Response of Soil Nitrogen Components and nirK- and nirS-Type Denitrifying Bacterial Community Structures to Drip Irrigation Systems in the Semi-Arid Area of Northeast China
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sample Collection
2.2. Measurement of Soil Physicochemical Properties and Acid Hydrolyzable Organic N Components
2.3. Measurement of Soil NR and NiR Activities
2.4. Total Soil DNA Extraction and Quantitative Fluorescence PCR of nirK and nirS Gene of Denitrifying Bacteria
2.5. Illumina MiSeq Sequencing and Bioinformatic Analysis
2.6. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties and Acid Hydrolyzable Organic N Components
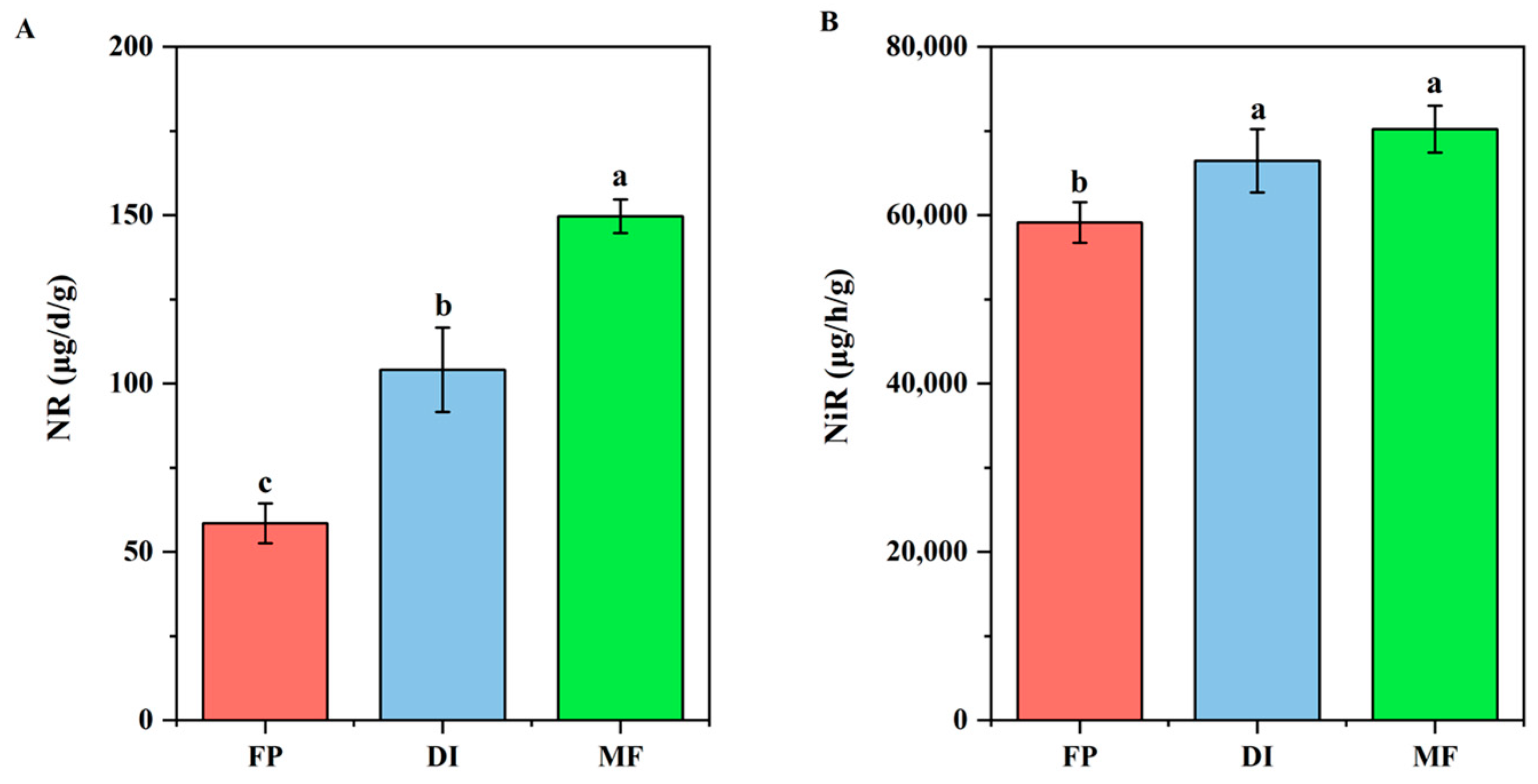
3.2. Soil NR and NiR Activities
3.3. The Abundance of nirK and nirS Genes
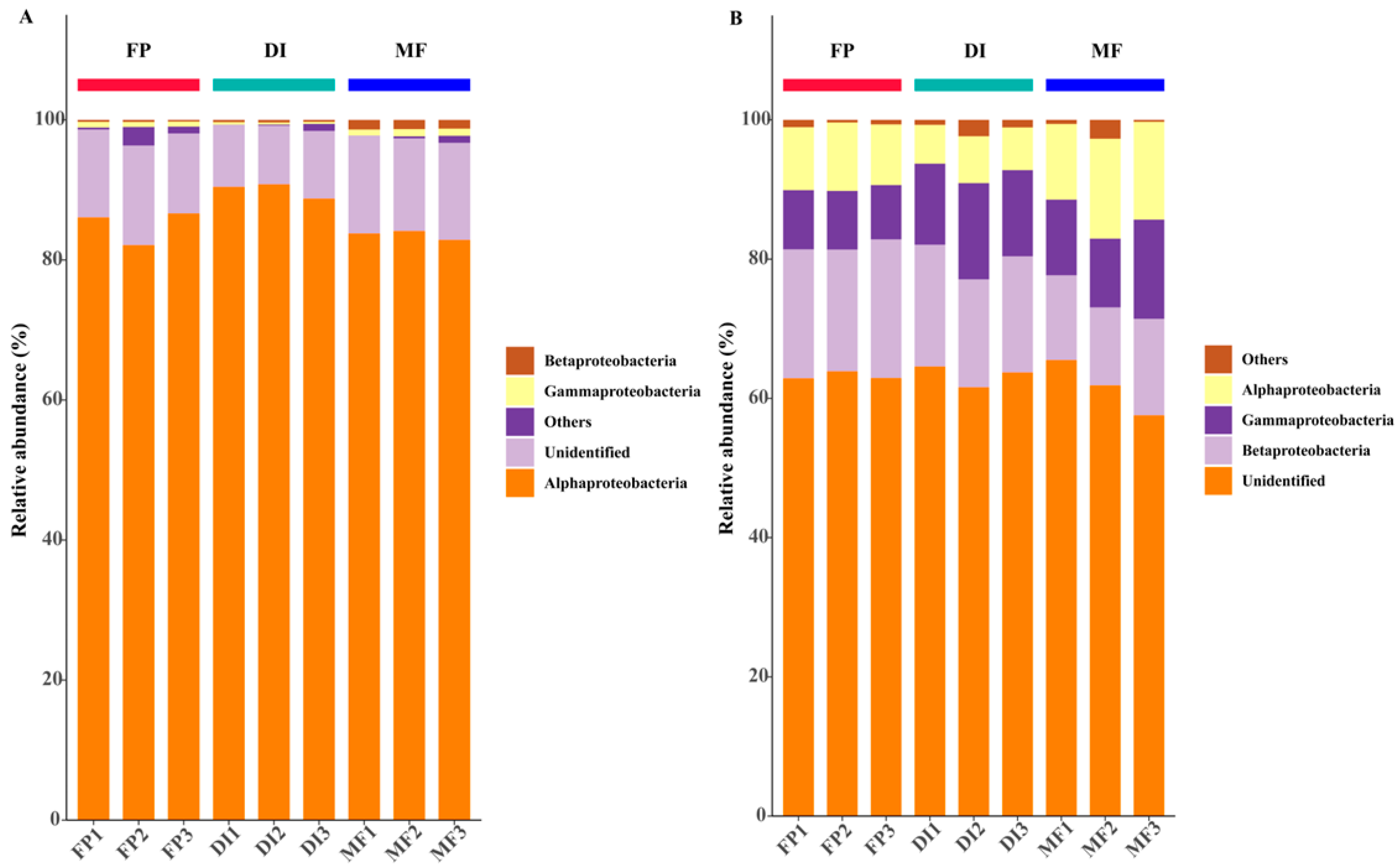
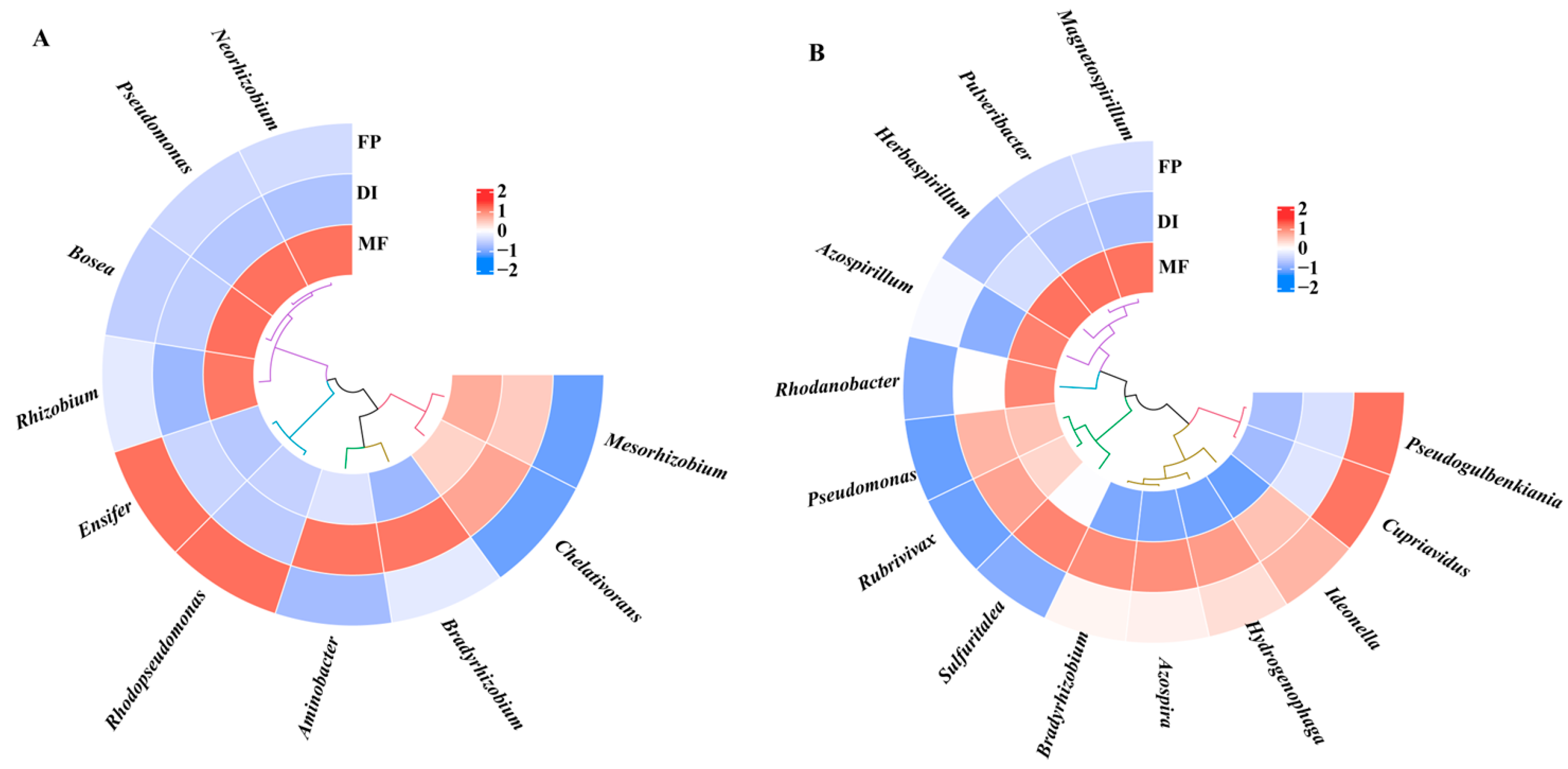
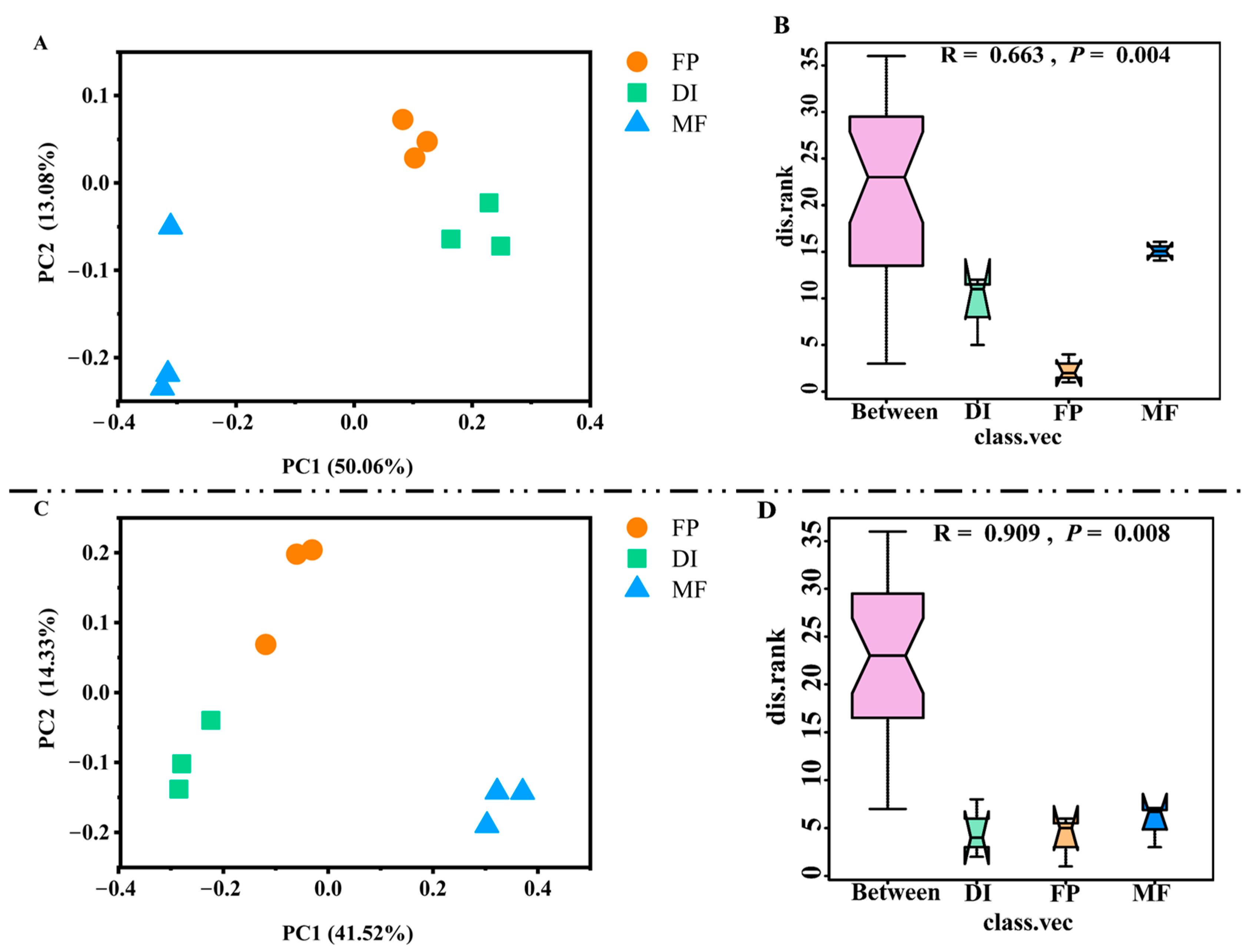
3.4. The Community Composition of nirK- and nirS-Type Denitrifying Bacteria
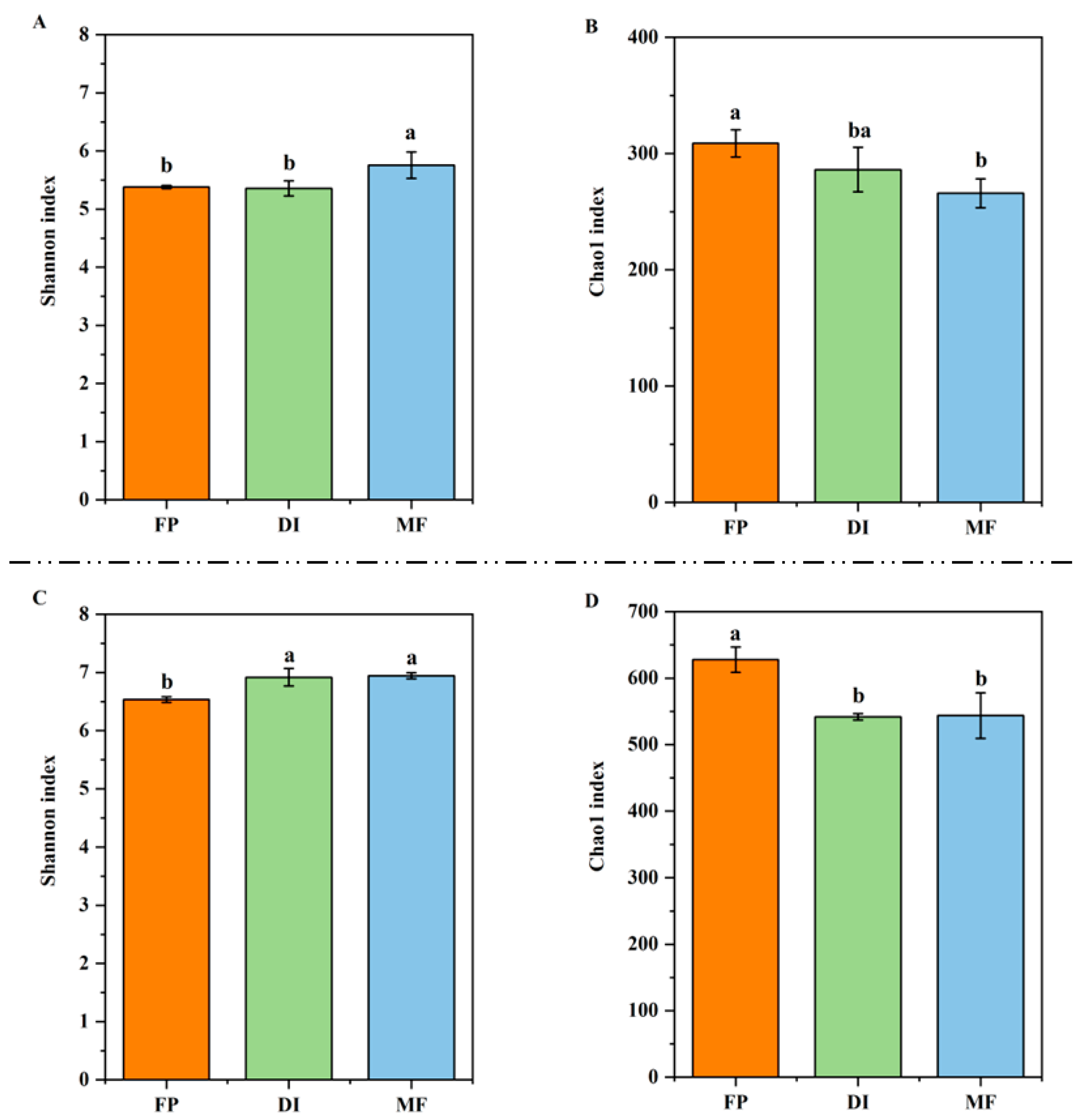
3.5. The Diversity of nirK- and nirS-Type Denitrifying Bacteria
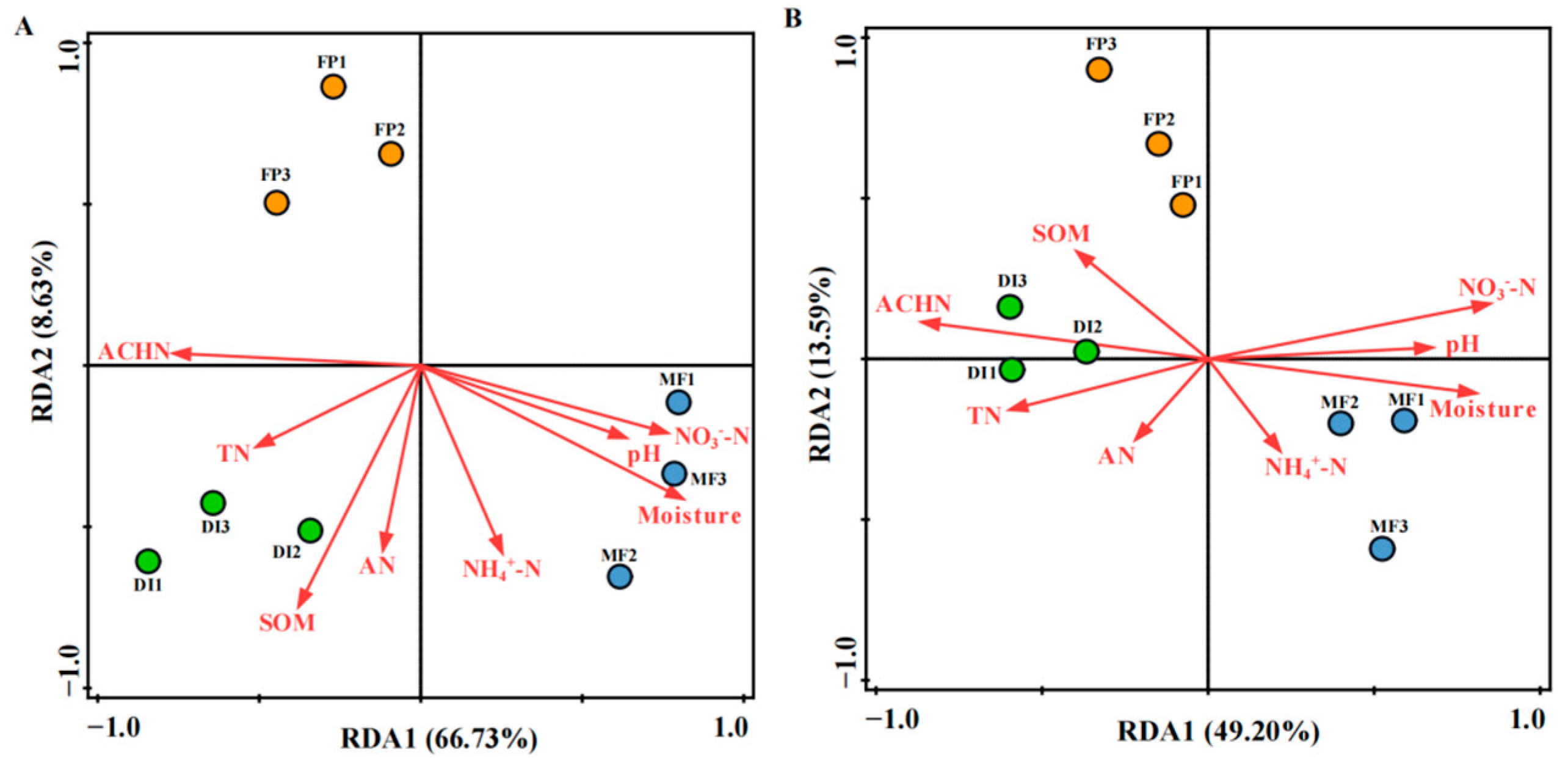
3.6. The Driving Factors Affecting the Denitrifying Bacterial Community Structure
4. Discussion
4.1. Impact of Irrigation Systems on the Soil Physicochemical Properties and Acid Hydrolyzable Organic N Components
4.2. Effects of Irrigation Systems on Enzyme Activities and the Abundance of nirK- and nirS-type Denitrifying Bacteria
4.3. Impact of Irrigation Systems on the nirK- and nirS-type Denitrifying Bacterial Community Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Philippot, L.; Spor, A.; Hénault, C.; Bru, D.; Bizouard, F. Loss in microbial diversity affects nitrogen cycling in soil. ISME J. 2013, 7, 1609–1619. [Google Scholar] [CrossRef]
- Feng, D.; Li, G.; Wang, D.; Wulazibieke, M.; Cai, M.; Kang, J. Evaluation of AquaCrop model performance under mulched drip irrigation for maize in Northeast China. Agric. Water Manag. 2022, 261, 107372. [Google Scholar] [CrossRef]
