Influence of Edible Potato Production Technologies with the Use of Soil Conditioner on the Nutritional Value of Tubers
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Field Experiment
2.2. Treatment Details
2.3. Potato Tuber Quality Characteristics
2.3.1. Procedure for Dry Matter Determination
- DM—dry matter content (g kg−1)
- SWB—sample weight before drying (g)
- SWA—sample weight after drying (g)
2.3.2. Procedure for Starch Determination
- SC—starch content (g kg−1 f. m.)
- a—weight of analyzed material (g)
- L—length of polarimeter tube (dm)
- α—measured rotation in degrees
2.3.3. Procedure for Total Sugar and Reducing Sugar Determination
2.3.4. Statistical Analysis
3. Results and Discussion
3.1. Dry Matter
3.2. Starch
3.3. Total and Reducing Sugars
3.4. Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devaux, A.; Goffart, J.P.; Petsakos, A.; Kromann, P.; Gatto, M.; Okello, J.; Hareau, G. Global food security, contributions from sustainable potato agri-food systems. In The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Campos, H., Ortiz, O., Eds.; Springer: New York, NY, USA, 2020; pp. 3–35. [Google Scholar] [CrossRef]
- Wijesinha-Bettoni, R.; Mouillé, B. The contribution of potatoes to global food security, nutrition and healthy diets. Am. J. Potato Res. 2019, 96, 139–149. [Google Scholar] [CrossRef]
- Ozturk, E.; Polat, T. The effect of long term storage on physical and chemical properties of potato. Turk. J. Field Crops 2016, 21, 218–223. [Google Scholar] [CrossRef]
- Dramićanin, A.M.; Andrić, F.L.; Poštić, D.; Momirović, N.M.; Milojković-Opsenica, D.M. Sugar profiles as a promising tool in tracing differences between potato cultivation systems, botanical origin and climate conditions. J. Food Compos. Anal. 2018, 72, 57–65. [Google Scholar] [CrossRef]
- Kazimierczak, R.; Średnicka-Tober, D.; Hallmann, E.; Kopczyńska, K.; Zarzyńska, K. The Impact of Organic vs. Conventional Agricultural Practices on Selected Quality Features of Eight Potato Cultivars. Agronomy 2019, 9, 799. [Google Scholar] [CrossRef]
- Ierna, A.; Parisi, B.; Melilli, M.G. Overall Quality of “Early” Potato Tubers as Affected by Organic Cultivation. Agronomy 2022, 12, 296. [Google Scholar] [CrossRef]
- Lang, T. Sustainable Diets: Hairshirts or a better food future? Development 2014, 57, 240–256. [Google Scholar] [CrossRef]
- Baudry, J.; Peneau, S.; Alles, B.; Touvier, M.; Hercberg, S.; Galan, P.; Amiot, M.J.; Lairon, D.; Mejean, C.; Kesse-Guyot, E. Food choice motives when purchasing in organic and conventional consumer clusters: Focus on sustainable concerns (The NutriNet-Sante Cohort Study). Nutrients 2017, 9, 88. [Google Scholar] [CrossRef]
- Grunert, K.G. Sustainability in the food sector: A consumer behaviour perspective. Int. J. Food Syst. Dyn. 2011, 2, 207–218. [Google Scholar] [CrossRef]
- Caradonia, F.; Ronga, D.; Tava, A.; Francia, E. Plant Biostimulants in Sustainable Potato Production: An Overview. Potato Res. 2022, 65, 83–104. [Google Scholar] [CrossRef]
- Ordóñez-Santos, L.E.; Arbones-Maciñeira, E.; Fernández-Perejón, J.; Lombardero-Fernández, M.; Vázquez-Odériz, L.; Romero-Rodríguez, A. Comparison of physicochemical, microscopic and sensory characteristics of ecologically and conventionally grown crops of two cultivars of tomato (Lycopersicon esculentum Mill.). J. Sci. Food Agric. 2009, 89, 743–749. [Google Scholar] [CrossRef]
- Pobereżny, J.; Wszelaczyńska, E.; Wichrowska, D.; Jaskulski, D. Content of nitrates in potato tubers depending on the organic matter, soil fertilizer, cultivation simplifications applied and storage. Chil. J. Agric. Res. 2015, 75, 42–49. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellin, A.; Martinetti, L.; Ferrante, A. Prodotti biostimolanti ed effetti sulle colture ortofloricole. Acta Ital. Horts. 2015, 15, 55–63. [Google Scholar]
- Jolayemi, O.L.; Malik, A.H.; Ekblad, T.; Fredlund, K.; Olsson, M.E.; Johansson, E. Protein-Based Biostimulants to Enhance Plant Growth—State-of-the-Art and Future Direction with Sugar Beet as an Example. Agronomy 2022, 12, 3211. [Google Scholar] [CrossRef]
- Kumari, M.; Swarupa, P.; Kesari, K.K.; Kumar, A. Microbial inoculants as plant biostimulants: A review on risk status. Life 2023, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.S.; Mithra, R.S.; Shiyal, V.N. Biostimulants for Sustainable Crop Production. In Advances in Agriculture Sciences Chief; Naresh, R.K., Ed.; AkiNik Publications: New Delhi, India, 2023; Volume 42, pp. 39–65. [Google Scholar] [CrossRef]
