Impact of Pyrolyzed and Unpyrolyzed Animal Manures on Soil Properties, Carbon Sequestration, and Clover Productivity in Andisol
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Feedstock and Biochars
2.3. Experimental Treatments and Sampling
2.4. Analyses of Chemical Composition in Soil Amendments, Soil Samples, and Foliar Content
2.5. Simulation of Biochar Movement in the Upper Layer of Soil
2.6. Statistical Analyses
3. Results
4. Discussion
4.1. Impact of Biochars and Manures on the Yield of Red Clover and Soil Characteristics
4.2. Impact of Biochars and Manures on the Content of Carbon in Soil
4.3. Movement of Biochar in the Uppermost Layer of Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lal, R. Digging deeper: A holistic perspective of factors affecting soil organic carbon sequestration in agroecosystems. Glob. Chang. Biol. 2018, 24, 3285–3301. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for policymakers. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Calvo Buendia, E., Masson-Delmotte, V., Portner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; IPCC: Geneva, Switzerland, 2019; pp. 3–36. [Google Scholar]
- Rombel, A.; Różyło, K.; Oleszczuk, P. The high dose of biochar reduces polycyclic aromatic hydrocarbons losses during co-composting of sewage sludge and wheat straw. J. Environ. Manag. 2024, 351, 119628. [Google Scholar] [CrossRef]
- Qin, Z.; Deng, S.; Dunn, J.B.; Smith, P.; Sun, W. Animal waste use and implications to agricultural greenhouse gas emissions in the United States. Environ. Res. Lett. 2021, 16, 064079. [Google Scholar] [CrossRef]
- Maillard, E.; Angers, D.A. Animal manure application and soil organic carbon stocks: A meta-analysis. Glob. Chang. Biol. 2014, 20, 666–679. [Google Scholar] [CrossRef]
- Ginebra, M.; Muñoz, C.; Zagal, E. Carbon stability and soil N2O emissions. Pyrolyzed or unpyrolyzed manure? J. Environ. Manag. 2022, 322, 116095. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, B.; Wang, S.; Zhu, X.; Vereecken, H.; Brueggemann, N. Stimulation of N2O emission by manure application to agricultural soils may largely offset carbon benefits: A global meta-analysis. Glob. Chang. Biol. 2017, 23, 4068–4083. [Google Scholar] [CrossRef]
