Abstract
Weeds are responsible for a significant proportion of agricultural production losses. Common ragweed (Ambrosia artemisiifolia L.) has become the dominant weed in much of the northern hemisphere over the last century and is projected to further expand northward with climate warming. Not only does it cause damage to agriculture, but it also poses a significant human health risk. With the increasing number of Ambrosia artemisiifolia, around 44 million more people will suffer from ragweed pollen-induced pollinosis in the future just in Europe. The following review provides an overview of the most important and recent research findings on the spread, morphology, life cycle, importance and damage, allelopathic effects, habitat and environmental requirements of common ragweed. These characteristics of the species may explain its success and, based on this knowledge, allow the development of methods for its control.
1. Introduction
Common ragweed (Ambrosia artemisiifolia L.) is one of the most important invasive plants, spreading widely in America, Asia and Europe. The secret of its success as an invader, apart from anthropogenic activity, is primarily its favorable invasive properties. Moreover, A. artemisiifolia can cause serious pollen allergy in sensitive people. Regarding interference among higher plants, its competitive ability and allelopathy are also considered essential factors. Because common ragweed (hereafter referred to as ragweed) has a broad ecological spectrum, it can adapt to various environmental factors [1]. A. artemisiifolia is considered an invasive summer annual, aggressive alien competitor weed species [2]. Ragweed is the most prominent agricultural weed and is responsible for large crop losses in many crops [3].
2. Spreading of the Species
The gene center of A. artemisiifolia is in North America, in Arizona (Sonora desert), which extends north and west of the Gulf of California. There are 10 Ambrosia species occurring in the Sonora Desert. It was first detected in 1838 and 1860, in the USA and Canada, respectively. It has recently become a widespread weed species in North America, reaching as far as the south of Canada. Out of North America, it can be found all over the world, in Europe, India, China, Japan, other Asian countries and also in Australia. To date, 41 species of the Ambrosia genus are known, out of which A. artemisiifolia, A. trifida and A. psylostachya pose threats to agricultural production [4].
A. artemisiifolia has no specific mechanism for its spreading. The seeds are dispersed by wind a few meters around the mother plants. Occasionally, it may spread by birds eating the seeds. Spreading by water has great importance, and rapid dispersal can be observed due to major floods. Anthropogenic spread has the greatest importance with contaminated seed, inadequately cleaned soil machinery and growing technological mistakes [5].
Chauvel et al. [6] examined the herbaria of botanic gardens, concluding that France was the first station of ragweed spreading in Europe. Based on the examined materials, it is almost certain that ragweed was present as early as 1763 in France. As an invasive alien weed species, its harmful effect on fields has been registered since 1863. In 1890, it was already detected in the neighboring countries of France. We have no detailed information on countries like Switzerland, Austria and Germany regarding the first detection of the species; however, some reports from the end of the 1800s suggest its presence in these countries. In Russia, the first detection of A. artemisiifolia was registered in 1918, and it is presumed that its introduction occurred through international trade [7]. Its first appearance was detected in Tupse and Vlagyikavkaz, along linear constructions (railway lines) and spread into the fields from there. In 1938, it had already been registered in the North-Caucasus and the eastern part of the Crimean Peninsula. A. artemisiifolia was detected in Ukraine in 1914 (Dnyepropetrovki). After that, it was identified in Kiev, Kharkiv, Odessa and Zaporozhye in 1925, 1929, 1936 and 1939, respectively [8]. Common ragweed has the largest abundance and pollen concentration in the region of middle Europe [4,9,10,11]. Its introduction into the region and Hungary is likely due to its settlement in France. The first descriptions regarding its occurrence are derived from herbarium samples from Orsova [4]. It is considered an introduced weed species in Hungary, France and Italy. Its spreading is extensive in Germany, Switzerland, Austria, Slovakia, Poland, Turkey and the Korean Peninsula [9,12]. Csontos et al. [13] examined the herbariums of more botanical museums, universities and botanical gardens. A considerable part of the herbariums was created in 1922. Therefore, they presumed that the spread of ragweed could be dated to that time. Based on the work of Béres–Hunyadi [14], A. artemisiifolia was first detected in Somogy county (Hungary), then later in Zala and Veszprém counties. In Hungary, ragweed spreads from the Szeged area to the middle of the Danube–Tisza region, and from there, it continues towards the northern areas. Following the Second World War (WWII), during Hungary’s reconstruction, it spread rapidly along agricultural transport routes, primarily along national roads and railways. Nowadays, it is considered a common and the number one weed species in Hungary. Case-Stinson [15] summarized the results of several studies on A. artemisiifolia and stated that ragweed is sensitive to drought stress. In Europe, where ragweed has not yet occupied its ecological niche, intense summer droughts can limit its spreading. It is believed to also be sensitive to extreme temperature values as well.
There are several monitoring systems for predicting plant pests in the world. The Hungarian arable weed survey system is unique in the world since the state and the changes in weed flora have been continuously recorded for years. In Hungary, the First National Weed Survey (NWS) was carried out between 1947 and 1953, and the fifth NWS was carried out between 2007 and 2008, while the latest was between 2018 and 2019 [16,17] (Table 1).

Table 1.
The coverage percentage and rank of ragweed in winter wheat and maize in Hungary, based on the national arable weed surveys (NWS) [16].
During the first, second, third, fourth, fifth and sixth NWS, the cover of A. artemisiifolia reached 0.39%, 0.87%, 2.57%, 4.7%, 5.3% and 4.85%, respectively, with regard to the combined data of wheat and maize. It has been considered to be the number one weed species in maize and cereal stubble for several decades.
3. Morphology
Ambrosia artemisiifolia L. is a summer annual herbaceous plant of the family Asteraceae. On average, it is a 120–140 cm tall, much-branched plant with densely haired, erect stems and short-stalked leaves, which can be sessile to double-stalked (Figure 1). The stalks are ovate and flaky. Male flowers are in racemes at the ends of the branches. Female flowers are lower down in the axils of the leaves. The fruit-bearing nests are uniflorous, and the fruit is enclosed in a hairy, six-toothed sheath that falls off with the fruit. Some individuals have few or no stamens. These have only female flowers. Some female flowers also occur in prosocial nests.
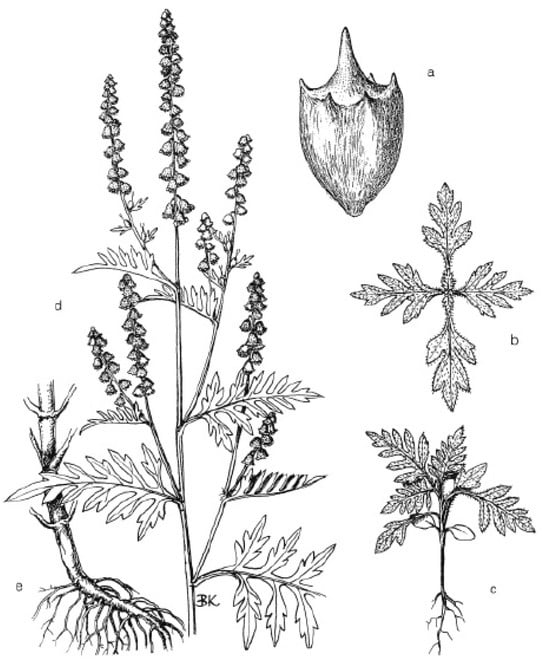
Figure 1.
Ambrosia artemisiifolia L. (common ragweed): (a) seed/achene; (b) seedling bottom view; (c) seedling; (d) flowering shoots; (e) stem with roots [18] (drawn by Krisztina Bíró, under legal protection of copyright).
Its fruit is a recurved ovate catkin, 2.5 mm wide and 3.5 mm long, with a terminal spike up to 2 mm long in the middle, usually surrounded by a ring of 1 mm long spines. The outer layer of the seed is hard. Inside is a whitish, oily and soft core surrounded by a brown membrane. Roots are deep-rooted stakes [2,18,19].
The A. artemisiifolia L. has three known varieties: var. artemisiifolia; var. elatior and var. paniculata (Table 2) [20].

Table 2.
The morphological stamp of the different varieties [20].
The petiole of the cotyledon is ovate and short-tongued. The first lobed leaves are transversely transverse and winged, and the later leaves are scattered [13]. The leaves are highly variable morphologically, which is influenced by leaf age. Because of its leaves, it is often confused with the ornamental plants commonly planted in gardens, as the stink-rose/flowering rose (Tagetes patula), tomato (Solanum lycopersicon) and the black wormwood (Artemisia vulgaris) [21].
