The Effects of Brassinosteroids on Nitrogen Utilization in Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Rice Seedling Culture
2.2. Extraction of RNA and Quantitative Real-Time PCR Analysis
2.3. Determination of Nitrate Content
2.4. Determination of Ammonium Content
2.5. Enzyme Activity Analysis
2.6. Determination of Total Free Amino Acids
2.7. Data Analysis
3. Results
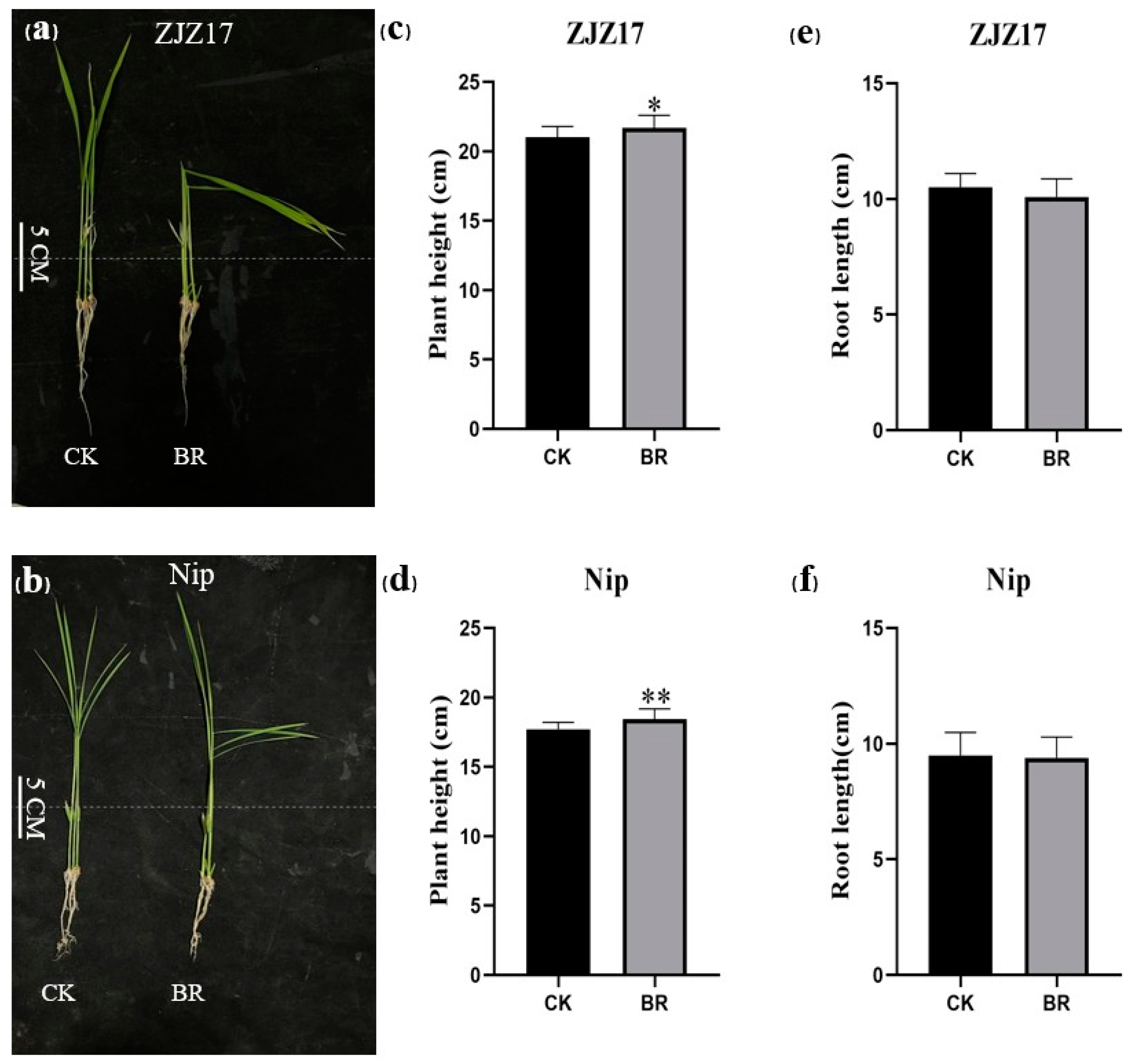
3.1. Rice Seedling Growth in Response to BRs
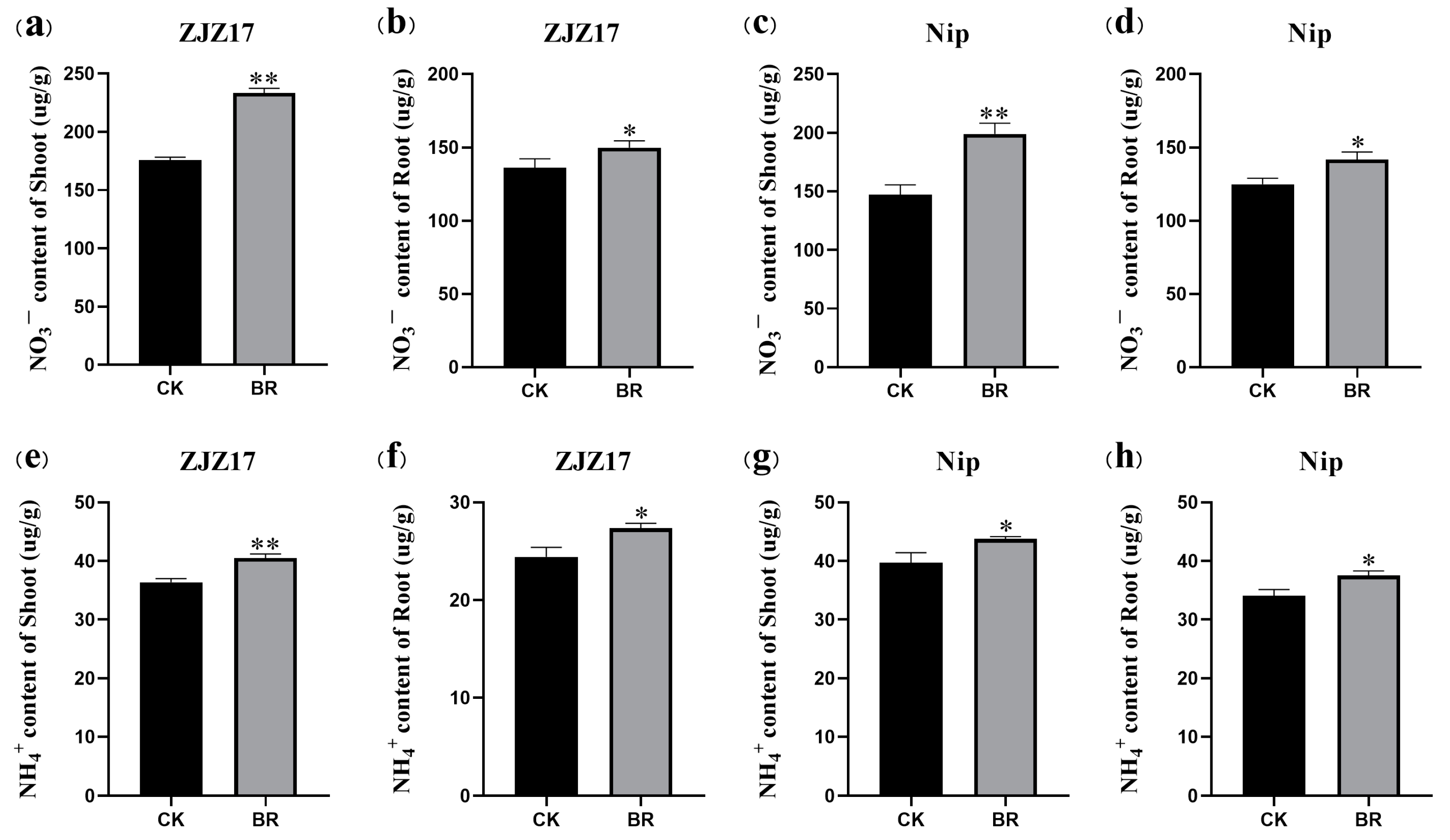
3.2. The Effects of BRs on Nitrate and Ammonium Accumulation in Rice
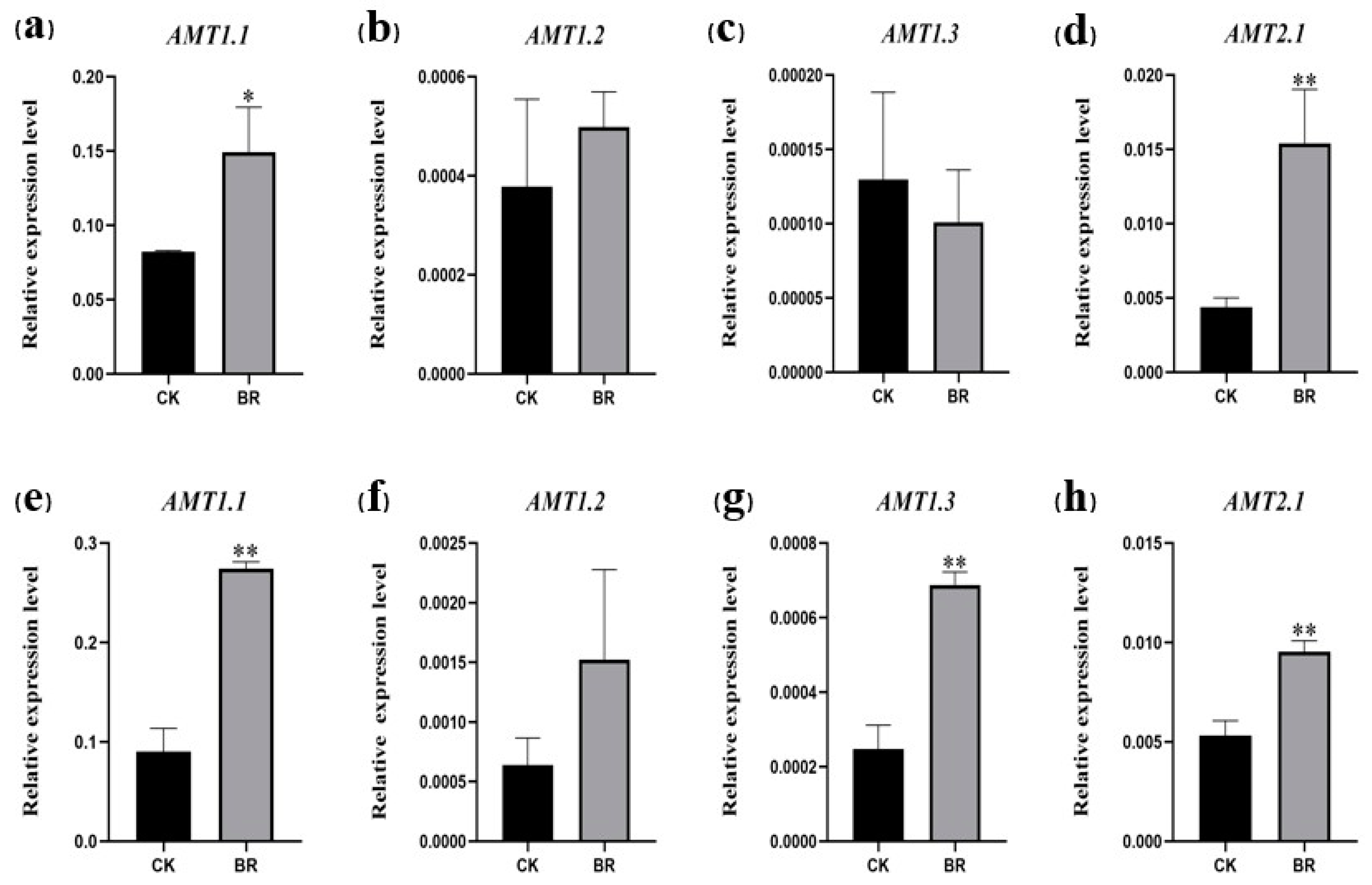
3.3. The Effects of BRs on the Expression of Rice Nitrogen Uptake-Related Genes
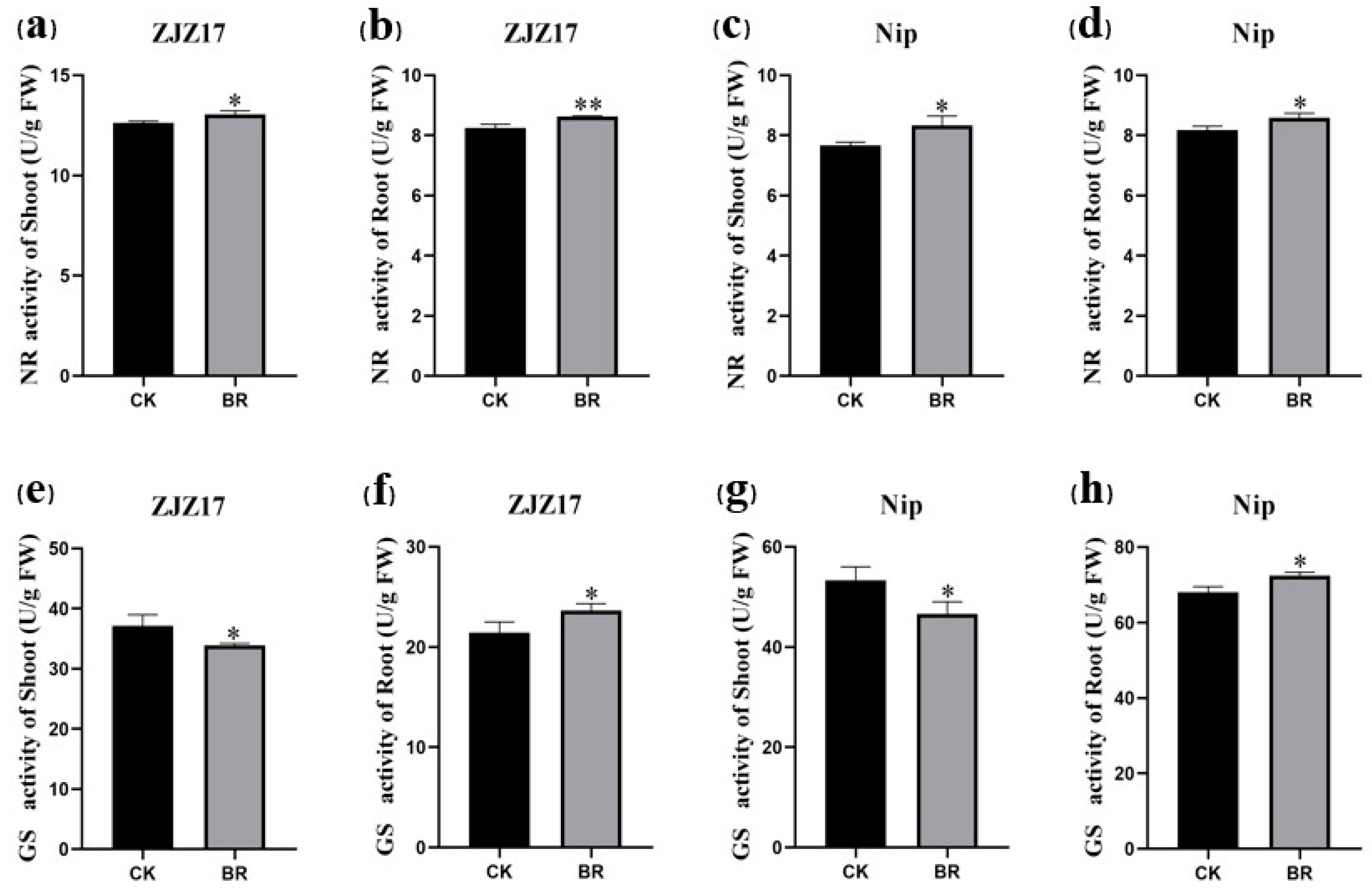
3.4. Influence of BRs on Nitrogen Assimilating Enzyme Activities in Rice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, N.-Q.; Lin, H.-X. Higher yield with less nitrogen fertilizer. Nat. Plants 2020, 6, 1078–1079. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, M.; Liu, Y.; Shi, L. Integration of the metabolome and transcriptome reveals the resistance mechanism to low nitrogen in wild soybean seedling roots. Environ. Exp. Bot. 2020, 175, 104043. [Google Scholar]
- Chai, Y.; Pannell, D.J.; Pardey, P.G. Nudging farmers to reduce water pollution from nitrogen fertilizer. Food Policy 2023, 120, 102525. [Google Scholar]
- Jain, A.K. Greenhouse gas emissions from nitrogen fertilizers. Nat. Food 2023, 4, 139–140. [Google Scholar] [PubMed]
- Gao, Z.; Wang, Y.; Chen, G.; Zhang, A.; Yang, S.; Shang, L.; Wang, D.; Ruan, B.; Liu, C.; Jiang, H.; et al. The indica nitrate reductase gene OsNR2 allele enhances rice yield potential and nitrogen use efficiency. Nat. Commun. 2019, 10, 5207. [Google Scholar]