- Sui, J.; Wang, J.; Gong, S.; Xu, D.; Zhang, Y.; Qin, Q. Assessment of maize yield-increasing potential and optimum N level under mulched drip irrigation in the Northeast of China. Field Crops Res. 2018, 215, 132–139. [Google Scholar] [CrossRef]
- Sanchez, L.; Meijide, A.; Garcia, L.; Vallejo, A. Combination of drip irrigation and organic fertilizer for mitigating emissions of nitrogen oxides in semiarid climate. Agric. Ecosyst. Environ. 2010, 137, 99–107. [Google Scholar] [CrossRef]
- Bronson, K.; Hunsaker, D.; Williams, C.; Thorp, K.; Rockholt, K. Nitrogen management affects nitrous oxide emissions under varying cotton irrigation systems in the desert southwest, USA. J. Environ. Qual. 2018, 47, 70–78. [Google Scholar] [CrossRef]
- Mehmood, F.; Wang, G.; Gao, Y.; Liang, Y.; Chen, J.; Si, Z. Nitrous oxide emission from winter wheat field as responded to irrigation scheduling and irrigation methods in the North China Plain. Agric. Water Manag. 2019, 222, 367–374. [Google Scholar] [CrossRef]
- Huang, J.; Dai, X.; Chen, X.; Ail, I.; Chen, H.; Gou, J. Combined forage grass-microbial for remediation of strontium-contaminated soil. J. Hazard. Mater. 2023, 450, 131013. [Google Scholar] [CrossRef]
- Sarula, C.; Yang, H.; Zhang, R.; Li, Y. Shallow-buried drip irrigation promoted the enrichment of beneficial microorganisms in surface soil. Rhizosphere 2023, 28, 100776. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, S.; Hu, T.; Li, H.; Sun, J. Responses of soil reactive nitrogen pools and enzyme activities to water and nitrogen levels and their relationship with apple yield and quality under drip fertigation. Sci. Hortic. 2024, 324, 112632. [Google Scholar] [CrossRef]
- Zong, R.; Wang, Z.; Li, W.; Li, H.; Ayantobo, O. Effects of practicing long-term mulched drip irrigation on soil quality in Northwest China. Sci. Total Environ. 2023, 878, 163247. [Google Scholar] [CrossRef]
- Ye, M.; Zheng, W.; Yin, C.; Fan, X.; Chen, H. The inhibitory efficacy of procyanidin on soil denitrification varies with N fertilizer type applied. Sci. Total Environ. 2022, 806, 150588. [Google Scholar] [CrossRef]