- Mostashari-Rad, F.; Ghasemi-Mobtaker, H.; Taki, M.; Ghahderijani, M.; Kaab, A.; Chau, K.; Nabavi-Pelesaraei, A. Exergoenvironmental damages assessment of horticultural crops using ReCiPe 2016 and cumulative exergy demand frameworks. J. Clean. Prod. 2021, 278, 123788. [Google Scholar] [CrossRef]
- Mangalassery, S.; Kalaivanan, D.; Philip, P.S. Effect of inorganic fertilisers and organic amendments on soil aggregation and biochemical characteristics in a weathered tropical soil. Soil Tillage Res. 2019, 187, 144–151. [Google Scholar] [CrossRef]
- Chadwick, D.; Sommer, S.; Thorman, R.; Fangueiro, D.; Cardenas, L.; Amon, B.; Misselbrook, T. Manure management: Implications for greenhouse gas emissions. Anim. Feed. Sci. Technol. 2011, 166–167, 514–531. [Google Scholar] [CrossRef]
- Wu, H.; Hanna, M.A.; Jones, D.D. Life cycle assessment of greenhouse gas emissions of feedlot manure management practices: Land application versus gasification. Biomass Bioenergy 2013, 54, 260–266. [Google Scholar] [CrossRef]
- Guo, X.-X.; Liu, H.-T.; Zhang, J. The role of biochar in organic waste composting and soil improvement: A review. Waste Manag. 2020, 102, 884–899. [Google Scholar] [CrossRef]
- Muñoz, C.; Ginebra, M.; Zagal, E. Variation of greenhouse gases fluxes and soil properties with addition of biochar from farm-wastes in volcanic and non-volcanic soils. Sustainability 2019, 11, 1831. [Google Scholar] [CrossRef]
- Gascó, G.; Paz-Ferreiro, J.; Cely, P.; Plaza, C.; Méndez, A. Influence of pig manure and its biochar on soil CO2 emissions and soil enzymes. Ecol. Eng. 2016, 95, 19–24. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management Science, Technology and Implementation, 2nd ed.; Routledge: London, UK, 2015. [Google Scholar]
- Ginebra, M.; Muñoz, C.; Calvelo Pereira, R.; Doussoulin, M.; Zagal, E. Biochar impacts on soil chemical properties, greenhouse gas emissions and forage productivity: A field experiment. Sci. Total Environ. 2022, 806, 150465. [Google Scholar] [CrossRef] [PubMed]
- Novotny, E.H.; Branco de Freitas Maia, C.M.; Thaís de Melo Carvalho, M.; Emöke Madari, B. Biochar: Pyrogenic carbon for agricultural use–A critical review. Rev. Bras. De Ciência Do Solo 2015, 39, 321–344. [Google Scholar] [CrossRef]
