Abstract
The impact of trap type and height on the captures of adults of the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), was assessed in cotton fields in three experimental sites (Nikaia, Nees Karyes, and Koilada) in Central Greece. Initially, the effectiveness of three different traps (white Delta, red Delta, and green Funnel) was compared. Subsequently, white Delta traps were positioned at three heights, i.e., 30, 60, and 90 cm, above ground level. Overall, captures notably increased in all traps from late July to mid-September. Funnel traps had poor performance for the captures of P. gossypiella adults, as compared with the respective figures for the other two Delta traps. Specifically, in two experimental sites (Nikaia and Koilada), both Delta traps cumulatively captured significantly more adults than the Funnel trap throughout the monitoring period. In the third site (Nees Karyes), the red Delta trap captured in total significantly more adults than the other two tested traps. Moreover, we found that traps in Nikaia placed at 90 cm captured significantly less adults as compared with the other two trap heights. The same trend was observed in Koilada; however, differences were significant only between traps at 30 and 90 cm. No significant differences were determined among different heights in the third site (Nees Karyes). These findings offer valuable insights for the monitoring protocols of P. gossypiella in cotton fields, on the basis of a standardized trapping strategy, that can take into account a wide range of factors, such as trap design and trap height.
1. Introduction
The pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), is a major pest of Malvaceae plants, particularly cotton [1,2]. This species has a global distribution and has been found to cause very serious infestation levels in many cotton-producing geographical zones [2,3]. In contrast with other moth species that infest cotton, the larval development of P. gossypiella is totally completed within the cotton boll, and the infestation is internal and cryptic, being visible from the exit hole, when it is too late to take control measures [4,5]. Moreover, it can rapidly build high population densities that completely destroy even 100% of the cotton production in a very short period of time. There are several reports that show that this species is expanding [6], despite the fact that it was recently successfully eradicated from the US [7].
Internal feeding necessitates the adoption of measures that control this species before entering the cotton boll; otherwise, any insecticidal application is likely to be ineffective. It is well established that P. gossypiella has developed a considerable level of resistance to several insecticides with different modes of action in many geographical zones [8]. At the same time, it has been found to be resistant to Cry1Ac- and Cry2Ab-expressing cotton [9,10,11]. In this context, control of this species should be based on a careful selection of measures that are to be taken, which, apart from the insecticides, should include other measures such as the application of plant growth regulators to accelerate cotton maturation [12], or the mechanical destruction of cotton residues in the field, to reduce the numbers of overwintering larvae [13].
Apart from the above, monitoring is considered as the keystone in any Integrated Pest Management (IPM)-based strategy against P. gossypiella [14,15]. This is usually carried out through samplings in the fruiting bodies of the plant, and, especially, the use of traps baited with the male attractant of the species, which is known commercially as gossyplure, i.e., Z,Z- and Z,E-hexadecadienyl acetate [16,17,18,19,20,21]. Athanassiou et al. [22] have shown that the species can be successfully monitored by both Funnel and sticky traps, but these trapping devices provided dissimilar results, in terms of the succession of the generations. In that study, the authors have underlined that Funnel traps, with the use of an insecticide as a killing agent, were more effective than sticky traps, but only when population densities were high, while trapping location and the amount of pheromone were less important [22]. Pheromone traps are capable of providing early detection estimates, while earlier studies have shown that trap captures can be used to predict infestation patterns in the cotton field [23,24], and, as a result, the concomitant insecticidal applications [25].
Although the trapping of moths in field studies is based on just trap deployment, this is only a theoretical approach, as improper trap placement may over- or underestimate population densities of a given species, leading to false estimations and predictions [26,27,28,29]. For another major pest of cotton, the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae), it was found that monitoring through pheromone traps was highly influenced by trap height, while this effect varied throughout the growing season [29]. Also, trap color is a critical parameter, a phenomenon that has not been clarified in detail in the case of moth species that are active during the dark hours of the day, such as P. gossypiella [30,31]. In this context, the availability of an extremely wide variety of commercially available traps and pheromones constitutes the selection of the devices that are to be used for a rather complicated procedure that requires additional attention.
Based on the above and considering that there is still inadequate information on the factors that affect the trapping of P. gossypiella, we performed a series of field tests to illustrate the influence of certain factors, such as trap type and trap height. For the experimentation, we selected the area of Thessaly, which is by far the most important cotton-producing geographical zone of the European Union (EU).