- Marmagne, A.; Masclaux-Daubresse, C.; Chardon, F. Modulation of plant nitrogen remobilization and postflowering nitrogen uptake under environmental stresses. J. Plant Physiol. 2022, 277, 153781. [Google Scholar] [PubMed]
- Li, Z.; Na Wu, X.; Jacquot, A.; Chaput, V.; Adamo, M.; Neuhäuser, B.; Straub, T.; Lejay, L.; Schulze, W.X. Phosphoregulation in the N-terminus of NRT2.1 affects nitrate uptake by controlling the interaction of NRT2.1 with NAR2.1 and kinase HPCAL1 in Arabidopsis. J. Exp. Bot. 2023, erad490. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, N. Molecular Mechanism Underlying the Plant NRT1.1 Dual-Affinity Nitrate Transporter. Front. Physiol. 2015, 6, 386. [Google Scholar] [PubMed]
- Hou, M.; Yu, M.; Li, Z.; Ai, Z.; Chen, J. Molecular Regulatory Networks for Improving Nitrogen Use Efficiency in Rice. Int. J. Mol. Sci. 2021, 22, 9040. [Google Scholar] [PubMed]
- Yang, S.; Yuan, D.; Zhang, Y.; Sun, Q.; Xuan, Y.H. BZR1 Regulates Brassinosteroid-Mediated Activation of AMT1;2 in Rice. Front. Plant Sci. 2021, 12, 665883. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Yang, S.; Huang, Y.; Su, Y. Identification of structural elements involved in fine-tuning of the transport activity of the rice ammonium transporter OsAMT1;3. Plant Physiol. Biochem. 2016, 108, 99–108. [Google Scholar] [CrossRef]
- Konishi, N.; Ishiyama, K.; Beier, M.P.; Inoue, E.; Kanno, K.; Yamaya, T.; Takahashi, H.; Kojima, S. Contributions of two cytosolic glutamine synthetase isozymes to ammonium assimilation in Arabidopsis roots. J. Exp. Bot. 2016, 68, 613–625. [Google Scholar] [CrossRef]
