Transcriptome and Metabolome Analyses Reveal Ascorbic Acid Ameliorates Cold Tolerance in Rice Seedling Plants
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Design
2.2. Measurement Indicators and Methods
2.2.1. Measurement of Chlorophyll Fluorescence Parameters
2.2.2. Measurement of Relative Electrical Conductivity (REC)
2.2.3. Measurement of H2O2 and Malondialdehyde (MDA) Content
2.2.4. Measurement of Antioxidant Enzyme Activity
2.2.5. Carbohydrate Measurement
2.2.6. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.2.7. RNA Sequencing (RNA-Seq) and Bioinformatics Analysis
2.2.8. Metabolomics Analysis
2.2.9. Exogenous Spraying of Sucrose and Ascorbic Acid (AsA) under Cold Stress
2.2.10. Measurement of AsA Content
2.2.11. Measurement of Ascorbate Oxidase Activity
2.2.12. Statistical Analysis
3. Results
3.1. Effects of Cold Stress on the Morphological and Physiological Characteristics of Rice Seedlings
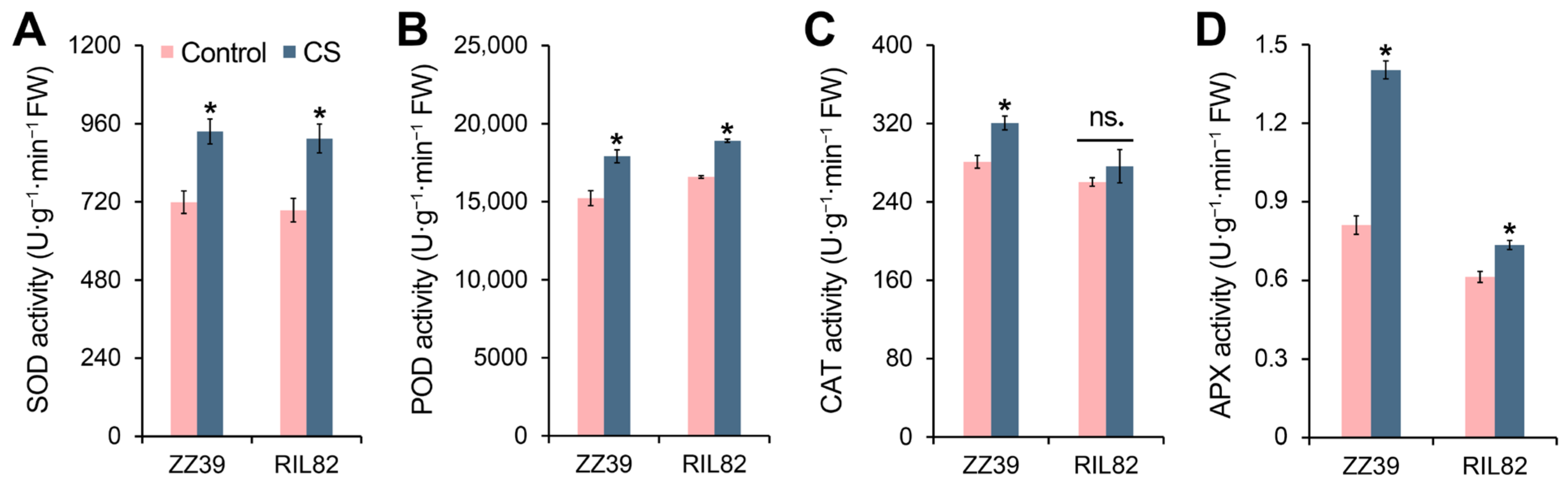
3.2. Effects of Cold Stress on Antioxidant Enzyme Activity in Rice Seedlings
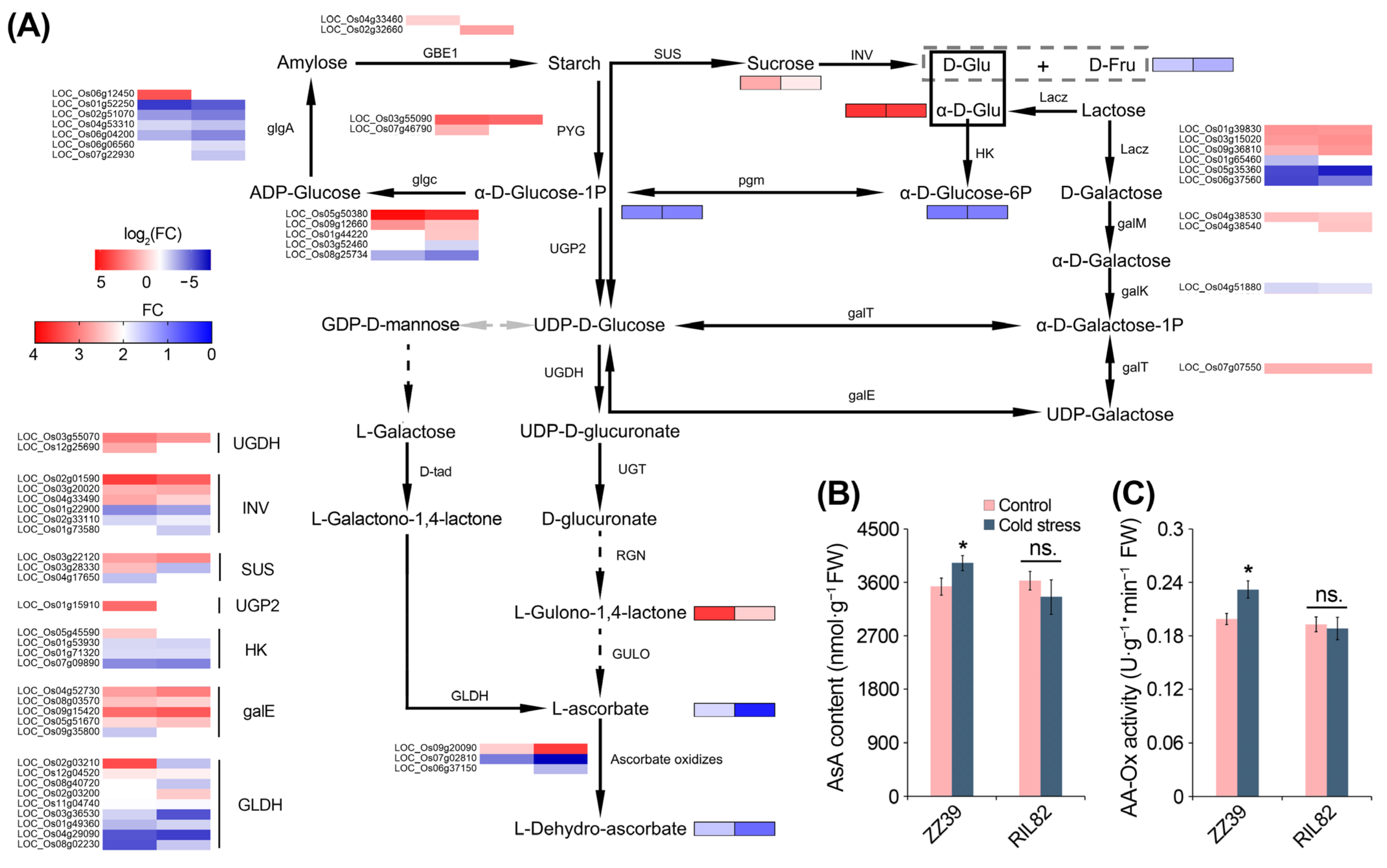
3.3. Comparative Transcriptome and Metabolome Profiling Revealed the Important Role of Ascorbic Acid in Cold Response of Rice
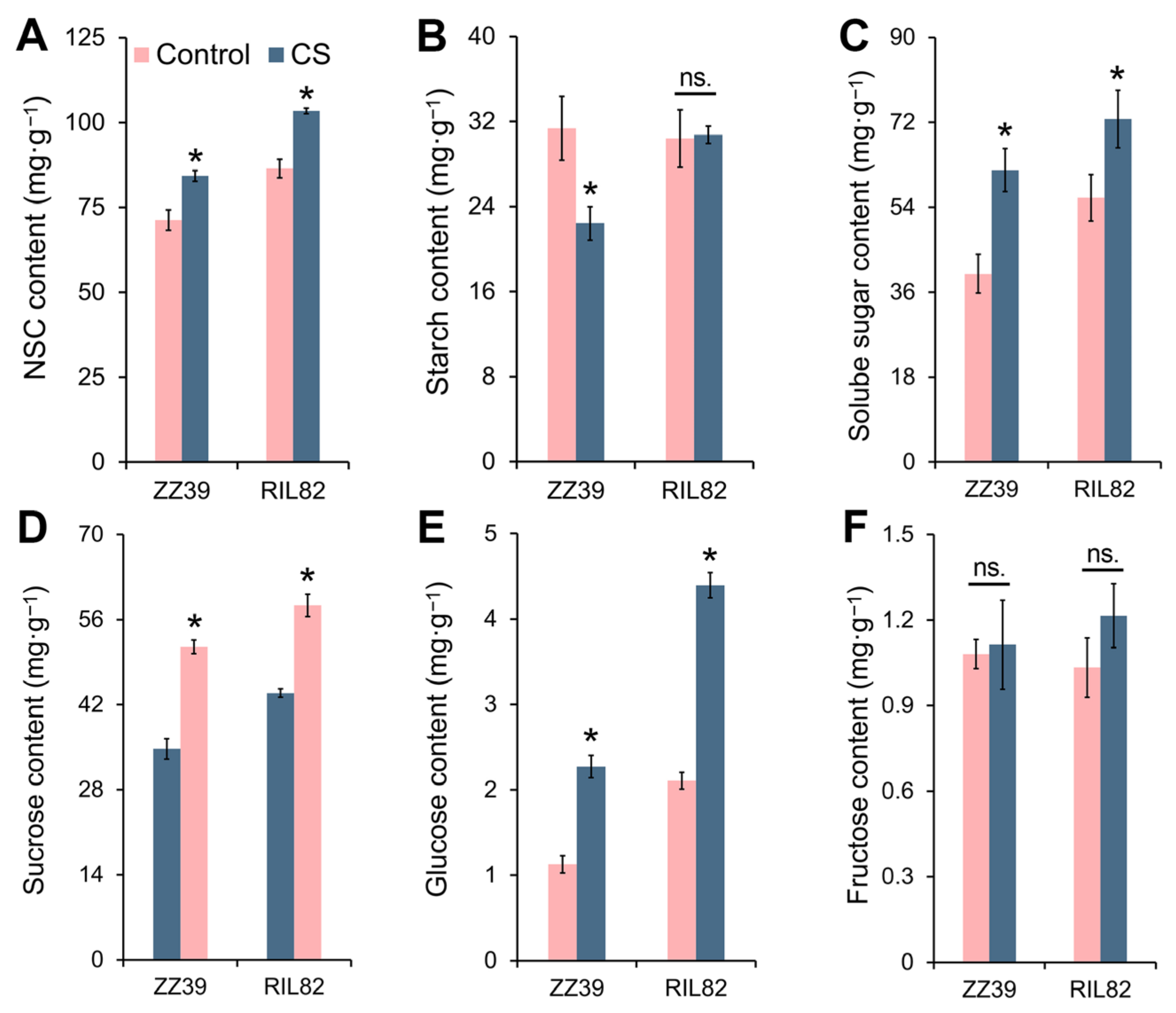
3.4. Effects of Cold Stress on the Carbohydrate Contents of Rice Seedlings
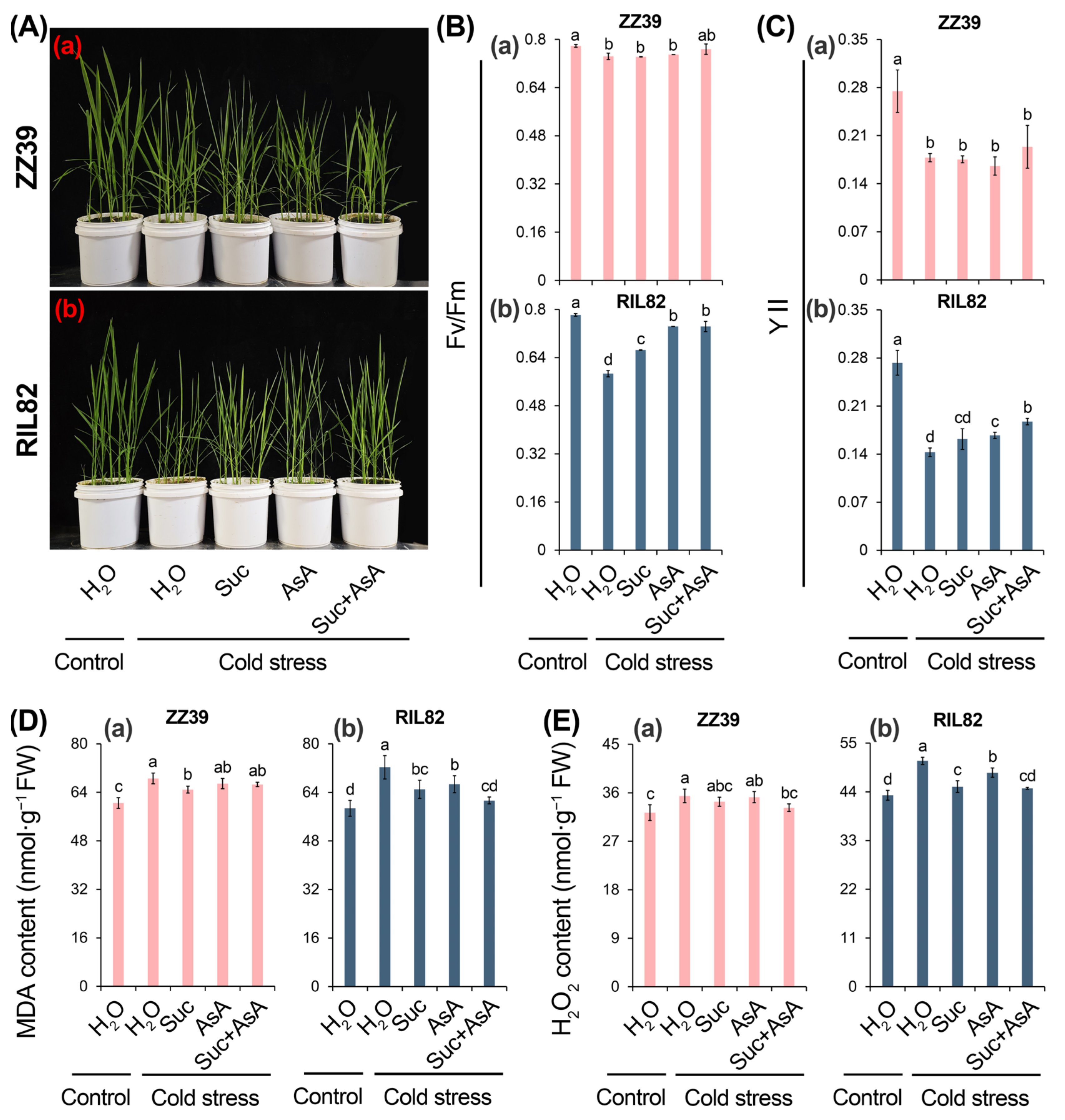
3.5. Effects of Sucrose and Ascorbic Acid Alone and in Combination on Cold Tolerance in Rice Seedlings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hasanuzzaman, M.; Nahar, K.; Fujit, M. Extreme temperature responses, oxidative stress and antioxidant defense in plants. In Abiotic Stress-Plant Responses and Applications in Agriculture; InTech: London, UK, 2013; pp. 169–205. [Google Scholar] [CrossRef]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef]