- Novak, J.; Cantrell, K.B.; Watts, D.W.; Busscher, W.J. Designing relevant biochars as soil amendments using lignocellulosic-based and manure-based feedstocks. J. Soils Sediments 2014, 14, 2. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. Biochar as an exceptional bioresource for energy, agronomy, carbon sequestration, activated carbon and specialty materials. Waste Biomass Valorization 2016, 7, 201–235. [Google Scholar] [CrossRef]
- Garbuz, S.; Camps-Arbestain, M.; Mackay, A.; DeVantier, B.; Minor, M. The interactions between biochar and earthworms, and their influence on soil properties and clover growth: A 6-month mesocosm experiment. Appl. Soil Ecol. 2020, 147, 103402. [Google Scholar] [CrossRef]
- Budai, A.; Rasse, D.P.; Lagomarsino, A.; Lerch, T.Z.; Paruch, L. Biochar persistence, priming and microbial responses to pyrolysis temperature series. Biol. Fertil. Soils 2016, 52, 749–761. [Google Scholar] [CrossRef]
- Singh, B.; Camps-Arbestain, M.; Lehmann, J. Biochar: A Guide to Analytical Methods; CRC Press: London, UK, 2017; pp. 23–38. [Google Scholar]
- Rehrah, D.; Reddy, M.R.; Novak, J.; Bansode, R. Production and characterization of biochars from agricultural by-products for use in soil quality enhancement. J. Anal. Appl. Pyrolysis 2014, 108, 301–309. [Google Scholar] [CrossRef]
- Wang, T.; Camps-Arbestain, M.; Hedley, M. Predicting C aromaticity of biochars based on their elemental composition. Org. Geochem. 2013, 62, 1–6. [Google Scholar] [CrossRef]
- Zimmerman, A.R. Abiotic and Microbial Oxidation of Laboratory-Produced Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef]
- Grutzmacher, P.; Puga, A.P.; Bibar, M.P.S.; Coscione, A.R.; Packer, A.P.; de Andrade, C.A. Carbon stability and mitigation of fertilizer induced N2O emissions in soil amended with biochar. Sci. Total Environ. 2018, 625, 1459–1466. [Google Scholar] [CrossRef]
- Ye, L.; Camps-Arbestain, M.; Shen, Q.; Lehmann, J.; Singh, B.; Sabir, M. Biochar Effects on Crop Yields with and without Fertilizer: A Meta-analysis of Field Studies Using Separate Controls. Soil Use Manag. 2020, 36, 2–18. [Google Scholar] [CrossRef]
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.C.; van Groenigen, J.W.; Hungate, B.A.; Verheijen, F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017, 12, 053001. [Google Scholar] [CrossRef]