- Tong, H.; Chu, C. Functional Specificities of Brassinosteroid and Potential Utilization for Crop Improvement. Trends Plant Sci. 2018, 23, 1016–1028. [Google Scholar]
- Luo, X.; Zheng, J.; Huang, R.; Huang, Y.; Wang, H.; Jiang, L.; Fang, X. Phytohormones signaling and crosstalk regulating leaf angle in rice. Plant Cell Rep. 2016, 35, 2423–2433. [Google Scholar]
- Yang, Y.; Chu, C.; Qian, Q.; Tong, H. Leveraging brassinosteroids towards the next Green Revolution. Trends Plant Sci. 2024, 29, 86–98. [Google Scholar] [PubMed]
- Gao, X.; Zhang, J.-Q.; Zhang, X.; Zhou, J.; Jiang, Z.; Huang, P.; Tang, Z.; Bao, Y.; Cheng, J.; Tang, H.; et al. Rice qGL3/OsPPKL1 Functions with the GSK3/SHAGGY-Like Kinase OsGSK3 to Modulate Brassinosteroid Signaling. Plant Cell 2019, 31, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Willige, B.C.; Jaillais, Y.; Geng, S.; Park, M.Y.; Gray, W.M.; Chory, J. BRASSINOSTEROID-SIGNALING KINASE 3, a plasma membrane-associated scaffold protein involved in early brassinosteroid signaling. PLoS Genet. 2019, 15, e1007904. [Google Scholar]
- Tian, P.; Liu, J.; Yan, B.; Zhou, C.; Wang, H.; Shen, R. BRASSINOSTEROID-SIGNALING KINASE1-1, a positive regulator of brassinosteroid signalling, modulates plant architecture and grain size in rice. J. Exp. Bot. 2022, 74, 283–295. [Google Scholar]
- Yin, W.; Li, L.; Yu, Z.; Zhang, F.; Liu, D.; Wu, H.; Niu, M.; Meng, W.; Zhang, X.; Dong, N.; et al. The divergence of brassinosteroid sensitivity between rice subspecies involves natural variation conferring altered internal auto-binding of OsBSK2. J. Integr. Plant Biol. 2022, 64, 1614–1630. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Y.; Xu, X.; Ran, M.; Zhang, J.; Deng, K.; Ji, G.; Xiao, L.; Zhou, X. Transcriptome and functional analysis revealed the intervention of brassinosteroid in regulation of cold induced early flowering in tobacco. Front. Plant Sci. 2023, 14, 1136884. [Google Scholar] [CrossRef]
- Wang, S.-q.; Zhao, H.-h.; Zhao, L.-m.; Gu, C.-m.; Na, Y.-g.; Xie, B.; Cheng, S.-h.; Pan, G.-j. Application of brassinolide alleviates cold stress at the booting stage of rice. J. Integr. Agric. 2020, 19, 975–987. [Google Scholar]
- Yu, B.; Wang, L.; Guan, Q.; Xue, X.; Gao, W.; Nie, P. Exogenous 24-epibrassinolide promoted growth and nitrogen absorption and assimilation efficiency of apple seedlings under salt stress. Front. Plant Sci. 2023, 14, 1178085. [Google Scholar]
- Lin, J.-H.; Xu, Z.-J.; Peng, J.-S.; Zhao, J.; Zhang, G.-B.; Xie, J.; Yi, Z.-X.; Zhang, J.-H.; Gong, J.-M.; Ye, N.-H.; et al. OsProT1 and OsProT3 Function to Mediate Proline- and γ-aminobutyric acid-specific Transport in Yeast and are Differentially Expressed in Rice (Oryza sativa L.). Rice 2019, 12, 79. [Google Scholar]
- Cataldo, D.A.; Haroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Schneidereit, J.; Häusler, R.E.; Fiene, G.; Kaiser, W.M.; Weber, A.P.M. Antisense repression reveals a crucial role of the plastidic 2-oxoglutarate/malate translocator DiT1 at the interface between carbon and nitrogen metabolism. Plant J. 2006, 45, 206–224. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H. A modified ninhydrin colorimetric analysis for amino acids. Arch. Biochem. Biophys. 1957, 67, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Flörchinger, M.; Kunz, H.-H.; Traub, M.; Wartenberg, R.; Jeblick, W.; Neuhaus, H.E.; Möhlmann, T. Uridine-ribohydrolase is a key regulator in the uridine degradation pathway of Arabidopsis. Plant Cell 2009, 21, 876–891. [Google Scholar] [CrossRef]