- Zhou, M.Q.; Shen, C.; Wu, L.H.; Tang, K.X.; Lin, J. CBF-dependent signaling pathway: A key responder to low temperature stress in plants. Crit. Rev. Biotechnol. 2011, 31, 186–192. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Guo, E.J.; Cheng, X.; Wang, L.Z.; Jiang, S.K.; Yang, X.L.; Ma, H.Y.; Zhang, T.Y.; Li, T.; Yang, X.G. Effects of chilling at different growth stages on rice photosynthesis, plant growth, and yield. Environ. Exp. Bot. 2022, 203, 105045. [Google Scholar] [CrossRef]
- Guo, E.J.; Wang, L.Z.; Jiang, S.K.; Xiang, H.T.; Shi, Y.Y.; Chen, X.; Wang, X.Y.; Zhang, T.Y.; Wang, L.M.; Feng, Y.J.; et al. Impacts of chilling at the tillering phases on rice growth and grain yield in Northeast China. J. Agron. Crop Sci. 2022, 208, 510–522. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Guo, E.J.; Shi, Y.Y.; Cheng, X.; Zhu, X.; Dong, X.; Li, T.; Wang, L.Z.; Jiang, S.K.; Xiang, H.T.; et al. Modelling the advancement of chilling tolerance breeding in Northeast China. J. Agron. Crop Sci. 2021, 6, 984–994. [Google Scholar] [CrossRef]
- Andaya, V.C.; Tai, T.H. Fine mapping of the qCTS4 locus associated with seedling cold tolerance in rice (Oryza sativa L.). Mol. Breeding 2007, 20, 349–358. [Google Scholar] [CrossRef]
- Lou, Q.; Chen, L.; Sun, Z.; Xing, Y.Z.; Li, J.; Xu, X.Y.; Mei, H.W.; Luo, L.J. A major QTL associated with cold tolerance at seedling stage in rice (Oryza sativa L.). Euphytica 2007, 158, 87–94. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, Y.; Fan, F.; Peng, J.; Zhang, J. Coordination of light, circadian clock with temperature: The potential mechanisms regulating chilling tolerance in rice. J. Integr. Plant Biol. 2020, 62, 737–760. [Google Scholar] [CrossRef]
- Fairhurst, T.H.; Dobermann, A. Rice in the global food supply. Better Crops Int. 2002, 16, 3–6. [Google Scholar]
- Ding, Y.; Yang, S. Surviving and thriving: How plants perceive and respond to temperature stress. Dev. Cell 2022, 57, 947–958. [Google Scholar] [CrossRef]
- Soualiou, S.; Duan, F.Y.; Li, X.; Zhou, W.B. Crop production under cold stress: An understanding of plant responses, acclimation processes, and management strategies. Plant Physiol. Biochem. 2022, 190, 47–61. [Google Scholar] [CrossRef]
