Tobacco/Salvia miltiorrhiza Intercropping Improves Soil Quality and Increases Total Production Value
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Measurement Items and Methods
2.3.1. Determination of Chemical Composition and Quality Evaluation of Tobacco Leaves after Flue-Curing
2.3.2. Soil Biological Shape Determination
2.4. Data Processing
3. Results
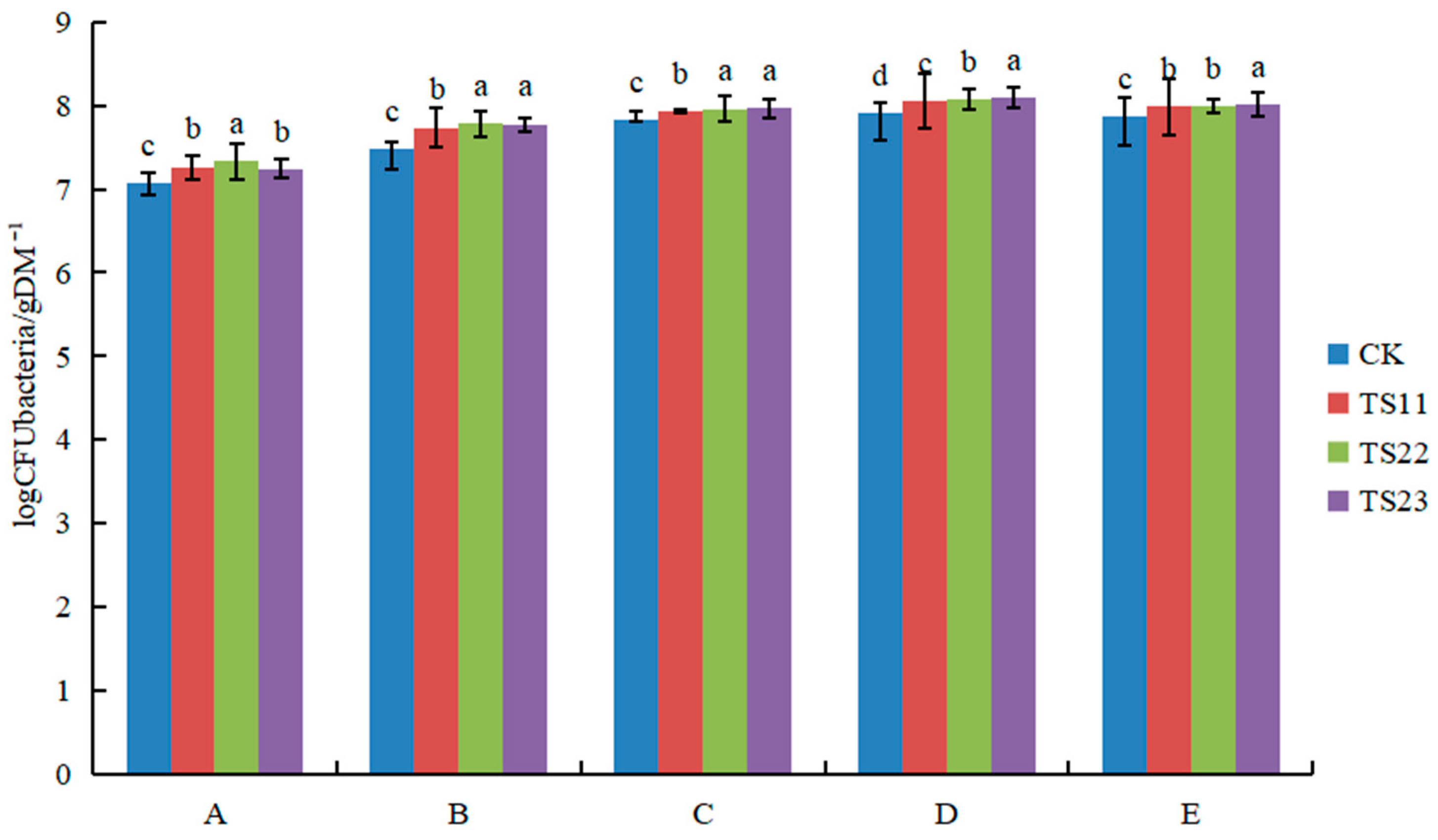
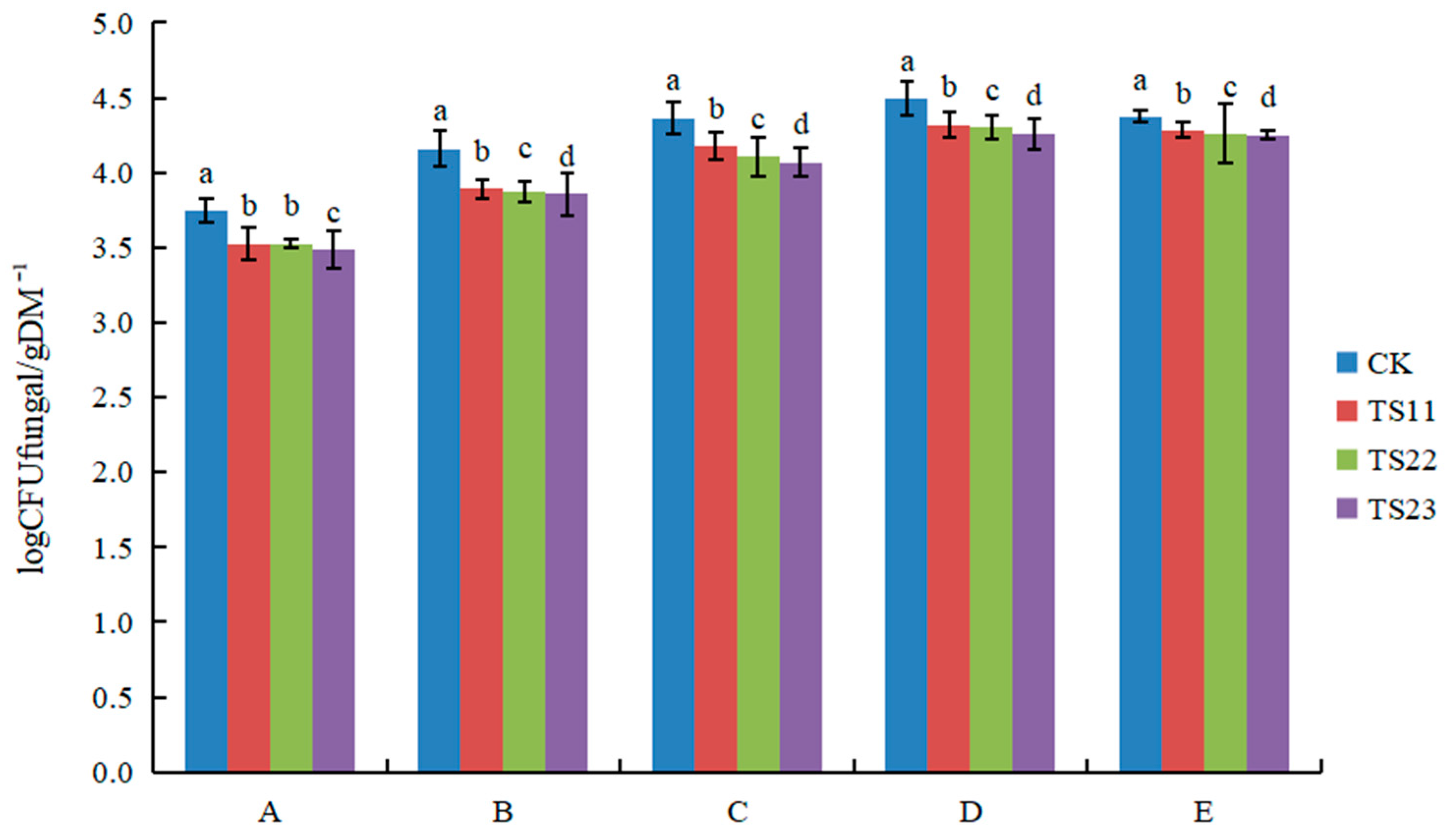
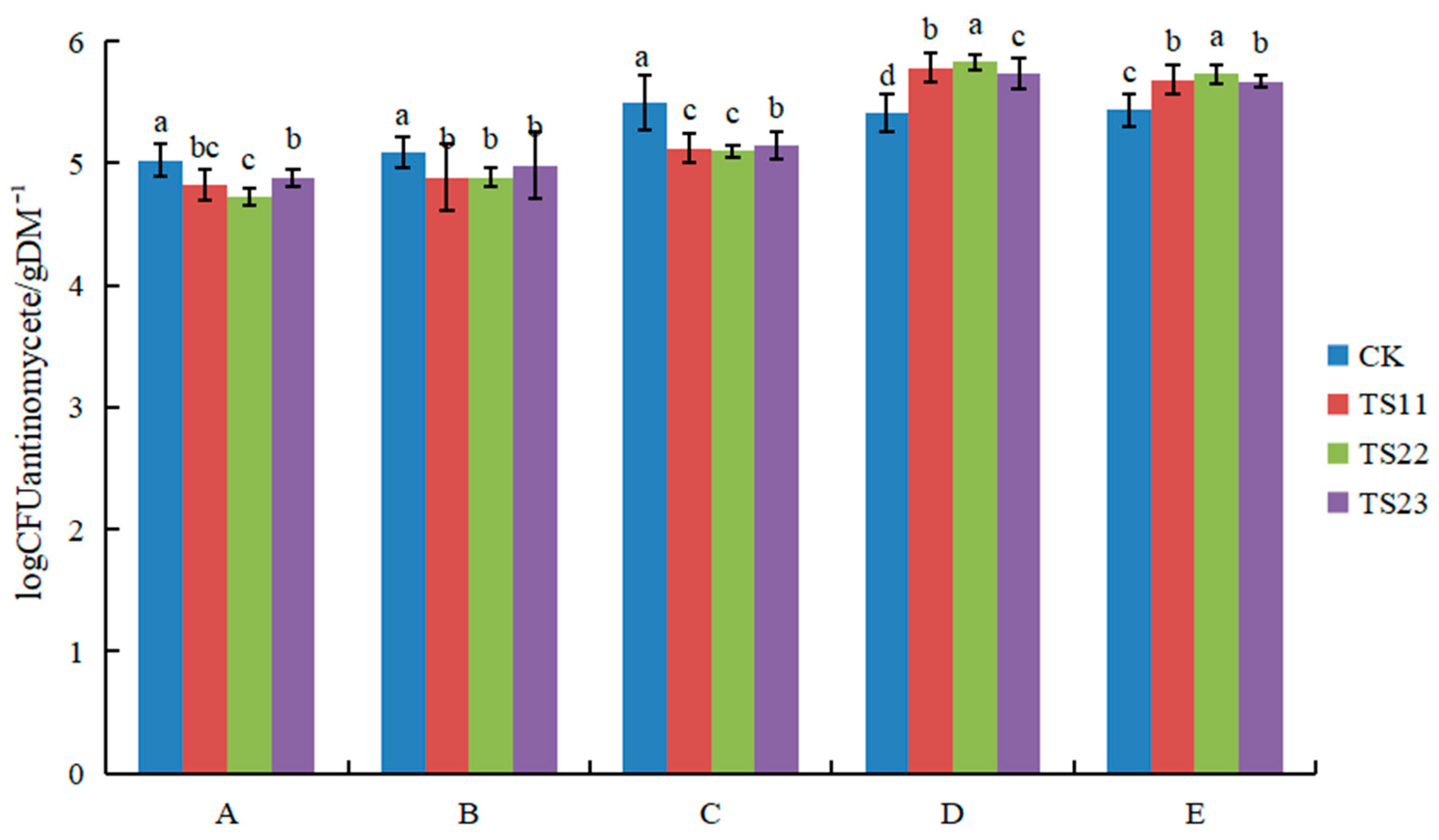
3.1. Effect of Intercropping of Flue-Cured Tobacco and Salvia miltiorrhiza on Soil Microbial Population
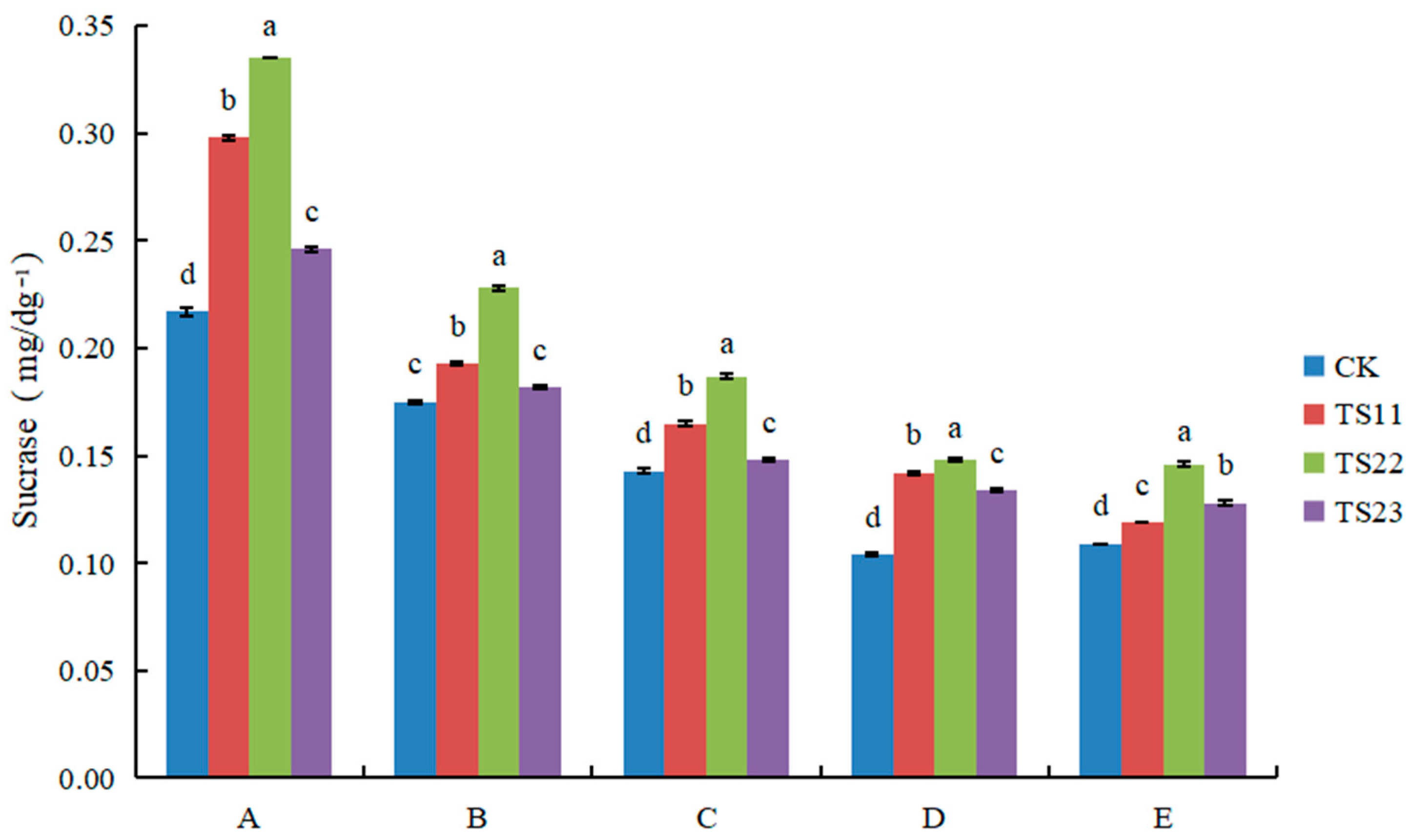
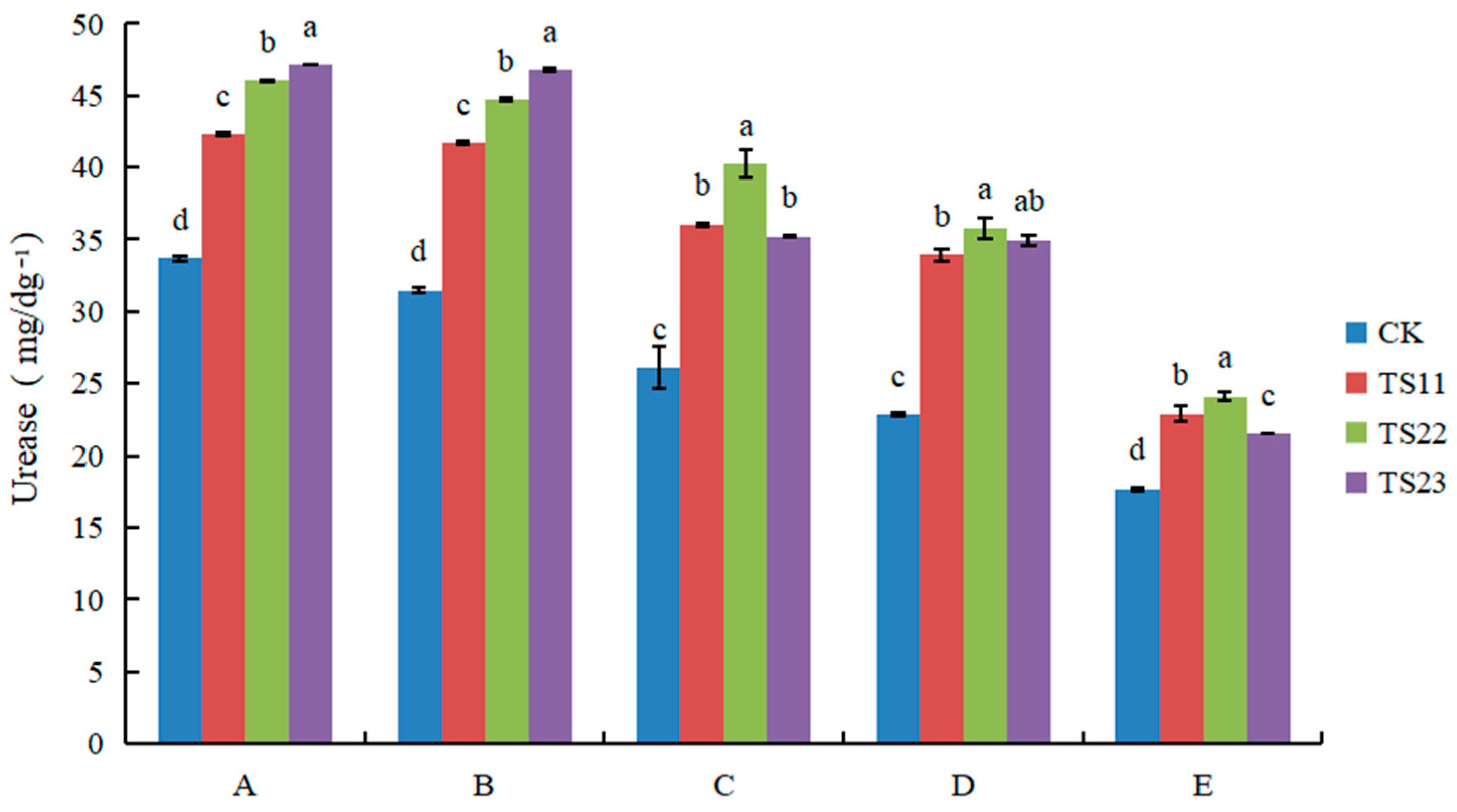
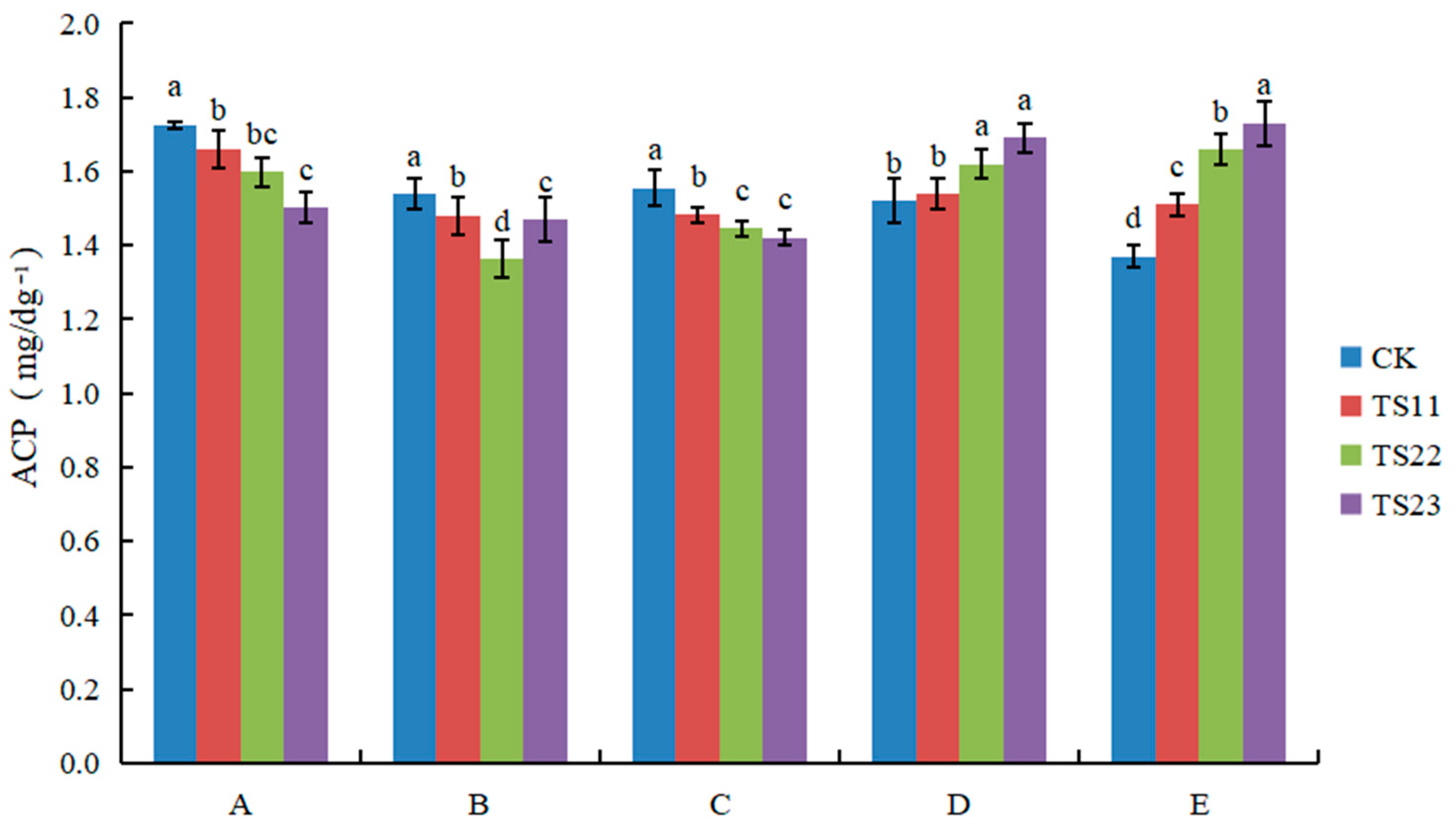
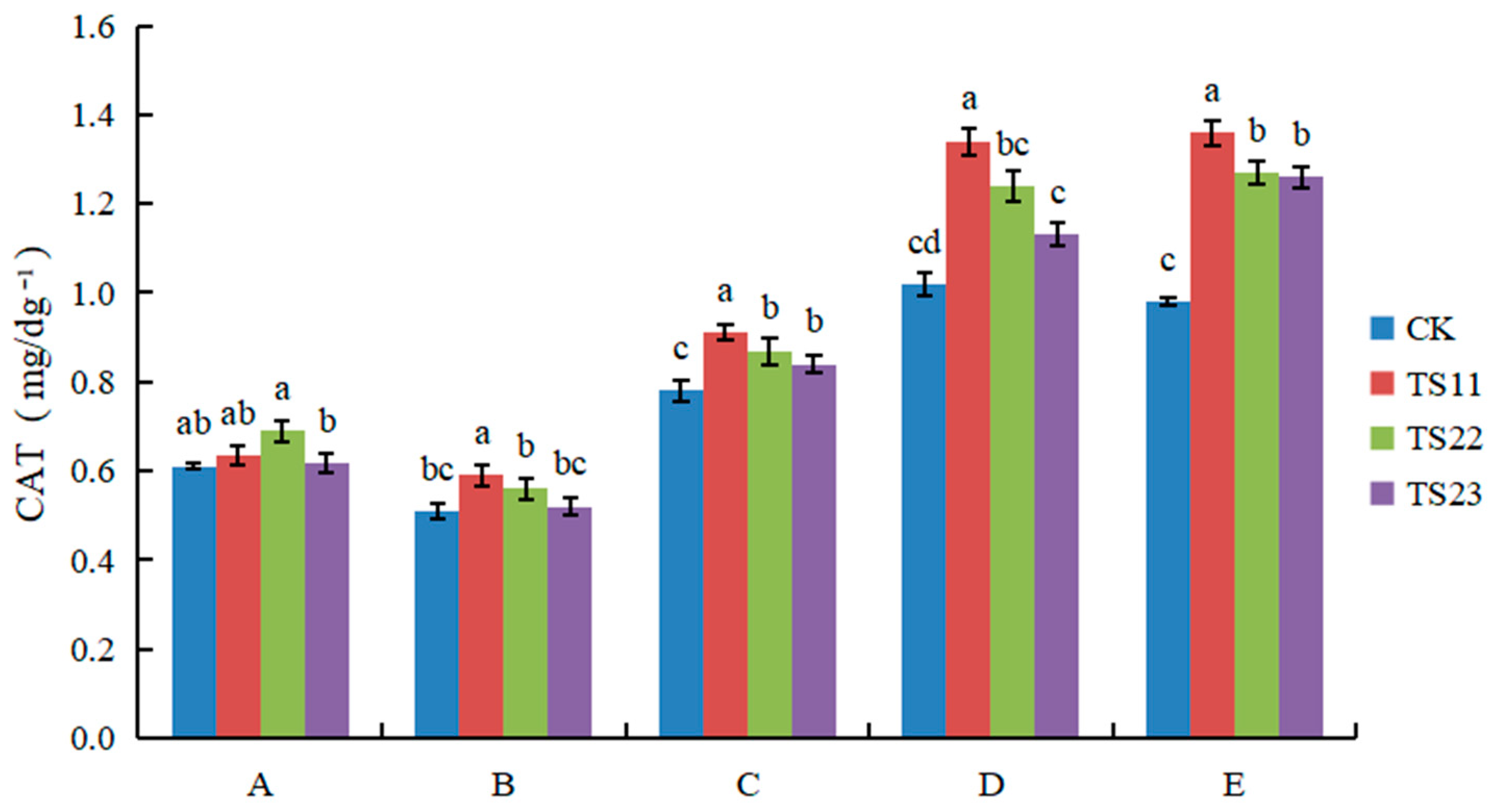
3.2. Effect of Intercropping of Flue-Cured Tobacco and Salvia miltiorrhiza on Soil Enzyme Activities
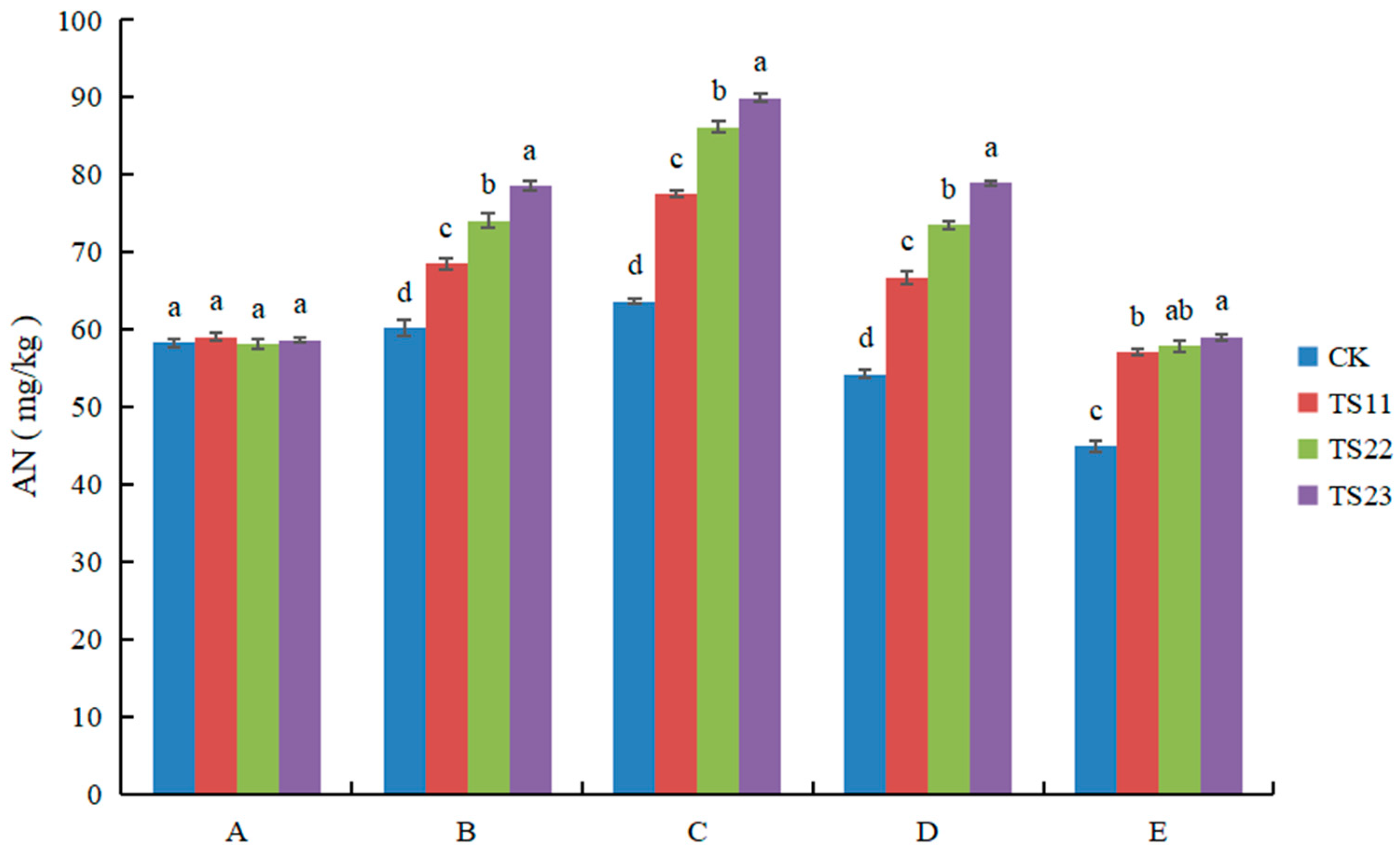
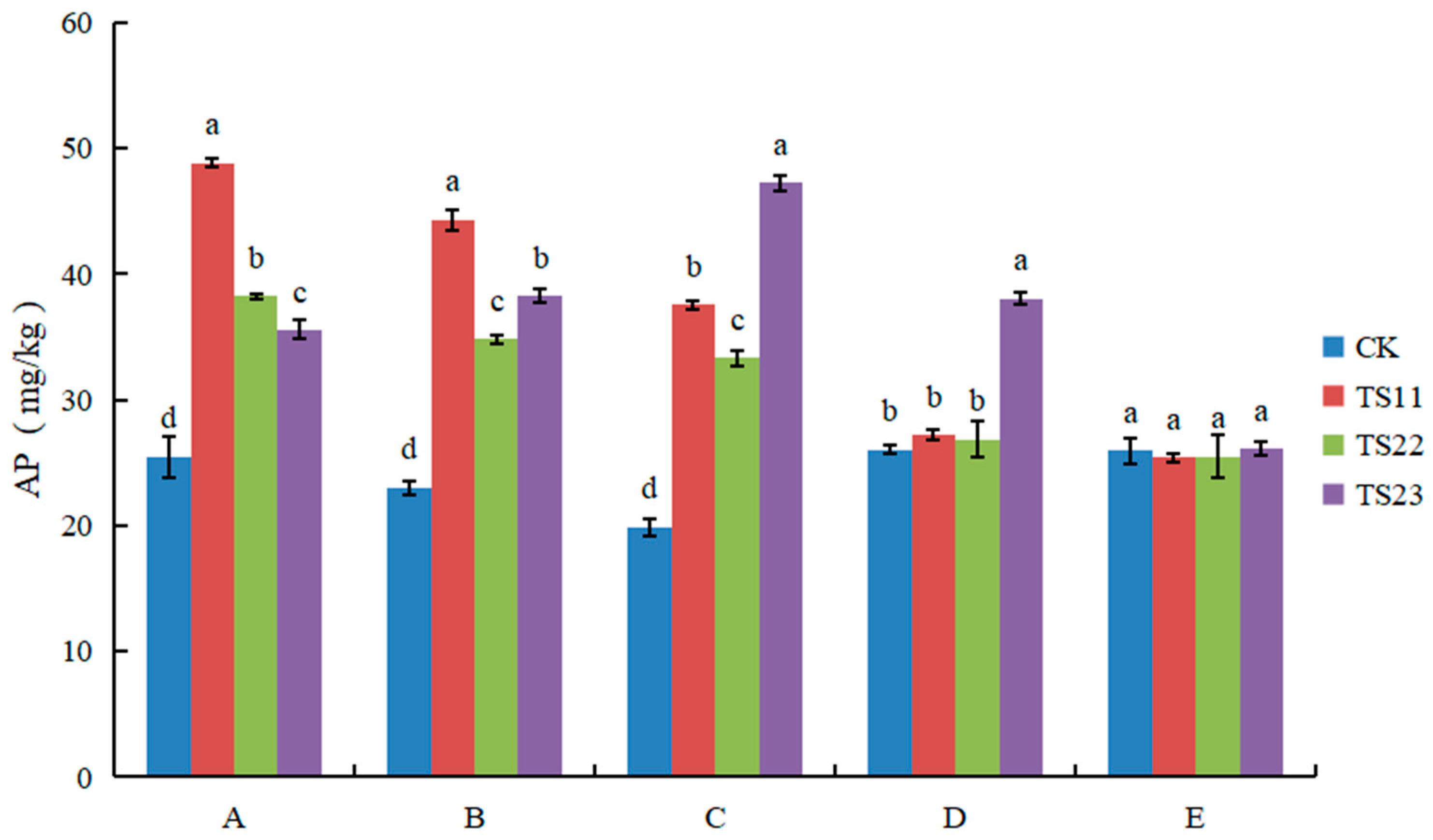
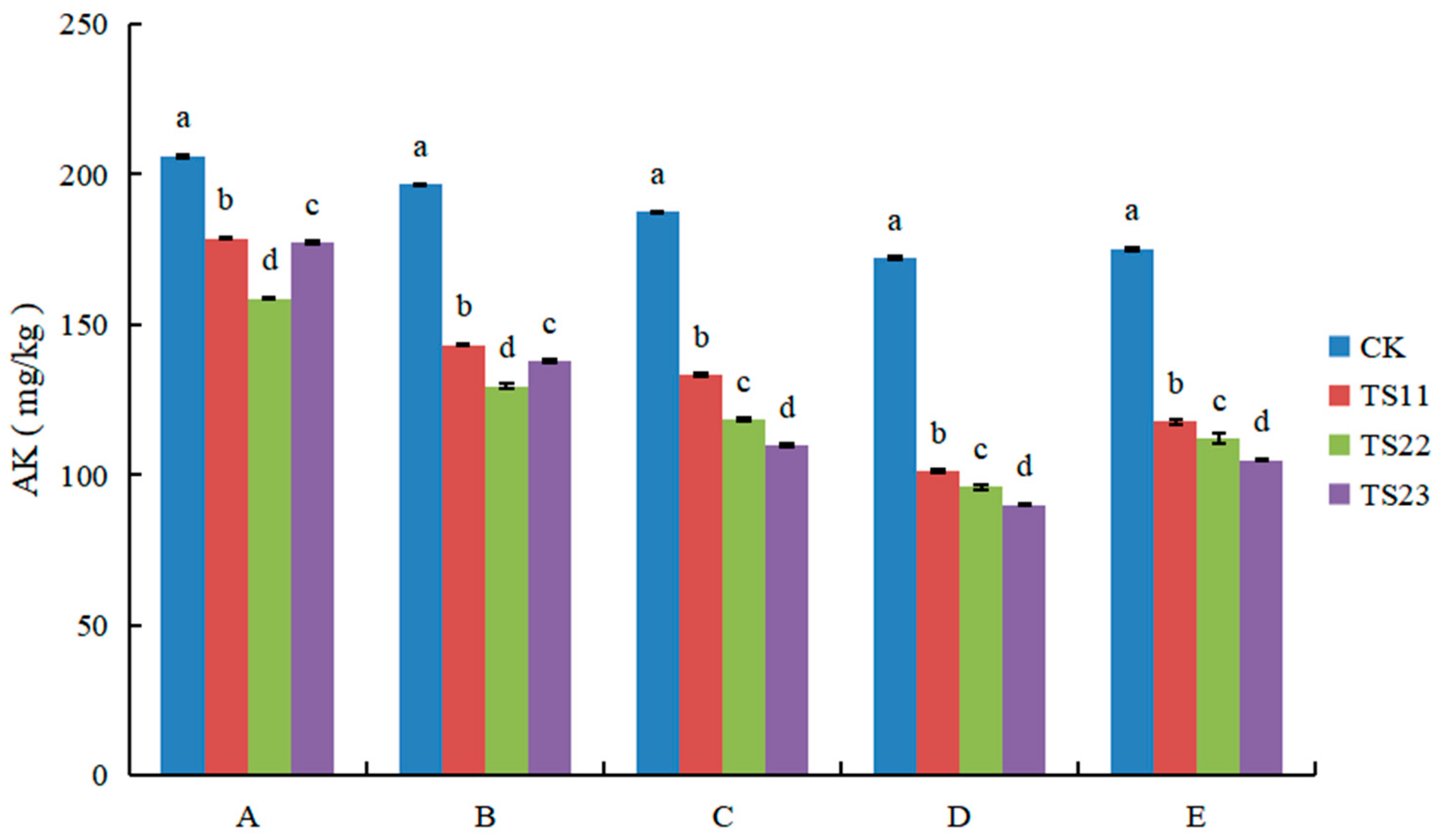