- Guo, N.; Zhang, S.; Gu, M.; Xu, G. Function, transport, and regulation of amino acids: What is missing in rice? Crop J. 2021, 9, 530–542. [Google Scholar]
- Wu, X.; Ding, C.; Baerson, S.R.; Lian, F.; Lin, X.; Zhang, L.; Wu, C.; Hwang, S.-Y.; Zeng, R.; Song, Y. The roles of jasmonate signalling in nitrogen uptake and allocation in rice (Oryza sativa L.). Plant Cell Environ. 2019, 42, 659–672. [Google Scholar] [CrossRef]
- Bai, L.; Deng, H.; Zhang, X.; Yu, X.; Li, Y. Gibberellin Is Involved in Inhibition of Cucumber Growth and Nitrogen Uptake at Suboptimal Root-Zone Temperatures. PLoS ONE 2016, 11, e0156188. [Google Scholar]
- Yan, Y.; Zhang, Z.; Sun, H.; Liu, X.; Xie, J.; Qiu, Y.; Chai, T.; Chu, C.; Hu, B. Nitrate confers rice adaptation to high ammonium by suppressing its uptake but promoting its assimilation. Mol. Plant 2023, 16, 1871–1874. [Google Scholar] [CrossRef]
- Bao, A.; Liang, Z.; Zhao, Z.; Cai, H. Overexpressing of OsAMT1-3, a High Affinity Ammonium Transporter Gene, Modifies Rice Growth and Carbon-Nitrogen Metabolic Status. Int. J. Mol. Sci. 2015, 16, 9037–9063. [Google Scholar] [CrossRef]
- Ferreira, L.M.; de Souza, V.M.; Tavares, O.C.H.; Zonta, E.; Santa-Catarina, C.; de Souza, S.R.; Fernandes, M.S.; Santos, L.A. OsAMT1.3 expression alters rice ammonium uptake kinetics and root morphology. Plant Biotechnol. Rep. 2015, 9, 221–229. [Google Scholar]