- Hara, P. The Role of Biostimulators in Potato Cultivation. Pol. Potato 2019, 29, 18–24. [Google Scholar]
- Głosek-Sobieraj, M.; Cwalina-Ambroziak, B.; Wierzbowska, J.; Waśkiewicz, A. The influence of biostimulants on the content of P, K, Ca, Mg, and Na in the skin and flesh of potato tubers. Pol. J. Environ. Stud. 2019, 28, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Zarzecka, K.; Gugała, M.; Grzywacz, K.; Domański, Ł. Changes in carbohydrate contents in table potato tubers under the influence of soil conditioner UGmax. Appl. Ecol. Environ. Res. 2019, 17, 2315–2324. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Pietraszko, M.; Jankowska, J.; Grudzinska, M.; Boguszewska-Mankowska, D. Integrated production of early potato varieties: Cyprian, Michalina and Viviana harvested in two dates. Acta Agrophys. 2016, 23, 129–142. [Google Scholar]
- Trawczyński, C. The influence of slow-release nitrogen fertilizer on the yield and quality tubers potato. Fragm. Agron. 2017, 34, 94–102. [Google Scholar]
- Shao, Z.; Mwakidoshi, E.R.; Muindi, E.M.; Soratto, R.P.; Ranjan, S.; Padhan, S.R.; Wamukota, A.W.; Sow, S.; Wasonga, D.O.; Nasar, J.; et al. Synthetic fertilizer application coupled with bioslurry optimizes potato (Solanum tuberosum) growth and yield. Agronomy 2023, 13, 2162. [Google Scholar] [CrossRef]
- Łagocka, A.; Kamiński, M.; Cholewiński, M.; Pospolita, W. Health and environmental benefits of utilization of post-fermentation pulp from agricultural biogas plants as a natural fertilizer. Kosmos 2016, 65, 601–607. [Google Scholar]
- Średnicka-Tober, D.; Kopczyńska, K.; Góralska-Walczak, R.; Hallmann, E.; Barański, M.; Marszałek, K.; Kazimierczak, R. Are organic certified carrots richer in health-promoting phenolics and carotenoids than the conventionally grown ones? Molecules 2022, 27, 4184. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. Optimizing Nitrogen Fertilization to Improve Qualitative Performances and Physiological and Yield Responses of Potato (Solanum tuberosum L.). Agronomy 2020, 10, 352. [Google Scholar] [CrossRef]
- Wadas, W.; Dziugieł, T. Quality of new potatoes (Solanum tuberosum L.) in response to plant biostimulants application. Agriculture 2020, 10, 265. [Google Scholar] [CrossRef]
- Głosek-Sobieraj, M.; Wierzbowska, J.; Cwalina-Ambroziak, B.; Waśkiewicz, A. Protein and sugar content of tubers in potato plants treated with biostimulants. J. Plant Protect. Res. 2022, 62, 370–384. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M.; Domański, Ł. Changes in sugars content in potato tubers under the effect of herbicide and biostimulants. Agron. Sci. 2022, 77, 1. [Google Scholar] [CrossRef]
- Göldel, B.; Lemic, D.; Bažok, R. Alternatives to synthetic insecticides in the control of the Colorado potato beetle (Leptinotarsa decemlineata Say) and their environmental benefits. Agriculture 2020, 10, 611. [Google Scholar] [CrossRef]
- Bisht, N.; Chauhan, P.S. Excessive and disproportionate use of chemicals cause soil contamination and nutritional stress. In Soil Contamination-Threats and Sustainable Solutions; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen Limited: London, UK, 2020; pp. 1–10. [Google Scholar] [CrossRef]
- Pietraszko, M. Profitability of fungicides application in control of potato late and early blight. Ziemn. Pol. 2021, 31, 3–10. [Google Scholar]
- Ahmad, U.; Sharma, L. A review of Best Management Practices for potato crop using Precision Agricultural Technologies. Smart Agric. Technol. 2023, 4, 100220. [Google Scholar] [CrossRef]
- Terry, L.A.; Medina, A.; Foukaraki, S.; Whitehead, P. Review of Factors Affecting Fruit and Vegetable Demand. In DEFRA (UK Government) Final Report FO0438; UK Government: London, UK, 2013. [Google Scholar]
- Alamar, M.C.; Tosetti, R.; Landahl, S.; Bermejo, A.; Terry, L.A. Assuring potato tuber quality during storage: A future perspective. Front. Plant Sci. 2017, 8, 2034. [Google Scholar] [CrossRef]
- American Association of Cereal Chemistry (AACC). Approved Method 44-15 A (Moisture-Air Oven Methods); AACC: St. Paul, MN, USA, 1993. [Google Scholar]
- Arbeitsgemeinschaft Getreideforschung, e.V. Standard Methoden für Getreide Mehl und Brot, 7th ed.; Verlag Moritz Schäfer: Detmold, Germany, 1994. [Google Scholar]