3.3. Effect of Intercropping of Flue-Cured Tobacco and Salvia miltiorrhiza on Available Nitrogen, Phosphorus and Potassium Content of Soil
3.4. Effects of Tobacco-Tansy Intercropping on Yield and Quality of Flue-Cured Tobacco
4. Discussion
4.1. Intercropping of Flue-Cured Tobacco and Salvia miltiorrhiza Changed Soil Microflora
4.2. Intercropping of Flue-Cured Tobacco and Salvia miltiorrhiza Enhanced the Absorption of Soil Nutrients
4.3. Intercropping Salvia miltiorrhiza Increased Total Output Value and Improved the Quality of Flue-Cured Tobacco
4.4. Intercropping Salvia miltiorrhiza Alleviates Continuous Cropping Obstacles and Reflects Intercropping Advantages
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- You, C.; Gao, F.; Wang, F.; Tang, S.; Gu, L.; Zhang, T.; Xu, Z.; Zhang, Z.; Institute of Tobacco Science and Technology; Fujian Agriculture and Forestry University; et al. Effect of continuous cropping on rhizosphere microecology and tobacco production quality of flue-cured tobacco in Yunnan. J. Chin. Tob. 2015, 21, 60–67. [Google Scholar]
- Chen, D. Ecological Regulation Mechanism of Tobacco Continuous Cropping Obstacle by Crop Diversity Cultivation. Ph.D. Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2010. [Google Scholar]
- Yan, J.; Yan, Y.; Duan, Y.; Long, Y.; Ye, C. Preliminary report on the influence of flue-cured tobacco on tobacco yield and quality. Tob. Technol. 2002, 41–45. [Google Scholar]
- Jiang, P. Preliminary study on the influence of different continuous cropping years on soil nutrients. Hubei Today 2015, 10, 92–93. [Google Scholar]
- Wang, Y.; Yu, H.; Li, X.; Lu, Z. Progress on continuous cropping disorders of Chinese medicinal materials. New Agric. 2023, 5, 27–28. [Google Scholar]
- Daniela, C.; Andrew, B.; Peter, H.; Thrall, G.B. Plantgenus (Acacia and Eucalyptus) alters soil microbial community structure and relative abundance within revegetated shelterbelts. Appl. Soil Ecol. 2019, 133, 1–11. [Google Scholar]