4. Life Cycle
4.1. Germination Biology
The field emergence of common ragweed in Hungary generally starts at the end of March. Its peak emergence occurs from mid-April to mid-May. After that, to a lesser extent, it can emerge until the first frosts under the moderate climate [14]. Temperature is believed to be the most important factor in germination regulation [22]. Emergence time significantly affects biomass, pollen and seed production. In agroecosystems, seeds that emerge in April are able to produce 3000–4000 seeds for an individual plant, while seeds that emerge later (in August) can produce only 12–16 seeds [23]. If young ragweed plants can develop without intra- and interspecific competition, their shoot dry biomass, pollen and seed production are much higher [3]. However, the emergence time also influences biomass production. Besides these, the temperature, rhythm of temperature change, light, humidity, and CO2 concentration also significantly affect germination [24]. Nevertheless, it is presumed that the leading cause of the rapid spreading of ragweed is anthropogenic in origin. Soil disturbance and plant production contribute essentially to the ragweed invasion [15]. Because hot, dry summers can cause secondary seed dormancy, field emergence does not occur during these hot, dry summers. Most of the freshly ripened seeds are in the dormant stage in the soil, while only a tiny proportion (ca. 10%) emerge within a year [3].
Germination is delayed by low temperature and wet conditions, low light intensity and high salt concentration [25]. Hall et al. [26] examined the seed weight of the populations derived from different countries and found that the seed weight of ragweed varied between 4.7 mg and 8.8 mg. Germination of various seed populations was also examined, and they concluded that neither seed weight nor carbon/nitrogen proportion significantly influenced germination rate [25,26].
Based on the work of Hall et al. [26], the place of origin of the seeds can also greatly influence the germination rate. Further, the age of the ragweed seeds also seems to be essential in germination. Younger seeds can germinate earlier as compared to older seeds. Regarding soil water potential, −0.06–0.00 mPa is favorable, while under −0.08 mPa means ragweed seeds are not able to germinate. Ragweed can also be found in saline and coastal areas, indicating that it can tolerate high concentrations of salt. However, seeds can germinate from a 7 cm soil layer, or even a deeper one, but seedlings cannot reach the soil surface (they germinate but do not emerge). These seedlings die in the soil after the endosperm’s food reserves are exhausted [5]. According to Guillemin and Chauvel [25], the maximum emergence depth is 8 cm. According to Sang et al. [27], seeds sown in 0–4 cm of soil depth germinated in 75% of cases, while seeds buried in 6 cm of soil depth germinated in only 2.5–0.5% of cases. Light seems to promote germination since seeds exposed to 12 h of light germinated in 97%, while seeds in the darkness just in 75%. Nevertheless, soil type and diffuse light availability can greatly influence weed seed germination. The seeds are able to germinate both in dark and light. The light requirement of germination strongly depends on the dormancy level, the place of origin, the age and the storage conditions before germination. Besides these, the germination temperature may be also an influencing factor [3,28].
Seeds of the persistent weed seed bank can remain viable for decades. Silc [29] reported that ragweed seeds can keep their viability in the soil for more than 20 years. Chikoye [30] and Oberdorfer [31] stated that seeds can keep their viability for 30 to 35 years, respectively. The time greatly depends on the soil type and burial depth. Farooq et al. [32] collected two populations of ragweed seeds, the first one derived from the western part of Turkey (Thrace), and the second from the north-eastern part of the country (next to the Black Sea). Collected seed samples were tested for different abiotic environmental factors for germination, e.g., temperature, pH, salt stress and alteration of light and dark periods. Ragweed gave the highest germination rate when the seeds were exposed to 12 h of light and germinated between 23 to 25 °C. Seed populations influenced the optimum pH for germination; seeds derived from the north-eastern part of the country (next to the Black Sea) were 7.43–6.69, while in the case of seeds derived from Thrace, it was between 7.72 and 8.22.
Based on Béres’s work, [33], its mass emergence in Hungary starts when the temperature of the upper soil layer is above 6 °C. Germination is significantly reduced at high temperatures. Shrestha et al. [34] reported 30.9 °C for optimum germination, while the upper maximum is 40 °C. Béres [33] reported that the optimum temperature for ragweed germination under laboratory conditions is the alternating 10/23 °C. Sang et al. [27] reported that 70% of the seeds can germinate at 200 mM/L NaCl concentrations, and a rapid decrease can be observed as the concentration increases up to 400 mM/L. In this case, the germination rate is 5–12%. When NaCl concentration is as high as 500 mM/L, ragweed seeds fail to germinate. Farooq et al. [32] characterized the effect of salt stress with the value of T50, which means the salt concentration is such that, as a result, half of the seeds cannot germinate.
Furthermore, there are significant intraspecific differences regarding the germination behavior between seed populations derived from the roadsides and arable fields. T50 values for roadside seeds are higher (2.327 mM/L for Black Sea seed and 332 mM/L for Thrace seed, respectively). T50 values of arable-field seeds are lower (201 mM/L for Black Sea seed and 229 mM/L for Thrace seed, respectively). This is probably related to the fact that due to the salting of roads at low temperatures, seed populations in such roadside areas have adapted better to salt stress. Based on the work of Picket-Baskin [35], the stratification time greatly influences germination. The soil temperature and wet conditions influence buried ragweed seeds’ viability [14]. Under natural conditions, the primary dormancy of the freshly harvested seeds in October generally ceases by January or February of next year [36]. Cold stratification for 15 weeks resulted in a higher germination rate at lower temperatures when seeds were stored for 11 weeks and germinated at a higher temperature. Willemsen [37] stated that 4 °C is the optimal temperature for seeds to break dormancy; it took only 2 weeks in wet and dark conditions. To fully ripen seeds, ragweed requires cold treatment between 4 and 11 °C for 15 weeks [38]. Kazinczi et al. [39] reported a decrease in ragweed germination rate when seeds were stored under dry conditions after 5 years of dry storage. Generally, it can be said that the treatments to overcome seed dormancy greatly depend on the weed species (or even intraspecifically), on the biotic and abiotic stresses on the mother plant, and on the level and type of seed dormancy.
4.2. Vegetative Development
A. artemisiifolia belongs to the C3 photosynthetic type of plants [40]. After its germination, an intensive vegetative development will start in the May and June months [14]. According to Szigetvári-Benkő [4], the peak of producing large vegetative biomass is in the middle of July and continues even until the peak of flowering in August and September. Weed individuals growing under warmer climatic conditions have been characterized by a longer vegetative phase [41]. The intensity of vegetative development greatly depends on temperature. Individuals after flowering can grow, but only with the elongation of the internodes. Shoot branching occurs 3–4 cm from the soil surface. Adventitious buds can reshoot and develop adventitious shoots on mechanically injured shoots [24] and adventitious roots from the shoots can also emerge (Figure 2). The maximum rate of vegetative development of ragweed occurs directly before the appearance of the male flowers. After flowering, the vegetative development slows down but does not stop [33]. The vegetative and generative productivity of ragweed plants is influenced mainly by some environmental factors such as the interaction of temperature, precipitation and soil type. The optimal shoot and root growth occur at 29.5 °C to 31.4 °C, respectively. The plant terminates its growth at temperatures above 43 °C [34]. The leaf area and dry shoot weight of an average developed ragweed plant are 12.000 cm2 and 450 g, respectively [33], but the emergence time and competitive conditions can greatly influence it [26]. Plants that emerge in April and develop without intra and interspecific competition could produce 1.550 g shoot dry biomass, while plants that emerged later (at the end of summer) could produce only 26 g shoot dry biomass.

Figure 2.
Adventitious root development from shoots (source: Ferenc Pál-Fám).
4.3. Generative Development
Flowering is induced by the continuously decreasing day length after the summer solstice. The flowering time is delayed if the photoperiod is longer than 14 h [24]. Within an individual plant, the proportion of the male and female flowers is approximately 50–50, but the ratio shifts due to the late emergence [14]. A complex regulation mechanism in the vegetative phase of ragweed determines the further development of floral meristems to male or female flowers. Based on the expression pattern of floral genes in this pathway, it was determined that ragweed is a short daylength species and the flowering induction depends on phytochrome B-related light signals. Floral identity in ragweed is decided just in vegetative growth. It is likely that in female flowers floral organ development is induced through the LMI-CAL regulation, while in male flowers the stamen and carpel forming AP2 and PI genes were found to be exclusively expressed in the generative phase [42].
Ragweed is a wind-pollinated species, and to get fully ripened seeds, it generally requires 40–60 days after fertilization [5]. Seed production can greatly vary (avg. 3.000–62.000 seeds/plant), depending on competition conditions and on biotic and abiotic factors. Regarding the fact that it has no special seed dispersal strategy, most of the ripened seeds fall within two meters of the individual [3,22]. Seed production is closely related to vegetative biomass [24,43,44]. The pollen size of ragweed is relatively large (15–25 µm) [14]. However, the volume of pollen and seed production depends mainly on vegetative production. Temperature and rainfall also play essential roles in their development [43]. Plants grown on wastelands can produce smaller amounts of pollen and seed, while those grown under arable conditions can be characterized by higher generative production. The generative production of ragweeds varies in the case of different populations from different European countries. The Romanian and Croatian ragweed populations achieved the highest pollen amount (104,109 pollen grains/m2) and seed production (67,103 seeds/m2). Out of the total populations from different countries (France, Switzerland, Italy, Slovenia, Croatia, Austria, Hungary, Slovakia, Poland, Montenegro, Romania, Turkey and Armenia), the achievement of the Romanian, Hungarian and Croatian ragweed populations is the largest [43].