- Lv, R.; Wang, Y.; Wang, Q.; Zeng, Y.; Shang, Q. Residual effect of straw biochar on grain yield and yield attributes in a double rice cropping system of subtropical China. Plant Soil Environ. 2022, 68, 328–337. [Google Scholar] [CrossRef]
- Yin, X.; Peñuelas, J.; Sardans, J.; Xu, X.; Chen, Y.; Fang, Y.; Wu, L.; Singh, B.P.; Tavakkoli, E.; Wang, W. Effects of nitrogen-enriched biochar on rice growth and yield, iron dynamics, and soil carbon storage and emissions: A tool to improve sustainable rice cultivation. Environ. Pollut. 2021, 287, 117565. [Google Scholar] [CrossRef]
- Yin, X.; Chen, J.; Cao, F.; Tao, Z.; Huang, M. Short-term application of biochar improves post-heading crop growth but reduces pre-heading biomass translocation in rice. Plant Prod. Sci. 2020, 23, 522–528. [Google Scholar] [CrossRef]
- Ashiq, W.; Nadeem, M.; Ali, W.; Zaeem, M.; Wu, J.; Galagedara, L.; Thomas, R.; Kavanagh, V.; Cheema, M. Biochar amendment mitigates greenhouse gases emission and global warming potential in dairy manure based silage corn in boreal climate. Environ. Pollut. 2020, 265, 114869. [Google Scholar] [CrossRef]
- Camps-Arbestain, M.; Shen, Q.; Wang, T.; van Zwieten, L.; Novak, J. Available nutrients in biochar. In Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CRC Press: London, UK, 2017; pp. 109–125. [Google Scholar]
- Subedi, R.; Taupe, N.; Pelissetti, S.; Petruzzelli, L.; Bertora, C.; Leahy, J.J.; Grignani, C. Greenhouse gas emissions and soil properties following amendment with manure-derived biochars: Influence of pyrolysis temperature and feedstock type. J. Environ. Manag. 2016, 166, 73–83. [Google Scholar] [CrossRef]
- Wang, T.; Camps Arbestain, M.; Hedley, M.; Bishop, P. Chemical and bioassay characterisation of nitrogen availability in biochar produced from dairy manure and biosolids. Org. Geochem. 2012, 51, 45–54. [Google Scholar] [CrossRef]
- Wang, T.; Camps-Arbestain, M.; Hedley, M.; Bishop, P. Predicting phosphorus bioavailability from high-ash biochars. Plant Soil 2012, 357, 173–187. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.; Naidu, R.; Parikh, S.J.; Du, J.; Qi, F.; Willett, I.R. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: A meta-analysis. Sci. Total Environ. 2020, 744, 140714. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil taxonomy: A basic system of soil classification for making and interpreting soil surveys. In Natural Resources Conservation Service, 2nd ed.; Department of Agriculture Handbook: Washington, DC, USA, 1999; Volume 436. [Google Scholar]