- Liu, W.; Zhou, B.; Wang, X.; Lu, H.; Guo, L.; Li, F. Effects of efficient ecological intercropping mode on the growth and active ingredient content of Salvia miltiorrhiza. Chin. Herb. Med. 2018, 41, 1027–1030. [Google Scholar]
- Deng, G.; Li, X.; Zhang, H.; Ren, W.; Zhang, J.; Wang, X.; Shan, C. Effects of intercropping maize on the growth of Salvia miltiorrhiza. Anhui Agric. Sci. 2017, 45, 122–123. [Google Scholar]
- He, X.; Xiao, C.; Ma, G.; Xu, A.; Dai, X. Effects of soybean in tobacco field on soil microorganisms and antagonistic microbial communities. In Proceedings of the Annual Conference of the Chinese Plant Protection Society, Beijing, China, 16–22 August 2009. [Google Scholar]
- Zhu, H. Tobacco sweet potato intercropping set-planting high-yield cultivation technology. Grassroots Agric. Technol. Promot. 2018, 10, 2. [Google Scholar]
- Jing, Y.; Guo, X.; Wang, X.; Niu, H.; Han, D.; Xu, Z. Effect of intercropping ginger on production quality, soil bacterial quantity and physical and chemical properties of flue-cured tobacco. Shandong Agric. Sci. 2022, 54, 86–94. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, X.; Xu, R.; Wang, X.; Niu, H.; Han, D.; Shao, H. Effect of flue-cured tobacco intercropping on the growth and yield and quality of flue-cured tobacco. China Agric. Sci. Technol. Her. 2023, 25, 161–169. [Google Scholar] [CrossRef]
- State Meteorological Administration. The China Meteorological Yearbook; Meteorological Publishing House: Beijing, China, 2019.
- Zhang, G.; Gong, Z. Methods for Laboratory Analysis of Soil Survey; Science Press: Beijing, China, 2012. [Google Scholar]
- World Reference Base for Soil Resources. World Reference Base for Soil Resources 2014; Version 3.03; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- GB2635-92; Flue-Cured Tobacco. China Standards Press: Beijing, China, 2003.
- Jia, M.; Jia, L.; Fu, W.; Chen, B.; Zhao, Q.; Liang, H.; Wang, J.; Liu, J.; Zhou, Q.; Wang, G. Effects of different well cellar depths on soil temperature and humidity and quality of roasted tobacco production. South. J. Agric. 2019, 50, 2141–2148. [Google Scholar]
- YC/T 159-2002; Determination of Water-Soluble Sugar in Tobacco and Tobacco Products Was Done by Continuous Flow Method. Standard Publishing House: Beijing, China, 2002.
- YC/T 160-2002; Determination of Total Alkaloid in Tobacco and Tobacco Products Was Done by Continuous Flow Method. Standard Publishing House: Beijing, China, 2002.
- YC/T 161-2002; Determination of Total Nitrogen in Tobacco and Tobacco Products Was Done by Continuous Flow Method. Standard Publishing House: Beijing, China, 2002.
- YC/T 217-2007; Determination of Potassium in Tobacco and Tobacco Products Was Done by Continuous Flow Method. Standard Publishing House: Beijing, China, 2007.