- Zheng, Q.; Ding, J.; Lin, W.; Yao, Z.; Li, Q. The influence of soil acidification on N2O emissions derived from fungal and bacterial denitrification using dual is otopocule map and acetylene inhibition. Environ. Pollut. 2022, 303, 119076. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Ma, L.; Li, D.; Liu, K.; Huang, Q. Denitrification potential of paddy and upland soils derived from the same parent material respond differently to long-term fertilization. Front. Environ. Sci. 2020, 8, 105. [Google Scholar] [CrossRef]
- Nie, J.; Yang, Y.; Wang, B.; Liu, Z.; Zhu, B. Stronger impact of urea application than incorporation of Chinese milk vetch (Astragalus sinicus L.) on nirK-denitrifying bacterial communities in a Chinese double-rice paddy. Acta Agric. Scand. Sect. B Soil Plant Sci. 2021, 71, 530–540. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Ciampitti, I.; He, P.; Xu, X. Response of soil denitrification potential and community composition of denitrifying bacterial to different rates of straw return in north-central China. Appl. Soil Ecol. 2022, 170, 104312. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, J.; Liu, Y.; Ma, J.; Yu, Q.; Zou, P. Differential responses of soil nirS-and nirK-type denitrifying microbial communities to long-term application of biogas slurry in a paddy soil. Appl. Soil Ecol. 2023, 182, 104711. [Google Scholar] [CrossRef]
- Hou, S.; Ai, C.; Zhou, W.; Liang, G.; He, P. Structure and assembly cues for rhizospheric nirK- and nirS-type denitrifier communities in long-term fertilized soils. Soil Biol. Biochem. 2018, 119, 32–40. [Google Scholar] [CrossRef]
- Azziz, G.; Monza, J.; Etchebehere, C.; Irisarri, P. nirS-and nirK-type denitrifier communities are differentially affected by soil type, rice cultivar and water management. Eur. J. Soil Biol. 2017, 78, 20–28. [Google Scholar] [CrossRef]
- Hu, X.; Liu, J.; Wei, D.; Zhu, P.; Cui, X. Chronic effects of different fertilization regimes on nirS-type denitrifier communities across the black soil region of Northeast China. Pedosphere 2020, 30, 73–86. [Google Scholar] [CrossRef]