2. Materials and Methods
2.1. Experimental Sites
This field study took place in the regional unit of Larissa (Region of Thessaly, Central Greece), in three experimental fields, in Nikaia, Nees Karyes, and Koilada, throughout the 2019 growing season. The experimental site in Nikaia (altitude: 90 m; 22°81′ E and 39°34′ N) was a 6 Ha field surrounded by cotton and wheat fields, whereas the 2 Ha experimental field in Nees Karyes (altitude: 146 m; 22°28′ E and 39°30′ N) was bordered only by cotton fields. Finally, the experimental field in Koilada (altitude: 140 m; 22°18′ E and 39°34′ N) was 4 Ha and in between maize, sunflower, and wheat fields. These three locations were selected due to their large cotton-producing areas. In all sites, cotton planting occurred in mid- or end-April (Nikaia and Nees Karyes: 20 April 2019, variety Celia; Koilada: 28 April 2019, variety Elpida), followed by standard cultivation practices (e.g., drip irrigation, standard fertilization scheme, etc.). Trapping activities commenced in late June and concluded in late September 2019. Throughout this period, the recorded temperatures in the region of Larissa ranged from 15.2 to 31.0 °C in June, 17.7 to 33.1 °C in July and 17.3 to 32.6 °C in August. Rainfall amounts for June, July, and August were 25.6, 19.0, and 16.4 mm, respectively. The meteorological conditions prevailing during the trials, in terms of temperature and precipitation, were typical for summer in the region of Larissa (high temperatures and low precipitation).
2.2. Trap Types and Pheromones
Three trap types were used, i.e., white Delta traps (Trécé, Inc., Adair, IA, USA), red Delta traps (Russell IPM, Deeside, UK), and green Funnel traps (Hellafarm S.A., Stylida, Greece). The bottom of the white and red Delta traps was covered with a sticky cardboard sheet for the capture of male moths that was replaced after each evaluation, whereas transfluthrin-impregnated papers (0.4% w/w, VAPONA, Sarantis SA, Athens, Greece) were introduced inside each Funnel trap as killing agents. The pheromone lures (PecGos) used contained the gossyplure sex-attractant pheromone [(Z,Z) and (Z,E)-7,11-hexadecadienyl acetate] and were provided by Novagrica Hellas S.A. (Athina, Greece).
2.3. Experimental Design
In a first field trial, one trap from each of the three different trap types, i.e., a white Delta trap, a red Delta trap, and a green Funnel trap, was installed in each of the three experimental sites with their lowest part at 60 cm from the ground level, taking care to place them at a distance of 50 m from each other. Traps were inspected at regular intervals (4–5 days) starting from 28 June to 12 September. Traps were rotated after each inspection to mitigate the individual location effect, whereas pheromone lures were replaced every three weeks. In a second trial, three white Delta traps were installed in each of the three experimental sites at three different heights, namely, with their lowest part at 30, 60, and 90 cm above the ground, and traps were inspected as previously described.
2.4. Statistical Analysis
Data were first checked for normality and homogeneity of variances using Shapiro–Wilk and Levene’s tests, respectively. Afterwards, data were subjected to One-way Analysis of Variance (ANOVA) with adult captures as the response variable and trap type and trap height as the main effects. For the comparison of the means, the Tukey–Kramer HSD test was used at the 0.05 significance level [32]. Additionally, to assess the synchronization between pairs of captures across the different trap types on the same evaluation date, correlation coefficient values were computed.
3. Results
3.1. Trial I: Trap Type Effect
Significant differences were noted among the three trap types used regarding the overall data (Table 1). In Nikaia, both Delta traps captured more adults than the Funnel trap throughout the monitoring period, but this trend was expressed much more vigorously in the case of the second half of the period, when P. gossypiella captures were high (Figure 1A). Similar results have been reported in Nees Karyes, where red Delta was by far much superior than the other two traps (Figure 1B). In Koilada, the two Delta traps provided similar captures throughout the entire monitoring period (Figure 1C). Considering the overall data, significant differences among traps were noted from the sixth trap-check date, and until the end of the monitoring period (Figure 1D).

Table 1.
Mean number (±SE) of Pectinophora gossypiella adults per trap captured in each trap type.
