Rhizosphere-Associated Microbiota Strengthen the Pathogenicity of Meloidogyne incognita on Arabidopsis thaliana
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Seedings and Nematode Juveniles
2.2. An Analysis of the Influence of Rhizosphere Microorganisms on M. incognita Pathogenicity
2.3. Isolation and Screening the Rhizosphere Bacteria Contributed to M. incognita Pathogenicity in A. thaliana
2.4. Influence of Single Strain in Nocardioides Combination on M. incognita Infection
2.5. Analysis of N. nematodiphilus R-N-C8 Increasing M. incognita Pathogenicity by Attracting J2s
2.6. Assay on Chemoattractant from N. nematodiphilus R-N-C8
2.7. Assay on L-Lysine Production by N. nematodiphilus R-N-C8
2.8. Statistical Analysis
3. Results
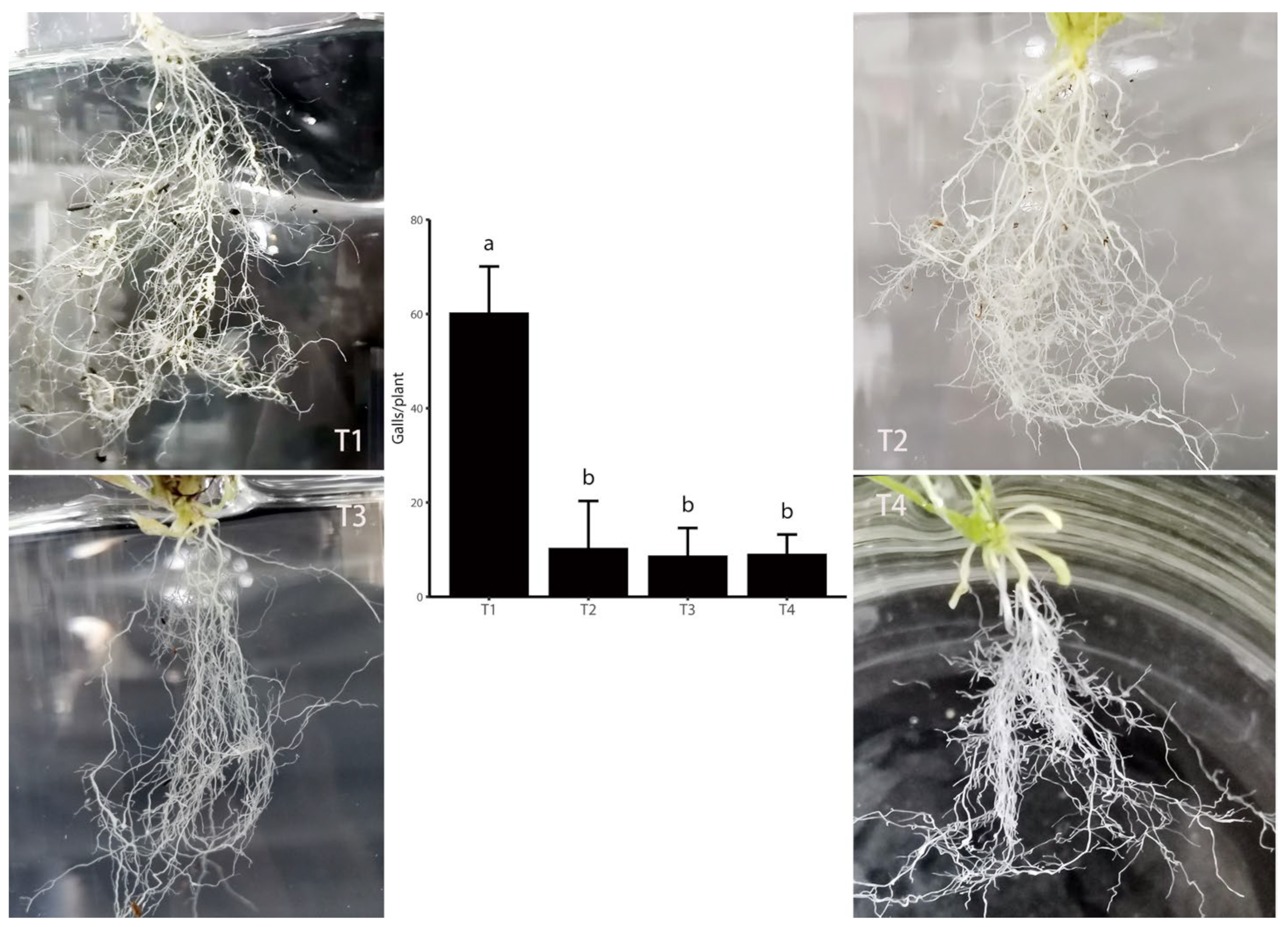
3.1. Elimination or Inhibition of Soil Bacteria Reduced M. incognita Pathogenicity in A. thaliana
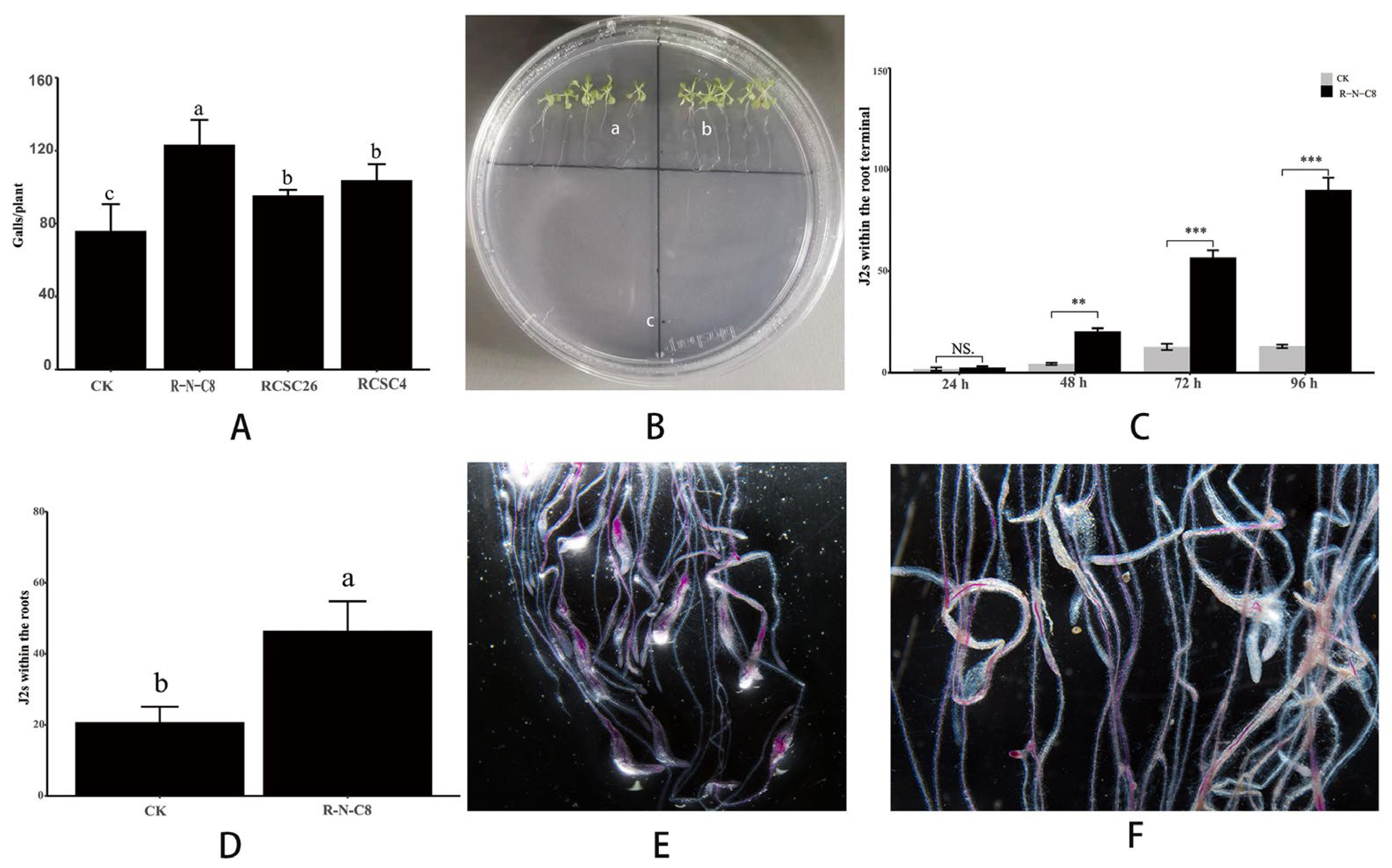
3.2. Sixteen Bacterial Combinations from Rhizosphere Soils Contributed to the M. incognita Pathogenicity in A. thaliana
3.3. N. nematodiphilus R-N-C8 Increased M. incognita Pathogenicity in A. thaliana by Attracting M. incognita J2s
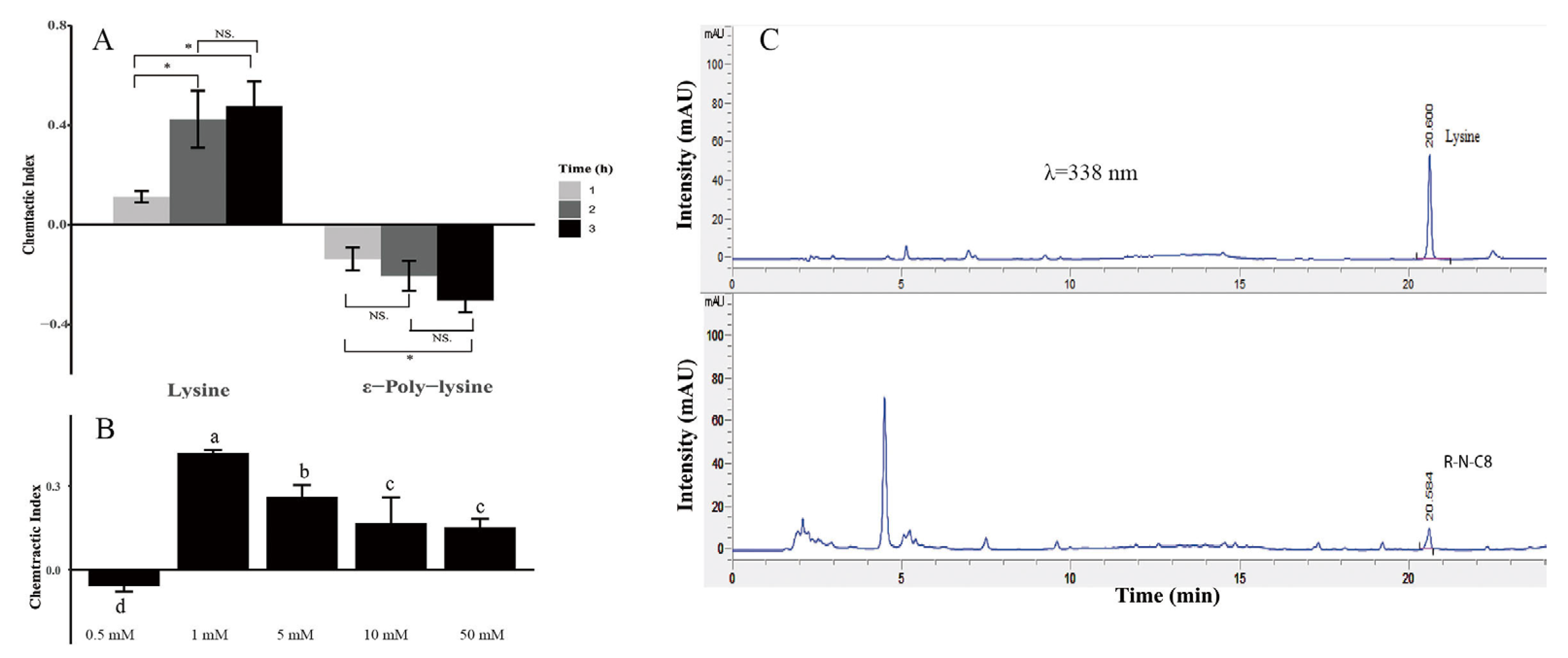
3.4. N. nematodiphilus R-N-C8 Producing L-Lysine to Attract M. incognita
4. Discussion
4.1. Microbes Increased Pathogenicity of RKNs
4.2. Host-Seeking and Locating Cues of RKNs
5. Conclusions and Future Studies
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montarry, J.; Mimee, B.; Danchin, E.G.J.; Koutsovoulos, G.D.; Ste-Croix, D.T.; Grenier, E. Recent Advances in Population Genomics of Plant-Parasitic Nematodes. Phytopathology 2021, 111, 40–48. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, H.J.; Kim, Y.H.; Jeon, J.M.; Song, H.S.; Kim, J.; No, S.Y.; Shin, J.H.; Choi, K.Y.; Park, K.M.; et al. Functional Study of Lysine Decarboxylases from Klebsiella pneumonia in Escherichia coli and Application of Whole Cell Bioconversion for Cadaverine Production. J. Microbiol. Biotechnol. 