- Talburt, W.; Smith, O. Potato Processing (No 6648 T3 1987); Van Nostrand Reinhold: New York, NY, USA, 1987; pp. 1–796. [Google Scholar]
- Koch, M.; Naumann, M.; Pawelzik, E. Cracking and fracture properties of potato (Solanum tuberosum L.) tubers and their relation to dry matter, starch, and mineral distribution. J. Sci. Food Agric. 2019, 99, 3149–3156. [Google Scholar] [CrossRef]
- Mystkowska, I. The effect of the use of biostimulators on dry matter and starch content of tuber potatoes. Fragm. Agron. 2019, 36, 45–53. [Google Scholar] [CrossRef]
- Naeem, M.; Caliskan, M. Comparison of methods for dry matter content determination in potato using multi-environments field data and stability statistics. Turk. J. Field Crops 2020, 25, 197–207. [Google Scholar] [CrossRef]
- Cieciura-Olczyk, M. Potato yielding on the effect of organic, natural and nitrogen fertilization. Fragm. Agron. 2019, 36, 7–17. [Google Scholar] [CrossRef]
- Djaman, K.; Sanogo, S.; Koudahe, K.; Allen, S.; Saibou, A.; Essah, S. Characteristics of organically grown compared to conventionally grown potato and the processed products: A review. Sustainability 2021, 13, 6289. [Google Scholar] [CrossRef]
- Manolov, I.; Neshev, N.; Chalova, V. Tuber quality parameters of potato varieties depend on potassium fertilizer rate and source. Agric. Sci. Procedia 2016, 10, 63–66. [Google Scholar] [CrossRef][Green Version]
- Bărăscu, N.; Ianoşi, M.; Duda, M.M.; Muntean, E. The NPK fertilization effects of tubers starch, dry matter and reducing sugar content. Sci. Pap. Ser. A Agron. 2016, 59, 194–199. [Google Scholar] [CrossRef]
- Koch, M.; Naumann, M.; Pawelzik, E.; Gransee, A.; Thiel, H. The importance of nutrient management for potato production Part I: Plant nutrition and yield. Potato Res. 2020, 63, 97–119. [Google Scholar] [CrossRef]
- Torabian, S.; Farhangi-Abriz, S.; Qin, R.; Noulas, C.; Sathuvalli, V.; Charlton, B.; Loka, D.A. Potassium: A vital macronutrient in potato production—A review. Agronomy 2021, 11, 543. [Google Scholar] [CrossRef]
- Devi, S.; Sharma, P.K.; Trivedi, J.; Kumar, L.; Shrivastava, S.A.; Kharshan, P.G.M. Effect of different levels of NPK fertilizer on quality parameters of potato (Solanum tuberosum L.). Pharm. Innov. J. 2023, 12, 5028–5032. [Google Scholar]
- Li, H.; Yang, X.; Kang, Y.; Li, W.; Li, H.; Qin, S. Effects of nitrogen, phosphorus and potassium combined fertilisation on the dry matter accumulation, distribution and yield of potato under ridge and furrow film mulch cropping. Potato Res. 2023, 66, 851–8718. [Google Scholar] [CrossRef]
- Kelling, K.A.; Stevenson, W.R.; Speth, P.E.; James, R.V. Interactive effects of fumigation and fungicides on potato response to nitrogen rate or timing. Am. J. Potato Res. 2016, 93, 533–542. [Google Scholar] [CrossRef]
- Bombik, A.; Rymuza, K.; Markowska, M.; Stankiewicz, C. Variability analysis of selected quantitative characteristics in edible potato varieties. Acta Sci. Pol. Agric. 2007, 6, 5–15. [Google Scholar]
- Milroy, S.P.; Wang, P.; Sadras, V.O. Defining upper limits of nitrogen uptake and nitrogen use efficiency of potato in response to crop N supply. Field Crops Res. 2019, 239, 38–46. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M.; Milewska, A. Effect of soil fertilizer UGmax on potato yielding and plant health. Prog. Plant Prot. 2011, 51, 153–157. [Google Scholar]
- Sosnowski, J. Reaction of Dactylis glomerata L., Festuca pratensis Huds. and Lolium perenne L. to microbiological fertilizer and mineral fertilization. Acta Sci. Pol. Agric. 2012, 11, 91–98. [Google Scholar]
- Zarzecka, K.; Gugała, M. Performance of one potato plant as influenced by soil conditioner UGmax. J. Ecol. Eng. 2013, 14, 45–49. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M.; Dołęga, H.; Zadrożniak, B. Modification of chemical composition of potato tubers as affected by insecticides. Fragm. Agron. 2014, 31, 129–137. [Google Scholar]
- Sayuk, O.; Plotnytska, N.; Troyachenko, R.; Ovezmyradova, O. Effect of fungicides on mycosis progression and potato yields. J. Agric. Sci. 2022, 33, 139–145. [Google Scholar] [CrossRef]
- Reyniers, S.; Ooms, N.; Gomand, S.V.; Delcour, J.A. What makes starch from potato (Solanum tuberosum L.) tubers unique: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2588–2612. [Google Scholar] [CrossRef]