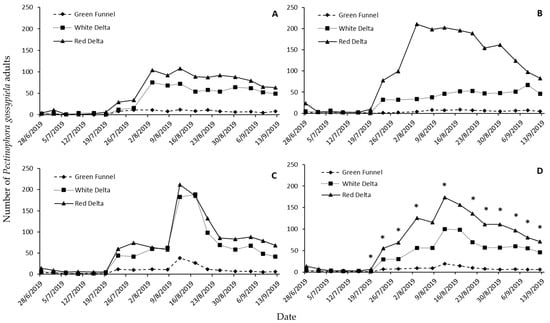
Figure 1.
Number of Pectinophora gossypiella adults per trap type (green Funnel, white Delta, red Delta) captured on each trap-inspection date in the experimental sites in Nikaia (A), Nees Karyes (B), Koilada (C), and cumulatively (D). Asterisks (*) denote significant differences among trap types.
Regarding the correlation coefficients’ values among pairs and traps in the different experimental sites, we found that in all cases these were positive and significant, given that their values ranged between 0.75 and 0.98 (Table 2). Finally, detection sensitivity data have shown that more than 11% of the Funnel traps captured no adults, while this percentage in the two types of Delta traps was zero (Table 3). Moreover, 11% of the red Delta traps captured more than 100 adults, while this percentage in the other two trap types was zero.

Table 2.
Correlation coefficient values for pairs of Pectinophora gossypiella captures between different trap types.

Table 3.
Trap sensitivity for captures of Pectinophora gossypiella adults in three types of traps (green Funnel, white Delta, red Delta) during the trial.
3.2. Trial II: Trap Height Effect
There were significant differences among treatments (Table 4). In Nikaia, the traps that had been placed at 30 and 60 cm had more adults than those that had been placed at 90 cm (Figure 2A). This difference was expressed more vigorously in the traps that had been placed in Nees Karyes, where captures in those that had been placed at 90 cm did not exceed 10 adults, for any of the dates examined (Figure 2B). In contrast, in Koilada captures had a pattern that was similar to that in Nikaia (Figure 2C). Comparing the overall data shows significant differences among the three trap heights, but only at the last five trap-check dates (Figure 2D).

Table 4.
Mean number (±SE) of Pectinophora gossypiella adults per trap captured in white Delta traps placed at three different heights (30, 60, and 90 cm).
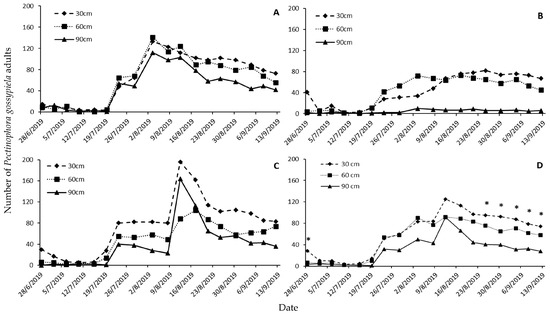
Figure 2.
Number of Pectinophora gossypiella adults per trap captured in white Delta traps placed at three different heights (30, 60, and 90 cm) on each trap-inspection date in the experimental sites in Nikaia (A), Nees Karyes (B), Koilada (C), and cumulatively (D). Asterisks (*) denote significant differences among trap heights.
Correlation coefficients’ values for the pairs of traps in the three areas were positive and significant (Table 5). Moreover, all traps contained at least one P. gossypiella adult throughout the monitoring period, and their detection sensitively was comparable, but the lowest percentage of traps that contained >100 adults was recorded for those that had been placed at 90 cm (Table 6).

Table 5.
Correlation coefficient values for pairs of white Delta traps placed at three different heights (30, 60, and 90 cm).

Table 6.
Trap sensitivity for captures of Pectinophora gossypiella adults in white Delta traps placed at three different heights (30, 60, and 90 cm) during the trial.
4. Discussion
Our results show that Funnel traps had poor performance on the captures of P. gossypiella adults, as compared with the respective figures for the other two trap types. This finding contradicts with data that had been previously reported from the same area (Thessaly) [22], where Funnel traps were more effective than the Delta traps. However, the Funnel traps (black stripe Funnel moth trap; Agrisense BCS, Pontypridd, UK) that had been used in those tests were different, and thus, these results may not be directly comparable with the findings of the current study. However, in sticky traps, the adults are captured on a sticky surface, and in many cases, Funnel traps require the addition of a killing agent, such as a tablet that contains insecticide, which may have a repulsive action to the adults that are attracted by the pheromonic source. Earlier studies have shown that the presence of an insecticide may moderate the capture capacity of trapping devices. For instance, the addition of plugs containing dichlorvos in McPhail traps reduced the captures of adults of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) [33].
