Effect of Microbial Inoculants Endowed with Multifarious Plant Growth-Promoting Traits on Grape Growth and Fruit Quality under Organic Fertilization Scenarios
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. PGP Trait Determination
2.2.1. Determination of Salt Tolerance of Strains
2.2.2. Determination of Alkali Resistance of Strains
2.2.3. Quantitative Measurement of Phosphate Solubilization
2.2.4. Quantification of IAA Production
2.2.5. Quantitative Measurement of Dissolved Potassium
2.2.6. Qualitative Siderophore Production
2.2.7. Nitrogen Fixation Assay
2.3. Field Trial
2.3.1. Overview of the Test Site
2.3.2. Test Material
2.3.3. Preparation of Microbial Inoculants
2.3.4. Field Experimental Design
2.4. Field Measurements
2.4.1. Determination of Grape Leaf Parameters
2.4.2. Determination of Grape Shoot Growth Parameters
2.4.3. Determination of Grape Fruit Quality Parameters
2.5. Data Analysis
3. Results
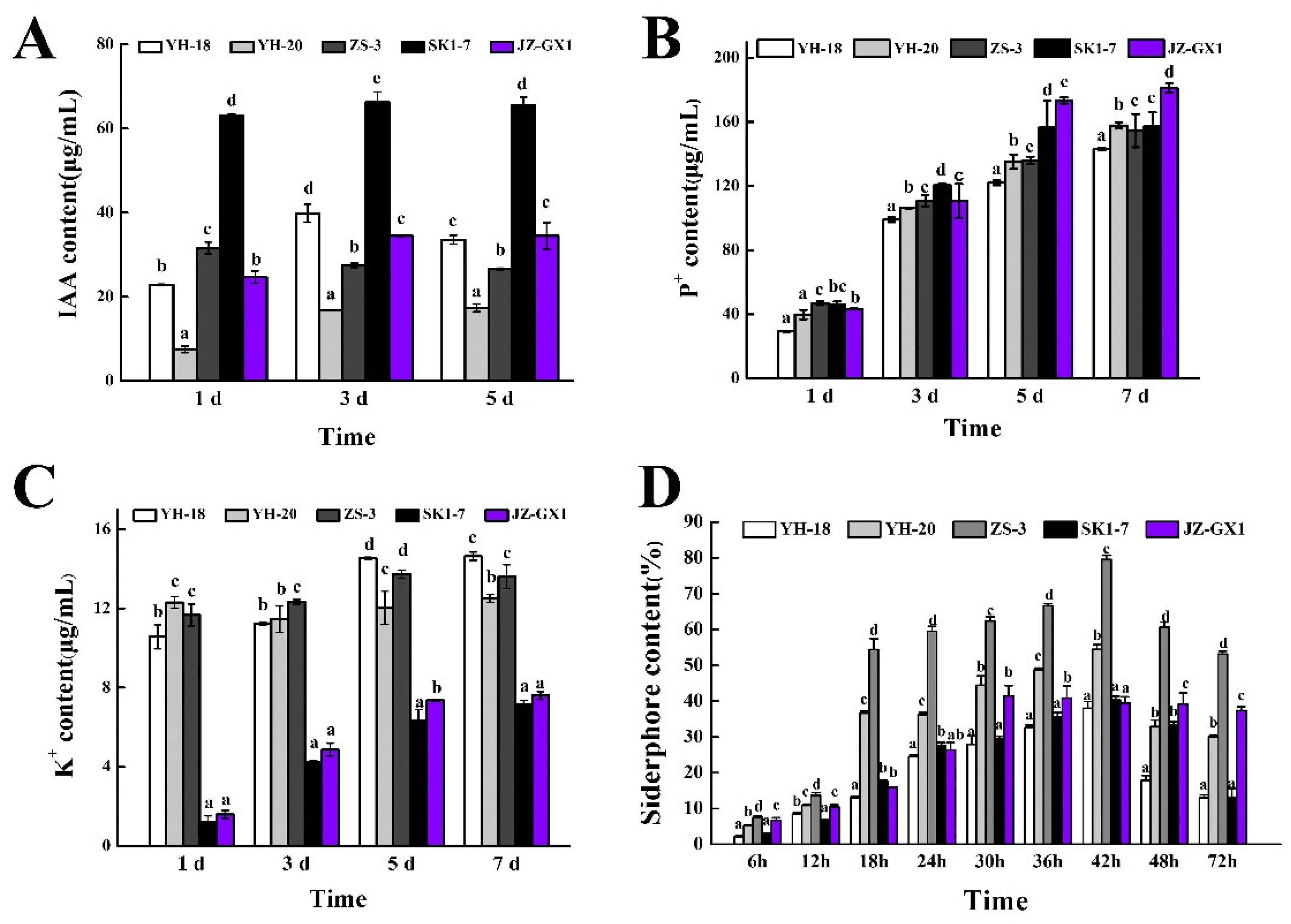
3.1. Plant Growth-Promoting Characteristics of the Five Strains
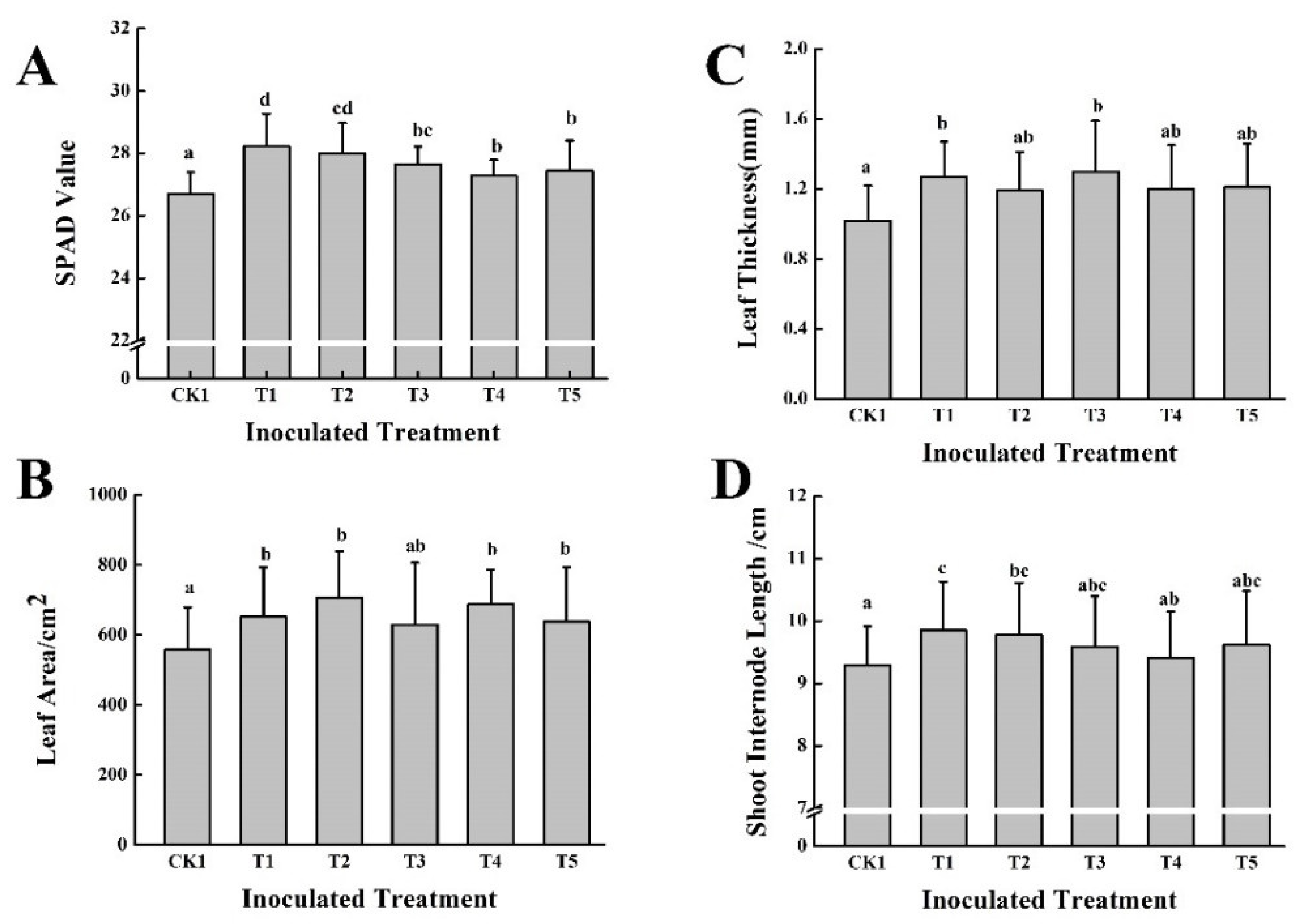
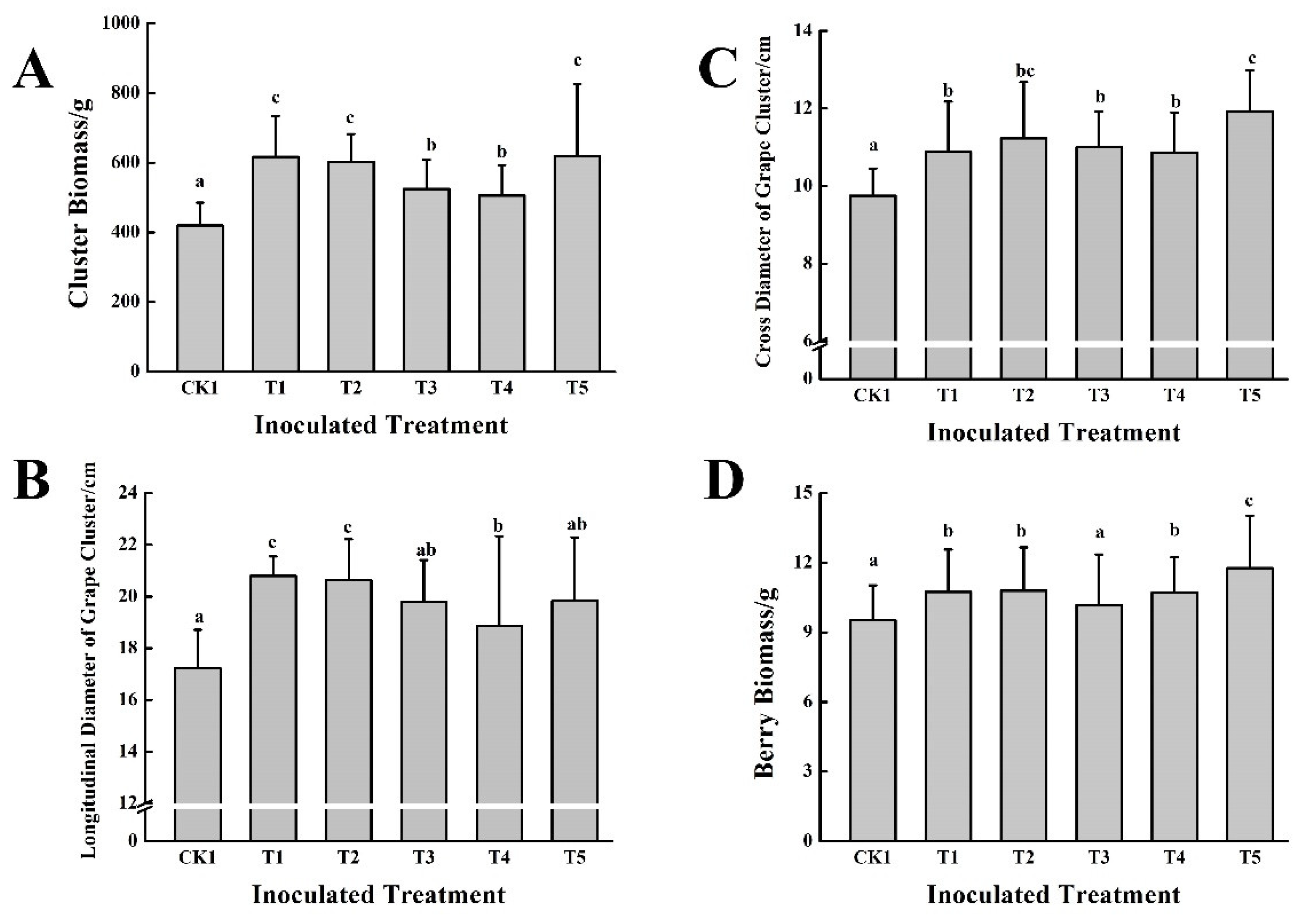
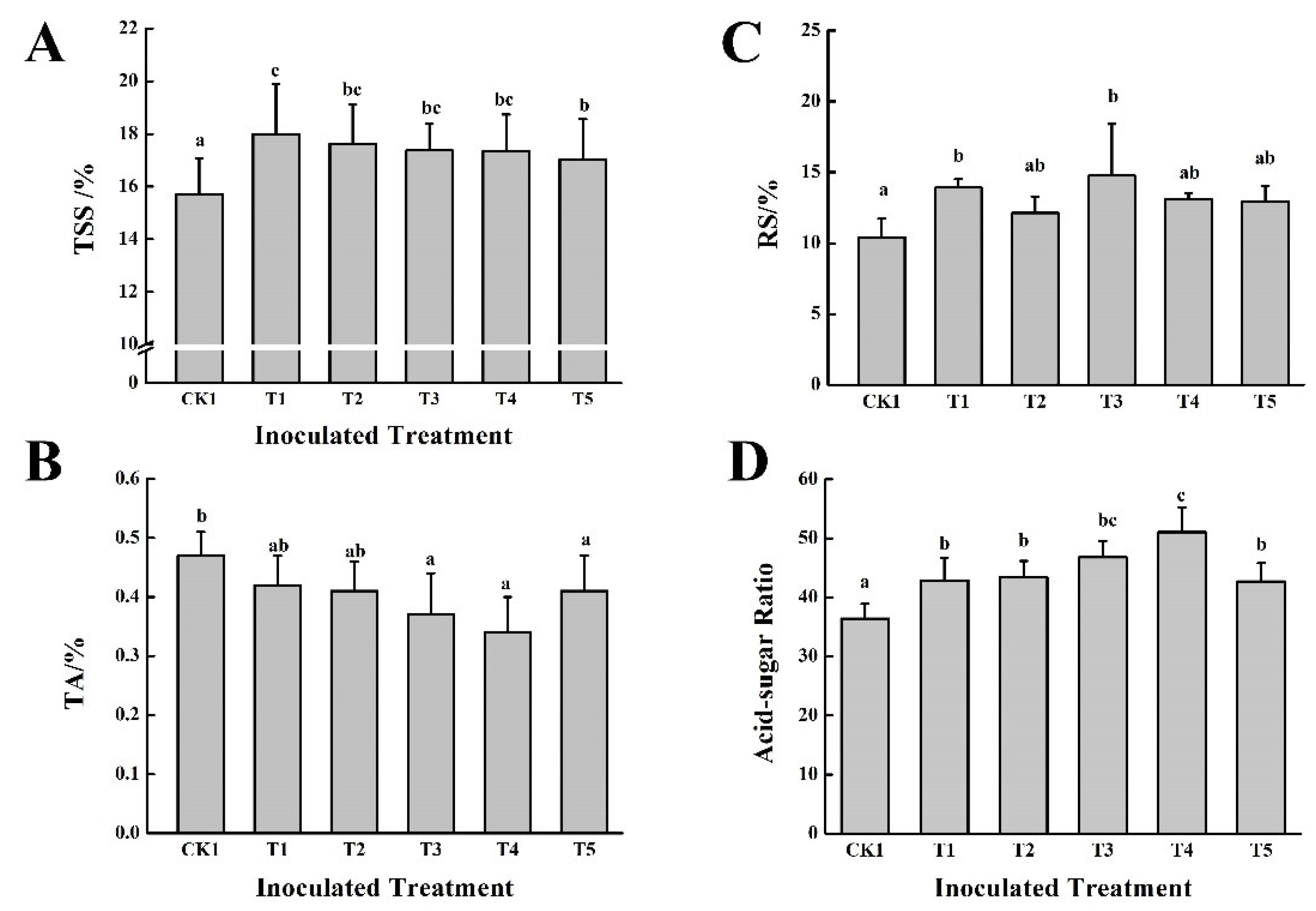
3.2. Effect of Five PGPB in Combination with Two Different Organic Fertilizers on Grape Growth and Fruit Quality in the First Year
3.3. Effect of the Five PGPB in Combination with Cow Dung Organic Fertilizers on Grape Growth and Fruit Quality over Two Consecutive Years