- Dupuis, J.H.; Liu, Q. Potato Starch: A Review of Physicochemical, Functional and Nutritional Properties. Am. J. Potato Res. 2019, 96, 127–138. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M.; Mystkowska, I.; Sikorska, A. Changes in dry weight and starch content in potato under the effect of herbicides and biostimulants. Plant Soil Environ. 2021, 67, 202–207. [Google Scholar] [CrossRef]
- Demidenko, G.A.; Turygina, O.V.; Martynova, O.V. The quality of potato tubers and yield by using fertilizer systems. IOP Conf. Ser. Earth Environ. Sci. 2022, 981, 022059. [Google Scholar] [CrossRef]
- Mareček, J.; Frančáková, H.; Bojňanská, T.; Fikselová, M.; Mendelová, A.; Ivanišová, E. Carbohydrates in varieties of stored potatoes and influence of storage on quality of fried products. J. Microbiol. Biotechnol. Food Sci. 2013, 2, 1744–1753. [Google Scholar]
- Leonel, M.; do Carmo, E.L.; Fernandes, A.M.; Soratto, R.P.; Ebúrneo, J.A.M.; Garcia, E.L.; dos Santos, T.P.R. Chemical composition of potato tubers: The effect of cultivars and growth conditions. J. Food Sci. Technol. 2017, 54, 2372–2378. [Google Scholar] [CrossRef]
- Pszczółkowski, P.; Sawicka, B.; Danilčenko, H. Effect of biopreparates on the dry matter, starching and vitamin C in potato tubers. Agron. Sci. 2019, 74, 47–56. [Google Scholar] [CrossRef]
- Liszka-Skoczylas, M. Effect of potato plants (Solanum tuberosum L.) fertilization on content and quality of starch in tubers. Food. Sci. Technol. Qual. 2020, 27, 31–46. [Google Scholar] [CrossRef]
- Yu, Y.; Han, F.; Huang, Y.; Xiao, L.; Cao, S.; Liu, Z.; Han, L. Physicochemical properties and molecular structure of starches from potato cultivars of different tuber colors. Starch-Stärke 2022, 74, 2200096. [Google Scholar] [CrossRef]
- Grudzińska, M.; Boguszewska-Mańkowska, D.; Zarzyńska, K. Drought stress during the growing season: Changes in reducing sugars, starch content and respiration rate during storage of two potato cultivars differing in drought sensitivity. J. Agron. Crop Sci. 2022, 208, 609–620. [Google Scholar] [CrossRef]
- Turska, E.; Wielogórska, G.; Rymuza, K. The influence of some agricultural factors on the quality of potato tubers. Fragm. Agron. 2009, 26, 156–161. [Google Scholar]
- Krzysztofik, B. Impact of soil cultivation on the extent of potato tuber size equalisation and starch yield. Acta Agrophys. 2009, 14, 355–365. [Google Scholar]
- Murawska, B.; Spychaj-Fabisiak, E.; Majcherczak, E.; Kozera, W.; Gaj, R.; Rozanski, S.; Jachymska, J. Importance of catch crops end microelements in potato cultivation. Zesz. Probl. Postęp. Nauk Rol. 2015, 580, 75–83. [Google Scholar]
- Koireng, R.J.; Singh, L.N.; Devi, K.P. Integration of different sources of organic manure and micro-nutrients on growth, yield and quality of potato (Solanum tuberosum L.) grown under new alluvial soil condition. Indian J. Agric. Res. 2018, 52, 172–176. [Google Scholar] [CrossRef]
- Sawicka, B.; Danilčenko, H.; Jariene, E.; Krochmal-Marczak, B. The phenotypic changeability of features of foreign cultivars of the potato in the Poland. Zesz. Probl. Post. Nauk Rol. 2009, 542, 447–463. [Google Scholar] [CrossRef]
- El-Zehery, T.M. Incorporated use impact of organic, bio and mineral fertilizers on potato (Solanum tuberosum L.) productivity and quality. J. Soil Sci. Agric. Eng. 2019, 10, 857–865. [Google Scholar] [CrossRef][Green Version]
- Hlisnikovský, L.; Menšík, L.; Kunzová, E. The effect of soil-climate conditions, farmyard manure and mineral fertilizers on potato yield and soil chemical parameters. Plants 2021, 10, 2473. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, A. Influence of pluvio-thermal conditions, growth biostimulators and herbicide on dry matter content and starch in edible potato tubers. Appl. Ecol. Environ. Res. 2019, 17, 1547–1557. [Google Scholar] [CrossRef]
- Maciejewski, T.; Szukała, J.; Jarosz, A. Influence of biostymulator Asahi SL i Atonik SL on qualitative tubers of potatoes. J. Res. Appl. Agric. Eng. 2007, 52, 109–112. [Google Scholar]
- Pereira, R.V.; Filgueiras, C.C.; Dória, J.; Peñaflor, M.F.G.; Willett, D.S. The effects of biostimulants on induced plant defense. Front. Agron. 2021, 3, 630596. [Google Scholar] [CrossRef]
- Monteiro, E.; Gonçalves, B.; Cortez, I.; Castro, I. The Role of Biostimulants as Alleviators of Biotic and Abiotic Stresses in Grapevine: A Review. Plants 2022, 11, 396. [Google Scholar] [CrossRef]
- Shailbala, M.; Pundhir, V.S. Integration of host resistance and fungicides for management of late blight of potato. Potato J. 2008, 35, 97–99. [Google Scholar]