2016, 26, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Jaouannet, M.; Perfus-Barbeoch, L.; Deleury, E.; Magliano, M.; Engler, G.; Vieira, P.; Danchin, E.G.J.; Rocha, M.D.; Coquillard, P.; Abad, P.; et al. A Root-Knot Nematode-Secreted Protein Is Injected into Giant Cells and Targeted to the Nuclei. New Phytol. 2012, 194, 924–931. [Google Scholar] [CrossRef]
- Elling, A.A. Major Emerging Problems with Minor Meloidogyne species. Phytopathology 2013, 103, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Westphal, A.; Hallmann, J.; Heuer, H. Specific Microbial Attachment to Root Knot Nematodes in Suppressive Soil. Appl. Environ. Microbiol. 2014, 80, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.Y.L.; Iwamoto, Y.; Tsumuraya, Y.; Oota, M.; Konishi, T.; Ito, S.; Kotake, T.; Ishikawa, H.; Sawa, S. Root-knot Nematode Chemotaxis is Positively Regulated by L-galactose Sidechains of Mucilage Carbohydrate Rhamnogalacturonan-I. Sci. Adv. 2021, 7, eabh4182. [Google Scholar] [CrossRef] [PubMed]
- Hewezi, T.; Baum, T.J. Manipulation of Plant Cells by Cyst and Root-Knot Nematode Effectors. Mol. Plant Microbe. Interact. 2013, 26, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Anjam, M.S.; Nawaz, M.A.; Lam, H.M.; Chung, G. Signal Transduction in Plant–Nematode Interactions. Int. J. Mol. Sci. 2018, 19, 1648. [Google Scholar] [CrossRef]
- Curtis, R.H.C. Plant-nematode Interactions: Environmental Signals Detected by the Nematode’s Chemosensory Organs Control Changes in the Surface Cuticle and Behavior. Parasite 2008, 15, 310–316. [Google Scholar] [CrossRef]
- Siddique, S.; Coomer, A.; Baum, T.; Williamson, V.M. Recognition and Response in Plant–Nematode Interactions. Annu. Rev. Phytopathol. 2022, 60, 143–162. [Google Scholar] [CrossRef]