Our overall trap performance demonstrated that Delta traps provided similar results, but at certain periods of our monitoring season, red traps performed better than the white ones. Several studies have shown that trap color is a critical parameter that determines trap performance for many moth species [30,31,34]. For the pine processionary moth, Thaumetopoea pityocampa (Denis & Schiffermüller) (Lepidoptera: Thaumetopoeidae), it has been found that white- and yellow-colored traps were more effective for the capture of adults when these traps had been placed in pine trees [35]. Similarly, Athanassiou et al. [36] found that Funnel traps of brighter colors were superior than those of darker colors for the capture of adults of the jasmine moth, Palpita unionalis (Hübner) (Lepidoptera: Pyralidae). However, traps performed in a dissimilar way only in some of the trap-check dates, which, in majority, were the dates when P. gossypiella captures were high. Apart from the effect of color as a visual stimulus to the insects that approach the trap, the color might have indirectly affected captures through its effect on non-target taxa. Indicatively, it has been reported that red- or green-colored pheromone sticky traps had less effect on non-target species, especially beneficial insects, as they caught fewer bees compared to the white, yellow, or blue traps, allowing more free space in the sticky surface for the target species [37]. Similar results have been reported by Bian et al. [38] who suggested that fewer natural enemies were captured on color-optimized sticky card traps compared to yellow commercial ones. Although we did not collect quantitative data for the captured non-target taxa individuals, we observed that red traps had lower non-target taxa numbers as compared with the white ones, which may be one of the main reasons for their highest efficacy on P. gossypiella. This effect may be important when P. gossypiella adult numbers were high, as this can be depicted in surfaces that are not saturated with large-bodied “visitor” species. Along these lines, it should be highlighted that since the use of traps for insect pest monitoring may exert a negative impact on beneficial insects (natural enemies, e.g., parasitoids and predators, pollinators, etc.), special care should be taken when designing monitoring strategies to mitigate their environmental impact.
Interestingly, the traps tested here exhibited a dissimilar performance in the different areas tested. Hence, both Delta traps performed in the same way in Nikaia and Koilada, but not in Nees Karyes. While we are unaware of the reasons that caused these dissimilarities, we hypothesize that this could be attributed to the non-target taxa numbers that might be higher in the case of the white traps. Also, these differences may have been influenced by the individual trapping locations, since some locations provided higher numbers of P. gossypiella adults than others. In fact, location is a critical parameter and should be taken into account when a trap-based monitoring protocol is planned. For instance, Karakantza et al. [39] reported high variation in the total captures of H. armigera adults among twenty cotton fields. The differences found among the traps that had been placed at different heights, although less pronounced as compared with those of the different trap types, were considerable during the second half of the monitoring period, when P. gossypiella adult numbers were high. We found that traps that had been placed at 90 cm captured less adults as compared with the other two trap categories. Given that most of the fruiting bodies of the cotton plants were, for most of the growing period, at low heights, it is likely that adults were mostly active in this zone. Also, trap performance in traps that had been placed at 90 cm might have been affected by factors that have less influence at lower heights (e.g., wind, etc.). Similarly, for H. armigera, it has been found that traps positioned at the canopy level in pigeon pea cultivation collected a greater quantity of male adults compared to traps positioned one and two feet above and below the canopy level [40]. However, this is not always the case, given that in another study, it was found that the installation of pheromone traps at different heights in cotton fields did not significantly affect adult H. armigera captures [29].
Apart from the differences noted here, all traps had a similar “synchronization” of captures, in terms of the population fluctuation during the entire experimental period. This means that all treatments tested provided similar information on the succession of generations of P. gossypiella throughout the monitoring period, and thus, all traps can be used with success to estimate the time of increased populations of this species. Nevertheless, trap performance was different in terms of early detection and trap sensitivity, i.e., the ability of certain traps to detect adults earlier than others. The detection sensitivity as categorized here underlines noticeable differences that also partially explain differential trap performance. For instance, a high number of the Funnel traps captured no P. gossypiella adults, which is indicative of their poorer performance for this species. Moreover, the fact that the red traps were the only ones that captured >100 adults underlines the ability of these traps to capture higher moth numbers when P. gossypiella population densities are high. High capture capacity and detection sensitivity may not be always combined in the same trap design, but there are previous studies that show that the detection sensitivity is a desirable characteristic, as they can time more accurately the decision for the application of control measures [22,29].