- Kovach, M.J.; Sweeney, M.T.; McCouch, S.R. New insights into the history of rice domestication. Trends Genet. 2007, 23, 578–587. [Google Scholar] [CrossRef]
- Gross, B.L.; Zhao, Z. Archaeological and genetic insights into the origins of domesticated rice. Proc. Natl. Acad. Sci. USA 2014, 111, 6190–6197. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, P.; Chen, Y.; Song, X.; Wei, X.; Shi, P.J. Global warming over 1960–2009 did increase heat stress and reduce cold stress in the major rice-planting areas across China. Eur. J. Agron. 2014, 59, 49–56. [Google Scholar] [CrossRef]
- Baier, M.; Bittner, A.; Prescher, A.; van Buer, J. Preparing plants for improved cold tolerance by priming. Plant Cell Environ. 2019, 42, 782–800. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Chong, K.; Xu, Y. Chilling tolerance in rice: Past and present. J. Plant Physiol. 2022, 268, 153576. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.H.; Jiang, N.; Fu, W.M.; Zheng, G.J.; Li, G.Y.; Feng, B.H.; Chen, T.T.; Ma, J.Y.; Li, H.B.; Tao, L.X.; et al. ATP hydrolysis determines cold tolerance by regulating available energy for glutathione synthesis in rice seedling plants. Rice 2020, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Feng, B.H.; Chen, T.T.; Zhang, C.X.; Tao, L.X.; Fu, G.F. Transcriptome analysis of pale-green leaf reveals photosynthetic regulatory pathways. Acta Physiol. Plant. 2017, 39, 274. [Google Scholar] [CrossRef]
- Zheng, S.; Su, M.; Wang, L.; Zhang, T.G.; Wang, J.; Xie, H.C.; Wu, X.X.; Haq, S.I.U.; Qiu, Q.S. Small signaling molecules in plant response to cold stress. J. Plant Physiol. 2021, 266, 153534. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Tominaga, Y.; Nakagawara, C.; Shigematsu, S.; Minami, A.; Kawamura, Y. Responses of the plasma membrane to low temperatures. Physiol. Plant. 2006, 126, 81–89. [Google Scholar] [CrossRef]
- Steponkus, P.L. Role of the plasma membrane in freezing injury and cold acclimation. Ann. Rev. Plant Physiol. 1984, 35, 543–584. [Google Scholar] [CrossRef]