- Niu, Q.; Huang, X.; Zhang, L.; Xu, J.; Yang, D.; Wei, K.; Niu, X.; An, Z.; Bennett, J.W.; Zou, C.; et al. A Trojan Horse Mechanism of Bacterial Pathogenesis against Nematodes. Proc. Natl. Acad. Sci. USA 2010, 107, 16631–16636. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yang, J.; Nie, Q.; Huang, D.; Yu, C.; Zheng, L.; Cai, M.; Thomashow, L.S.; Weller, D.M.; Yu, Z.; et al. Volatile Organic Compounds from Paenibacillus polymyxa KM2501-1 Control Meloidogyne incognita by Multiple Strategies. Sci. Rep. 2017, 7, 16213. [Google Scholar] [CrossRef]
- Topalović, O.; Vestergård, M. Can Microorganisms Assist the Survival and Parasitism of Plant-Parasitic Nematodes? Trends Parasitol. 2021, 37, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Y.; Tian, X.L.; Wang, Y.S.; Lin, R.M.; Mao, Z.C.; Chen, N.; Xie, B.Y. Metagenomic Analysis of the Pinewood Nematode Microbiome Reveals a Symbiotic Relationship Critical for Xenobiotics Degradation. Sci. Rep. 2013, 3, 1869. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tian, B.; Ji, X.; Shang, S.; Lu, C.; Zhang, K. Associated Bacteria of Different Life Stages of Meloidogyne incognita Using Pyrosequencing-Based Analysis. J. Basic Microbiol. 2015, 55, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.Y.; Cao, Y.; Zhang, K.Q. Metagenomic Insights into Communities, Functions of Endophytes, and Their Associates with Infection by Root-Knot Nematode, Meloidogyne incognita, in Tomato Roots. Sci. Rep. 2015, 5, 17087. [Google Scholar] [CrossRef] [PubMed]
- Yergaliyev, T.M.; Alexander-Shani, R.; Dimerets, H.; Pivonia, S.; Bird, D.M.; Rachmilevitch, S.; Szitenberg, A. Bacterial Community Structure Dynamics in Meloidogyne incognita-Infected Roots and Its Role in Worm-Microbiome Interactions. mSphere 2020, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lei, S.; Cheng, Z.; Jin, L.; Zhang, T.; Liang, L.M.; Cheng, L.; Zhang, Q.; Xu, X.; Lan, C.; et al. Microbiota and Functional Analyses of Nitrogen-fixing Bacteria in Root-Knot Nematode Parasitism of Plants. Microbiome 2023, 11, 48. [Google Scholar] [CrossRef]