Pasqualini et al. [45] examined the effect of ozone on ragweed pollen. They simulated the ozone effect, which often occurs at higher concentrations under natural conditions, especially in the air of large towns. Pollen viability (germinability) was 39 and 55.9% 7 days after ozone treatment and without it, respectively. The quantity of the reactivated oxygen forms was also measured. Still, there was not a significant increase in the examined period, so oxidative stress in the pollen grains was not observed. Despite this, the lower viability of ozone-treated pollens was most likely due to membrane injuries.
The pollen production of an averagely developed ragweed individual may vary between 2 and 8 million pollen grains. Male flowers create bell-shaped nest whorls (inflorescence). The diameter of a male inflorescence is about 2–3 mm. The inflorescence is opened, the flowers are located spirally and they open from the outside to the inside. Generally, flowers can develop continuously, and flowering can take a long time. On the male flowers, we can observe a strongly retarded female derivate, the so-called pistillodium. After the primary pollen spreads, it grows and pushes out the remaining pollen grains from the ports. Male flowering generally starts in the middle of July and takes a long time: over 2–2.5 months. Female flowers begin to bloom two weeks after the males and continue until the first frosts [46]. Generally, there are 17 male flowers within an inflorescence. One flower contains about 7000 pollen grains, so 119 thousand pollen grains can scatter from 1 male inflorescence [3].
Ragweeds developed on wheat stubbles can produce lower amounts of pollens and seeds. At a weed density of ragweed individuals/m2, pollen production is 3996 billion, while seed production is 16 thousand per square meter [47]. Seeds that emerge later (in June and July) can flower for 80 days and need 155 days until they ripen their seeds.
The male flowers open 7–10 days earlier than the female ones, and flowering starts earlier on the main shoot than on the branches [48]. In the case of some European populations, the occurrence of female flowers on an individual plant is exclusive; the presence of the male ones cannot be detected [24,49].
Under glasshouse conditions, Wayne et al. [50] examined the enhanced level of CO2 concentration on the pollen production of ragweed. The results of the tests showed that, although the size of the pollen grains did not increase due to the increased carbon dioxide level, the amount of pollen production proved to be 61% higher with the increased concentration of atmospheric CO2. Due to the different ecological factors in the different habitats, significant differences exist regarding the flowering period. Under the cooler climate, the plants start to flower earlier, and the pollen season takes longer [41]. Gentili et al. [51] studied the effect of temperature on pollen production. Temperature regimes were 14–18 °C (low), 20–24 °C (medium) and 26–30 °C (high). It was concluded that the growth rate was larger at higher temperatures (26–30 °C). Consequently, the proportion of male flowers and pollen production was higher. The higher temperature also promoted the synthesis of the allergenic proteins. Due to the enhanced temperature and higher pollen production, climate change can significantly contribute to the enhanced level of allergenic diseases. Although higher temperatures favor ragweed development, extreme meteorological events hinder its spread and development. Observations of the latest years indicated in Hungary that the increasing spread of A. artemisiifolia var. elatior (vs. A. artemisiifolia var. artemisiifolia) makes the start of the pollen season three weeks earlier [17].
4.4. Effect of Climate Change on the Spread and Harmful Effect of Ragweed
Climate change can induce physiological, biochemical and phenological changes in a plant species occurring in a given area [52,53]. As a result, the spread of A. artemisiifolia can be expected to the north, as the unfavorable conditions that have existed thus far will gradually change, and parallel with this, ragweed populations adapt to those conditions. So, we can conclude that the potential spreading area of ragweed will grow on all continents [12,15,53,54,55,56,57,58]. Out of the changing environmental factors, the enhanced level of CO2 can significantly contribute to its future successful spread [15,50,59]. Consequently, pollen production will be higher, leading to an increase in the number of respiratory diseases [50,59]. Aside from this, due to the higher temperature occurring in the spring, the germination of ragweed will happen earlier. So, the flowering time and pollen scattering will start earlier, and the pollen dispersal period will be extended [60]. The expression level of the Amb a 1 gene will be enhanced due to the enhanced levels of CO2 and drought stress, and the allergenic effect of the pollen will be stronger [60,61].
An enhanced level of temperature (minimum, maximum and average) results in a higher amount of pollen production [51,57]. Due to the additive effect of a higher temperature and CO2 level, the vegetative biomass production will increase, and this fact will also contribute to higher pollen production. Pollen production increases as individual flowers emit more pollen or larger flowers develop [43,62]. However, intense summer droughts and extreme temperature values due to climate change can be serious limiting factors for ragweed.
August minimum precipitation is a determining factor in its spread [15]. Under arid conditions, the expression of the gene responsible for pollinosis increases [63]. In the drier regions of China, the spread of ragweed is limited by low rainfall [58]. The amount of precipitation in April is believed to be a determining factor regarding ragweed infestation in Hungary [64]. The interactions between ragweed and its natural enemies—e.g., Ophraella communa—can change. Ragweed phenological stages shift and changes and shifts in natural enemy generation may occur.
Regarding its life cycle, considerable field emergence may occur even in autumn, when the temperature and soil moisture content are enough for its germination. In spite of the fact that young ragweed seedlings do not survive winter frosts, they may be potential competitive partners of late summer, autumn-sown crops, such as winter rape and winter cereals (Figure 3) [52].
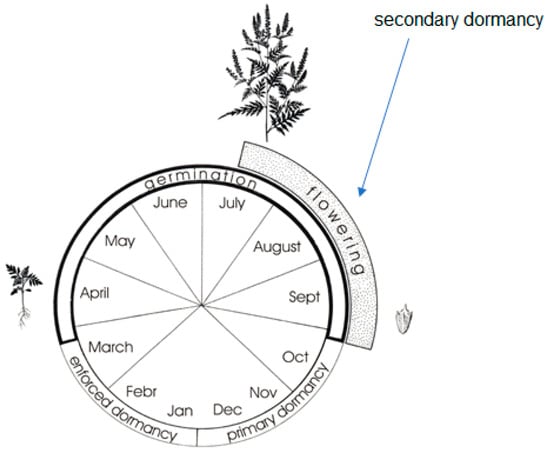
Figure 3.
Life cycle of common ragweed in Hungary (modified after Béres-Bíró [18]) (drawn by Krisztina Bíró, under legal protection of copyright).
5. Importance and Harmful Effects
5.1. Economic Importance
A. artemisiifolia is believed to be one of Europe’s most noxious invasive weed species. It has a strong competitive ability and allelopathy too. Its wide distribution is due to its good adaptation ability to different ecological conditions [1]. It causes the biggest problem in spring-sown wide-row crops. Out of them, the maize sowing area is the largest in Hungary (ca. 1 million ha) [65]. A. artemisiifolia is considered an invasive plant of the most significant economic importance [66]. It can be found in almost all arable crops and can cause considerable yield losses. Besides agriculture, it can be found in other habitats as well, like non-cultivated fields, stubbles and along linear constructions (railway, highroads, etc.), and in interior areas around construction sites, where soil disturbance occurs regularly [21]. A lot of biotic and abiotic factors play an important role in the spreading of ragweed. The most important ones are the fact that there are a few natural host-specific enemies in Europe, human faults (like the increase of uncultivated lands and the lack of professional knowledge), increased hobby gardens, crop seeds infested with ragweed seeds, expensive weed management technologies and the appearance of herbicide-resistant biotypes [3]. Kolejanisz [67] proved that management factors proved to be stronger predictors in terms of ragweed infestation than environmental ones. This information may be important regarding the more accurate prediction of the future spread of ragweed since so far the forecasts have been largely based on climatic data. Ragweed is a low-demand species. Its occurrence is not relevant in the forest. Although forest plantings cause soil disturbance, machine-maintained forest roads, mowed roadsides and ditches are suitable for ragweed when sufficient light reaches the soil [68].
Without weed management, ragweed could cover 340,000 ha of Hungary’s 6.5 million ha arable land (5.33%). Based on an 862 EUR/ha income, the infestation should have caused a EUR 293 million loss of income in 2012 [3]. Except in cases of delayed crop emergence and arid conditions, ragweed has no detrimental effect on cereals. In undeveloped stands (because of internal water, frostbite, pest injuries, etc.) and at the end of vegetation due to the lower weed suppression ability of wheat, it may cause problems. It causes the biggest problem in row crops, where the protection against them already starts in the forecrop or on the stubble of the forecrop [68,69].