- Smirnoff, N. BOTANICAL BRIEFING: The function and metabolism of ascorbic acid in plants. Ann. Bot. 1996, 78, 661–669. [Google Scholar] [CrossRef]
- Gallie, D.R. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot. 2013, 64, 433–443. [Google Scholar] [CrossRef]
- Ajila Celi, G.E.; Gratão, P.L.; Lanza, M.G.D.B.; Reis, A.R.D. Physiological and biochemical roles of ascorbic acid on mitigation of abiotic stresses in plants. Plant Physiol. Biochem. 2023, 202, 107970. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Barth, C.; De Tullio, M.; Conklin, P.L. The role of ascorbic acid in the control of flowering time and the onset of senescence. J. Exp. Bot. 2006, 57, 1657–1665. [Google Scholar] [CrossRef]
- Liao, G.L.; Xu, Q.; Allan, A.C.; Xu, X.B. L-Ascorbic acid metabolism and regulation in fruit crops. Plant Physiol. 2023, 192, 1684–1695. [Google Scholar] [CrossRef]
- Feng, B.H.; Li, G.Y.; Islam, M.; Fu, W.M.; Zhou, Y.Q.; Chen, T.T.; Tao, L.X.; Fu, G.F. Strengthened antioxidant capacity improves photosynthesis by regulating stomatal aperture and ribulose-1,5-bisphosphate carboxylase/oxygenase activity. Plant Sci. 2020, 290, 110245. [Google Scholar] [CrossRef]
- Kotchoni, S.O.; Larrimore, K.E.; Mukherjee, M.; Kempinski, C.F.; Barth, C. Alterations in the endogenous ascorbic acid content affect flowering time in Arabidopsis. Plant Physiol. 2009, 149, 803–815. [Google Scholar] [CrossRef]
- Majzoobi, M.; Beparva, P.; Farahnaky, A.; Badii, F. Physicochemical properties of cross-linked wheat starch affected by L-ascorbic acid. J. Agric. Sci. Technol. 2014, 16, 355–364. [Google Scholar]
- Gęgotek, A.; Skrzydlewska, E. Chapter nine—Ascorbic acid as antioxidant. Vitam. Horm. 2023, 121, 247–270. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Alhasnawi, A.N.; Kadhimi, A.A.; Isahak, A.; Mohamad, A.; Yusoff, W.M.W.; Zain, C.R.C.M. Exogenous application of ascorbic acid ameliorates detrimental effects of salt stress in rice (MRQ74 and MR269) seedlings. Asian J. Crop Sci. 2015, 7, 186–196. [Google Scholar] [CrossRef]