- Maul, J.; Cavigelli, M.; Vinyard, B.; Jeffrey, S. Crop system history and crop rotation phase drive the abundance of soil denitrification genes nirK, nirS and nosZ in conventional and organic grain agroecosystems. Agric. Ecosyst. Environ. 2019, 273, 95–106. [Google Scholar] [CrossRef]
- Bowen, H.; Maul, J.; Cavigelli, M.; Yarwood, S. Denitrifier abundance and community composition linked to denitrification activity in an agricultural and wetland soil. Appl. Soil Ecol. 2020, 151, 103521. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Cheng, Z. Changes in soil bacterial and fungal community characteristics in response to long-term mulched drip irrigation in oasis agroecosystems. Agric. Water Manag. 2023, 279, 108178. [Google Scholar] [CrossRef]
- Lu, R. Soil and Agro-Chemical Analytical Methods; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Throbäck, I.; Enwall, K.; Jarvis, A.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.; Biasi, C.; Horn, M. Contrasting denitrifier communities relate to contrasting N2O emission patterns from acidic peat soils in Arctic tundra. ISME J. 2012, 6, 1058–1077. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Haas, B.; Clemente, J.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Si, W.; Yan, S.; Wu, L.; Zhao, W.; Zhang, J. Water consumption, soil nitrate-nitrogen residue and fruit yield of drip-irrigated greenhouse tomato under various irrigation levels and fertilization practices. Agric. Water Manag. 2023, 277, 108092. [Google Scholar] [CrossRef]
- Yu, Y.; Neil, C.; Gong, Y.; Feng, M.; Fang, C. Benefits and limitations to straw- and plastic-film mulch on maize yield and water use efficiency: A meta-analysis across hydrothermal gradients. Eur. J. Agron. 2018, 99, 138–147. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, B.; Wu, S.; Feng, H. Soil carbon and nitrogen of wheat–maize rotation system under continuous straw and plastic mulch. Nutr. Cycl. Agroecosyst. 2021, 119, 181–193. [Google Scholar] [CrossRef]