- Xuan, Y.H.; Duan, F.Y.; Je, B.I.; Kim, C.M.; Li, T.Y.; Liu, J.M.; Park, S.J.; Cho, J.H.; Kim, T.H.; von Wiren, N.; et al. Related to ABI3/VP1-Like 1 (RAVL1) regulates brassinosteroid-mediated activation of AMT1;2 in rice (Oryza sativa). J. Exp. Bot. 2016, 68, 727–737. [Google Scholar]
- Lamig, L.; Moreno, S.; Álvarez, J.M.; Gutiérrez, R.A. Molecular mechanisms underlying nitrate responses in plants. Curr. Biol. 2022, 32, R433–R439. [Google Scholar] [CrossRef]
- Galangau, F.; Daniel-vedele, F.; Moureaux, T.; Dorbe, M.F.; Leydecker, M.T.; Caboche, M. Expression of leaf nitrate reductase genes from tomato and tobacco in relation to light-dark regimes and nitrate supply. Plant Physiol. 1988, 88, 383–388. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kwon, Y.J.; Kim, S.-I.; Kim, D.Y.; Song, J.T.; Seo, H.S. Ammonium Inhibits Chromomethylase 3-Mediated Methylation of the Arabidopsis Nitrate Reductase Gene NIA2. Front. Plant Sci. 2016, 6, 168430. [Google Scholar] [CrossRef]
- Han, R.-c.; Xu, Z.-r.; Li, C.-y.; Rasheed, A.; Pan, X.-h.; Shi, Q.-h.; Wu, Z.-m. The removal of nitrate reductase phosphorylation enhances tolerance to ammonium nitrogen deficiency in rice. J. Integr. Agric. 2022, 21, 631–643. [Google Scholar] [CrossRef]
- He, W.; Wang, L.; Lin, Q.; Feng, Y. Rice seed storage proteins: Biosynthetic pathways and the effects of environmental factors. J. Integr. Plant Biol. 2021, 63, 1999–2019. [Google Scholar] [CrossRef]
- Kulaeva, O.N.; Burkhanova, E.A.; Fedina, A.B.; Khokhlova, V.A.; Bokebayeva, G.A.; Vorbrodt, H.-M.; Adam, G.N. Effect of brassinosteroids on protein synthesis and plant-cell ultrastructure under stress conditions. ACS Symp. Ser. 1991, 474, 141–155. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Xie, Q.; Tong, H.; Chu, C. Crosstalk between brassinosteroid signaling and variable nutrient environments. Sci. China Life Sci. 2023, 66, 1231–1244. [Google Scholar]