- Zhang, X.M.; Mo, W.T.; Wei, Y.Q.; Li, X.; Ma, G.M.; Yan, Y.S.; Wang, X.J.; Liu, J.J.; Liu, Z.Y.; Zhou, X.K.; et al. Nocardioides nematodiphilus sp. nov., Isolated from Rhizosphere of Arabidopsis thaliana. Int. J. Syst. Evol. Microbiol. 2022, 72, 005271. [Google Scholar] [CrossRef]
- Lu, C.J.; Meng, Y.; Wang, Y.L.; Zhang, T.; Yang, G.F.; Mo, M.H.; Ji, K.F.; Liang, L.M.; Zou, C.G.; Zhang, K.Q. Survival and Infectivity of Second-Stage Root-Knot Nematode Meloidogyne incognita Juveniles Depend on Lysosome-Mediated Lipolysis. J. Biol. Chem. 2022, 298, 101637. [Google Scholar] [CrossRef]
- Kyndt, T.; Goverse, A.; Haegeman, A.; Warmerdam, S.; Wanjau, C.; Jahani, M.; Engler, G.; Engler, J.d.A.; Gheysen, G. Redirection of Auxin Flow in Arabidopsis thaliana Roots After Infection by Root-Knot Nematodes. J. Exp. Bot. 2016, 67, 4559–4570. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Fan, S.; Sun, H.; Chen, C.; Wamg, A.; Jiang, L.; Ma, D. Rhizosphere-Associated Microbiomes of Rice (Oryza sativa L.) Under the Effect of Increased Nitrogen Fertilization. Front. Microbiol. 2021, 12, 730506. [Google Scholar] [CrossRef] [PubMed]
- Fudali, S.L.; Wang, C.; Williamson, V.M. Ethylene Signaling Pathway Modulates Attractiveness of Host Roots to the Root-Knot Nematode Meloidogyne hapla. Mol. Plant Microbe Interact. 2013, 26, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Wei, L.; Kaloshian, I. Root-knot Nematodes Induce Pattern-Triggered Immunity in Arabidopsis thaliana Roots. New Phytol. 2016, 211, 276–287. [Google Scholar] [CrossRef]
- Oota, M.; Tsai, A.Y.L.; Aoki, D.; Matsushita, Y.; Toyoda, S.; Fukushima, K.; Saeki, K.; Toda, K.; Perfus-Barbeoch, L.; Favery, B.; et al. Identification of Naturally Occurring Polyamines as Root-Knot Nematode Attractants. Mol. Plant 2020, 13, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Y.; Sun, Y.; Yang, L.; Hu, Y.; Li, J.; Wang, J.; Zhang, F.; Liu, Y. Chemotactic Responses of the Root-Knot Nematode Meloidogyne incognita to Streptomyces plicatus. FEMS Microbiol. Lett. 2019, 366, fnz234. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.K.; Wang, Y.; Chen, Y.; Lou, K.; Cao, L.L.; Xu, L.H.; Li, W.J. Zhihengliuella alba sp. nov., and Emended Description of the Genus Zhihengliuella. Int. J. Syst. Evol. Microbiol. 2009, 59, 2025–2031. [Google Scholar] [CrossRef]
- Iwasaki, S.; Kobayashi, H.; Furukawa, J.; Namikoshi, M.; Okuda, S.; Sato, Z.; Matsuda, I.; Noda, T. Studies on Macrocyclic Lactone Antibiotics. VII. Structure of a Phytotoxin “Rhizoxin” Produced by Rhizopus chinensis. J. Antibiot. 1984, 37, 354–362. [Google Scholar] [CrossRef]
- Martinez, L.P.; Hertweck, C. Pathogenic Fungus Harbours Endosymbiotic Bacteria for Toxin Production. Nature 2005, 437, 884–888. [Google Scholar] [CrossRef]
- Dewey, F.M.; Wong, Y.L.; Seery, R.; Hollins, T.W.; Gurr, S.J. Bacteria Associated with Stagonospora (Septoria) nodorum Increase Pathogenicity of the Fungus. New Phytol. 1999, 144, 489–497. [Google Scholar] [CrossRef]
- Ahmed, W.; Dai, Z.; Zhang, J.; Li, S.; Ahmed, A.; Munir, S.; Liu, Q.; Tan, Y.; Ji, G.; Zhao, Z. Plant-Microbe Interaction: Mining the Impact of Native Bacillus amyloliquefaciens WS-10 on Tobacco Bacterial Wilt Disease and Rhizosphere Microbial Communities. Microbiol. Spectr. 2022, 10, e0147122. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The Rhizosphere: A Playground and Battlefield for Soilborne Pathogens and Beneficial Microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Castillo, J.D.; Vivanco, J.M.; Manter, D.K. Bacterial Microbiome and Nematode Occurrence in Different Potato Agricultural Soils. Microb. Ecol. 2017, 74, 888–900. [Google Scholar] [CrossRef]
- Sikder, M.M.; Vestergård, M.; Kyndt, T.; Topalović, O.; Kudjordjie, E.N.; Nicolaisen, M. Genetic Disruption of Arabidopsis Secondary Metabolite Synthesis Leads to Microbiome-Mediated Modulation of Nematode Invasion. ISME J. 2022, 16, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Gowda, M.T.; Prasanna, R.; Rao, U.; Somvanshi, V.S.; Singh, P.K.; Sing, A.K.; Chawla, G. Microbiome Transplant Can Effectively Manage Root-Knot Nematode Infectivity in Tomato. Appl. Soil Ecol. 2023, 190, 105020. [Google Scholar] [CrossRef]