In additive competition studies, the effect of weed density on crop yield can be studied. Lehoczky et al. [48] examined the ragweed density on wheat stubble three weeks after harvest. Ragweed was able to create an average density of 20.9 individuals per m2. Varga et al. [70] studied the competition between ragweed and sunflower. Based on their results, in the case of 10 individual ragweeds per m2, the yield quantity of maize was reduced by 37%. In an experiment in Keszthely (Hungary), 1 individual per square meter caused a 25% yield loss. As the weed density increased (2–10 plants/m2), the yield loss also increased by 30–33%. Hall et al. [26] studied the effect of A. artemisiifolia on soybean. A total of 5 ragweed in the pot decreased the cv. Albena variety biomass production by 11%, and the cv. Mentor variety biomass production by 26% compared to the control. At harvest time, cv. Albena had 51% less aboveground dry weight when 5 ragweed were in the pot as compared to the control. The “Mentor” variety had the lowest (33%) loss in the aboveground dry weight mass when 5 ragweed individuals were in the pot.
5.2. Human Health Injuries
Ragweed was introduced into Europe and found to have favorable conditions in the moderate climate, spreading widely in these areas. The highly allergenic ragweed pollen quickly induced public health problems [71]. Wyman [72] was the first to describe allergenic disease due to ragweed pollen. Ragweed is responsible for more allergenic disease than the other allergenic plant species [73]. A medium concentration can cause allergic reactions in people susceptible to ragweed pollen, whereas a high concentration causes allergic reactions in all patients. In Hungary, countrywide regular pollen monitoring has been working for a long time (81) and a ragweed pollen alarm system also exists. Regarding the period between 1992 and 2008, the pollen concentration increased until 1999 but then decreased until 2007. It can be explained by the fact that the summer periods between 1999 and 2007 were hot and dry, which led to a reduced pollen concentration [74]. The ragweed pollen concentration increases in the air nowadays, which is often at on extremely high level [75]. Untreated or improperly treated ragweed allergies can turn into asthma [76].
The Amb a 1 gene is firstly responsible for forming an allergy [77]. Wopfner et al. [78] inform us about nine genes of ragweed accountable for pollen allergy (see Table 3).

Table 3.
Allergens of ragweed (after Woepfner et al. [78] and Buters et al. [76]).
Buters et al. [76] described 11 allergenic genes. Amb a 11—next to the Amb a 1—is considered a critical gene to induce allergy. Farkas et al. [79] examined the expression of allergenic genes derived from the populations collected in Hungary. They stated that in the male flowers, the expression levels of the Amb a 1, Amb a 3, Amb a 4, Amb a 5, Amb a 6 and Amb a 8 genes are incredibly high. The highest was in the case of the Amb 5 gene in the male flowers. Amb a 1, Amb a 3 and Amb a 8 were expressed in the female flowers, but to a lesser extent. All genes, except Amb 6, were expressed in the leaf samples, with Amb 12 expression being twice as high as in the male flowers. These results suggest that those allergenic genes expressed in the leaves can play a role in metabolic processes and stress responses. Its significance for human health is supported by numerous publications on pollen pollution in populated areas and ragweed weed management methods [80]. High pollen concentrations cause severe problems in pollen-sensitive populations [81]. Ragweed pollen can induce an allergic reaction in a lot of people. In Europe, ca. 23.2 million people show sensitivity to ragweed pollen. The highest pollen concentrations are detected in the Great Pannonian Plain, the Po Plain and the Rhone Valley (Figure 4) [82].
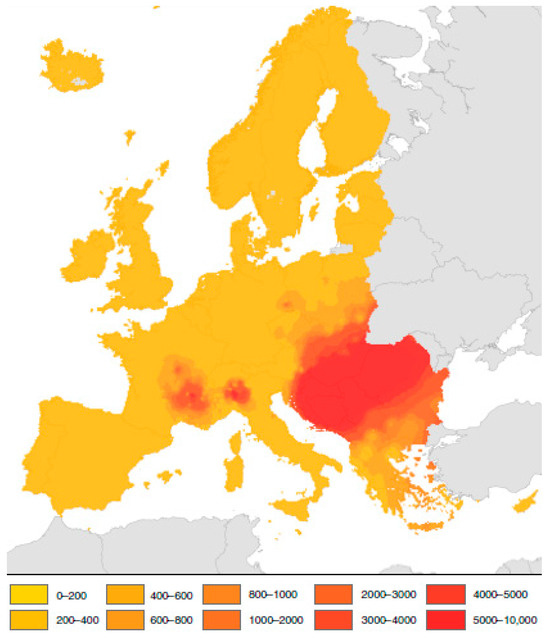
Figure 4.
Pollen scattering of ragweed in Europe (number of grains per cubic meter of air) (after Schaffner et al. [82]).
The Bratislava air pollen concentration was analyzed between 2002 and 2007 by Chrenová et al. [83]. During the main flowering period of ragweed, the pollen concentration exceeded 150 pollen grains/m3 for some days and remained consistently above 50 grains/m3. In many cases, its concentration was above 300 grains per m3 in the air. Pollen allergy to ragweed is probably one of the most common health problems in Europe. The number of people showing pollen allergenicity will increase twice in the future, and it may rise from 33 million to 77 million between 2041 and 2060. Climate change will increase the pollen concentration and extend the pollen season, which can last until October [84]. Ragweed can produce about 1.2 billion pollen grains for an individual plant. Ragweed pollen causes hay fever during its flowering period in late summer and early autumn, making it the leading cause of hay fever caused by allergens [85]. Jager [86] stated that the maximum pollen concentration was at night in Vienna in the 1980s; at the end of the 1990s, the maximum pollen concentration was in the late afternoon and early evening periods. The reason for this change is that the primary pollen source becomes located closer to the city. It is presumed that the spreading speed of pollen is 20 km/h from the Carpathian Basin towards western parts of Europe. This hypothesis was proven later when Kazinczi et al. [9] confirmed that its intensive spread could be detected in Austria, Germany and Switzerland since the end of the 2000s. Pollen concentrations in the air are 10 g/m3, 30 g/m3 and 100 g/m3 (medium, high and extremely high, respectively).
According to Juhász-Juhász [87], ragweed pollens account for the majority of seasonal pollen concentration (60–71%). There are 30–35 million pollen grains in 1 g of pollen. The primary cause—at least 50 percent of pollinosis—is due to the presence of ragweed pollen and other co-factors. According to some surveys, pollen density during the main flowering period can exceed 20,000 pollen grains per m3. In Hungary, 20–25% of the population shows sensitivity to ragweed pollen; therefore, this is considered one of the major research areas in medical sciences [21]. Considering the Great Pannonian Plain, the highest pollen concentration occurs in the southern part (Vajdasag), around Szeged town, and in the southern part of the trans-Danube region (around Pécs town) [4].
6. Habitat and Environmental Requirements of Ragweed
Ragweed prefers open habitats. It can be found under all environmental conditions, except extreme environmental factors and a lack of light. It spreads almost immediately in large, bare areas. It is believed to be the most characteristic plant species in the first years of disturbed areas. It is a permanent companion plant for construction and fieldwork. Its permanent presence is typical in areas where soil disturbance is frequent (arable fields, along roads) and where human interventions are regular. Today, it is also present in significant quantities in narrow-row cultures [88]. It is absent from semi-natural habitats without soil or other human disturbance [48]. The extremely high prevalence of ragweed is due to its excellent adaptation ability to various environmental factors. Sandy soils are most favorable for ragweed, followed by Ramann’s brown forest soils and then meadow soils [1]. A slightly acidic pH is favorable for ragweed development, but at a lower pH (around 5), the allergenic effect of pollen increases [51]. Ragweed favors calcareous soils, but the lower calcium concentration is not a limiting factor for its occurrence in acidic soils [89]. From the point of view of ragweed infestation; soil mineral composition, like Mn‒, K‒ and Na‒content; May temperatures and annual and April precipitation are also important factors [64]. A. artemisiifolia is a nitrophylous plant species, and its development is considerably promoted after nitrogen application. Among the forms of nitrogen applied on meadow soils, the Péti salt (ammonium-nitrate + dolomite powder) had the strongest effect, while urea proved to be the most effective on brown forest soils and sandy soils [1].
Dickerson [22] studied the effect of shading on the ragweed and found that 70% of the shadow was not efficient for the ragweed. The 30% shadow had no negative impact on the development. Ragweed belongs to the so-called ‘arid tolerant species’, meaning that the water-saturated leaves can lose 70% of their maximum water content without irreversible injuries. The sublethal water saturation deficit of ragweed (WSDsubl) is above 70% [3]. Ragweed can survive on soils contaminated with heavy metals (Ni, Zn, Cu and Cd). Tóth and colleagues [90] and Pichtel et al. [91] conducted research in which the accumulation of lead (Pb) from electronic waste was simulated to see how A. artemisiifolia behaves in the event of lead accumulation in the soil. Ragweed showed a high tolerance to lead. The roots could accumulate 1600 mg/kg without lethal effects. Ragweed has been characterized by a broad ecological amplitude and a high light requirement during the intensive growth phase; after that (during the flowering period), it does not require as much light.