- Wang, D.; Mo, Y.; Li, G.; Wilkerson, J.; Hoogenboom, G. Improving maize production and decreasing nitrogen residue in soil using mulched drip fertigation. Agric. Water Manag. 2021, 251, 106871. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, M.; Zhu, B.; Fan, J.; Lin, H. Drip fertigation sustains crop productivity while mitigating reactive nitrogen losses in Chinese agricultural systems: Evidence from a meta-analysis. Sci. Total Environ. 2023, 886, 163804. [Google Scholar] [CrossRef]
- Li, R.; Wu, X.; Zhang, Y.; Wang, L.; Li, X. Nitrate nitrogen contents and quality of greenhouse soil applied with different N rates under drip irrigation. J. Plant Nutr. 2015, 21, 1642–1651. [Google Scholar] [CrossRef]
- Chen, N.; Li, X.; Jiri, I.; Shi, H.; Zhang, Y. The effects of biodegradable and plastic film mulching on nitrogen uptake, distribution, and leaching in a drip-irrigated sandy field. Agric. Ecosyst. Environ. 2020, 292, 106817. [Google Scholar] [CrossRef]
- Qiang, D.; Ting, H.; Shen, G.; Ming, H. Effect of different mulching measures on nitrate nitrogen leaching in spring maize planting system in south of Loess Plateau. Agric. Water Manag. 2019, 213, 654–658. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, M.; Fu, J.; Zhang, X.; Li, Y.; Xing, Y. Effects of drip irrigation on yield, soil fertility and soil enzyme activity of different potato varieties in Northwest China. Front. Plant Sci. 2023, 14, 1240196. [Google Scholar] [CrossRef]
- Yang, W.; Shao, F.; Li, Z.; Li, W.; Yong, W.; Wen, X. Drip irrigation incorporating water conservation measures: Effects on soil water–nitrogen utilization, root traits and grain production of spring maize in semi-arid areas. J. Integr. Agric. 2021, 20, 16. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Li, Y. Effects of drip irrigation system uniformity and nitrogen applied on deep percolation and nitrate leaching during growing seasons of spring maize in semi-humid region. Irrig. Sci. 2014, 32, 221–236. [Google Scholar] [CrossRef]
- Tao, R.; Wakelin, S.; Liang, Y.; Baowei, H.; Chu, G. Nitrous oxide emission and denitrifier communities in drip-irrigated calcareous soil as affected by chemical and advances in organic chemistry. Sci. Total Environ. 2018, 612, 739–749. [Google Scholar] [CrossRef]