- Qin, X.; Li, X.; Xiao, J.; Wu, Q.; Li, Y.; Li, C.; Jiang, D.; Tang, T.; Nan, W.; Liang, Y.; et al. Transcriptomic and Physiological Analyses of Two Rice Restorer Lines under Different Nitrogen Supplies Provide Novel Insights into Hybrid Rice Breeding. Plants 2023, 12, 2276. [Google Scholar] [CrossRef]
- Qiao, Y.; Yin, L.; Wang, B.; Ke, Q.; Deng, X.; Wang, S. Melatonin promotes plant growth by increasing nitrogen uptake and assimilation under nitrogen deficient condition in winter wheat. Plant Physiol. Biochem. 2019, 139, 342–349. [Google Scholar] [CrossRef]
- Mohn, M.A.; Thaqi, B.; Fischer-Schrader, K. Isoform-Specific NO Synthesis by Arabidopsis thaliana Nitrate Reductase. Plants 2019, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. NO source in higher plants: Present and future of an unresolved question. Trends Plant Sci. 2022, 27, 116–119. [Google Scholar] [CrossRef]
- Astier, J.; Gross, I.; Durner, J. Nitric oxide production in plants: An update. J. Exp. Bot. 2018, 69, 3401–3411. [Google Scholar] [PubMed]
- Piacentini, D.; Della Rovere, F.; Lanni, F.; Cittadini, M.; Palombi, M.; Fattorini, L.; Cecchetti, V.; Altamura, M.M.; Falasca, G. Brassinosteroids interact with nitric oxide in the response of rice root systems to arsenic stress. Environ. Exp. Bot. 2023, 209, 105287. [Google Scholar] [CrossRef]
- Arredondo-Peter, R.; Moran, J.F.; Sarath, G. Rice (Oryza) hemoglobins. F1000Res 2014, 3, 253. [Google Scholar]