7. Allelopathic Effect
Out of interference among higher plants, not only competition but allelopathy—a type of chemical interaction—is also characteristic of ragweed [92]. Ragweed can produce water-soluble extracts, which can influence the development and physiological processes of the donor plant species [49,92]. Earlier studies proved that ragweed as a donor plant may play an important role in agroecosystems, but its role as a test plant is less important. For example, ragweed is able to utilize minerals from sunflower water extracts, which are known for their inhibitory effect [65]. Brückner [93] examined the effect of ragweed on the development of donor plants. The leaf extracts showed the highest inhibitory effect out of the plant parts. Allelopathy may play an essential role in the spreading of a plant species, especially in the case of some invasive alien species, such as A. artemisiifolia. Not only water but organic extracts can significantly retard the germination of the test species by 20–54% in maize, soybean, bean, pea and sunflower [94].
Kazinczi et al. [65] examined the effect of Helianthus annuus, Convolvulus arvensis, Abutilon theophrasti and ragweed extracts on the development of ragweed. They found that neither root nor shoot biomass reduction happened as compared to control. Despite this, the sunflower extracts significantly enhanced the ragweed biomass. The opposite effect was observed in the cases of velvetleaf (Abutilon theophrasti) and C. arvensis extracts and, based on the results of Szabó et al. [95], ragweed plant residues and its water extracts (when residues were incorporated into the soil or the extracts were used for irrigation, respectively) proved autotoxic for themselves. The experiments of Su et al. [96] show that A. artemisiifolia and A. trifida also have a quantity-dependent autotoxicity. The 0.1 g 100 mL−1; 1 g 100 mL−1 and 2 g 100 mL−1 plant extract concentrations have positive effects on germination of themselves. But 5 g 100 mL−1; 10 g 100 mL−1 concentrations inhibited the germination. In the case of A. artemisiifolia, 5 g 100 mL−1 has a 2% inhibition rate and 10 g 100 mL−1 has a 38% inhibition rate for germination. Vidotto et al. [97] investigated ragweed’s allelopathic effect on barley, lettuce, tomato, alfalfa and wheat. Different parts of ragweed at different concentrations were incorporated into the soil of the pots. Methyl coffeateant residues stimulated seed germination but reduced the development. Ragweed’s allelopathic effects are primarily due to phenolic and terpenoid compounds [93]. Šucur et al. [98] identified the phenolic compounds of A. trifida and studied the effect on sunflower, maize and soybean in three-term ratios 1:3, 1:1 and 3:1 (weed: crop). The highest lipid peroxidation was detected in the sunflower leaves at a 3:1 ratio. The maize lipid peroxidation was moderated and an increase was detected 10 days after sowing. But 14 days later, there was no significant increase in lipid peroxidation. In soybean, no lipid peroxidation increase was detected. The maize and soybean increased antioxidant enzyme activity, which could be the explanation for the moderation and no lipid peroxidation increase. The identified components were 8.9 µg/g protocatechuic acid, 4.5 µg/p-hydroxybenzoic acid, 3.55 µg/g vanillic acid, 1.75 µg/g syringic acid, 0.96 µg/g p-cummaric acid and 0.7 µg/g ferulic acid. A. trifida and A. artemisiifolia have almost the same allelopathic effect on the crops. Šcepanovic et al. [99] examined the allelopathic effect of some phenolic acids on A. artemisiifolia. These phenolic acids are gallic acid, caffeic acid, ferulic acid, vanillic acid, syringic acid, p-hydroxybenzoic acid, protocatechuic acid, chlorogenic acid, p-cumaric acid and a mixture of these. The lowest concentration was the natural phenolic concentration found in cover crop plants’ dry tissue (Sinapis alba, Raphanus sativus and Camelina sativa). The experiments showed that caffeic acid, gallic acid and syringic acid have inhibitory effects only at higher dosages. The mixed components had the highest inhibition effect on A. artemisiifolia. Su et al.’s [96] experiments showed that the two main germination inhibitors were chlorogenic acid and vanillin. A total of 20 μg 100 mL−1 vanillin has a 2% germination inhibitory effect, and 1000 and 2000 μg 100 mL−1 vanillin had a 16% germination inhibitor effect. A total of 1500 μg 100 mL−1 chlorogenic acid had a 2% germination inhibitory effect, and 3000 μg 100 mL−1 chlorogenic acid had a 38% germination inhibitory effect.
8. Beneficial Effects of Ragweed
Although ragweed is known for its harmful effects, it has some favorable characteristics as well. Pichtel et al. [91] showed that the high Pb accumulation of ragweed makes it capable of eliciting Pb content from the soil without causing plant injury. Ragweed is believed to be a medicinal plant; tea made from the flowering shoots is effective against stomach diseases and local bleeding. Leaf cooking is suitable for treating skin diseases and preventing eye irritation. It can be used for animal feeding, and its seeds (achenes) are used for the feeding and winter survival of birds because the seeds are rich in oil and nutrients [100].
9. Conclusions
The common ragweed (Ambrosia artemisiifolia L.) originated from the Sonoran Desert, and spread over a large area on several continents around the world. It is spreading rapidly, mainly due to anthropogenic influences, and in the northern hemisphere, its spread is expected to continue northwards. Its invasion success is mainly due to its favorable biological characteristics such as long seed viability, high seed production, rapid vegetative growth, etc. Its spread is also likely to be aided in the future by the effects of climate change, in particular the rising temperature and the increase in anthropogenic CO2 in the atmosphere, which is also the main cause of climate change.
Since ragweed arrived in Europe in the 20th century, it has now become the most important weed. In addition to its damage to agriculture, it causes major health problems due to the allergies induced by its pollen during the flowering period for those who are sensitive to it. With the increase in the number of individuals and their northward spread, the pollen concentration in the atmosphere will increase, with a concomitant increase in the number of people becoming ill. Overall, the success of ragweed is mainly connected to anthropogenic activity (soil disturbance and CO2 increase in the atmosphere). To reduce its adverse effects on agriculture and human health, the number of ragweed individuals should be reduced as much as possible.
Author Contributions
Creating a literature database, B.K.; database analysis and evaluation, I.J.; specialist advice, J.T.; database organization and evaluation, S.K.; as a supervisor, verification of content and form requirements and providing expert advice, G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Hungarian Government and the European Union, with the co-funding of the European Regional Development Fund in the frame of the Széchenyi 2020 Programme GINOP-2.3.2-15-2016-00054 project.