- Gugala, M.; Zarzecka, K.; Dolega, H.; Baranowska, A. Efficacy of herbicides in potato crop. Ann. Univ. Mariae Curie-Sklodowska Sect. E Agric. 2012, 67, 45–51. [Google Scholar]
- El-Ganainy, S.M.; Abbas, A.O.; El-Hefny, D.; Abdallah, I.S. Efficacy of the New Herbicide Clomazone Against Weeds in Potato (Solanum tuberosum L.), Its Effect on Quality and Its Residues in Tubers and Soil. Gesunde Pflanz. 2023, 75, 67–75. [Google Scholar] [CrossRef]
- Kaliyeva, L.T.; Kushenbekova, A.K.; Tulegenova, D.K.; Kuanaliyeva, M.K. Influence of insecticides on the harvest and quality of potato stubs in the conditions of West Kazakhstan region. IOP Conf. Ser. Earth Environ. Sci. 2022, 979, 012041. [Google Scholar] [CrossRef]
- Rady, A.; Guyer, D.; Lu, R. Evaluation of sugar content of potatoes using hyperspectral imaging. Food Bioprocess Technol. 2015, 8, 995–1010. [Google Scholar] [CrossRef]
- Xing, Y.; Niu, X.; Wang, N.; Jiang, W.; Gao, Y.; Wang, X. The correlation between soil nutrient and potato quality in Loess Plateau of China based on PLSR. Sustainability 2020, 12, 1588. [Google Scholar] [CrossRef]
- Mona, E.E.; Ibrahim, S.A.; Manal, F.M. Combined effect of NPK levels and foliar nutritional compounds on growth and yield parameters of potato plants (Solanum tuberosum L.). Afr. J. Microbiol. Res. 2012, 6, 5100–5109. [Google Scholar] [CrossRef]
- AbdEl-Nabi, H.M.E.; El-Gamily, E.; Keshta, N.A. Response of potato plants to organic, bio and mineral fertilization. J. Plant Prod. 2016, 7, 861–867. [Google Scholar] [CrossRef]
- Jatav, A.; Kushwah, S.; Naruka, I. Performance of potato varieties for growth, yield, quality and economics under different levels of nitrogen. Adv. Res. 2017, 9, 1–9. [Google Scholar] [CrossRef]
- El-Ghamriny, E.A.; Saeed, M.N.A. Effect of irrigation intervals, Mineral fertilizers and biofertilizers on potato plants grown under sandy soil conditions. I-Growth, water relations, chemical contents and leaf anatomy. Egypt. J. Appl. Sci. 2007, 22, 480–511. [Google Scholar]
- Haider, M.W.; Ayyub, C.M.; Pervez, M.A.; Asad, H.U.; Manan, A.; Raza, S.A.; Ashraf, I. Impact of foliar application of seaweed extract on growth, yield and quality of potato (Solanum tuberosum L.). Soil Environ. 2012, 31, 157–162. [Google Scholar]
- Zarzecka, K.; Gugała, M. The effect of herbicides and biostimulants on sugars content in potato tubers. Plant Soil Environ. 2018, 64, 82–87. [Google Scholar] [CrossRef]
- Trawczyński, C. The impact of amino acid-based biostimulant–tecamin–on the yield and quality of potatoes. Pol. Potato. 2014, 3, 29–34. [Google Scholar]
- Karak, S.; Thapa, U.; Hansda, N.N. Impact of Biostimulant on Growth, Yield and Quality of Potato (Solanum tuberosum L.). Biol. Forum 2023, 15, 297–302. [Google Scholar] [CrossRef]
- Ezzat, A.S.; Asfour, H.E.-S.; Tolba, M.H. Improving yield and quality of some new potato varieties in winter plantation using organic stimulators. J. Plant Prod. 2011, 2, 653–671. [Google Scholar] [CrossRef]
- Arafa, A.A.; Hussien, S.F.M. Response of tuber yield quantity and quality of potato plants and its economic consideration to certain bioregulators or effective microorganisms under potassium fertilization. J. Plant Prod. 2012, 3, 131–150. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.L.; Love, S.L.; Sowokinos, J.R.; Thornton, M.K.; Shock, C.C. Review of the sugar end disorder of potato (Solanum tuberosum L.). Am. J. Potato Res. 2008, 85, 375–386. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B.P.; Kumar, P. An overview of the factors affecting sugar content of potatoes. Ann. Appl. Biol. 2004, 145, 247–256. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M.; Mystkowska, I.; Baranowska, A.; Sikorska, A. Effect of herbicides on the content dry matter and sugars in edible potato tubers. Rom. Agric. Res. 2017, 34, 371–375. [Google Scholar]
- Baranowska, A.; Mystkowska, I. The effect of growth biostimulators and herbicide on the content of sugars in tubers of edible potato (Solanum tuberosum L.). Appl. Ecol. Environ. Res. 2019, 17, 3457–3468. [Google Scholar] [CrossRef]
- Kogovšek, P.; Pompe-Novak, M.; Petek, M.; Fragner, L.; Weckwerth, W.; Gruden, K. Primary metabolism, phenylpropanoids and antioxidant pathways are regulated in potato as a response to Potato virus Y infection. PLoS ONE 2016, 11, e0146135. [Google Scholar] [CrossRef]