- Bird, D.M.; Opperman, C.H.; Davies, K.G. Interactions Between Bacteria and Plant-Parasitic Nematodes: Now and Then. Int. J. Parasitol. 2003, 33, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Shi, H.; Tao, J.; Jin, J.; Wang, S.; Zheng, Q.; Liu, P.; Xiang, B.; Chen, Q.; Xu, Y.; et al. Metagenomic Insights into the Changes in the Rhizosphere Microbial Community Caused by the Root-Knot Nematode Meloidogyne incognita in Tobacco. Environ. Res. 2023, 216, 114848. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; He, D.; Shi, T.; Zhang, Y.; Liu, S.; Yan, X.; Huang, L. Responses of Plant Immune System and Rhizosphere Soil Microbiome to the Elicitor BAR11 in Arabidopsis thaliana. Sci. Total Environ. 2024, 914, 169920. [Google Scholar] [CrossRef]
- Chen, X.; Li, F.L.; Kong, C.H. Rhizosphere Bacteria Mediate Flowering Time of Two Genotypes of Arabidopsis with and without Root-Secreted Signaling (−)-loliolide. Rhizosphere 2023, 27, 100774. [Google Scholar] [CrossRef]
- Idbella, M.; Bonanomi, G.; Filippis, F.D.; Foscari, A.; Zotti, M.; Abd-ElGawad, A.M.; Fechtali, T.; Incerti, G.; Mazzoleni, S. Negative Plant-Soil Feedback in Arabidopsis thaliana: Disentangling the Effects of Soil Chemistry, Microbiome, and Extracellular Self-DNA. Microbiol. Res. 2024, 281, 127634. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; del Rio, T.G.; et al. Defining the Core Arabidopsis thaliana Root Microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Kim, K.K.; Kim, J.S.; Kim, D.S.; Ko, S.H.; Yang, S.H.; Shin, Y.K.; Lee, J.S. Nocardioides baekrokdamisoli sp. nov., Isolated from Soil of a Crater Lake. Int. J. Syst. Evol. Microbiol. 2016, 66, 4231–4235. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Lee, J.S.; Shin, Y.K.; Park, Y.H.; Lee, S.T. Reclassification of Nocardioides simplex ATCC 13260, ATCC19565, and ATCC 19566 as Rhodococcus erythropolis. Int. J. Syst. Evol. Microbiol. 1997, 47, 904–907. [Google Scholar]