Data Availability Statement
Data available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nádasy, E.; Kazinczi, G. Growth of common ragweed (Ambrosia artemisiifolia) on different soil types with various nitrogen supplies. In Proceedings of the 10th Slovenian Conference on Plant Protection with International Participation, Podčetrtek, Slovenia, 1–2 March 2011. [Google Scholar]
- Ujvárosi, M. Gyomnövények; Mezőgazdasági Kiadó: Budapest, Hungary, 1973; pp. 432–500. [Google Scholar]
- Szigetvári, C.; Benkő, Z.R. Biológiai Inváziók Magyarországon. Özönnövények; TermészetBÚVÁR Alapítvány Kiadó: Budapest, Hungary, 2004; pp. 337–371. (In Hungarian) [Google Scholar]
- Kazinczi, G.; Novák, R. (Eds). Integrated Methods for the Control of Ragweed; Gyommentes Környezetét Alapítvány: Budapest, Hungary, 2012; pp. 30–98. [Google Scholar]
- Makra, L.; Matyasovszky, I.; Hufnagel, L.; Tusnády, G. The history of ragweed in the world. Appl. Ecol. Enviromental Res. 2015, 13, 489–500. [Google Scholar] [CrossRef]
- Chauvel, B.; Dessaint, F.; Cardinal-Legrand, C.; Bretagnolle, F. The historical spread of Ambrosia artemisiifolia L. in France. Herb. Rec. J. Biogeogr. 2006, 33, 665–673. [Google Scholar] [CrossRef]
- Genton, B.J.; Shykoff, J.A.; Giraud, T. High genetic diversity in French invasive populations of common ragweed, Ambrosia artemisiifolia, as a result of multiple sources of introduction. Mol. Ecol. 2005, 14, 4275–4285. [Google Scholar] [CrossRef] [PubMed]
- Afonin, A.N.; Luneva, N.N.; Fedorova, Y.A.; Kletchkovskiy, E.; Chebanovskaya, A.F. History of introduction and distribution of common ragweed (Ambrosia artemisiifolia L.) in the European part of the Russian Federation and in the Ukraine. EPPO Bull. 2018, 48, 266–273. [Google Scholar] [CrossRef]
- Kazinczi, G.; Béres, I.; Novák, R.; Bíró, K.; Pathy, Z. Common ragweed (Ambrosia artemisiifolia): A review with special regards to the results in Hungary. I. Taxonomy, origin and distribution, morphology, life cycle and reproduction strategy. Herbologia 2008, 9, 55–91. [Google Scholar]
- Clot, B.; Pietragalla, B. Aerobiological markers of ragweed invasion in Switzerland. In Proceedings of the First International Ragweed Conference, Budapest, Hungary, 10–13 September 2008. [Google Scholar]
- Thibaudon, M.; Šikoparija, B.; Oliver, G.; Smith, M.; Skjøth, C.A. Ragweed pollen source inventory for France the second largest centre of Ambrosia in Europe. Atmos. Environ. 2014, 83, 62–71. [Google Scholar] [CrossRef]
- Keszthelyi, S.; Kazinczi, G.; Somfalvi-Tóth, K. Geographical dispersion of ragweed leaf beetle (Ophraella communa) based on climatic and biological characters in the Palearctic habitats. Agric. For. Entomol. 2022, 25, 165–185. [Google Scholar] [CrossRef]
- Csontos, P.; Vitalos, M.; Barina, Z.; Kiss, L. Early distribution and spread of Ambrosia artemisiifolia in Central and Eastern Europe. Bot. Helv. 2010, 120, 75–78. [Google Scholar] [CrossRef]
- Béres, I.; Hunyadi, K. A parlagfű (Ambrosia elatior L.) biológiája. Növényvédelem 1980, 16, 109–116. [Google Scholar]
- Case, M.J.; Stinson, K.A. Climate change impacts on the distribution of the allergenic plant, common ragweed (Ambrosia artemisiifolia) in the eastern United States. PLoS ONE 2018, 13, e0205677. [Google Scholar] [CrossRef]
- Novák, R.; Magyar, M.; Simon, G.; Kadaravek, B.; Kadaravekné Guttyán, A.; Blazsek, K.; Erdélyi, K.; Farkas, G.; Gyulai, B.; Hornyák, A.; et al. A Hatodik Országos Szántóföldi Gyomfelvételezés előzetes eredményei. In Proceedings of the 66. Növényvédelmi Tudományos Napok, Budapest, Hungary, 18–19 February 2020. (In Hungarian). [Google Scholar]
- Novák, R. Az Ötödik Országos Gyomfelvételezés Magyarország szántóföldjein. In Proceedings of the Magyar Gyomkutató Társaság 18, Konferenciája, Balatonszemes, Hungary, 8 March 2012. (In Hungarian). [Google Scholar]
- Hunyadi, K.; Béres, I.; Kazinczi, G. ; Gyomnövények, Gyomirtás, Gyombiológia; Mezőgazda Kiadó: Budapest, Hungary, 2000; pp. 477–585. (In Hungarian) [Google Scholar]
- Bassett, I.J.; Crompton, C.W. The biology of Canadian weeds. Ambrosia artemisiifolia L. and A. psilostachya Dc. Can. J. Plant Sci. 1975, 55, 463–476. [Google Scholar]
- Hrabovský, M.; Micieta, K. Review of the taxonomic concepts of the invasive species Ambrosia artemisiifolia l. as the basis for the evaluation of variability of this species in Europe. Acta Bot. Univ. Comen. 2013, 48, 9–13. [Google Scholar]
- Kazinczi, G.; Béres, I.; Novák, R.; Karmán, J. Újra fókuszban az ürömlevelű parlagfű. Növényvédelem 2009, 45, 389–403, (In Hungarian with an English summary). [Google Scholar]
- Dickerson, C.T. Studies on the Germination, Growth, Development and Control of Common Ragweed (Ambrosia artemisiifolia L.). Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 1968. [Google Scholar]
- Béres, I.; Bíró, K. A parlagfû (Ambrosia elatior L.) életciklusa és fenofázisának idôtartama. Növényvédelem 1993, 29, 148–151, (In Hungarian with an English summary). [Google Scholar]
- Essl, F.; Biró, K.; Brandes, D.; Broennimann, O.; Bullock, J.M.; Chapman, D.S.; Chauvel, B.; Dullinger, S.; Fumanal, B.; Guisan, A.; et al. Biological Flora of the British Isles: Ambrosia artemisiifolia. J. Ecol. 2015, 103, 1069–1098. [Google Scholar] [CrossRef]
- Guillemin, J.-P.; Chauvel, B. Effects of the seed weight and burial depth on the seed behavior of common ragweed (Ambrosia artemisiifolia). Weed Biol. Manag. 2011, 11, 217–223. [Google Scholar] [CrossRef]
- Hall, R.M.; Urban, B.; Wagentristl, H.; Karrer, G.; Winter, A.; Czerny, R.; Kaul, H.-P. Common ragweed (Ambrosia artemisiifolia L.) Causes Severe Yield Losses in Soybean and Impairs Bradyrhizobium japonicum Infection. Agronomy 2021, 11, 1616. [Google Scholar] [CrossRef]
- Sang, W.; Liu, X.; Axmacher, J.C. Germination and emergence of Ambrosia artemisiifolia L. under changing environmental conditions in China. Plant Species Biol. 2011, 26, 125–133. [Google Scholar] [CrossRef]
- Magyar, L. A Gyommagvak Terjedése; Universitias-Győr Nonprofit Kft.: Győr, Hungary, 2023; pp. 282–364. [Google Scholar]
- Silc, U. Odontito-Ambrosietum Jarolimek et al. 1997—A ruderal association new to Slovenia. Acta Bot. Croat. 2002, 61, 179–198. [Google Scholar]
- Chikoye, D.; Weise, S.F.; Swanton, C.J. Influence of common ragweed (Ambrosia artemisiifolia) time of emergence and density on white bean (Phaseolus vulgaris). Weed Sci. 1995, 43, 375–380. [Google Scholar] [CrossRef]
- Oberdorfer, E. Pflanzensoziologische Exkursionsflora für Deutschland und angrenzende Gebiete, 8th ed.; Eugen Ulmer: Stuttgart, Germany, 2001; p. 1051. [Google Scholar]
- Farooq, S.; Onen, H.; Ozaslan, C.; Baskin, C.C.; Gunal, H. Seed germination niche for common ragweed (Ambrosia artemisiifolia L.) populations naturalized in Turkey. S. Afr. J. Bot. 2019, 123, 361–371. [Google Scholar] [CrossRef]
- Béres, I. A Parlagfû (Ambrosia elatior L.) Hazai Elterjedése, Biológiája és a Védekezés Lehetôségei. Candidate’s Thesis, Agricultural University, Keszthely, Hungary, 1981. (In Hungarian). [Google Scholar]
- Shrestha, A.; Roman, E.S.; Thomas, A.G.; Swanton, C.J. Modeling germination and shoot-radicle elongation of Ambrosia artemisiifolia. Weed Sci. 1999, 47, 557–562. [Google Scholar] [CrossRef]
- Pickett, S.T.; Baskin, J.M. The Role of Temperature and Light in the Germination Behavior of Ambrosia artemisiifolia. Bull. Torrey Bot. Club 1973, 100, 165. [Google Scholar] [CrossRef]
- Béres, I.; Hunyadi, K. Dormancy and germination of common ragweed (Ambrosia elatior L.) seeds in the field in Hungary. Acta Agron. Acad. Sci. Hung. 1984, 33, 383–387, (In Hungarian with an English summary). [Google Scholar]