- Křížkovská, B.; Viktorova, J.; Lipov, J. Approved genetically modified potatoes (Solanum tuberosum) for improved stress resistance and food safety. J. Agric. Food Chem. 2022, 70, 11833–11843. [Google Scholar] [CrossRef]
- Islam, M.M.; Naznin, S.; Naznin, A.; Uddin, M.N.; Amin, M.N.; Rahman, M.M.; Tipu, M.M.H.; Alsuhaibani, A.M.; Gaber, A.; Ahmed, S. Dry matter, starch content, reducing sugar, color and crispiness are key parameters of potatoes required for chip processing. Horticulturae 2022, 8, 362. [Google Scholar] [CrossRef]
- Pandey, V.; Kumar, V.A.; Brar, A. Biochemical behaviour of potato tubers during storage. Chem. Sci. Rev. Lett. 2017, 6, 1818–1822. [Google Scholar]
- Siddiqui, S.; Ahmed, N.; Phogat, N. Potato Starch as Affected by Varieties, Storage Treatments and Conditions of Tubers. In Starch-Evolution and Recent Advances; Ochubiojo Emeje, M., Ed.; Biochemistry; IntechOpen: Bristol, UK, 2021. [Google Scholar]
- Sahin, U.; Kiziloglu, F.M.; Angin, I. Changes in Some Quality Properties after Different Storage Periods of Potato Tubers Grown under Well and Deficit Irrigation Conditions. Bulg. J. Agric. Sci. 2006, 12, 673–682. [Google Scholar]
- Poberezny, J.; Wszelaczynska, E. Effect of bioelements (N, K, Mg) and long-term storage of potato tubers on quantitative and qualitative losses Part II. Content of dry matter and starch. J. Elem. 2011, 16, 237–246. [Google Scholar] [CrossRef]
- Amjad, A.; Javed, M.S.; Hameed, A.; Hussain, M.; Ismail, A. Changes in sugar contents and invertase activity during low temperature storage of various chipping potato cultivars. Food Sci. Technol. 2019, 40, 340–345. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen-Xiang, L. Effects of storage temperature and duration on carbohydrate metabolism and physicochemical properties of potato tubers. J. Food Nutr. 2021, 7, 1–8. [Google Scholar] [CrossRef]
- Özcan, S.; Șanlı, A.; Ok, F.Z. Determination of storage responses and quality changes of some potato (Solanum tuberosum L.) cultivars during storage. Turk. J. Agric. Food Sci. Technol. 2019, 7, 59–66. [Google Scholar]
- Wszelaczyńska, E.; Pobereżny, J.; Gościnna, K.; Szczepanek, M.; Tomaszewska-Sowa, M.; Lemańczyk, G.; Lisiecki, K.; Trawczyński, C.; Boguszewska-Mańkowska, D.; Pietraszko, M. Determination of the effect of abiotic stress on the oxidative potential of edible potato tubers. Sci. Rep. 2023, 13, 9999. [Google Scholar] [CrossRef] [PubMed]
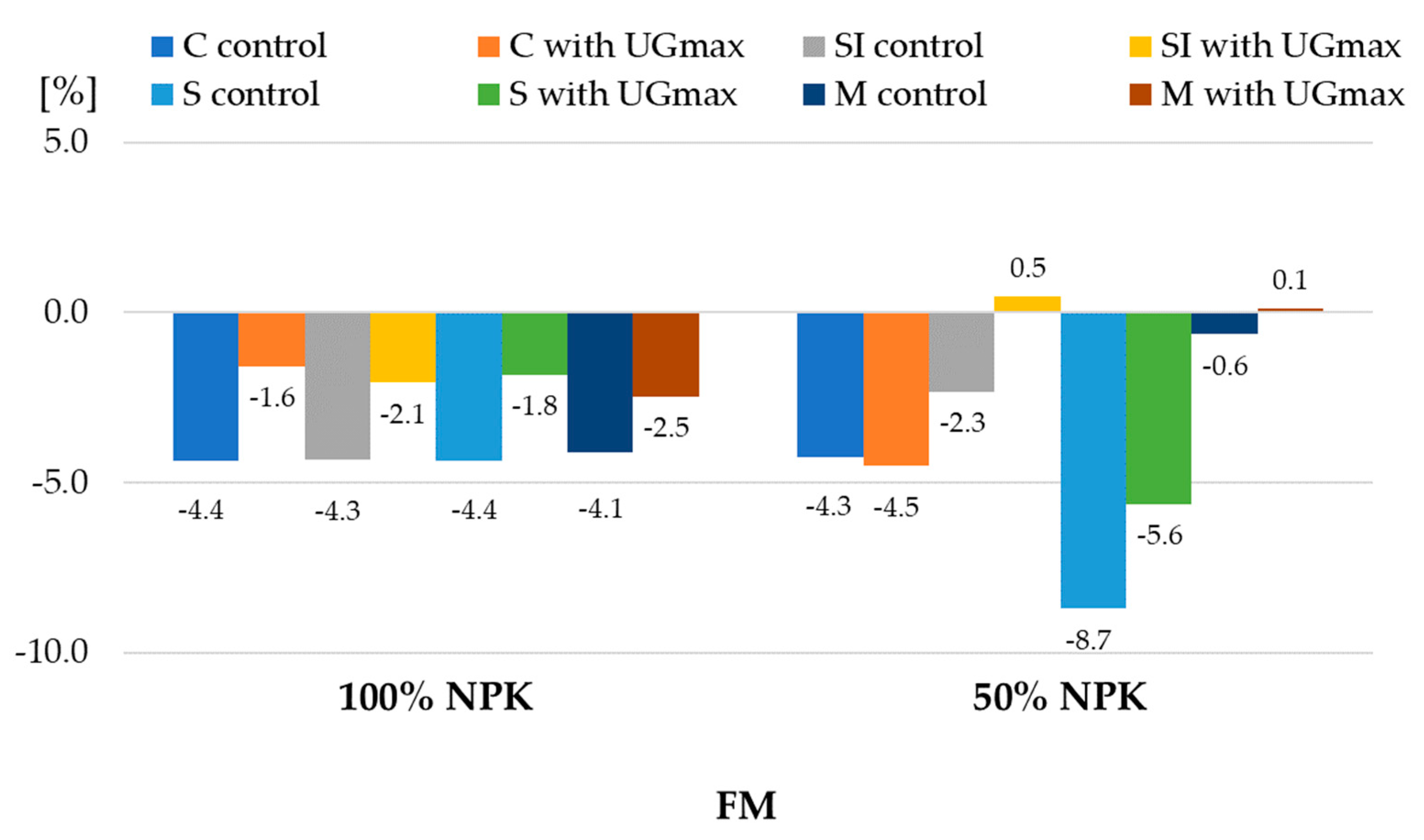
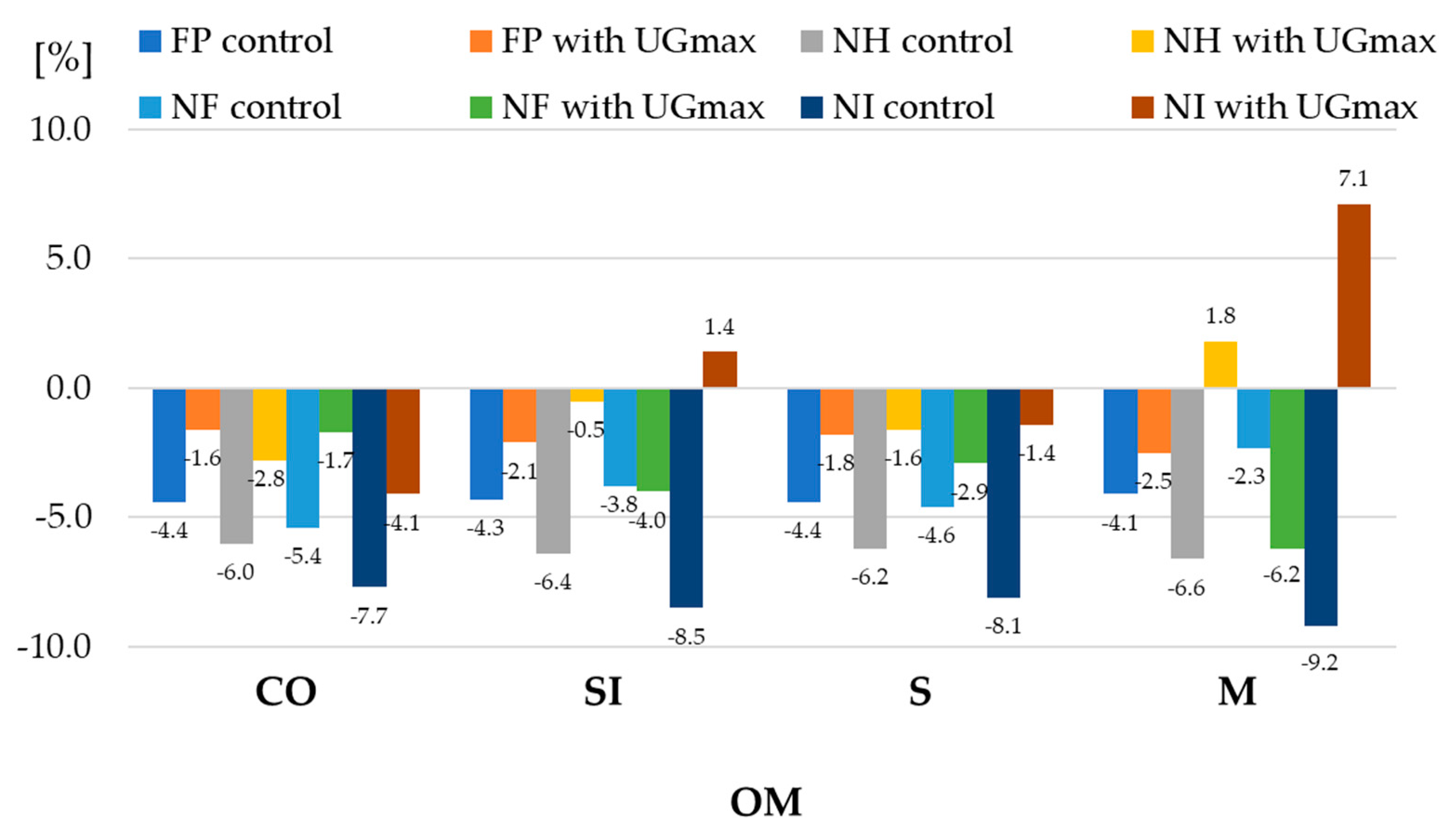
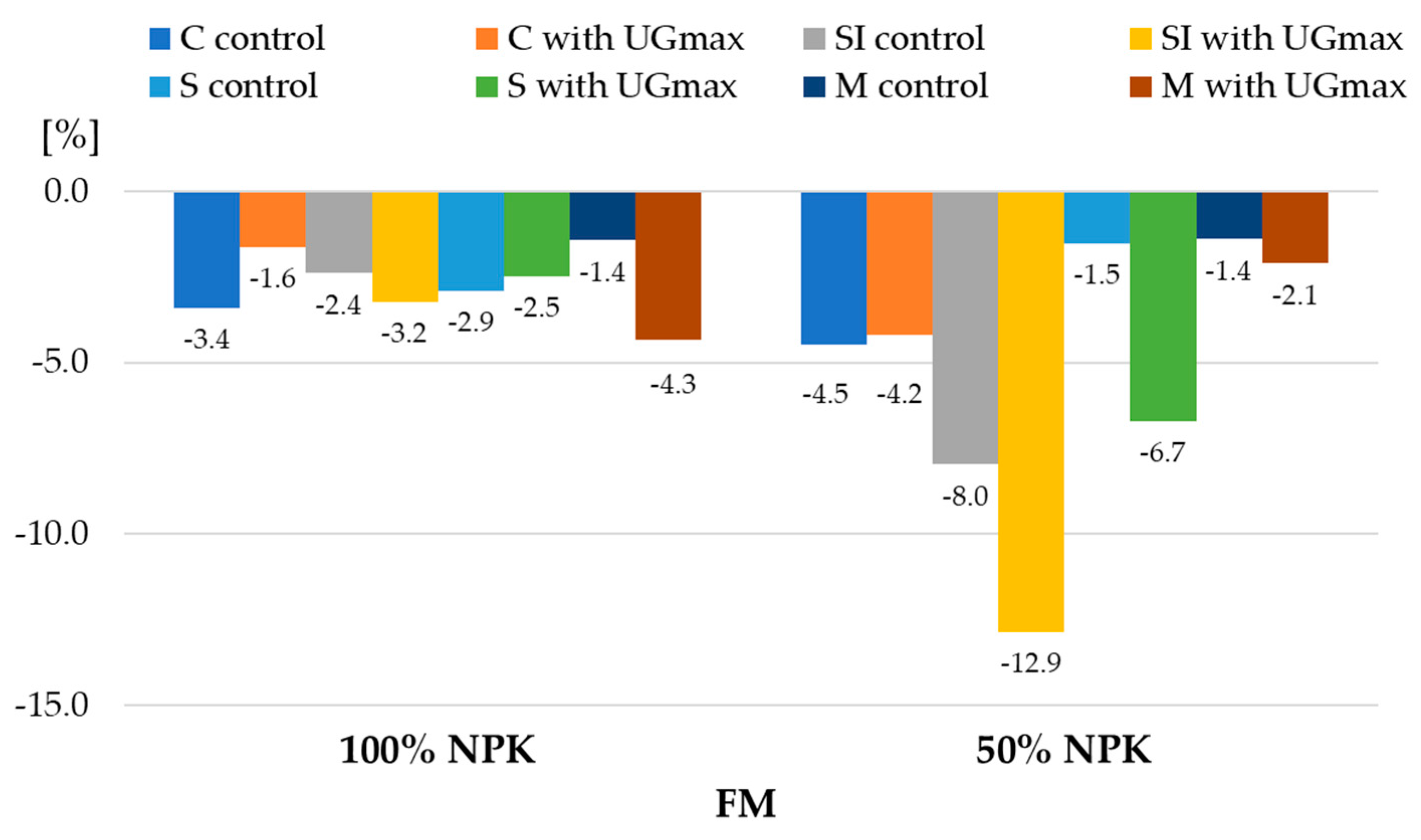
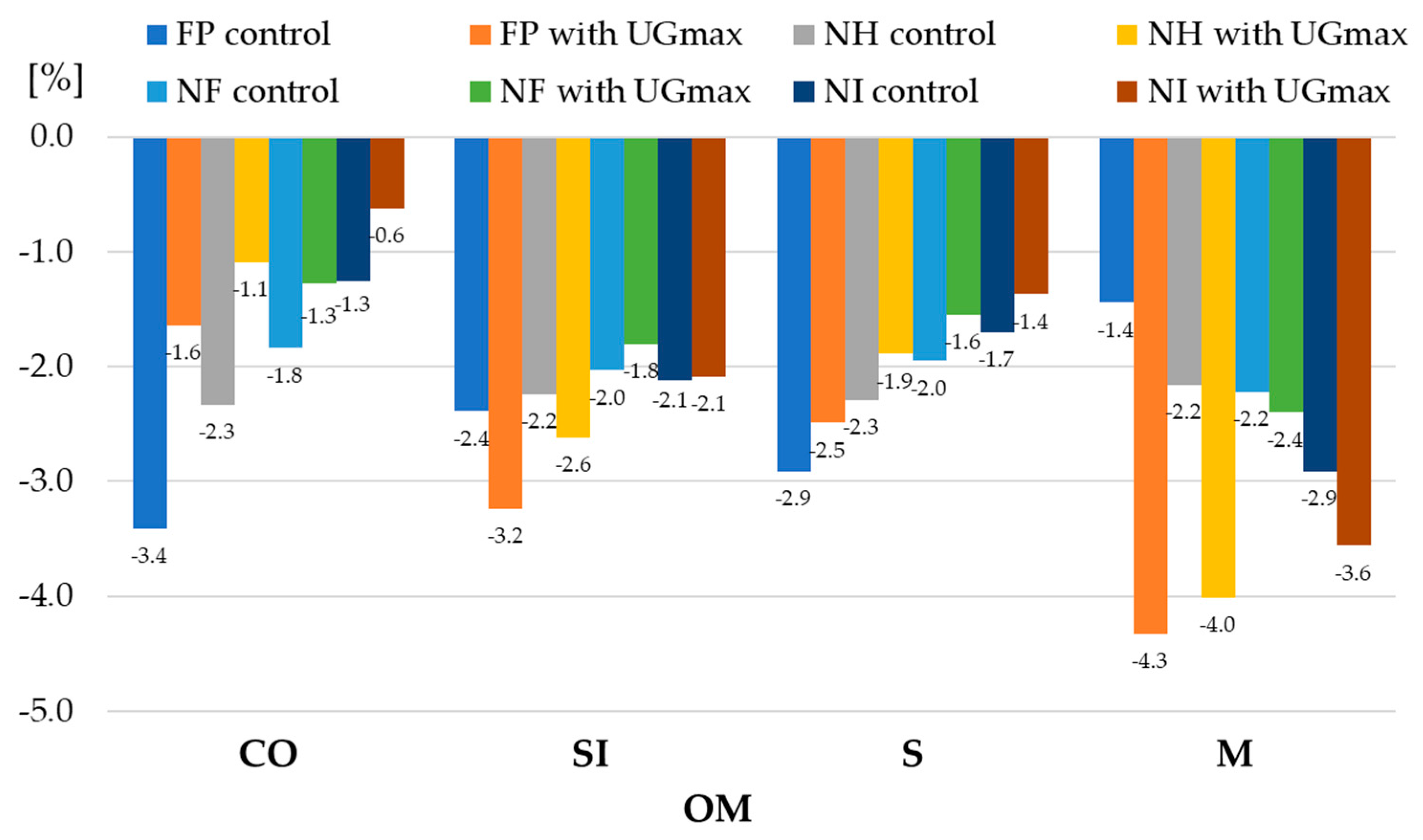
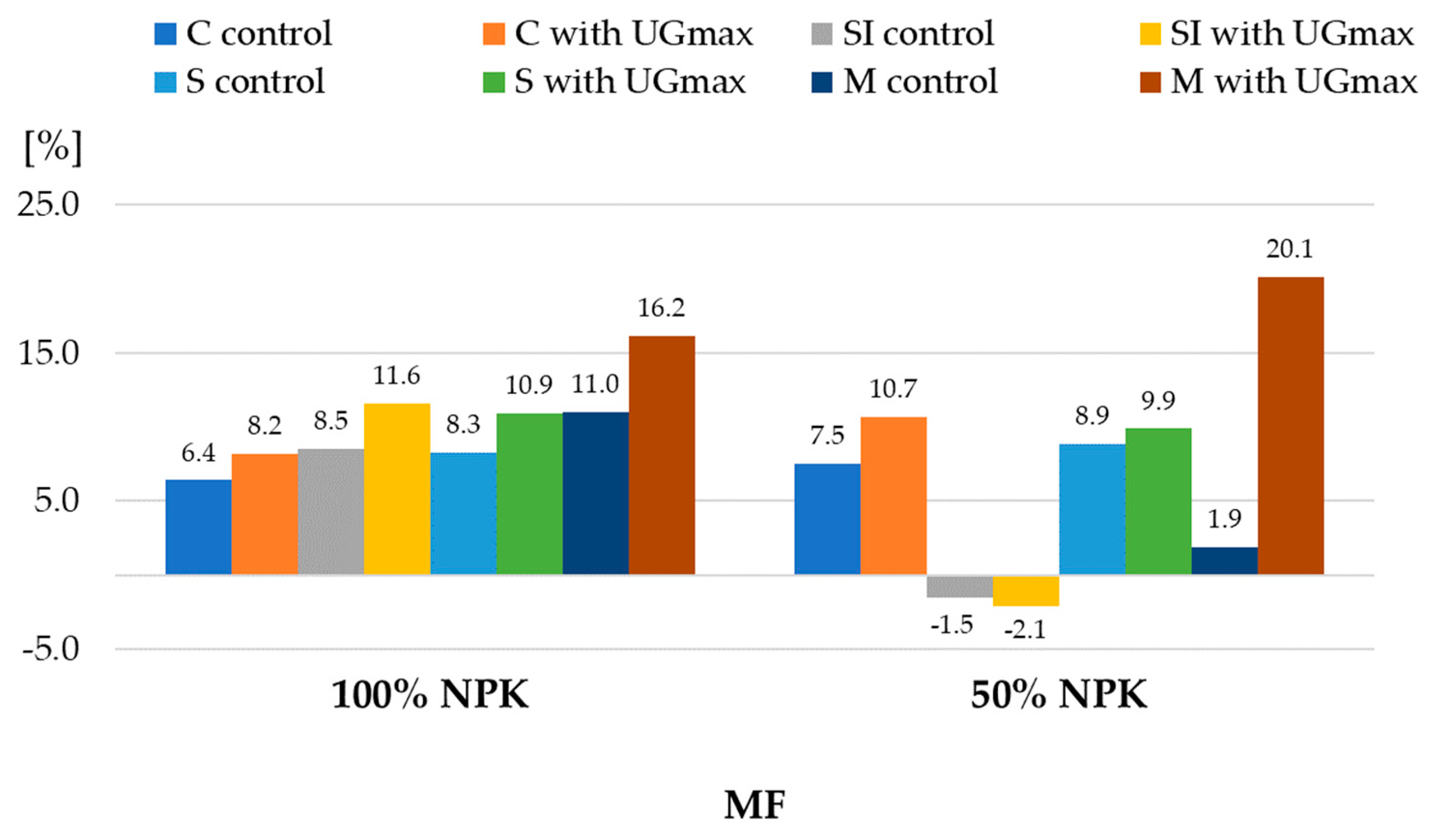
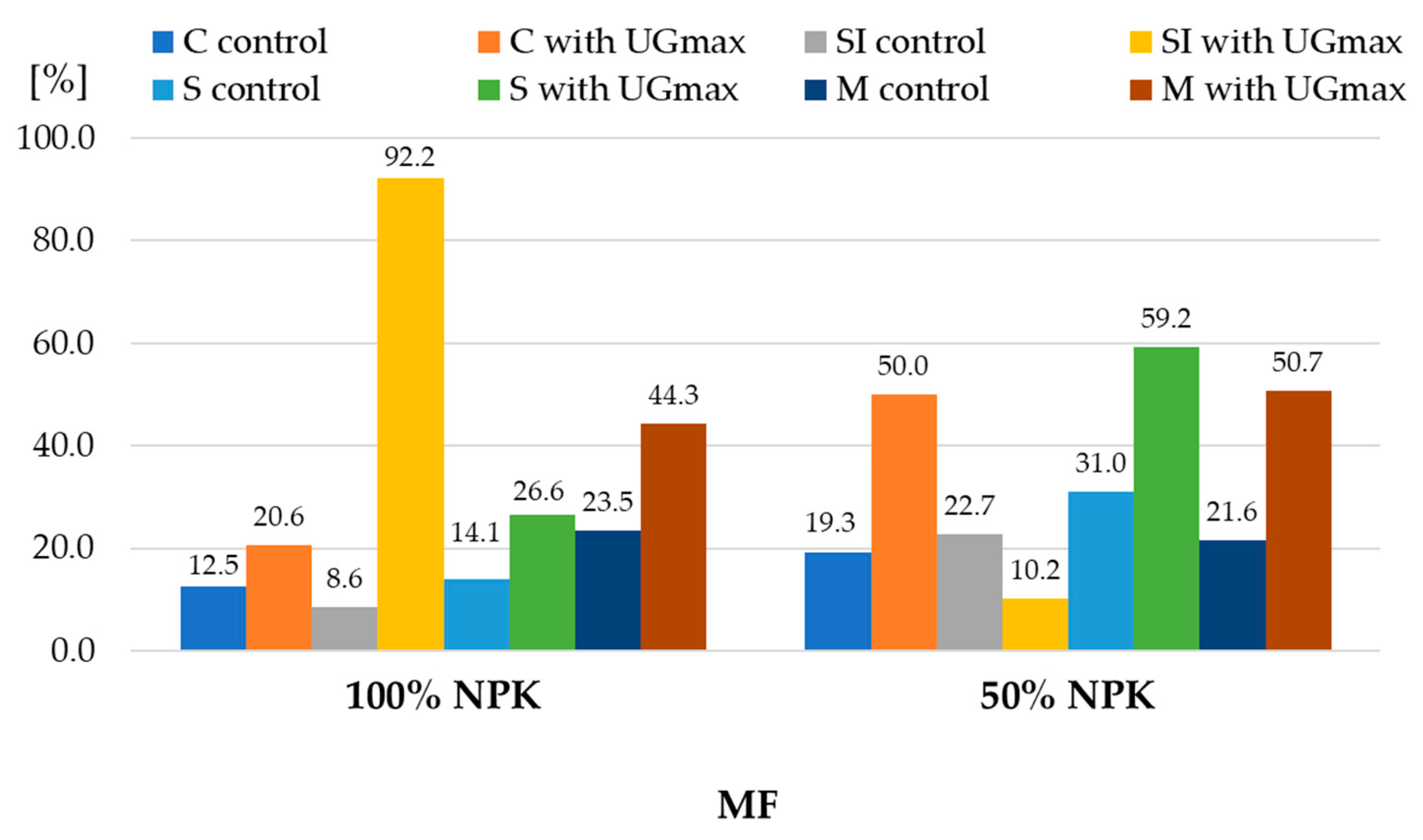

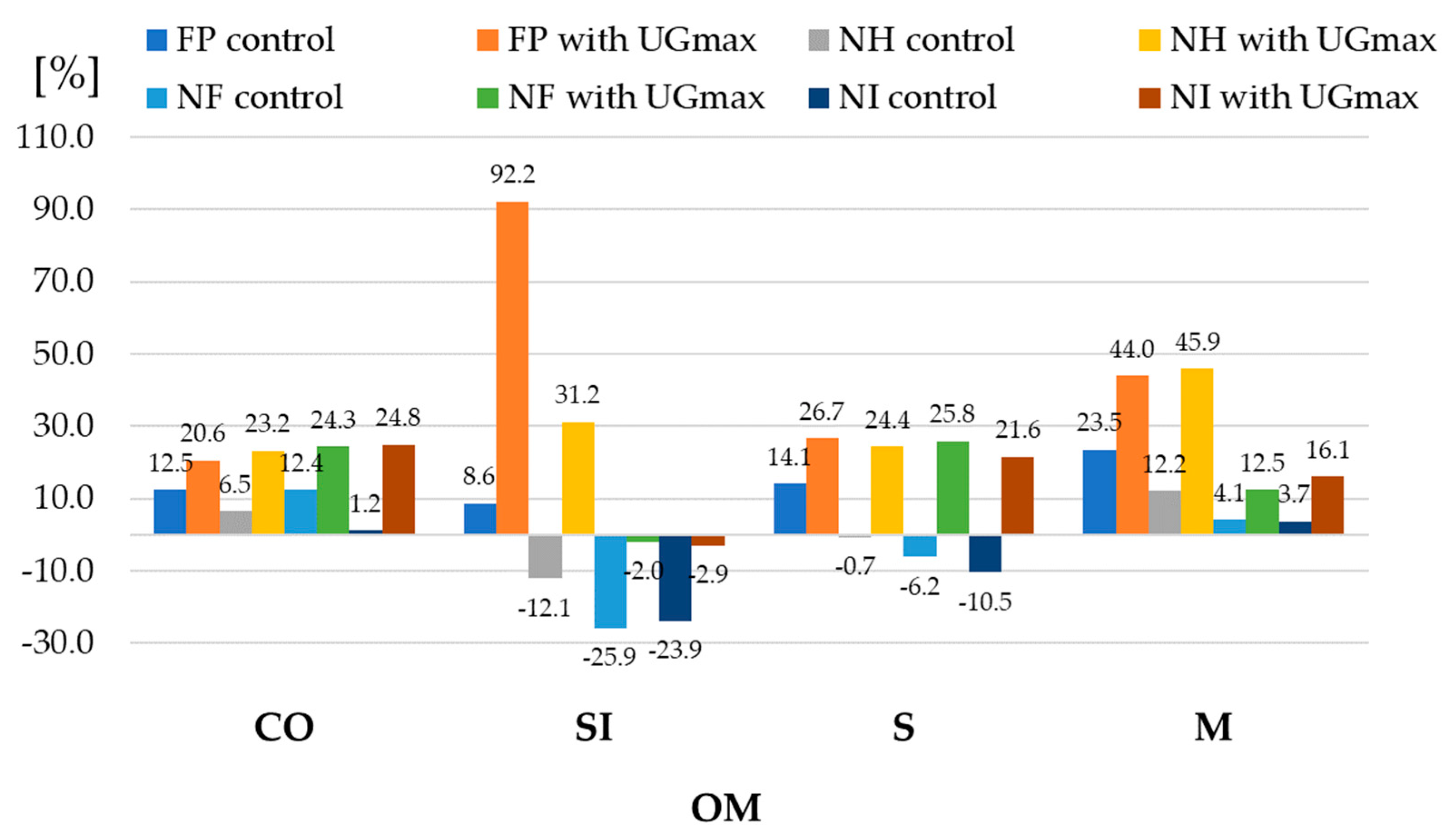
| 1 Experiment Factors | Dry Matter | Starch | |||||
|---|---|---|---|---|---|---|---|
| MF (NPK) | |||||||
| OM | SC | 100% | 50% | Mean | 100% | 50% | Mean |
| CO | control | 149.1 ± 2.9 | 145.2 ± 2.8 | 147.2 ± 3.2 | 96.7 ± 2.3 | 91.3 ± 1.2 | 94.0 ± 3.3 |
| with UGmax | 153.0 ± 3.4 | 152.9 ± 2.8 | 153.0 ± 2.8 | 103.7 ± 2.4 | 100.2 ± 2.6 | 102.0 ± 2.9 | |
| Mean | 151.0 ± 3.6 | 149.0 ± 4.8 | 150.0 ± 4.2 | 100.2 ± 4.4 | 95.8 ± 5.2 | 98.0 ± 5.1 | |
| SI | control | 153.3 ± 2.9 | 146.0 ± 4.1 | 149.7 ± 5.1 | 100.4 ± 1.6 | 99.0 ± 3.0 | 99.7 ± 2.2 |
| with UGmax | 163.2 ± 2.5 | 150.0 ± 3.3 | 156.6 ± 7.6 | 114.2 ± 2.4 | 109.6 ± 3.2 | 111.9 ± 3.6 | |
| Mean | 158.2 ± 5.9 | 148.0 ± 4.0 | 153.1 ± 7.2 | 107.3 ± 7.8 | 104.3 ± 6.5 | 105.8 ± 7.0 | |
| S | control | 150.7 ± 2.9 | 148.5 ± 3.0 | 149.6 ± 2.9 | 98.1 ± 1.9 | 96.0 ± 0.9 | 97.0 ± 1.7 |
| with UGmax | 157.6 ± 2.9 | 145.9 ± 1.2 | 151.8 ± 6.7 | 108.5 ± 2.3 | 111.7 ± 3.0 | 110.1 ± 3.0 | |
| Mean | 154.1 ± 4.6 | 147.2 ± 2.5 | 150.7 ± 5.1 | 103.3 ± 6.0 | 106.3 ± 8.8 | 104.8 ± 7.2 | |
| M | control | 157.5 ± 3.1 | 139.92.3 | 148.7 ± 9.9 | 104.1 ± 1.1 | 97.4 ± 1.9 | 100.7 ± 3.9 |
| with UGmax | 173.4 ± 1.7 | 145.9 ± 1.2 | 159.7 ± 15.1 | 124.6 ± 3.2 | 108.8 ± 1.7 | 116.7 ± 9.0 | |
| Mean | 165.4 ± 9.0 | 142.9 ± 3.7 | 154.2 ± 13.5 | 114.3 ± 11.5 | 103.1 ± 6.5 | 108.7 ± 10.6 | |
| Mean | control | 152.7 ± 4.1 | 144.9 ± 4.2 | 148.8 ± 5.7 | 99.8 ± 3.3 | 95.9 ± 3.4 | 97.9 ± 3.8 |
| with UGmax | 161.8 ± 8.3 | 148.7 ± 3.7 | 155.2 ± 9.2 | 112.7 ± 8.4 | 107.6 ± 5.1 | 110.2 ± 7.3 | |
| Mean | 157.2 ± 7.9 | 146.8 ± 4.3 | 152.0 ± 8.2 | 106.3 ± 9.1 | 101.7 ± 7.3 | 104.0 ± 8.5 | |
| 2 LSD α = 0.05 | A—6.83; B—3.71; C—4.42 | A—6.37; B—4.80; C—3.39 | |||||
| A/B—3.85; A/C—3.26; B/C—2.46; A/B/C—2.58 | A/B—3.47; A/C—2.57; B/C—n. s.; A/B/C—2.62 | ||||||