- de Carvalho, C.C.C.R. Adaptation of Rhodococcus erythropolis Cells for Growth and Bioremediationunder Extreme Conditions. Res. Microbiol. 2012, 163, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Dam, N.M.V.; Bouwmeester, H.J. Metabolomics in the Rhizosphere: Tapping into Belowground Chemical Communication. Trends Plant Sci. 2016, 21, 256–265. [Google Scholar] [PubMed]
- Perry, R.N. Chemoreception in Plant-Parasitic Nematodes. Annu. Rev. Phytopathol. 1996, 34, 181–199. [Google Scholar] [CrossRef]
- Farnier, K.; Bengtsson, M.; Becher, P.G.; Witzell, J.; Witzgall, P.; Manduríc, S. Novel Bioassay Demonstrates Attraction of the White Potato Cyst Nematode Globodera pallida (Stone) to Non-Volatile and Volatile Host Plant Cues. J. Chem. Ecol. 2012, 38, 795–801. [Google Scholar] [CrossRef]
- Ali, J.G.; Alborn, H.T.; Stelinski, L.L. Constitutive and Induced Subterranean Plant Volatiles Attract both Entomopathogenic and Plant Parasitic Nematodes. J. Ecol. 2011, 99, 26–35. [Google Scholar] [CrossRef]
- Dong, L.; Li, X.; Huang, L.; Gao, Y.; Zhong, L.; Zheng, Y.; Zuo, Y. Lauric Acid in Crown Daisy Root Exudate Potently Regulates Root-Knot Nematode Chemotaxis and Disrupts Mi-ffp-18 Expression to Block Infection. J. Exp. Bot. 2014, 65, 131–141. [Google Scholar] [CrossRef]
- Kihika, R.; Murungi, L.K.; Coyne, D.; Ng’ang’a, M.; Hassanali, A.; Teal, P.E.A.; Torto, B. Parasitic Nematode Meloidogyne incognita Interactions with Different Capsicum annum Cultivars Reveal the Chemical Constituents Modulating Root Herbivory. Sci. Rep. 2017, 7, 2903. [Google Scholar] [CrossRef]