- Liu, T.; Hu, X.; Zhang, J. H2O2 mediates ALA-induced glutathione and ascorbate accumulation in the perception and resistance to oxidative stress in Solanum lycopersicum at low temperatures. BMC Plant Biol. 2018, 18, 34. [Google Scholar] [CrossRef]
- Monajjem, S.; Soltani, E.; Zainali, E.; Esfahani, M.; Ghaderi-Far, F.; Chaleshtori, M.H.; Rezaei, A. Seed priming improves enzymatic and biochemical performances of rice during seed germination under low and high temperatures. Rice Sci. 2023, 30, 335–347. [Google Scholar] [CrossRef]
- Hatsugai, N.; Katagiri, F. Quantification of plant cell death by electrolyte leakage assay. Bio. Protoc. 2018, 8, e2758. [Google Scholar] [CrossRef]
- Brennan, T.; Frenkel, C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Z.; Zhang, B.L. Measurements of proline and malondialdehyde content and antioxidant enzyme activities in leaves of drought stressed cotton. Bio. Protoc. 2016, 6, e1913. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Maehly, A.C. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [CrossRef]
- Bonnecarrère, V.; Borsani, O.; Díaz, P.; Capdevielle, F.; Blanco, P.; Monza, J. Response to photoxidative stress induced by cold in japonica rice is genotype dependent. Plant Sci. 2011, 180, 726–732. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zhang, C.X.; Feng, B.H.; Chen, T.T.; Fu, W.M.; Li, H.B.; Li, G.Y.; Jin, Q.Y.; Tao, L.X.; Fu, G.F. Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source-sink relationship and sugars allocation. Environ. Exp. Bot. 2018, 155, 718–733. [Google Scholar] [CrossRef]
- Feng, B.H.; Yang, Y.; Shi, Y.F.; Shen, H.C.; Wang, H.M.; Huang, Q.N.; Xu, X.; Lü, X.G.; Wu, J.L. Characterization and genetic analysis of a novel rice spotted-leaf mutant HM47 with broad-spectrum resistance to Xanthomonas oryzae pv. oryzae. J. Integr. Plant Biol. 2013, 55, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Yu, Z.G.; Qiao, L.Y. Study on the relationship between the activity of ascorbate oxidase and disease resistance in wheat. Hubei Agric. Sci. 2003, 1, 31–33. Available online: http://www.cqvip.com/qk/93129x/200301/7365556.html (accessed on 12 January 2024).