- YC/T 162-2002; Determination of Chlorine in Tobacco and Tobacco Products Was Done by Continuous Flow Method. Standard Publishing House: Beijing, China, 2002.
- Guan, S.-Y. Soil Enzymes and Their Research Methods; Agricultural Press: Beijing, China, 1982; pp. 168–171. [Google Scholar]
- Xu, G.; Zheng, H. Handbook of Soil Microbiological Methods; Agricultural Press: Beijing, China, 1986; pp. 102–110. [Google Scholar]
- Rukun, L. Methods of Soil Agrochemical Analysis; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Liu, S.; Xia, X.; Chen, G.; Mao, D.; Che, S.; Li, Y. Progress in the study of soil enzymes. China Agric. Bull. 2011, 27, 1–7. [Google Scholar]
- Chen, W.; Jiang, Z.; Hu, Y.; Shu, H. The ecological characteristics of soil microorganisms in apple orchards. J. Soil Water Conserv. 2008, 3, 168–171. [Google Scholar]
- Zhang, D.; Wang, J.; Yang, S.; Zhang, X.; Liu, J.; Zhao, J.; He, D.; Yang, H.; Mo, J.; Gou, J.; et al. Effects of genotypes involved in tobacco intercropping on soil bacterial community structure. J. Grass Ind. 2017, 26, 120–130. [Google Scholar]
- Lai, R. Impacts of Planting Garlic on Tobacco Biotopes. Ph.D. Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2011. [Google Scholar]
- Zhan, J.; Chen, Z. Tobacco Cultivation; Yunnan Science and Technology Press: Kunming, China, 1998; pp. 67–69. [Google Scholar]
- Meng, P.; Wang, J.; Song, Z.; Dong, L.; Zhang, J.; Yang, S. Characteristics of nitrogen, phosphorus and potassium accumulation and distribution in Salvia miltiorrhiza and its relationship with dry matter and salvinorin B accumulation. J. Plant Nutr. Fertil. 2013, 19, 940–945. [Google Scholar]
- Wang, P.; Zhu, L.; Chen, X.; Feng, H.; Sun, W.; Qin, N. Effects of intercropping Eustoma and shallot on soil nutrients, microbiota and enzyme activities. J. Plant Nutr. Fertil. 2018, 24, 668–675. [Google Scholar]
- Tofinger, M.P.; Snaydom, R.W. The root activity of cereals and peas when grown in pure stands and mixtures. Plant Soil 1992, 142, 281–285. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, M.; Liang, Z.; Ru, M.; Liu, Y.; Liu, F. Logistic modelling of root growth pattern of Salvia miltiorrhiza. Hubei Agric. Sci. 2014, 53, 1583–1589. [Google Scholar]
- Fan, F.; Zhang, F.; Lu, Y. Linking plant identity and interspecific competitionto soil nitrogen cycling through ammonia oxidiser communities. Soil Biol. Biochem. 2011, 43, 46–54. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, G.; Sun, C.; Jiang, Y.; Jiang, Y.; Liu, X. Dynamics of soil microbial nitrogen and its bioavailability. J. Plant Nutr. Fertil. 2003, 9, 87–90. [Google Scholar]
- Wang, H.; Wang, H.; Zhao, Q.; Zhuang, H.; Song, Y.; Zhu, Z. Effects of intercropping betel nut with vanilla orchid at different spacing on soil nutrients and microorganisms. J. Plant Nutr. Fertil. 2013, 19, 988–994. [Google Scholar]
- Sun, H.; Zhang, F. Acid phosphatase activity in wheat roots under phosphorus deficiency. J. Appl. Ecol. 2002, 13, 379–381. [Google Scholar]
- Shi, A.-D.; Li, J.-W.; Yuan, L. Effects of rotational intercropping system on yield, quality and soil nutrients of roasted tobacco. J. Plant Nutr. Fertil. 2011, 17, 411–418. [Google Scholar]
- Zhang, D. Effects of Crop Rotation and Intercropping on the Growth Status and Yield Quality of Roasted Tobacco KRK26. Ph.D. Thesis, Hunan Agricultural University, Changsha, China, 2012. [Google Scholar]
- Li, T.; Ma, G. Effects of seed amaranth-tobacco intercropping on the content and quality of some mineral elements in tobacco leaves. J. Soil Water Conserv. 2004, 18, 138–141. [Google Scholar]
- Yang, T.; Xia, W.; Fan, J. Study on the characteristics of potassium accumulation in tobacco under different levels of potassium supply. Henan Sci. 2005, 23, 375–378. [Google Scholar]
- Mulchi, C.L. Chloride effects on agronomic and physical properties of Maryland tobacco. Tob. Sci. 1982, 26, 13–16. [Google Scholar]
- Wang, P. Progress of research on continuous cropping obstacles and control of tobacco. Anhui Agric. Bull. 2023, 29, 24–26+32. [Google Scholar] [CrossRef]
- Hao, W.Y.; Ren, L.X.; Ran, W.; Shen, Q. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f. sp. niveum. Plant Soil 2010, 336, 485–497. [Google Scholar] [CrossRef]
- Janvier, C.; Villeneuve, F.; Alabouvette, C.; Edel-Hermann, V.; Mateille, T.; Steinberg, C. Soil health through soil disease suppression: Which strategy from descriptors to indicators? Soil Biol. Biochem. 2007, 39, 12–23. [Google Scholar] [CrossRef]
- Insam, H.; Mitchell, C.C.; Dormaar, J.F. Relationship of soil microbial biomass and activity with fertilization practice and crop yield of three Ultisols. Soil Biol. Biochem. 1991, 23, 459–464. [Google Scholar] [CrossRef]