- Murungi, L.K.; Kirwa, H.; Coyne, D.; Teal, P.E.A.; Beck, J.J.; Torto, B. Identification of Key Root Volatiles Signaling Preference of Tomato over Spinach by the Root Knot Nematode Meloidogyne incognita. J. Agric. Food Chem. 2018, 66, 7328–7336. [Google Scholar] [CrossRef]
- Strehmel, N.; Böttcher, C.; Schmidt, S.; Scheel, D. Profiling of Secondary Metabolites in Root Exudates of Arabidopsis thaliana. Phytochemistry 2014, 108, 35–46. [Google Scholar] [CrossRef]
- Ferkey, D.M.; Sengupta, P.; L’Etoile, N.D. Chemosensory Signal Transduction in Caenorhabditis elegans. Genetics 2021, 217, iyab004. [Google Scholar] [CrossRef]
- Shingai, R.; Wakabayashi, T.; Sakata, K.; Matsuura, T. Chemotaxis of Caenorhabditis elegans during Simultaneous Presentation of Two Water-Soluble Attractants, L-lysine and Chloride Ions. Comp. Biochem. Physiol. 2005, 142, 308–317. [Google Scholar] [CrossRef]
- Li, B.; Wang, P.; Yang, L.; Rang, X.; Zhou, W.; Liu, Y. Chemotaxis of Meloidogyne incognita Response to Rhizosphere Bacteria. Microorganisms 2023, 11, 2271. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.-K.; Ma, L.; Yang, Z.-X.; Bao, L.-F.; Mo, M.-H. Rhizosphere-Associated Microbiota Strengthen the Pathogenicity of Meloidogyne incognita on Arabidopsis thaliana. Agronomy 2024, 14, 664. https://doi.org/10.3390/agronomy14040664
Zhou X-K, Ma L, Yang Z-X, Bao L-F, Mo M-H. Rhizosphere-Associated Microbiota Strengthen the Pathogenicity of Meloidogyne incognita on Arabidopsis thaliana. Agronomy. 2024; 14(4):664. https://doi.org/10.3390/agronomy14040664
Chicago/Turabian StyleZhou, Xing-Kui, Li Ma, Zi-Xiang Yang, Ling-Feng Bao, and Ming-He Mo. 2024. "Rhizosphere-Associated Microbiota Strengthen the Pathogenicity of Meloidogyne incognita on Arabidopsis thaliana" Agronomy 14, no. 4: 664. https://doi.org/10.3390/agronomy14040664
APA StyleZhou, X.-K., Ma, L., Yang, Z.-X., Bao, L.-F., & Mo, M.-H. (2024). Rhizosphere-Associated Microbiota Strengthen the Pathogenicity of Meloidogyne incognita on Arabidopsis thaliana. Agronomy, 14(4), 664. https://doi.org/10.3390/agronomy14040664