- Su, L.Y.; Dai, Z.W.; Li, S.H.; Xin, H.P. A novel system for evaluating drought–cold tolerance of grapevines using chlorophyll fluorescence. BMC Plant Biol. 2015, 15, 82. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Breusegem, F.V. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjarvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.H.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, A.; Dietz, K.J. Reactive oxygen species and the redox-regulatory network in cold stress acclimation. Antioxidants 2018, 7, 169. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhu, J.H.; Gong, Z.Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 5, 856–867. [Google Scholar] [CrossRef]
- Gusain, S.; Joshi, S.; Joshi, R. Sensing, signalling, and regulatory mechanism of cold-stress tolerance in plants. Plant Physiol. Biochem. 2023, 197, 107646. [Google Scholar] [CrossRef]
- Leisner, C.P.; Potnis, N.; Sanz-Saez, A. Crosstalk and trade-offs: Plant responses to climate change-associated abiotic and biotic stresses. Plant Cell Environ. 2023, 46, 2946–2963. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Saito, K. Integrated metabolomics for abiotic stress responses in plants. Curr. Opin. Plant Biol. 2015, 24, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Li, X.M.; Lin, H.X.; Chong, K. Crop improvement through temperature resilience. Annu. Rev. Plant Biol. 2019, 70, 753–780. [Google Scholar] [CrossRef]
- Huang, X.; Wu, Y.Q.; Zhang, S.S.; Yang, H.; Wu, W.L.; Lyu, L.F.; Li, W.L. Changes in antioxidant substances and antioxidant enzyme activities in raspberry fruits at different developmental stages. Sci. Hortic. 2023, 321, 12314. [Google Scholar] [CrossRef]