| Dry Matter | Starch | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Experiment Factors | OM | ||||||||||
| ChP | SC | CO | SI | S | M | Mean | CO | SI | S | M | Mean |
| FP | control | 149.1 ± 16.8 | 153.3 ± 2.9 | 150.7 ± 2.9 | 157.5 ± 3.1 | 152.7 ± 4.1 | 96.7 ± 10.5 | 100.4 ± 1.6 | 98.1 ± 1.9 | 104.1 ± 1.1 | 99.8 ± 3.3 |
| with UGmax | 153.0 ± 3.4 | 163.2 ± 2.5 | 157.6 ± 2.9 | 173.4 ± 1.7 | 161.8 ± 8.3 | 103.7 ± 2.4 | 114.2 ± 2.4 | 108.5 ± 2.3 | 124.6 ± 3.2 | 112.7 ± 8.4 | |
| Mean | 151.0 ± 3.6 | 158.2 ± 5.9 | 154.1 ± 4.6 | 165.4 ± 9.0 | 157.2 ± 7.9 | 100.2 ± 4.4 | 107.3 ± 7.8 | 103.3 ± 6.0 | 114.3 ± 11.5 | 106.3 ± 9.1 | |
| NH | control | 146.4 ± 3.3 | 150.3 ± 2.9 | 147.8 ± 3.0 | 154.3 ± 3.3 | 149.7 ± 4.1 | 96.0 ± 2.0 | 99.8 ± 1.3 | 97.4 ± 1.6 | 103.5 ± 1.2 | 99.2 ± 3.2 |
| with UGmax | 147.0 ± 3.5 | 151.5 ± 2.6 | 148.7 ± 3.1 | 156.1 ± 2.0 | 150.8 ± 4.3 | 100.5 ± 0.9 | 107.4 ± 1.8 | 103.5 ± 1.2 | 114.2 ± 2.9 | 106.4 ± 5.6 | |
| Mean | 146.7 ± 3.1 | 150.9 ± 2.6 | 148.3 ± 2.8 | 155.2 ± 2.6 | 150.3 ± 4.2 | 98.3 ± 2.8 | 103.6 ± 4.4 | 100.4 ± 3.6 | 108.9 ± 6.2 | 102.8 ± 5.8 | |
| NF | control | 132.4 ± 2.4 | 135 ± 1.4 | 133.2 ± 1.9 | 137.5 ± 0.5 | 134.5 ± 2.5 | 92.5 ± 1.2 | 93.51 ± 0.8 | 92.5 ± 1.4 | 94.6 ± 2.6 | 93.3 ± 1.8 |
| with UGmax | 134.9 ± 3.0 | 140.1 ± 2.6 | 137.0 ± 2.6 | 145.2 ± 3.4 | 139.3 ± 4.8 | 93.5 ± 3.2 | 94.6 ± 3.2 | 93.6 ± 3.2 | 95.7 ± 3.4 | 94.3 ± 2.9 | |
| Mean | 133.6 ± 2.8 | 137.5 ± 3.3 | 135.1 ± 2.9 | 141.4 ± 4.8 | 136.9 ± 4.6 | 93.0 ± 2.2 | 94.1 ± 2.4 | 93.1 ± 2.3 | 95.1 ± 2.8 | 93.8 ± 2.4 | |
| NI | control | 143.6 ± 3.8 | 147.4 ± 2.9 | 145.0 ± 3.2 | 151.1 ± 3.6 | 146.8 ± 4.1 | 95.4 ± 2.7 | 99.2 ± 1.6 | 96.8 ± 2.1 | 103.0 ± 1.4 | 98.6 ± 3.4 |
| with UGmax | 140.9 ± 3.6 | 139.8 ± 2.8 | 139.9 ± 3.2 | 138.7 ± 2.3 | 139.8 ± 2.7 | 97.4 ± 3.0 | 100.6 ± 1.7 | 98.5 ± 2.2 | 103.8 ± 2.6 | 100.1 ± 3.3 | |
| Mean | 142.3 ± 3.7 | 143.6 ± 4.9 | 142.5 ± 4.0 | 144.9 ± 7.3 | 143.3 ± 4.9 | 96.4 ± 2.8 | 99.9 ± 1.7 | 97.7 ± 2.1 | 103.4 ± 1.9 | 99.3 ± 3.4 | |
| Mean | control | 142.9 ± 7.1 | 146.5 ± 7.6 | 144.2 ± 7.4 | 150.1 ± 8.3 | 145.9 ± 7.9 | 95.2 ± 2.5 | 98.2 ± 3.2 | 96.2 ± 2.7 | 101.3 ± 4.3 | 97.7 ± 3.9 |
| with UGmax | 143.9 ± 7.6 | 148.7 ± 10 | 145.8 ± 8.8 | 153.3 ± 13.8 | 147.9 ± 10.7 | 98.8 ± 4.5 | 104.2 ± 7.9 | 101.0 ± 6.1 | 109.6 ± 11.7 | 103.4 ± 8.8 | |
| Mean | 143.4 ± 7.3 | 147.6 ± 8.9 | 145.0 ± 8.0 | 151.7 ± 11.3 | 146.9 ± 9.4 | 97.0 ± 4.0 | 101.2 ± 6.6 | 98.6 ± 5.2 | 105.4 ± 9.6 | 100.6 ± 7.3 | |
| 2 LSD α = 0.05 | A—3.20; B—5.16; C—3.81 | A—3.32; B—3.84; C—2.75 | |||||||||
| A/B—4.65; A/C—n. s.; B/C—3.77; A/B/C—2.65 | A/B—3.34; A/C—2.85; B/C—3.11; A/B/C—3.02 | ||||||||||
| Parameters | Starch | Total Sugars | Reducing Sugars | ||
|---|---|---|---|---|---|
| Experiment | 1 Assessment Date | ||||
| Dry matter | I | ah | ** 0.509 ** 0.667 | ||
| as | |||||
| II | ah | ** 0.716 ** 0.884 | ** −0.551 ** −0.774 | * −0.262 ** −0.426 | |
| as | |||||
| Starch | I | ah | ** −0.569 ** −0.561 | ** −0.407 | |
| as | |||||
| II | ah | ** 0.587 ** −0.768 | ** −0.449 ** −0.364 | ||
| as | |||||
| Total sugars | I | ah | * 0.354 ** 0.521 | ||
| as | |||||
| II | ah as | ** 0.418 ** 0.600 | |||
| 1 Experiment Factors | Total Sugars | Reducing Sugars | |||||
|---|---|---|---|---|---|---|---|
| MF (NPK) | |||||||
| OM | SC | 100% | 50% | Mean | 100% | 50% | Mean |
| CO | control | 5.77 ± 0.47 | 5.19 ± 0.44 | 5.48 ± 0.51 | 2.88 ± 0.15 | 2.07 ± 0.06 | 2.48 ± 0.47 |
| with UGmax | 5.13 ± 0.06 | 4.77 ± 0.50 | 4.95 ± 0.38 | 1.31 ± 0.02 | 0.98 ± 0.10 | 1.15 ± 0.68 | |
| Mean | 4.73 ± 0.46 | 4.98 ± 0.48 | 4.86 ± 0.51 | 1.63 ± 1.09 | 1.04 ± 0.57 | 1.34 ± 0.88 | |
| SI | control | 5.06 ± 0.12 | 5.19 ± 0.44 | 5.13 ± 0.29 | 2.10 ± 0.10 | 1.28 ± 0.47 | 1.69 ± 0.64 |
| with UGmax | 4.39 ± 0.06 | 4.77 ± 0.50 | 4.58 ± 0.37 | 1.16 ± 0.30 | 1.01 ± 0.06 | 1.09 ± 0.45 | |
| Mean | 4.73 ± 0.36 | 4.98 ± 0.48 | 4.86 ± 0.42 | 1.63 ± 0.67 | 1.04 ± 0.30 | 1.34 ± 0.58 | |
| S | control | 4.92 ± 0.25 | 4.61 ± 0.42 | 4.76 ± 0.35 | 1.99 ± 0.10 | 1.29 ± 0.06 | 1.64 ± 0.41 |
| with UGmax | 4.26 ± 0.06 | 4.21 ± 0.01 | 4.23 ± 0.05 | 1.75 ± 0.12 | 1.11 ± 0.20 | 1.43 ± 0.39 | |
| Mean | 4.92 ± 0.40 | 4.61 ± 0.35 | 4.76 ± 0.37 | 1.99 ± 0.16 | 1.29 ± 0.16 | 1.64 ± 0.40 | |
| M | control | 4.35 ± 0.59 | 4.210.17 | 4.28 ± 0.40 | 1.83 ± 0.06 | 1.48 ± 0.26 | 1.65 ± 0.25 |
| with UGmax | 3.65 ± 0.12 | 3.48 ± 0.10 | 3.56 ± 0.13 | 1.32 ± 0.19 | 0.77 ± 0.01 | 1.05 ± 0.36 | |