- Willernsen, R.W. Effect of stratification temperature and germination termperature on germination and the induction of secondary dormancy in common ragweed seeds. Am. J. Bot. 1975, 62, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Baskin, C.C. Temperature requirements for after ripening in buried seeds of four summer annual weeds. Weed Res. 1987, 27, 385–389. [Google Scholar] [CrossRef]
- Kazinczi, G.; Béres, I.; Fischl, G.; Horváth, J. Adatok néhány inváziós gyomnövényfaj csírzásbiológiájához. Növenyvédelem 2011, 47, 89–106, (In Hungarian with an English summary). [Google Scholar]
- Fumanal, B.; Girod, C.; Fried, G.; Bretagnolle, F.; Chauvel, B. Can the large ecological amplitude of Ambrosia artemisiifolia explain its invasive success in France? Weed Res. 2008, 48, 349–359. [Google Scholar] [CrossRef]
- Stinson, K.A.; Wheeler, J.A.; Record, S.; Jennings, J.L. Regional variation in timing, duration, and production of flowers by allergenic ragweed. Plant Ecol. 2018, 219, 1081–1092. [Google Scholar] [CrossRef]
- Mátyás, K.K.; Hegedűs, G.; Taller, J.; Farkas, E.; Decsi, K.; Kutasy, B.; Kálmán, N.; Nagy, E.; Kolics, B.; Virág, E. Different expression pattern of flowering pathway genes contribute to male or female organ development during floral transition in the monoecious weed Ambrosia artemisiifolia L. (Asteraceae). PeerJ 2019, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Lommen, S.T.E.; Hallmann, C.A.; Jongejans, E.; Chauvel, B.; Leitsch-Vitalos, M.; Aleksanyan, A.; Tóth, P.; Preda, C.; Scepanovic, M.; Onen, H.; et al. Explaining variability in the production of seed and allergenic pollen by invasive Ambrosia artemisiifolia across Europe. Biol. Invasions 2018, 20, 1475–1491. [Google Scholar] [CrossRef]
- Basky, Z. A Magyarországon őshonos levéltetvek hatása a parlagfű (Ambrosia artemisiifolia L.) fejlődésére. Magy. Gyomkutatás És Technológia 2007, 8, 21–40, (In Hungarian with an English summary). [Google Scholar]
- Pasqualini, S.; Tedeschini, E.; Frenguelli, G.; Wopfner, N.; Ferreira, F.; D’Amato, G.; Ederli, L. Ozone affects pollen viability and NAD(P)H oxidase release from Ambrosia artemisiifolia pollen. Environ. Pollut. 2010, 159, 2823–2830. [Google Scholar] [CrossRef] [PubMed]
- Mátyás, K.K.; Bódis, J.; Virág, E.; Taller, J.; Pintér, C. Az ürömlevelű parlagfű (Ambrosia artemisiifolia L.) virágzatának részletes leírása sztereomikroszkópos rétegfotózás használatával. Bot. Közlemények 2020, 107, 103–109, (In Hungarian with an English summary). [Google Scholar] [CrossRef]
- Szigetvári, C.; Benkő, Z.R. Common ragweed (Ambrosia elatior L.). In The Most Important Invasive Plants in Hungary; Botta-Dukát, Z., Balogh, L., Eds.; Institute of Ecology and Botany, Hungarian Academy of Sciences: Vácrátot, Hungary, 2008; pp. 183–203. [Google Scholar]
- Lehoczky, É.; Kerekes, B.; Szabó, R.; Busznyák, J.; Gólya, G. Study on the biomass and seed production of ragweed (Ambrosia artemisiifolia L.) on winter wheat stubble. Növénytermelés 2011, 60, 57–60, (In Hungarian with an English summary). [Google Scholar]
- Tóth, C.T.; Szabó, Z.; Csubák, M. Biológiailag aktív növényi extraktum antifungális hatásának vizsgálata talajgombákon. Agrártudományi Közlemények 2012, 50, 247–252, (In Hungarian with an English summary). [Google Scholar]
- Wayne, P.; Foster, S.; Connolly, J.; Bazzaz, F.; Epstein, P. Production of allergenic pollen by ragweed (Ambrosia artemisiifolia L.) is increased in CO2-enriched atmospheres. Ann. Allergy Asthma Immunol. 2002, 88, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Gentili, R.; Asero, R.; Caronni, S.; Guarino, M.; Montagnani, C.; Mistrello, G.; Citterio, S. Ambrosia artemisiifolia L. temperatureresponsive traits influencing the prevalence and severity of pollinosis: A study in controlled conditions. BMC Plant Biol. 2019, 19, 155. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ding, J.; Siemann, E.; Keller, S.P. Biocontrol of invasive weeds under climate change: Progress, challenges and management implications. Curr. Opin. Insect Sci. 2020, 38, 72–78. [Google Scholar] [CrossRef]
- Behrendt, H.; Ring, J. Climate Change, Environment and Allergy. Chem. Immunol. Allergy 2012, 96, 7–14. [Google Scholar] [CrossRef]
- Cunze, S.; Leiblein, M.C.; Tackenberg, O. Range Expansion of Ambrosia artemisiifolia in Europe Is Promoted by Climate Change. ISRN Ecol. 2013, 2013, 610126. [Google Scholar] [CrossRef]
- Storkey, J.; Stratonovitch, P.; Chapman, D.S.; Vidotto, F.; Semenov, M.A. A process-based approach to predicting the effect of climate change on the distribution of an invasive allergenic plant in Europe. PLoS ONE 2014, 9, e88156. [Google Scholar] [CrossRef]
- Mattia, I.; De Simone, W.; D’Alessandro, P.; Console, G.; Biondi, M. Investigating the current and future co-occurrence of Ambrosia artemisiifolia and Ophraella communa in Europe through ecological modelling and remote sensing data analysis. Int. J. Environ. Res. Public Health 2019, 16, 3416. [Google Scholar]
- Ianovici, N.; Bîrsan, M.-V. The influence of meteorological factors on the dynamic of Ambrosia artemisiifolia pollen in an invaded area. Not. Bot. Horti Agrobot. 2020, 48, 752–769. [Google Scholar] [CrossRef]
- Xiao-Li, L.; Hong-Qun, L.; Jian-Hua, W.; Xie-Ping, S. The current and future potential geographical distribution of common ragweed, Ambrosia artemisiifolia in China. Pak. J. Bot. 2021, 53, 167. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.A.; Wayne, P.M.; Macklin, E.A.; Muilenberg, M.L.; Wagner, C.J.; Epstein, R.P.; Bazzaz, F.A. Interaction of the Onset of Spring and Elevated Atmospheric CO2 on Ragweed (Ambrosia artemisiifolia L.) Pollen Production. Environ. Health Perspect. 2006, 114, 865–869. [Google Scholar] [CrossRef]
- El Kelish, A.; Zhao, F.; Heller, W.; Durner, J.; Winkler, J.B.; Behrendt, H.; Traidl-Hoffmann, T.; Horres, R.; Pfeifer, M.; Frank, U.; et al. Ragweed (Ambrosia artemisiifolia) pollen allergenicity: SuperSAGE transcriptomic analysis upon elevated CO2 and drought stress. BMC Plant Biol. 2014, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Singer, B.D.; Ziska, L.H.; Frenz, D.A.; Gebhard, D.E.; Straka, J.G. Increasing Amb a 1 content in common ragweed (Ambrosia artemisiifolia) pollen as a function of rising atmospheric CO2 concentration. Funct. Plant Biol. 2005, 32, 667–670. [Google Scholar] [CrossRef]
- Damialis, A.; Traidl-Hoffmann, C.; Treudler, R. Climate Change and Pollen Allergies. In Biodiversity and Health in the Face of Climate Change; Marselle, M.R., Stadler, J., Korn, H., Irvine, K.N., Bonn, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 47–66. [Google Scholar] [CrossRef]
- Rauer, D.; Gilles, S.; Wimmer, M.; Frank, U.; Mueller, C.; Musiol, S.; Vafadari, B.; Aglas, L.; Ferreira, F.; Schmitt-Kopplin, P.; et al. Ragweed plants grown under elevated CO2 levels produce pollen which elicit stronger allergic lung inflammation. Allergy 2021, 76, 1718–1730. [Google Scholar] [CrossRef]
- Pinke, G.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. Environmental and land-use variables determining the abundance of Ambrosia artemisiifolia in arable fields in Hungary. Preslia 2011, 83, 219–235. [Google Scholar]
- Kazinczi, G.; Béres, I.; Onorfi, A.; Nádasy, E.; Takács, A.; Horváth, J.; Torma, M. Allelopathic effect of plant extracts on common ragweed (Ambrosia artemisiifolia L.). J. Plant Dis. Prot. 2008, 21, 335–340. [Google Scholar]