In summary, our tests underline the need for the adoption of a standardized trapping protocol, especially in area-wide management strategies against P. gossypiella in cotton. We demonstrated that specific traps are more effective than others, while trap height is an important parameter at certain periods of the growing season. Hence, unless these parameters are taken into account, the results of trapping may be inaccurate and lead to over- or underestimation of the population densities of this species. Given that larvae of this species are “internal feeders”, the early detection of adult activity is essential for the application of insecticides before the increase in the infestation level that is likely to constitute any measures considered ineffective.
5. Conclusions
To conclude, our results have shown significant differences in terms of captures of P. gossypiella adults between Funnel and Delta traps, with the latter performing better throughout the monitoring period. Additionally, we found that trap performance can be affected by the height of trap placement; however, this effect was not consistent across the experimental sites. Our findings aim to contribute to the optimization of the use of pheromone-baited traps for the monitoring of P. gossypiella adults as a tool to efficiently time control measures against this major pest of cotton.
Author Contributions
Conceptualization, C.G.A.; methodology, C.G.A.; investigation, G.C.K., T.N.V. and C.I.R.; resources, C.G.A.; data curation, G.C.K., T.N.V., and C.I.R.; writing—original draft preparation, C.G.A. and C.I.R.; writing—review and editing, C.G.A. and C.I.R.; visualization, C.I.R.; supervision, C.G.A.; funding acquisition, C.G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deguine, J.P.; Ferron, P.; Russell, D. Sustainable pest management for cotton production. A review. Agron. Sustain. Dev. 2008, 28, 113–137. [Google Scholar] [CrossRef]
- CABI Compendium. Pectinophora gossypiella (Pink Bollworm); CABI Head Office: Wallingford, UK, 2021; Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.39417 (accessed on 18 March 2024).
- Matheson, P.; Parvizi, E.; Fabrick, J.A.; Siddiqui, H.A.; Tabashnik, B.E.; Walsh, T.; McGaughran, A. Genome-wide analysis reveals distinct global populations of pink bollworm (Pectinophora gossypiella). Sci. Rep. 2023, 13, 11762. [Google Scholar] [CrossRef]
- Fand, B.B.; Nagrare, V.S.; Deshmukh, V.; Naikwadi, B.V.; Gokte-Narkhedkar, N.; Waghmare, V.N. A simple & low-cost laboratory rearing technique for cotton pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechidae) using detached green bolls of cotton. Phytoparasitica 2020, 48, 25–33. [Google Scholar] [CrossRef]
- Busnoor, A.V.; Wadaskar, R.M.; Fand, B.B.; Tambe, V.J.; Pillai, T.; Mahule, D.J.; Nagrare, V.S.; Prasad, Y.G. Laboratory evaluation of toxicity of selected insecticides against egg and larval stages of cotton pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae). J. Cotton Res. 2024, 7, 2. [Google Scholar] [CrossRef]
- Naik, V.B.; Pusadkar, P.P.; Waghmare, S.T.; Raghavendra, K.P.; Kranthi, S.; Kumbhare, S.; Nagrare, V.S.; Kumar, R.; Prabhulinga, T.; Gokte-Narkhedkar, N.; et al. Evidence for population expansion of Cotton pink bollworm Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae) in India. Sci. Rep. 2020, 10, 4740. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Liesner, L.R.; Ellsworth, P.C.; Unnithan, G.C.; Fabrick, J.A.; Naranjo, S.E.; Li, X.; Dennehy, T.J.; Antilla, L.; Staten, R.T.; et al. Transgenic cotton and sterile insect releases synergize eradication of pink bollworm a century after it invaded the United States. Proc. Natl. Acad. Sci. USA 2021, 118, e2019115118. [Google Scholar] [CrossRef]
- Kranthi, K.R.; Jadhav, D.R.; Kranthi, S.; Wanjari, R.R.; Ali, S.S.; Russell, D.A. Insecticide resistance in five major insect pests of cotton in India. Crop Prot. 2002, 21, 449–460. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Liu, Y.B.; de Maagd, R.A.; Dennehy, T.J. Cross-resistance of pink bollworm (Pectinophora gossypiella) to Bacillus thuringiensis toxins. Appl. Environ. Microbiol. 2000, 66, 4582–4584. [Google Scholar] [CrossRef]
- Dhurua, S.; Gujar, G.T. Field-evolved resistance to Bt toxin Cry1Ac in the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), from India. Pest Manag. Sci. 2011, 67, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Fabrick, J.A.; Li, X.; Carrière, Y.; Tabashnik, B.E. Molecular genetic basis of lab- and field-selected Bt resistance in pink bollworm. Insects 2023, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Kittock, D.L.; Mauney, J.R.; Arle, H.F.; Bariola, L.A. Termination of late season cotton fruiting with growth regulators as an insect-control technique. J. Environ. Qual. 1973, 2, 405–408. [Google Scholar] [CrossRef]
- Grefenstette, B.; El-Lissy, O.; Staten, R.T. Pink Bollworm Eradication Plan in the U.S. 2009. Available online: https://www.aphis.usda.gov/plant_health/plant_pest_info/cotton_pests/downloads/pbw-erad-plan2-08.pdf (accessed on 24 February 2024).