- Ma, C.; Song, H.; Fang, J.; Zhang, J.; Wei, S.; Liu, X.; Chen, K.; Zhang, W. Development of a process-based N2O emission model for natural forest and grassland ecosystems. J. Adv. Model. Earth Syst. 2022, 14, e2021MS002460. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, F.; Jia, X.; Zhang, H.; Lin, C.; Chen, L.; Chen, J. Differential response of denitrifying community to the application of green manure and reduced chemical fertilizer in a paddy soil. Chil. J. Agric. Res. 2020, 80, 393–404. [Google Scholar] [CrossRef]
- Duan, R.; Long, X.; Tang, Y.F.; Wen, J.; Su, S.; Bai, L.; Liu, R.; Zeng, X. Effects of different fertilizer application methods on the community of nitrifiers and denitrifiers in a paddy soil. Soils Sediments 2018, 18, 24–38. [Google Scholar] [CrossRef]
- Jin, W.; Cao, W.; Liang, F.; Wen, Y.; Wang, F.; Dong, Z.; Song, H. Water management impact on denitrifier community and denitrification activity in a paddy soil at different growth stages of rice. Agric. Water Manag. 2020, 241, 106354. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Hu, F.; Shi, W. Soil nitrous oxide emissions following crop residue addition: A meta-analysis. Glob. Chang. Biol. 2013, 19, 2956–2964. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, C.; Wei, X.; Liu, Y.; Chen, X.; Qin, H.; Wu, J.; Su, Y.; Ge, T.; Hu, H. Characterization of nirS- and nirK-containing communities and potential denitrification activity in paddy soil from eastern China. Agric. Ecosyst. Environ. 2021, 319, 107561. [Google Scholar] [CrossRef]
- Christopher, M.; Blaz, S.; Magnus, R.; Sare, H. Phylogenetic analysis of nitrite, nitric oxide, and nitrous oxide respiratory enzymes reveal a complex evolutionary history for denitrification. Mol. Biol. Evol. 2008, 25, 1955–1966. [Google Scholar] [CrossRef]
- Christopher, M.; Ayme, S.; Fiona, P.; Marie-Christine, B. Recently identified microbial guild mediates soil N2O sink capacity. Nat. Clim. Chang. 2014, 4, 801–805. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, T.; Zhang, C.; Fang, K.; Xia, C.; Bai, S.; Zeng, M.; Qiu, X. Illumina MiSeq sequencing reveals the community composition of nirS-type and nirK-type denitrifiers in Zhoucun reservoir-a large shallow eutrophic reservoir in norther China. Rsc. Adv. 2016, 6, 91517–91528. [Google Scholar] [CrossRef]