- Huwald, D.; Schrapers, P.; Kositzki, R.; Haumann, M.; Hemschemeier, A. Characterization of unusual truncated hemoglobins of Chlamydomonas reinhardtii suggests specialized functions. Planta 2015, 242, 167–185. [Google Scholar]
- Sanz-Luque, E.; Ocaña-Calahorro, F.; Galván, A.; Fernández, E. THB1 regulates nitrate reductase activity and THB1 and THB2 transcription differentially respond to NO and the nitrate/ammonium balance in Chlamydomonas. Plant Signal. Behav. 2015, 10, e1042638. [Google Scholar] [CrossRef]
- Calatrava, V.; Chamizo-Ampudia, A.; Sanz-Luque, E.; Ocaña-Calahorro, F.; Llamas, A.; Fernandez, E.; Galvan, A. How Chlamydomonas handles nitrate and the nitric oxide cycle. J. Exp. Bot. 2017, 68, 2593–2602. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Wan, G.-F.; Zhou, J.-Q.; Song, G.-C.; Zhao, J.; Huang, F.-L.; Meng, S. The Effects of Brassinosteroids on Nitrogen Utilization in Rice. Agronomy 2024, 14, 604. https://doi.org/10.3390/agronomy14030604
Yang W, Wan G-F, Zhou J-Q, Song G-C, Zhao J, Huang F-L, Meng S. The Effects of Brassinosteroids on Nitrogen Utilization in Rice. Agronomy. 2024; 14(3):604. https://doi.org/10.3390/agronomy14030604
Chicago/Turabian StyleYang, Wei, Guo-Feng Wan, Jia-Qi Zhou, Gen-Cai Song, Jing Zhao, Feng-Lin Huang, and Shuan Meng. 2024. "The Effects of Brassinosteroids on Nitrogen Utilization in Rice" Agronomy 14, no. 3: 604. https://doi.org/10.3390/agronomy14030604
APA StyleYang, W., Wan, G.-F., Zhou, J.-Q., Song, G.-C., Zhao, J., Huang, F.-L., & Meng, S. (2024). The Effects of Brassinosteroids on Nitrogen Utilization in Rice. Agronomy, 14(3), 604. https://doi.org/10.3390/agronomy14030604