| Mean | 4.00 ± 0.54 | 3.84 ± 0.40 | 3.92 ± 0.46 | 1.58 ± 0.34 | 1.12 ± 0.42 | 1.35 ± 0.43 | |
| Mean | control | 5.02 ± 0.62 | 4.80 ± 0.55 | 4.91 ± 0.51 | 2.20 ± 0.42 | 1.53 ± 0.44 | 1.86 ± 0.47 |
| with UGmax | 4.36 ± 0.55 | 4.31 ± 0.62 | 4.33 ± 0.38 | 1.39 ± 0.60 | 0.97 ± 0.15 | 1.18 ± 0.20 | |
| Mean | 4.69 ± 0.67 | 4.55 ± 0.63 | 4.62 ± 0.64 | 1.79 ± 0.25 | 1.25 ± 0.20 | 1.52 ± 0.61 | |
| 2 LSD α = 0.05 | A—0.369; B—n. s.; C—0.339 | A—0.499; B—0.320; C—0.307 | |||||
| A/B—n. s.; A/C—n. s.; B/C—n. s.; A/B/C—n. s. | A/B—n. s.; A/C—0.355; B/C—n. s.; A/B/C—n. s. | ||||||
| Total Sugars | Reducing Sugars | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Experiment Factors | OM | ||||||||||
| ChP | SC | CO | SI | S | M | Mean | CO | SI | S | M | Mean |
| FP | control | 5.77 ± 0.49 | 5.06 ± 0.14 | 4.91 ± 0.24 | 4.35 ± 0.62 | 5.02 ± 0.64 | 2.88 ± 0.04 | 2.10 ± 0.06 | 1.99 ± 0.09 | 1.83 ± 0.06 | 2.20 ± 0.43 |
| with UGmax | 5.13 ± 0.03 | 4.39 ± 0.07 | 4.26 ± 0.04 | 3.65 ± 0.13 | 4.36 ± 0.55 | 1.31 ± 0.06 | 1.16 ± 0.08 | 1.75 ± 0.11 | 1.32 ± 0.11 | 1.39 ± 0.66 | |
| Mean | 5.45 ± 0.47 | 4.73 ± 0.38 | 4.59 ± 0.39 | 4.00 ± 0.56 | 4.69 ± 0.68 | 2.10 ± 0.18 | 1.63 ± 0.67 | 1.37 ± 0.16 | 1.58 ± 0.59 | 1.67 ± 0.71 | |
| NH | control | 5.78 ± 0.42 | 4.76 ± 0.20 | 4.77 ± 0.22 | 4.61 ± 0.19 | 4.98 ± 0.54 | 3.13 ± 0.13 | 2.92 ± 0.14 | 2.53 ± 0.02 | 2.19 ± 0.01 | 2.69 ± 0.39 |
| with UGmax | 5.32 ± 0.49 | 5.02 ± 0.19 | 4.67 ± 0.34 | 3.84 ± 0.34 | 4.71 ± 0.65 | 1.69 ± 0.06 | 1.62 ± 0.23 | 1.66 ± 0.17 | 1.27 ± 0.22 | 1.56 ± 0.30 | |
| Mean | 5.55 ± 0.48 | 4.89 ± 0.23 | 4.72 ± 0.26 | 4.23 ± 0.49 | 4.84 ± 0.60 | 2.41 ± 1.08 | 2.27 ± 0.67 | 2.09 ± 0.16 | 1.73 ± 0.59 | 2.12 ± 0.67 | |
| NF | control | 6.04 ± 0.72 | 5.66 ± 0.15 | 5.35 ± 0.37 | 5.27 ± 0.29 | 5.58 ± 0.49 | 2.74 ± 0.10 | 3.40 ± 0.21 | 2.57 ± 0.35 | 2.21 ± 0.23 | 2.73 ± 0.55 |
| with UGmax | 5.49 ± 0.46 | 5.18 ± 0.36 | 4.84 ± 0.37 | 4.87 ± 0.56 | 5.09 ± 0.47 | 1.85 ± 0.25 | 1.98 ± 0.13 | 1.42 ± 0.25 | 1.44 ± 0.17 | 1.67 ± 0.39 | |
| Mean | 5.78 ± 0.62 | 5.42 ± 0.31 | 5.10 ± 0.41 | 5.07 ± 0.46 | 5.34 ± 0.52 | 2.29 ± 0.52 | 2.69 ± 0.94 | 1.99 ± 0.69 | 1.83 ± 0.46 | 2.20 ± 0.71 | |
| NI | control | 5.78 ± 0.35 | 4.45 ± 0.27 | 4.62 ± 0.20 | 4.87 ± 0.26 | 4.93 ± 0.59 | 3.39 ± 0.27 | 3.73 ± 0.33 | 3.06 ± 0.12 | 2.54 ± 0.08 | 3.18 ± 0.50 |
| with UGmax | 5.51 ± 0.99 | 5.65 ± 0.44 | 5.08 ± 0.70 | 4.03 ± 0.71 | 5.07 ± 0.91 | 2.06 ± 0.47 | 2.09 ± 0.30 | 1.58 ± 0.37 | 1.55 ± 0.25 | 1.82 ± 0.41 | |
| Mean | 5.65 ± 0.68 | 5.05 ± 0.73 | 4.85 ± 0.53 | 4.45 ± 0.67 | 5.00 ± 0.75 | 2.72 ± 0.80 | 2.91 ± 0.95 | 2.32 ± 0.85 | 2.04 ± 0.57 | 2.50 ± 0.83 | |
| Mean | control | 5.84 ± 0.46 | 4.98 ± 0.45 | 4.91 ± 0.34 | 4.78 ± 0.48 | 5.13 ± 0.60 | 3.04 ± 0.30 | 3.04 ± 0.71 | 2.54 ± 0.43 | 2.19 ± 0.29 | 2.70 ± 0.57 |
| with UGmax | 5.36 ± 0.53 | 5.06 ± 0.54 | 4.71 ± 0.48 | 4.10 ± 0.64 | 4.81 ± 0.71 | 1.73 ± 0.60 | 1.71 ± 0.56 | 1.60 ± 0.25 | 1.40 ± 0.37 | 1.61 ± 0.48 | |
| Mean | 5.60 ± 0.54 | 5.02 ± 0.49 | 4.81 ± 0.42 | 4.44 ± 0.65 | 4.97 ± 0.67 | 2.38 ± 0.81 | 2.38 ± 0.92 | 2.07 ± 0.59 | 1.79 ± 0.55 | 2.15 ± 0.77 | |
| 2 LSD α = 0.05 | A—0.369; B—0.306; C—0.267 | A—0.420; B—0.422; C—0.215 | |||||||||
| A/B—n. s.; A/C—0.294; B/C—0.362; A/B/C—n. s. | A/B—n. s.; A/C—n. s.; B/C—n. s; A/B/C—n. s. | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gościnna, K.; Retmańska, K.; Wszelaczyńska, E.; Pobereżny, J. Influence of Edible Potato Production Technologies with the Use of Soil Conditioner on the Nutritional Value of Tubers. Agronomy 2024, 14, 549. https://doi.org/10.3390/agronomy14030549
Gościnna K, Retmańska K, Wszelaczyńska E, Pobereżny J. Influence of Edible Potato Production Technologies with the Use of Soil Conditioner on the Nutritional Value of Tubers. Agronomy. 2024; 14(3):549. https://doi.org/10.3390/agronomy14030549
Chicago/Turabian StyleGościnna, Katarzyna, Katarzyna Retmańska, Elżbieta Wszelaczyńska, and Jarosław Pobereżny. 2024. "Influence of Edible Potato Production Technologies with the Use of Soil Conditioner on the Nutritional Value of Tubers" Agronomy 14, no. 3: 549. https://doi.org/10.3390/agronomy14030549
APA StyleGościnna, K., Retmańska, K., Wszelaczyńska, E., & Pobereżny, J. (2024). Influence of Edible Potato Production Technologies with the Use of Soil Conditioner on the Nutritional Value of Tubers. Agronomy, 14(3), 549. https://doi.org/10.3390/agronomy14030549