- Demanèche, S.; Philippot, L.; David, M.; Navarro, E.; Vogel, T.; Simonet, P. Characterization of denitrification gene clusters of soil bacteria via a metagenomic approach. Appl. Environ. Microb. 2009, 75, 534–537. [Google Scholar] [CrossRef]
- Kornaros, M.; Zafiri, C.; Lyberatos, G. Kinetics of denitrification by Pseudomonas denitrificans under growth conditions limited by carbon and/or nitrate or nitrite. Water Environ. Res. 1996, 68, 934–945. [Google Scholar] [CrossRef]
- Guo, Y.; Geng, J.; Cheng, S.; Fang, H.; Li, Y.; Yang, Y. Soil acidification and ammonia-oxidizing archaeal abundance dominate the contrasting responses of soil N2O emissions to NH4+ and NO3− enrichment in a subtropical plantation. Eur. J. Soil Biol. 2023, 116, 103491. [Google Scholar] [CrossRef]
- Francis, C.; O’Mullan, G.; Cornwell, J.; Ward, B. Transitions in nirS type denitrifier diversity community composition and biogeochemical activity along the Chesapeake Bay estuary. Front. Microbiol. 2013, 4, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Braker, G.; Farias, L.; Ulloa, O. Communities of nirS-type denitrifiers in the water column of the oxygen minimum zone in the eastern South Pacific. Environ. Microbiol. 2005, 7, 1298–1306. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Moisture | pH | SOM | TN | AVN | NO3−-N | NH4+-N |
|---|---|---|---|---|---|---|---|
| (%) | (g kg−1) | (g kg−1) | (g kg−1) | (mg kg−1) | (mg kg−1) | ||
| FP | 10.84 ± 0.11 c | 6.75 ± 0.11 a | 17.06 ± 0.06 ab | 1.05 ± 0.02 a | 95.54 ± 0.51 b | 7.93 ± 1.42 b | 0.66 ± 0.03 a |
| DI | 11.59 ± 0.45 b | 6.71 ± 0.35 a | 19.54 ± 0.71 a | 1.08 ± 0.02 a | 115.29 ± 1.63 a | 10.36 ± 1.11 a | 0.52 ± 0.07 b |
| MF | 13.81 ± 0.22 a | 7.04 ± 0.08 a | 17.81 ± 1.59 b | 1.04 ± 0.03 a | 106.38 ± 8.06 a | 10.44 ± 1.03 a | 0.54 ± 0.01 b |
| Treatment | ACHN | AAN | AN | ASN | AUN | AIN |
|---|---|---|---|---|---|---|