- Li, L.; Li, S.M.; Sun, J.H.; Zhou, L.L.; Bao, X.G.; Zhang, H.G.; Zhang, F.S. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc. Natl. Acad. Sci. USA 2007, 104, 11192–11196. [Google Scholar] [CrossRef] [PubMed]
- Li, F.D.; Meng, P.; Fu, D.L.; Wang, B. Light distribution, photosynthetic rate and yield in a paulownia- wheat intercropping system in China. Agrofor. Syst. 2008, 74, 163–174. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, S.; Xie, J.; Yan, B.; Hou, J.; Wang, W.; Song, Y.; Zhang, X.; Li, J. Changing law of biomass accumulation and active components of Salvia miltiorrhiza in different growth periods. Chin. Mod. Tradit. Med. 2015, 25, 1171–1176. [Google Scholar]
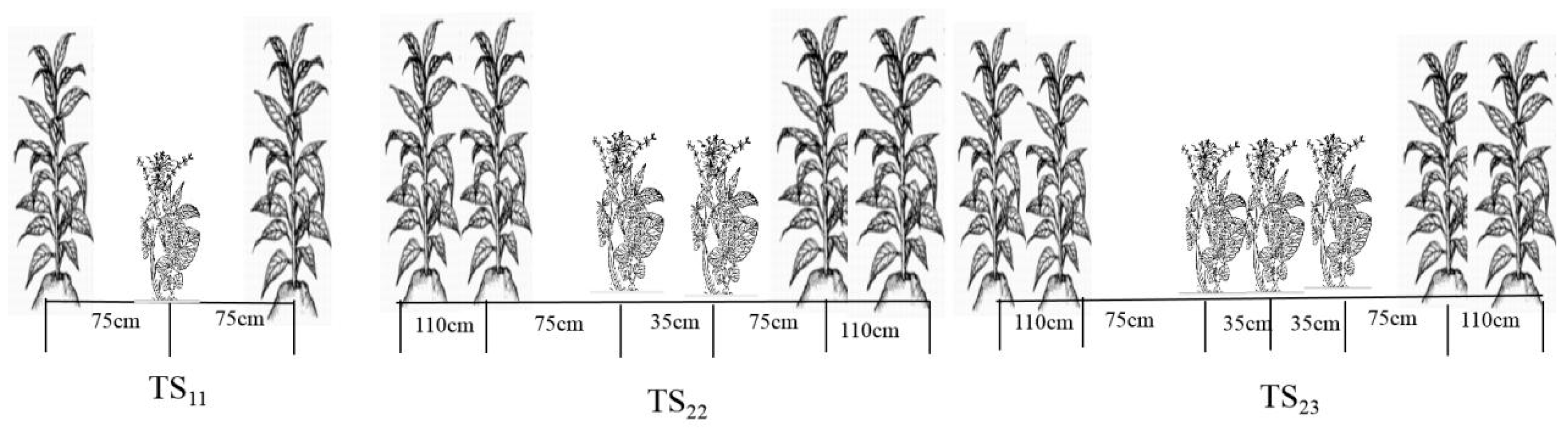
| Year | Month | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|
| 2018 | monthly average temperature (°C) | 15.1 | 20.2 | 24.6 | 26.5 |
| monthly precipitation (mm) | 57 | 63 | 132 | 246 | |
| Month | 8 | 9 | 10 | 11 | |
| monthly average temperature (°C) | 26.2 | 20.3 | 13.9 | 7.6 | |
| monthly precipitation (mm) | 180 | 76 | 12 | 34 | |
| 2019 | Month | 4 | 5 | 6 | 7 |
| monthly average temperature (°C) | 14.5 | 22 | 25.5 | 28 | |
| monthly precipitation (mm) | 50 | 43 | 120 | 231 | |
| Month | 8 | 9 | 10 | 11 | |
| monthly average temperature (°C) | 25.5 | 22 | 15 | 10.5 | |
| monthly precipitation (mm) | 153 | 66 | 13 | 34 |
| Treatment | Type | Top-Quality Tobacco (%) | Production (kg·ha−1) | Production Value (CNY·ha−1) | Total Production Value (CNY·ha−1) | |
|---|---|---|---|---|---|---|
| 2018 | CK | T | 48.05 | 2178.41 | 48,343.99 | 48,469.64 |
| TS11 | T | 49.41 | 1991.2 | 44,572.46 | 52,561.59 | |
| S | 826.44 | 7989.13 | ||||
| TS22 | T | 49.12 | 1969.47 | 44,769.57 | 58,799.49 | |
| S | 1451.52 | 14,029.92 | ||||
| TS23 | T | 49.59 | 1841.94 | 41,369.97 | 56,650.36 | |
| S | 1591.71 | 15,280.39 | ||||
| 2019 | CK | T | 48.22 | 2140.93 | 47,635.68 | 47,635.68 |
| TS11 | T | 49.88 | 2106.46 | 47,152.13 | 54,367.72 | |
| S | 751.62 | 7215.59 | ||||
| TS22 | T | 49.58 | 2092.95 | 46,850.08 | 58,315.15 | |
| S | 1177.91 | 11,465.07 | ||||
| TS23 | T | 49.51 | 1870.87 | 42,019.64 | 54,985.44 | |
| S | 1177.91 | 12,965.8 |
| Treatment | Grade | Total Sugar | Reducing Sugar | Total Nicotine | Total Nitrogen | K | CI | K/CI | Glycemic Ratio | Ratio of Nitrogen to Alkali |
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | |||||
| CK | B2F | 22.71 a | 16.31 b | 3.03 c | 2.32 a | 2.06 c | 0.15 a | 13.73 c | 7.50 b | 0.77 a |
| TS11 | B2F | 24.02 b | 17.74 a | 3.11 b | 2.28 ab | 2.21 a | 0.13 ab | 16.46 a | 7.72 a | 0.72 b |
| TS22 | B2F | 21.72 c | 15.95 c | 3.14 b | 2.21 b | 1.85 d | 0.12 b | 15.42 b | 6.92 c | 0.70 b |
| TS23 | B2F | 22.15 ab | 15.46 c | 3.21 a | 2.18 b | 2.14 b | 0.14 a | 15.79 b | 6.90 c | 0.68 b |
| CK | C3F | 24.53 b | 17.02 b | 3.19 b | 2.07 b | 1.58 d | 0.18 a | 8.78 d | 7.69 c | 0.65 a |
| TS11 | C3F | 26.06 a | 19.03 a | 3.12 b | 2.04 b | 2.32 a | 0.17 b | 13.65 a | 8.35 a | 0.65 a |
| TS22 | C3F | 24.52 b | 17.59 b | 3.04 c | 2.01 bc | 1.91 b | 0.16 c | 11.94 b | 8.07 b | 0.66 a |
| TS23 | C3F | 23.86 c | 16.47 c | 3.39 a | 2.12 a | 1.91 b | 0.18 a | 10.61 c | 7.04 d | 0.63 a |
| CK | X2F | 33.55 a | 26.30 a | 1.24 d | 1.55 c | 1.42 d | 0.15 a | 9.47 d | 27.06 a | 1.25 a |
| TS11 | X2F | 30.92 b | 23.09 b | 1.40 c | 1.58 c | 2.32 a | 0.14 a | 16.57 a | 22.09 b | 0.68 d |
| TS22 | X2F | 25.79 c | 19.26 c | 2.74 a | 1.87 a | 1.64 c | 0.15 a | 10.93 c | 9.41 d | 1.13 b |
| TS23 | X2F | 27.78 bc | 20.68 c | 2.12 b | 1.75 b | 1.83 b | 0.13 ab | 14.08 a | 13.10 c | 0.83 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Guo, X.; Chen, Q.; Sun, Z.; Shang, X.; Gao, Y.; Yu, T.; Zhang, L.; Yang, L.; Hou, X. Tobacco/Salvia miltiorrhiza Intercropping Improves Soil Quality and Increases Total Production Value. Agronomy 2024, 14, 598. https://doi.org/10.3390/agronomy14030598
Su X, Guo X, Chen Q, Sun Z, Shang X, Gao Y, Yu T, Zhang L, Yang L, Hou X. Tobacco/Salvia miltiorrhiza Intercropping Improves Soil Quality and Increases Total Production Value. Agronomy. 2024; 14(3):598. https://doi.org/10.3390/agronomy14030598
Chicago/Turabian StyleSu, Xueqi, Xiaomeng Guo, Qian Chen, Zheng Sun, Xianchao Shang, Yun Gao, Tao Yu, Li Zhang, Long Yang, and Xin Hou. 2024. "Tobacco/Salvia miltiorrhiza Intercropping Improves Soil Quality and Increases Total Production Value" Agronomy 14, no. 3: 598. https://doi.org/10.3390/agronomy14030598
APA StyleSu, X., Guo, X., Chen, Q., Sun, Z., Shang, X., Gao, Y., Yu, T., Zhang, L., Yang, L., & Hou, X. (2024). Tobacco/Salvia miltiorrhiza Intercropping Improves Soil Quality and Increases Total Production Value. Agronomy, 14(3), 598. https://doi.org/10.3390/agronomy14030598