- Henneberry, T.J. Integrated systems for control of the pink bollworm Pectinophora gossypiella in cotton. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 567–580. [Google Scholar]
- Roelofs, W.L.; Carde, R.T. Responses of Lepidoptera to synthetic sex pheromone chemicals and their analogues. Annu. Rev. Entomol. 1977, 22, 377–405. [Google Scholar] [CrossRef]
- Carrière, Y.; Ellers-Kirk, C.; Pedersen, B.; Haller, S.; Antilla, L. Predicting spring moth emergence in the pink bollworm (Lepidoptera: Gelechiidae): Implications for managing resistance to transgenic cotton. J. Econ. Entomol. 2001, 94, 1012–1021. [Google Scholar] [CrossRef]
- Tamhankar, A.J.; Rajendran, T.P.; Mamdapur, V.R. Evaluation of a pheromone trap for the cotton pink bollworm Pectinophora gossypiella Saunders. Int. J. Pest Manag. 2001, 47, 179–180. [Google Scholar] [CrossRef]
- Byers, J.A.; Naranjo, S.E. Detection and monitoring of pink bollworm moths and invasive insects using pheromone traps and encounter rate models. J. Appl. Ecol. 2014, 51, 1041–1049. [Google Scholar] [CrossRef]
- Haynes, K.F.; Miller, T.A.; Staten, R.T.; Li, W.-G.; Baker, T.C. Pheromone trap for monitoring insecticide resistance in the pink bollworm moth (Lepidoptera: Gelechiidae): New tool for resistance management. Environ. Entomol. 1987, 16, 84–89. [Google Scholar] [CrossRef]
- Neumark, S.; Jacobson, M.; Teich, I. Evaluation of gossyplure, compared with hexalure, for monitoring pink bollworm infestations in cotton fields of Israel. Environ. Lett. 1975, 10, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kabissa, J.C.B. Seasonal occurrence and damage by Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae) to cotton in Eastern Tanzania. Trop. Pest Manag. 1990, 36, 356–358. [Google Scholar] [CrossRef]
- Athanassiou, C.; Kavallieratos, N.; Gravanis, F.; Koukounitsas, N.A.; Roussou, D.E. Influence of trap type, pheromone quantity and trapping location on capture of the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae). Appl. Entomol. Zool. 2002, 37, 385–391. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Ahmad, N.; Hussain, T. Pheromone trap catches as a means of predicting damage by pink bollworm larvae in cotton. Crop Prot. 1993, 12, 597–600. [Google Scholar] [CrossRef]
- Buchelos, C.T.; Athanassiou, C.G.; Papapostolou, C.C.; Georgiou, A. Correlation between the number of adult male Pectinophora gossypiella (Saund.) (Lep., Gelechidae) catches on pheromone traps and the rate of infestation in fruiting bodies of cotton plants by young larvae in three regions of central Greece. J. Appl. Entomol. 1999, 123, 433–436. [Google Scholar] [CrossRef]
- Milonas, P.; Gogou, C.; Papadopoulou, A.; Fountas, S.; Liakos, V.; Papadopoulos, N.T. Spatio-temporal distribution of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) and Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae) in a cotton production area. Neotrop. Entomol. 2016, 45, 240–251. [Google Scholar] [CrossRef]
- Krishnananda, N.; Satyanarayana, S.V.V. Effect of height of pheromone trap on the capture of Spodoptera litura moths in tobacco nurseries. Phytoparasitica 1985, 13, 59–62. [Google Scholar] [CrossRef]
- Elkinton, J.S.; Cardé, R.T. Effects of intertrap distance and wind direction on the interaction of gypsy moth (Lepidoptera: Lymantriidae) pheromone- baited traps. Environ. Entomol. 1988, 17, 764–769. [Google Scholar] [CrossRef]
