plant-soil-enzyme C–N–P stoichiometry and microbial nutrient limitation. Soil Biol. Biochem. 2022, 170, 108714. [Google Scholar] [CrossRef]
- Maarastawi, S.A.; Frindte, K.; Bodelier, P.L.E.; Knief, C. Rice straw serves as the additional carbon source for rhizosphere microorganisms and reduces root exudate consumption. Soil Biol. Biochem. 2019, 135, 235–238. [Google Scholar] [CrossRef]
- Curtright, A.J.; Tiemann, L.K. Chemical identity of carbon substrates drives differences in denitrification and NO reduction within agricultural soils. Soil Biol. Biochem. 2023, 184, 109078. [Google Scholar] [CrossRef]
- Peng, X.Q.; Wang, W. Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of northern China. Soil Biol. Biochem. 2016, 98, 74–84. [Google Scholar] [CrossRef]
- Manzoni, S.; Jackson, R.B.; Trofymow, J.A.; Porporato, A. The global stoichiometry of litter nitrogen mineralization. Science 2008, 321, 684–686. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, S. Seasonal changes of soil carbon fractions and enzyme activities in response to winter cover crops under long-term rotation and tillage systems. Eur. J. Soil Sci. 2020, 72, 886–899. [Google Scholar] [CrossRef]
- Brown, R.W.; Jones, D.L. Plasticity of microbial substrate carbon use efficiency in response to changes in plant carbon input and soil organic matter status. Soil Biol. Biochem. 2024, 188, 109230. [Google Scholar] [CrossRef]
- Ding, X.L.; He, H.B.; Zhang, B.; Zhang, X.D. Plant-N incorporation into microbial amino sugars as affected by inorganic N addition: A microcosm study of 15 N-labeled maize residue decomposition. Soil Biol. Biochem. 2011, 43, 1968–1974. [Google Scholar] [CrossRef]
- Bai, Z.; Bodé, S.; Huygens, D.; Zhang, X.D.; Boeckx, P. Kinetics of amino sugar formation from organic residues of different quality. Soil Biol. Biochem. 2013, 57, 814–821. [Google Scholar] [CrossRef]
- Yeboah, S.; Zhang, R.Z.; Cai, L.Q.; Jun, W. Different carbon sources enhance system productivity and reduce greenhouse gas intensity. Plant Soil Environ. 2018, 64, 463–469. [Google Scholar] [CrossRef]
- Kominoski, J.S.; Rosemond, A.D.; Benstead, J.P.; Ladislavgulis, V.; Maerz, J.C.; Manning, D.W.P. Low-to-moderate nitrogen and phosphorus concentrations accelerate microbially driven litter breakdown rates. Ecol. Appl. 2015, 25, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Miltner, A.; Bombach, P.; Schmidt-Brücken, B.; Kästner, M. SOM genesis: Microbial biomass as a significant source. Biogeochemistry 2012, 111, 41–55. [Google Scholar] [CrossRef]
- Liu, Z.H.; Huang, F.Y.; Wang, B.F.; Li, Z.Y.; Zhao, C.X.; Ding, R.X.; Yang, B.P.; Zhang, P.; Jia, Z.K. Soil respiration in response to biotic and abiotic factors under different mulching measures on rain-fed farmland. Soil Tillage Res. 2023, 232, 105749. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhang, J.L.; Zhao, X.J.; Liao, Y.C.; Jing, X.L.; Xue, J. Effects of different tillages on soil CO2 flux, and its relation to soil moisture and soil temperature in dry-land maize field. Agric. Res. Arid. Areas 2018, 36, 88–93. [Google Scholar]
- Potts, J.; Brown, R.W.; Jones, D.L.; Cross, P. Seasonal variation is a bigger driver of soil faunal and microbial community composition than exposure to the neonicotinoid acetamiprid within Brassica napus production systems. Soil Biol. Biochem. 2023, 184, 109088. [Google Scholar] [CrossRef]
- Su, F.; Wei, Y.; Wang, F.; Guo, J.; Zhang, J.; Wang, Y.; Guo, H.; Hu, S. Sensitivity of plant species to warming and altered precipitation dominates the community productivity in a semiarid grassland on the Loess Plateau. Ecol. Evol. 2019, 9, 7628–7638. [Google Scholar] [CrossRef] [PubMed]
| Item | CK | Y4C0 | Y3C1 | Y2C2 | Y1C3 | Y0C4 |
|---|---|---|---|---|---|---|
| SOC (g·kg−1) | — | 415.39 ± 5.22 ns | 420.91 ± 3.37 | 423.08 ± 5.82 | 424.39 ± 3.49 | 415.47 ± 3.39 |
| TN (g·kg−1) | 7.71 ± 0.16 e | 9.65 ± 0.17 d | 11.57 ± 0.18 c | 15.95 ± 0.23 b | 21.94 ± 0.14 a | |
| C: N | 53.85 ± 1.45 a | 43.63 ± 1.12 b | 36.57 ± 1.15 c | 26.61 ± 1.22 d | 18.94 ± 1.13 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Liu, J.; Zhang, Z.; Liu, W.; Zhang, Q.; Wang, X.; Ren, C.; Yang, G.; Han, X. The Impact of Combining Robinia pseudoacacia Leaves and Corn Straw on Soil Carbon Content and Corn Yield in Loess Plateau. Agronomy 2024, 14, 689. https://doi.org/10.3390/agronomy14040689
Liu H, Liu J, Zhang Z, Liu W, Zhang Q, Wang X, Ren C, Yang G, Han X. The Impact of Combining Robinia pseudoacacia Leaves and Corn Straw on Soil Carbon Content and Corn Yield in Loess Plateau. Agronomy. 2024; 14(4):689. https://doi.org/10.3390/agronomy14040689
Chicago/Turabian StyleLiu, Hanyu, Jianjian Liu, Zhenjiao Zhang, Weichao Liu, Qi Zhang, Xing Wang, Chengjie Ren, Gaihe Yang, and Xinhui Han. 2024. "The Impact of Combining Robinia pseudoacacia Leaves and Corn Straw on Soil Carbon Content and Corn Yield in Loess Plateau" Agronomy 14, no. 4: 689. https://doi.org/10.3390/agronomy14040689
APA StyleLiu, H., Liu, J., Zhang, Z., Liu, W., Zhang, Q., Wang, X., Ren, C., Yang, G., & Han, X. (2024). The Impact of Combining Robinia pseudoacacia Leaves and Corn Straw on Soil Carbon Content and Corn Yield in Loess Plateau. Agronomy, 14(4), 689. https://doi.org/10.3390/agronomy14040689