- Stolpe, N. Descripciones de los Principales Suelos de la VIII Región de Chile; Departamento de Suelos, Universidad de Concepción: Concepción, Chile, 2006. [Google Scholar]
- IBI-International Biochar Initiative. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. 2013. [WWW Document]. Available online: http://www.biochar-international.org/characterizationstandard (accessed on 1 February 2024).
- Davies, R.; Di Sacco, A.; Newton, R. Germination Testing: Procedures and Evaluation. Millennium Seed Bank Partnership; Wakehurst Place: Ardingly, UK, 2015. [Google Scholar] [CrossRef]
- Sadzawka, A.; Carrasco, M.A.; Grez, R.; de la Mora, M.L.; Flores, H.; Neaman, A. Métodos de Análisis Recomendados para los Suelos de Chile: Revisión 2006; Serie Actas INIA No34; INIA: Santiago, Chile, 2006. [Google Scholar]
- Robarge, W.P.; Edwards, A.; Johnson, B. Water and waste water analysis for nitrate via nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1983, 14, 1207–1215. [Google Scholar] [CrossRef]
- Longeri, L.; Etchevers, J.; Venegas, J. Metodología de perfusión para estudios de nitrificación en suelos. Ciencia Investig. Agraria 1979, 6, 295–299. [Google Scholar] [CrossRef]
- Sepúlveda, M.Á.; Hidalgo, M.; Araya, J.; Casanova, M.; Muñoz, C.; Doetterl, S.; Wasner, D.; Colpaert, B.; Bodé, S.; Boeckx, P.; et al. Near-infrared spectroscopy: Alternative method for assessment of stable carbon isotopes in various soil profiles in Chile. Geoderma Reg. 2021, 25, e00397. [Google Scholar] [CrossRef]
- Yamamoto, A.; Akiyama, H.; Kojima, M.; Osaki, A. Nitrous oxide emissions from an andosol upland field amended with four different types of biochars. Nutr. Cycl. Agroecosyst. 2019, 113, 323–335. [Google Scholar] [CrossRef]
- Nkoh, J.N.; Baquy, M.A.A.; Mia, S.; Shi, R.Y.; Kamran, M.A.; Mehmood, K.; Xu, R.K. A critical-systematic review of the interactions of biochar with soils and the observable outcomes. Sustainability 2021, 13, 3726. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Soja, G.; Bárta, J.; Chen, W.-H.; Amirahmadi, E. How do different feedstocks and pyrolysis conditions effectively change biochar modification scenarios? A critical analysis of engineered biochars under H2O2 oxidation. Energy Convers. Manag. 2024, 300, 117924. [Google Scholar] [CrossRef]