- Kong, W.N.; Hu, R.S.; Zhao, Z.G.; Li, J.; Zhang, Z.W.; Li, S.C.; Ma, R.Y. Effects of trap height, location, and spacing on pheromone-baited trap catch efficacy for oriental fruit moths (Lepidoptera: Tortricidae) in a peach orchard. Can. Entomol. 2014, 146, 684–692. [Google Scholar] [CrossRef]
- Karakasis, A.; Lampiri, E.; Rumbos, C.I.; Athanassiou, C.G. Factors Affecting Adult captures of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in pheromone-baited traps. Agronomy 2021, 11, 2539. [Google Scholar] [CrossRef]
- Mitchell, E.R.; Agee, H.R.; Heath, R.R. Influence of pheromone trap color and design on capture of male velvet bean caterpillar and fall armyworm moths (Lepidoptera: Noctuidae). J. Chem. Ecol. 1989, 15, 1775–1784. [Google Scholar] [CrossRef]
- Kwon, J.H.; Huh, M.J.; Lee, D.H.; Seo, S.-M.; Park, I.K. Effect of pheromone blends, trap type and color on the capture of male clearwing moths, Synanthedon bicingulata (Lepidoptera: Sesiidae). Chemoecology 2021, 31, 289–299. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry, 3rd ed.; Freeman: New York, NY, USA, 1995; p. 877. [Google Scholar]
- Katsoyannos, B.; Papadopoulos, N.; Heath, R.; Hendrichs, J.; Kouloussis, N. Evaluation of synthetic food-based attractants for female Mediterranean fruit flies (Dipt., Tephritidae) in McPhail type traps. J. Appl. Entomol. 1999, 123, 607–612. [Google Scholar] [CrossRef]
- Sukovata, L.; Dziuk, A.; Parratt, M.; Bystrowski, C.; Dainton, K.; Polaszek, A.; Moore, R. The importance of trap type, trap colour and capture liquid for catching Dendrolimus pini and their impact on by-catch of beneficial insects. Agric. For. Entomol. 2020, 22, 319–327. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Gakis, S.F.; Kyrtsa, L.A.; Mazomenos, B.E.; Gravanis, F.T. Influence of trap type, trap colour, and trapping location on the capture of the pine moth, Thaumetopoea pityocampa. Entomol. Exp. Appl. 2007, 122, 117–123. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Mazomenos, B.E. Effect of trap type, trap color, trapping location, and pheromone dispenser on captures of male Palpita unionalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 2004, 97, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Clare, G.; Suckling, D.M.; Bradley, S.J.; Walker, J.T.S.; Shaw, P.W.; Daly, J.M.; McLaren, G.F.; Wearing, C.H. Pheromone trap colour determines catch of non-target insects. N. Z. Plant Prot. 2000, 53, 216–220. [Google Scholar] [CrossRef]
- Bian, L.; Yang, P.X.; Yao, Y.J.; Luo, Z.X.; Cai, X.M.; Chen, Z.M. Effect of trap color, height, and orientation on the capture of yellow and stick tea thrips (Thysanoptera: Thripidae) and nontarget insects in tea gardens. J. Econ. Entomol. 2016, 109, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Karakantza, E.; Rumbos, C.I.; Cavalaris, C.; Athanassiou, C.G. Different trap types depict dissimilar spatio-temporal distribution of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in cotton fields. Agronomy 2023, 13, 1256. [Google Scholar] [CrossRef]
- Priyanka, L.S.; Saminathan, V.R.; Sithanantham, S.; Ambethgar, V.; Manivannan, N. Evaluation of pheromone traps for the management of pod borer, Helicoverpa armigera (Hubner) in Redgram [Cajanus cajan (L.) Millsp.] ecosystem. Res. Biot. 2020, 2, 8–10. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).