- Martin, R.E.; Postiglione, A.E.; Muday, G.K. Reactive oxygen species function as signaling molecules in controlling plant development and hormonal responses. Curr. Opin. Plant. Biol. 2022, 69, 102293. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Khan, M.S.S.; Ahmed, S.; Abdullah, M.; Hannan, F.; Chen, J. OsLPXC negatively regulates tolerance to cold stress via modulating oxidative stress, antioxidant defense and JA accumulation in rice. Free Radic. Biol. Med. 2023, 199, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Karami-Moalem, S.; Maali-Amiri, R.; Kazemi-Shahandashti, S.-S. Effect of cold stress on oxidative damage and mitochondrial respiratory properties in chickpea. Plant Physiol. Biochem. 2018, 122, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N. Ascorbic acid: Metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant Biol. 2000, 3, 229–235. [Google Scholar] [CrossRef]
- Macknight, R.C.; Laing, W.A.; Bulley, S.M.; Broad, R.C.; Johnson, A.A.; Hellens, R.P. Increasing ascorbate levels in crops to enhance human nutrition and plant abiotic stress tolerance. Curr. Opin. Biotechnol. 2017, 44, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Gamboa, L.M.; Liu, S.X.; Jarrod, W.C.; Zachary, C.C.; Raquel, T.; Jessica, P.Y.; Argelia, L. Characterization of the response to abiotic stresses of high ascorbate Arabidopsis lines using phenomic approaches. Plant Physiol. Biochem. 2020, 151, 500–515. [Google Scholar] [CrossRef]
- Aboobucker, S.I.; Lorence, A. Recent progress on the characterization of aldonolactone oxidoreductases. Plant Physiol. Biochem. 2016, 98, 171–185. [Google Scholar] [CrossRef]
- Pignocchi, C.; Foyer, C.H. Apoplastic ascorbate metabolism and its role in the regulation of cell signalling. Curr. Opin. Plant Biol. 2003, 6, 379–389. [Google Scholar] [CrossRef]
- Wheeler, G.; Jones, M.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Baig, M.M.; Kelly, S.; Loewus, F. L-ascorbic acid biosynthesis in higher plants from L-Gulono-1,4-lactone and L-Galactono-1, 4-lactone. Plant Physiol. 1970, 46, 277–280. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Pastori, G.M.; Foyer, C.H. Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol. 2000, 123, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Schertl, P.; Sunderhaus, S.; Klodmann, J.; Grozeff, G.E.G.; Bartoli, C.G.; Braun, H.P. L-galactono-1,4-lactone dehydrogenase (GLDH) forms part of three subcomplexes of mitochondrial complex I in Arabidopsis thaliana. J. Biol. Chem. 2012, 287, 14412–14419. [Google Scholar] [CrossRef] [PubMed]
- Munne-Bosch, S.; Queval, G.; Foyer, C.H. The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol. 2013, 161, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, J.; Park, S.W. Role of L-ascorbate in alleviating abiotic stresses in crop plants. Bot. Stud. 2014, 55, 38. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Kyndt, T.; Hancock, R.D. Vitamin C in plants: Novel concepts, new perspectives, and outstanding issues. Antioxid. Redox Signal 2020, 32, 463–485. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Lu, T.; Yan, W.; Yu, P.; Fu, W.; Li, J.; Su, X.; Chen, T.; Fu, G.; Wu, Z.; et al. Transcriptome and Metabolome Analyses Reveal Ascorbic Acid Ameliorates Cold Tolerance in Rice Seedling Plants. Agronomy 2024, 14, 659. https://doi.org/10.3390/agronomy14040659
Wang H, Lu T, Yan W, Yu P, Fu W, Li J, Su X, Chen T, Fu G, Wu Z, et al. Transcriptome and Metabolome Analyses Reveal Ascorbic Acid Ameliorates Cold Tolerance in Rice Seedling Plants. Agronomy. 2024; 14(4):659. https://doi.org/10.3390/agronomy14040659
Chicago/Turabian StyleWang, Huanran, Tingting Lu, Wenhui Yan, Pinghui Yu, Weimeng Fu, Juncai Li, Xiaona Su, Tingting Chen, Guanfu Fu, Zhihai Wu, and et al. 2024. "Transcriptome and Metabolome Analyses Reveal Ascorbic Acid Ameliorates Cold Tolerance in Rice Seedling Plants" Agronomy 14, no. 4: 659. https://doi.org/10.3390/agronomy14040659
APA StyleWang, H., Lu, T., Yan, W., Yu, P., Fu, W., Li, J., Su, X., Chen, T., Fu, G., Wu, Z., & Feng, B. (2024). Transcriptome and Metabolome Analyses Reveal Ascorbic Acid Ameliorates Cold Tolerance in Rice Seedling Plants. Agronomy, 14(4), 659. https://doi.org/10.3390/agronomy14040659