- Caddel, J.L.; Zhang, H.; Wise, K. Responses of alfalfa, red clover, and white clover to soil pH and lime treatments. Forage Grazinglands 2004, 2, 1–8. [Google Scholar] [CrossRef]
- Nelson, N.O.; Agudelo, S.C.; Yuan, W.; Gan, J. Nitrogen and phosphorus availability in biochar-amended soils. Soil Sci. 2011, 176, 218–226. [Google Scholar] [CrossRef]
- Basalirwa, D.; Shigeto, S.; Cosmas, W.; Fuyumi, A.; Aung, Z.O.; Sho, K.; Daisuke, S.; Sadahiro, Y.; Tsugiyuki, M.; Eiji, N. Assessment of crop residue and palm shell biochar incorporation on greenhouse gas emissions during the fallow and crop growing seasons of broccoli (Brassica oleracea var. italica). Soil Tillage Res. 2020, 196, 104435. [Google Scholar] [CrossRef]
- Masud, M.M.; Li, J.Y.; Xu, R.K. Use of alkaline slag and crop residue biochars to promote base saturation and reduce acidity of an acidic Ultisol. Pedosphere 2014, 24, 791–798. [Google Scholar] [CrossRef]
- Camps-Arbestain, M.; Amonette, J.E.; Singh, B.; Wang, T.; Schmidt, H.-P. A biochar classification and associated test methods. In Biochar Environmental Management: Science, Technology and Implementation; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2015; pp. 164–194. [Google Scholar]
- Calvelo Pereira, R.; Kaal, J.; Camps Arbestain, M.; Pardo Lorenzo, R.; Aitkenhead, W.; Hedley, M.; Macías, F.; Hindmarsh, J.; Maciá-Agulló, J.A. Contribution to characterisation of biochar to estimate the labile fraction of carbon. Org. Geochem. 2011, 42, 1331–1342. [Google Scholar] [CrossRef]
- Bender, A.; Tamm, S. Seed yield of tetraploid red clover as influenced by cover crop management. Zemdirb. Agric. 2018, 105, 133–140. [Google Scholar] [CrossRef]
- Yuan, S.; Zhao, L.; Meng, H.; Shen, Y. The main types of biochar and their properties and expectative researches. J. Plant Nutr. Fertil. 2016, 22, 1402–1417. [Google Scholar] [CrossRef]
- Cely, P.; Tarquis, A.M.; Paz-Ferreiro, J.; Méndez, A.; Gascó, G. Factors driving the carbon mineralization priming effect in a sandy loam soil amended with different types of biochar. Solid Earth 2014, 5, 585–594. [Google Scholar] [CrossRef]