- Skjøth, C.A.; Sun, Y.; Karrer, G.; Sikoparija, B.; Smith, M.; Schaffner, U.; Müller-Schärer, H. Predicting abundances of invasive ragweed across Europe using a “top-down” approach. Sci. Total Environ. 2019, 686, 212–222. [Google Scholar] [CrossRef]
- Kolejanisz, T. Az Ürömlevelű Parlagfű (Ambrosia artemisiifolia L.) Térfoglalását Befolyásoló Ökológiai és Agrotechnikai Tényezők az Osztrák-Magyar Határ Térségében. Ph.D. Thesis, Széchenyi István Egyetem, Mosonmagyaróvár, Hungary, 2022. (In Hungarian with an English summary). [Google Scholar]
- Hirka, A.; Csóka, G. A Parlagfű (Ambrosia artemisiifolia L.) a hazai erdőkben. Növényvédelem 2009, 45, 389–403, (In Hungarian with an English summary). [Google Scholar]
- Hódi, L. Integrált védelem a parlagfű ellen. Növényvédelem 2009, 45, 485–489, (In Hungarian with an English summary). [Google Scholar]
- Varga, P.; Kazinczi, G.; Béres, I.; Kovács, I. Competition between sunflower and Ambrosia artemisiifolia in additive experiments. In Proceedings of the V, Alps-Adria Scientific Workshop, Opatija, Croatia, 4 December 2006. [Google Scholar]
- Páldy, A.; Apatini, D.; Collinsné, H.Z. Magyarország parlagfű-szennyezettsége 2000-2005. Egészségtudomány 2006, 50, 39–60, (In Hungarian with an English summary). [Google Scholar]
- Wyman, M. Autumnal catarrh. Boston Med. J. 1875, 93, 209–212. [Google Scholar] [CrossRef][Green Version]
- Szentes, D.; Lehoczky, É. Az ürömlevelű parlagfű (Ambrosia artemisiifolia L.) elterjedése, biológiája, mezőgazdasági és humánegészségügyi kártétele. Magy. Gyomkutatás És Technológia 2017, 17, 3–24, (In Hungarian with an English summary). [Google Scholar]
- Apatini, D.; Magyar, D.; Novák, E.; Páldy, A. Parlagfû (Ambrosia artemisiifolia L.) pollenszezonok vizsgálata az ÁNTSZ aerobiológiai hálózat adatai alapján (1992–2008). Növényvédelem 2009, 45, 449–454, (In Hungarian with an English summary). [Google Scholar]
- Matyasovszky, I.; Makra, L.; Tusnády, G.; Csépe, Z.; Nyúl, L.G.; Chapman, D.S.; Sümeghy, Z.; Szűcs, G.; Páldy, A.; Magyar, D.; et al. Biogeographical drivers of ragweed pollen concentrations in Europe. Theor. Appl. Climatol. 2018, 133, 277–295. [Google Scholar] [CrossRef]
- Buters, J.; Alberternst, B.; Nawrath, S.; Wimmer, M.; Traidl-Hoffmann, C.; Starfinger, U.; Behrendt, H.; Schmidt-Weber, C.; Bergmann, K.C. Ambrosia artemisiifolia (ragweed) in Germany—Current presence, allergological relevance and containment procedures. Allergo J. Int. 2015, 24, 108–120. [Google Scholar] [CrossRef]
- Lockey, R.F.; Bukantz, S.C.; Bousqet, J. Allergens and Allergen Immunotherapy; Marcel Decker: New York, NY, USA, 2004; pp. 65–85. [Google Scholar]
- Wopfner, N.; Gadermaier, G.; Egger, M.; Asero, R.; Ebner, C.; Jahn-Schmid, B.; Ferreira, F. The spectrum of allergens in ragweed and mugwort pollen. Int. Arch. Allergy Immunol. 2005, 138, 337–346. [Google Scholar] [CrossRef]
- Farkas, E.; Farkas, Z.; Hegedűs, G.; Kutasy, B.; Kolics, B.; Mátyás, K.K.; Parrag, T.; Solti, I.; Virág, E.; Taller, J. Identification and expression of pollen allergen transcripts in different organs of the common ragweed (Ambrosia artemisiifolia L.). Georg. Agric. Multidiscip. J. Agric. Sci. 2019, 23, 116–130. [Google Scholar]
- Katz, D.S.W.; Connor Barrie, B.T.; Carey, T.S. Urban ragweed populations in vacant lots: An ecological perspective on management. Urban For. Urban Green. 2014, 13, 756–760. [Google Scholar] [CrossRef]
- Katz, D.S.W.; Batterman, S.A. Allergenic pollen production across a large city for common ragweed (Ambrosia artemisiifolia). Landsc. Urban Plan. 2019, 190, 103615. [Google Scholar] [CrossRef]
- Schaffner, U.; Steinbach, S.; Sun, Y.; Skjøth, C.A.; de Weger, L.A.; Lommen, S.T.; Augustinus, B.A.; Bonini, M.; Karrer, G.; Šikoparija, B.; et al. Biological weed control to relieve millions from Ambrosia allergies in Europe. Nat. Commun. 2020, 11, 1745. [Google Scholar] [CrossRef]
- Chrenová, J.; Micieta, K.; Scevkova, J. Monitoring of Ambrosia pollen concentration in the atmosphere of Bratislava (Slovakia) during years 2002–2007. Aerobiologia 2010, 26, 83–88. [Google Scholar] [CrossRef]
- Lake, I.R.; Jones, N.R.; Agnew, M.; Goodess, C.M.; Giorgi, F.; Hamaoui-Laguel, L.; Semenov, M.A.; Solomon, F.; Storkey, J.; Vautard, R.; et al. Climate Change and Future Pollen Allergy in Europe. Environ. Health Perspect. 2017, 25, 385–391. [Google Scholar] [CrossRef] [PubMed]
- McGoey, B.M.; Stinchcombe, J.R. Introduced populations of ragweed show as much evolutionary potential as native populations. Evol. Appl. 2021, 14, 1436–1449. [Google Scholar] [CrossRef]
- Jager, S. Ragweed (Ambrosia) sensitisation rates correlate with the amount of inhaled airborne pollen. A 14-year study in Vienna, Austria. Aerobiologia 2000, 16, 149–153. [Google Scholar] [CrossRef]
- Juhász, M.; Juhász, I.E. A hazai gyomnövények aeropollinológiai jelentősége. In Környezeti Ártalmak és a Légzőrendszer; Szabó, T., Bártfai, I., Somlai, J., Eds.; Környezetvédelmi és Vízügyi Minisztérium: Budapest, Hungary, 2002; Volume 12, pp. 149–161. (In Hungarian) [Google Scholar]
- Bašić, F.; Đikić, M.; Gadžo, D. Appearance and spreading of common ragweed (Ambrosia artemisiifolia L.) in Bosnia and Herzegovina. Folia Biol. Et Geol. 2017, 58, 1475–1550. [Google Scholar]
- Pinke, G.; Karácsony, P.; Botta-Dukát, Z.; Czúcz, B. Relating Ambrosia artemisiifolia and other weeds to the management of Hungarian sunflower crops. J. Pest. Sci. 2013, 86, 621–631. [Google Scholar] [CrossRef]
- Tóth, M.D.; Puskás, G.S.; Rohr, R.; Balázsy, S. A parlagfű (Ambrosia elatior L.) kadmium-, réz-, nikkel- és cinktartalma ruderáliákon. Agrokémia És Talajt. 2005, 54, 403–416, (In Hungarian with an English summary). [Google Scholar] [CrossRef]
- Pichtel, J.; Kuroiwa, K.; Sawyerr, H.T. Distribution of Pb, Cd and Ba in soils and plants of two contaminated sites. Environ. Pollut. 2000, 110, 171–178. [Google Scholar] [CrossRef]
- Chengxu, W.; Mingxing, M.; Xuhui, C.; Bo, Q. Review on Allelopathy of Exotic Invasive Plants. Procedia Eng. 2011, 18, 240–246. [Google Scholar] [CrossRef]
- Brückner, D.J. A parlagfű allelopátiás hatása a kultúrnövények csírázására. Növénytermelés 1998, 47, 635–644, (In Hungarian with an English summary). [Google Scholar]
- Béres, I.; Kazinczi, G.; Narwal, S.S. Allelopathic plants. 4. Common ragweed (Ambrosia elatior L. syn.: A. artemisiifolia). Allelopath. J. 2002, 9, 27–34. [Google Scholar]
- Szabó, C.Z.; Pölös, E.; Palkovics, A.; Ágoston, J.; Vojnich, V.J. Autotoxicitási vizsgálatok a parlagfűn (Ambrosia artemisiifolia L.). Gradius 2019, 6, 39–46. [Google Scholar]
- Su, P.; Liu, X.; Wang, R.; Liu, T.; Zhao, W.; Sun, M.; Wang, H.; Liu, Y.; Wu, Q. Autotoxicity of Ambrosia artemisiifolia and Ambrosia trifida and its significance for the regulation of intraspecific populations density. Sci. Rep. 2022, 12, 17424. [Google Scholar] [CrossRef]
- Vidotto, F.; Tesio, F.; Ferrero, A. Allelopathic effects of Ambrosia artemisiifolia L. in the invasive process. Crop Prot. 2013, 54, 161–167. [Google Scholar] [CrossRef]
- Šućur, J.; Konstantinović, B.; Crnković, M.; Bursić, V.; Samardžić, N.; Malenčić, Đ.; Prvulović, D.; Popov, M.; Vuković, G. Chemical Composition of Ambrosia trifida L. and Its Allelopathic Influence on Crops. Plants 2021, 10, 2222. [Google Scholar] [CrossRef]
- Šćepanović, M.; Košćak, L.; Šoštarčić, V.; Pismarović, L.; Milanović-Litre, A.; Kljak, K. Selected Phenolic Acids Inhibit the Initial Growth of Ambrosia artemisiifolia L. Biology 2022, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Pölös, E.; Baglyas, F.; Vojnich, V.F. A parlagfű káráról és hasznáról. Gradius 2015, 2, 312–317. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).