| FP | 507.12 ± 25.75 a | 68.32 ± 15.68 a | 280.75 ± 34.29 a | 48.16 ± 16.15 b | 109.90 ± 26.39 a | 507.97 ± 39.19 b |
| DI | 537.88 ± 65.26 a | 54.13 ± 20.33 a | 309.87 ± 64.82 a | 68.32 ± 9.76 ab | 105.56 ± 26.25 a | 580.10 ± 37.12 ab |
| MF | 403.14 ± 17.16 b | 40.69 ± 18.24 a | 195.63 ± 3.42 b | 73.55 ± 4.66 a | 93.27 ± 14.26 a | 602.97 ± 36.36 a |
| Treatment | AAN | AN | ASN | AUN | ACHN | AIN |
|---|---|---|---|---|---|---|
| FP | 6.52 ± 1.63 a | 26.78 ± 3.82 a | 4.59 ± 1.45 a | 10.48 ± 2.31 b | 48.37 ± 2.93 a | 48.45 ± 3.26 b |
| DI | 4.99 ± 1.79 a | 28.57 ± 5.46 a | 6.30 ± 0.82 a | 9.73 ± 2.65 ab | 49.59 ± 5.08 a | 53.48 ± 3.90 ab |
| MF | 3.91 ± 1.84 b | 18.82 ± 0.32 a | 7.08 ± 0.33 b | 8.97 ± 1.08 a | 38.78 ± 1.39 a | 58.01 ± 5.21 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiang, R.; Wang, M.; Li, Q.; Li, Y.; Sun, H.; Liang, W.; Li, C.; Zhang, J.; Liu, H. Response of Soil Nitrogen Components and nirK- and nirS-Type Denitrifying Bacterial Community Structures to Drip Irrigation Systems in the Semi-Arid Area of Northeast China. Agronomy 2024, 14, 577. https://doi.org/10.3390/agronomy14030577
Qiang R, Wang M, Li Q, Li Y, Sun H, Liang W, Li C, Zhang J, Liu H. Response of Soil Nitrogen Components and nirK- and nirS-Type Denitrifying Bacterial Community Structures to Drip Irrigation Systems in the Semi-Arid Area of Northeast China. Agronomy. 2024; 14(3):577. https://doi.org/10.3390/agronomy14030577
Chicago/Turabian StyleQiang, Ruowen, Meng Wang, Qian Li, Yingjie Li, Huixian Sun, Wenyu Liang, Cuilan Li, Jinjing Zhang, and Hang Liu. 2024. "Response of Soil Nitrogen Components and nirK- and nirS-Type Denitrifying Bacterial Community Structures to Drip Irrigation Systems in the Semi-Arid Area of Northeast China" Agronomy 14, no. 3: 577. https://doi.org/10.3390/agronomy14030577
APA StyleQiang, R., Wang, M., Li, Q., Li, Y., Sun, H., Liang, W., Li, C., Zhang, J., & Liu, H. (2024). Response of Soil Nitrogen Components and nirK- and nirS-Type Denitrifying Bacterial Community Structures to Drip Irrigation Systems in the Semi-Arid Area of Northeast China. Agronomy, 14(3), 577. https://doi.org/10.3390/agronomy14030577