- Stylianou, M.; Christou, A.; Dalias, P.; Polycarpou, P.; Michael, C.; Agapiou, A.; Papanastasiou, P.; Fatta-Kassinos, D. Physicochemical and structural characterization of biochar derived from the pyrolysis of biosolids, cattle manure and spent coffee grounds. J. Energy Inst. 2020, 93, 2063–2073. [Google Scholar] [CrossRef]
- Smith, P.; Calvin, K.; Nkem, J.; Campbell, D.; Cherubini, F.; Grassi, G.; Korotkov, V.; Le Hoang, A.; Lwasa, S.; McElwee, P.; et al. Which practices co-deliver food security, climate change mitigation and adaptation, and combat land degradation and desertification? Glob. Chang. Biol. 2020, 26, 1532–1575. [Google Scholar] [CrossRef]
- Kätterer, T.; Roobroeck, D.; Kimutai, G.; Karltun, E.; Nyberg, G.; Sundberg, C.; de Röing Nowina, K. Maize grain yield responses to realistic biochar application rates on smallholder farms in Kenya. Agron. Sustain. Dev. 2022, 42, 63. [Google Scholar] [CrossRef]
- Liu, Z.; Dugan, B.; Masiello, C.A.; Gonnermann, H.M. Biochar particle size, shape, and porosity act together to influence soil water properties. PLoS ONE 2017, 12, e0179079. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Van Zwieten, L.; Singh, B.; Tavakkoli, E.; Joseph, S.; Macdonald, L.M.; Rose, T.J.; Rose, M.T.; Kimber, S.W.L.; Morris, S.; et al. Biochar built soil carbon over a decade by stabilizing rhizodeposits. Nat. Clim Chang. 2017, 7, 371–376. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, G.; Huang, D.; Lai, C.; Chen, M.; Cheng, M.; Tang, W.; Tang, L.; Dong, H.; Huang, B.; et al. Biochar for environmental management: Mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chem. Eng. J. 2019, 373, 902–922. [Google Scholar] [CrossRef]
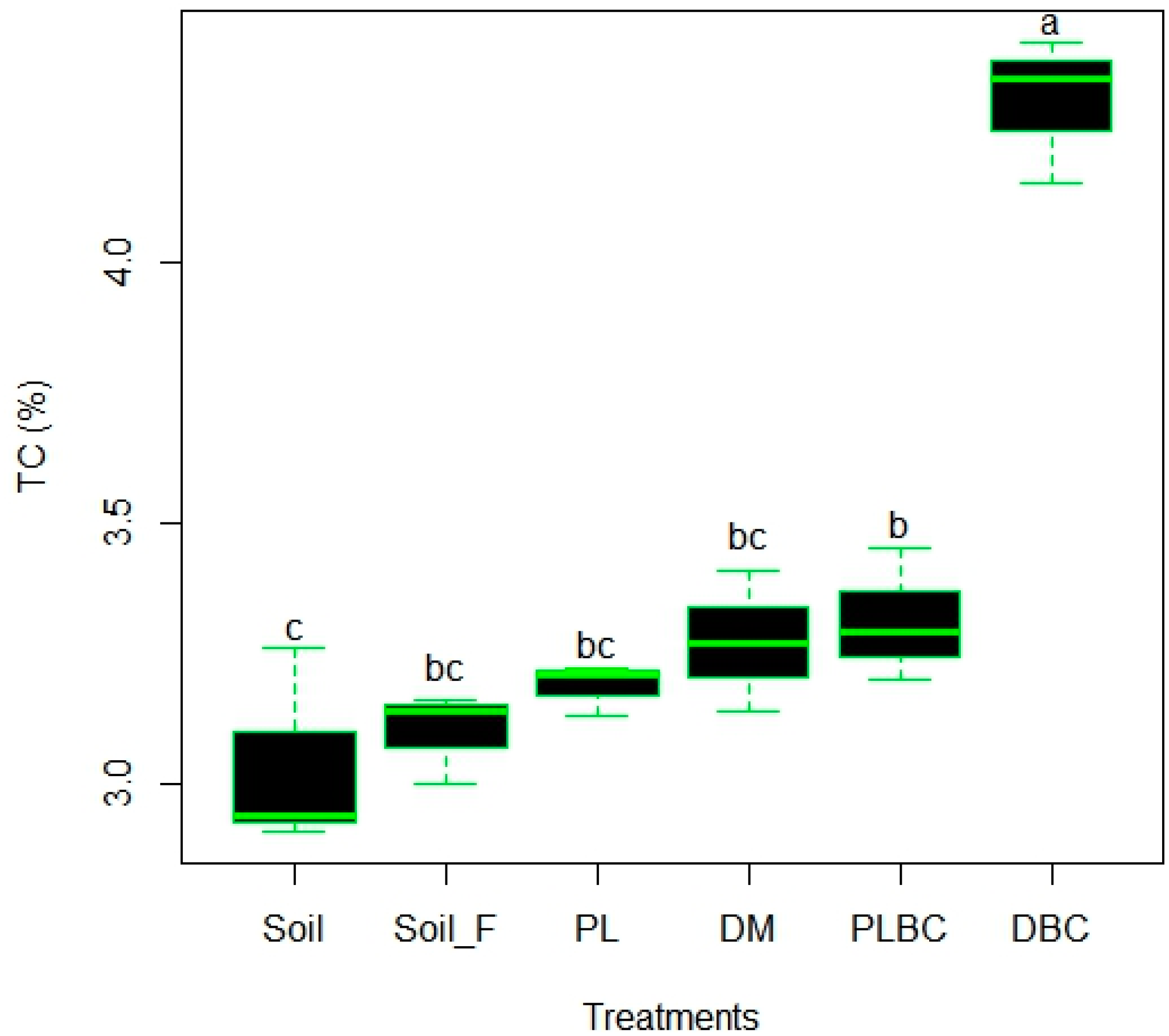

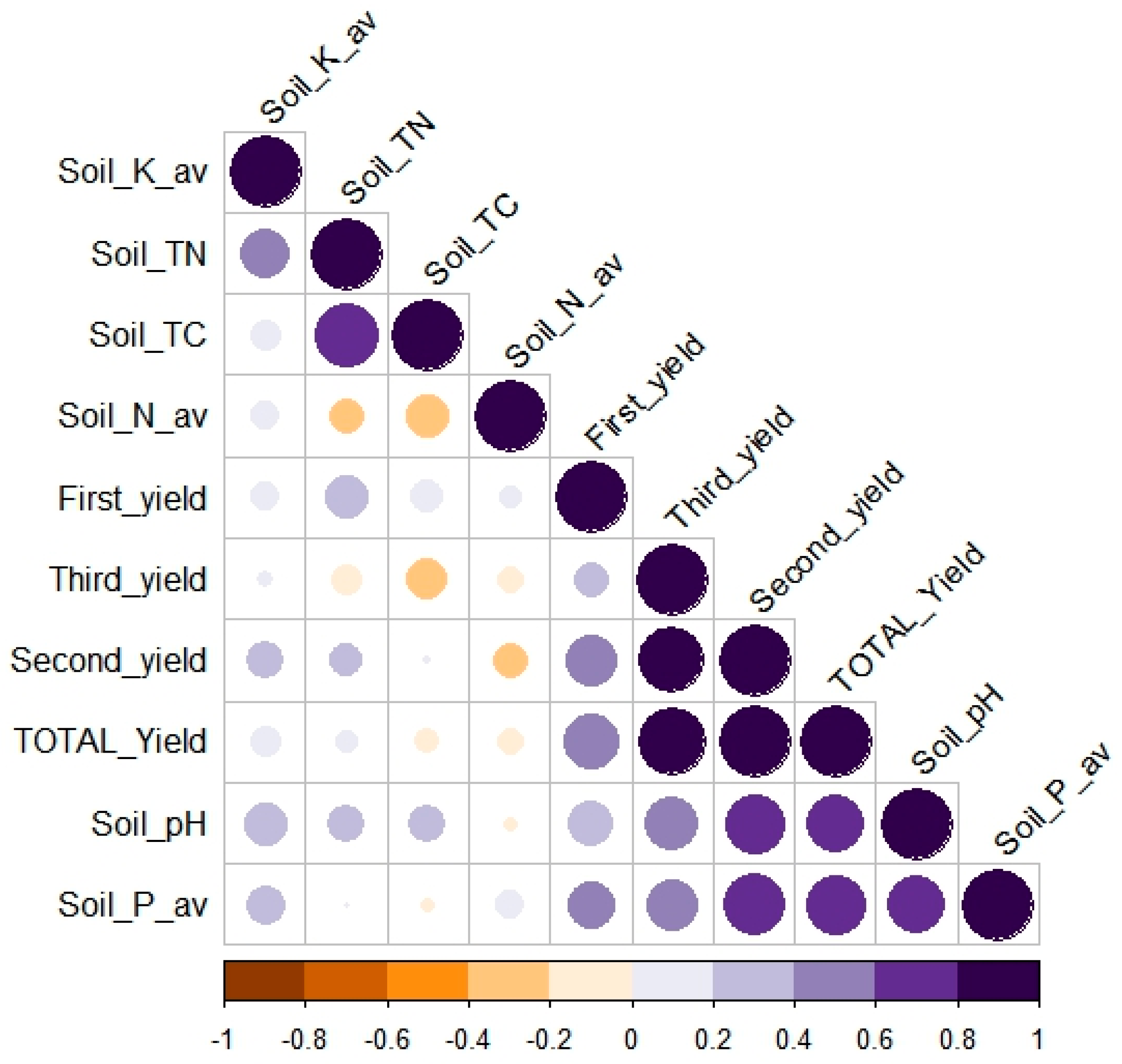
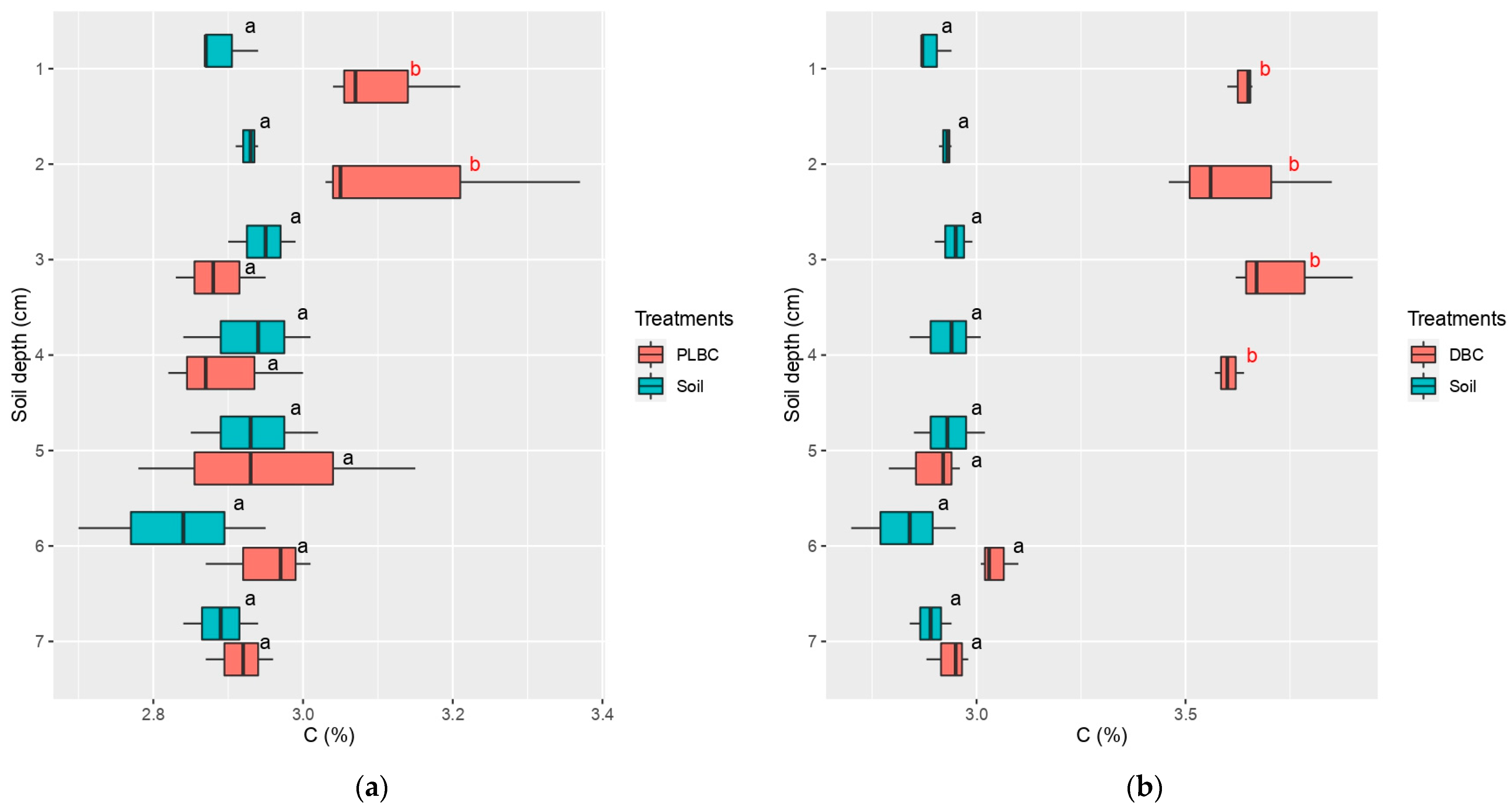
| Treatments | TC (%) | TN (%) | Corg (%) | H:Corg * | pH | NO3− N (mg kg−1) | NH4+ N (mg kg−1) | P2O5 (%) | K2O (%) |
|---|---|---|---|---|---|---|---|---|---|
| PL | 16.2 | 1.2 | - | - | 8.7 | 612.9 | 157.3 | 4.83 | 1.37 |
| DM | 30.5 | 1.2 | - | - | 8.21 | 47.3 | 101.4 | 0.43 | 0.3 |
| PLBC | 11.5 | 0.6 | 6.84 | 0.07 | 10.5 | 2.27 | 9.6 | 2.26 | 1.34 |
| DBC | 38.0 | 1.4 | 36.96 | 0.28 | 10.7 | 3.53 | 5.1 | 0.39 | 1.14 |
| Treatment | Foliar N | Foliar P | Foliar K | Soil NO3− | Soil NH4+ | Soil av. N | Soil av. P | Soil av. K | Soil pH | Soil N |
|---|---|---|---|---|---|---|---|---|---|---|
| Units | (%) | (%) | (%) | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | - | (%) |
| Soil | 3.98 (0.43) a | 0.34 (0.02) a | 3.44 (0.33) a | 10.27 (3.1) a | 15.50 (2.79) a | 25.77 (5.2) a | 41.77 (1.3) b | 374.1 (6.4) a | 5.94 (0.05) c | 0.22 (0.02) a |
| Soil_F | 3.71 (0.11) ab | 0.31 (0.01) a | 3.95 (0.58) a | 8.50 (1.5) a | 12.57 (3.05) a | 21.1 (1.6) a | 43.27 (4.1) b | 373.8 (72.7) a | 5.87 (0.07) c | 0.22 (0.01) a |
| PL | 3.53 (0.15) b | 0.33 (0.04) a | 3.94 (0.24) a | 9.57 (5.1) a | 10.73 (3.57) a | 20.30 (4.4) a | 50.27 (3.9) a | 379.1 (70.0) a | 6.19 (0.12) b | 0.22 (0.01) a |
| DM | 3.50 (0.10) b | 0.34 (0.03) a | 3.46 (0.27) a | 10.93 (5.8) a | 10.90 (4.96) a | 21.77 (9.2) a | 40.03 (3.2) b | 362.7 (25.2) a | 5.95 (0.06) c | 0.23 (0.01) a |
| PLBC | 3.99 (0.29) a | 0.34 (0.03) a | 3.59 (0.26) a | 10.37 (4.88) a | 11.90 (4.59) a | 22.27 (9.2) a | 50.33 (3.9) a | 408.4 (25.3) a | 6.44 (0.23) a | 0.23 (0.01) a |
| DBC | 3.42 (0.09) b | 0.320 (0.02) a | 3.76 (0.14) a | 7.57 (1.68) a | 9.87 (1.78) a | 17.4 (2.62) a | 43.90 (3.1) b | 12.3 (122.4) a | 6.22 (0.13) ab | 0.25 (0.02) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, C.; Ginebra, M.; Zagal, E. Impact of Pyrolyzed and Unpyrolyzed Animal Manures on Soil Properties, Carbon Sequestration, and Clover Productivity in Andisol. Agronomy 2024, 14, 592. https://doi.org/10.3390/agronomy14030592
Muñoz C, Ginebra M, Zagal E. Impact of Pyrolyzed and Unpyrolyzed Animal Manures on Soil Properties, Carbon Sequestration, and Clover Productivity in Andisol. Agronomy. 2024; 14(3):592. https://doi.org/10.3390/agronomy14030592
Chicago/Turabian StyleMuñoz, Cristina, Milagros Ginebra, and Erick Zagal. 2024. "Impact of Pyrolyzed and Unpyrolyzed Animal Manures on Soil Properties, Carbon Sequestration, and Clover Productivity in Andisol" Agronomy 14, no. 3: 592. https://doi.org/10.3390/agronomy14030592
APA StyleMuñoz, C., Ginebra, M., & Zagal, E. (2024). Impact of Pyrolyzed and Unpyrolyzed Animal Manures on Soil Properties, Carbon Sequestration, and Clover Productivity in Andisol. Agronomy, 14(3), 592. https://doi.org/10.3390/agronomy14030592