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, K.; Xing, H.; Guo, Y.; Zhao, F.; Liu, Z.; Li, K.; Li, Y.; Guo, X. High-density genetic linkage map construction and cane cold hardiness QTL mapping for Vitis based on restriction site-associated DNA sequencing. BMC Genom. 2020, 21, 419. [Google Scholar] [CrossRef]
- Qian, X.; Sun, L.; Xu, X.Q.; Zhu, B.Q.; Xu, H.Y. Differential expression of VvLOXA Diversifies C6 volatile profiles in some Vitis vinifera table grape cultivars. Int. J. Mol. Sci. 2017, 18, E2705. [Google Scholar] [CrossRef]
- Farhan, M.; Butt, Z.A.; Khan, A.U.; Wahid, A.; Ahmad, M.; Kanwal, A. Restoration of pesticide contaminated agriculture soil through bio-augmentation and its kinetics. Pak. J. Agric. Sci. 2017, 54, 547–551. [Google Scholar]
- Strafella, S.; Simpson, D.J.; Khanghahi, M.Y.; De Angelis, M.; Ganzle, M.; Minervini, F.; Crecchio, C. Comparative genomics and in vitro plant growth promotion and biocontrol traits of lactic acid bacteria from the wheat rhizosphere. Microoganisms 2021, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, X.; Yang, Q.; Chi, X.; Pan, L.; Chen, N.; Study, L.; Wang, T.; Wang, M.; Yu, S. Soil eukaryotic microorganism succession as affected by continuous cropping of peanut—Pathogenic and beneficial fungi were selected. PLoS ONE 2012, 7, e40659. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Chen, B.; Li, X. Relationship between soil chemical properties and microbial metabolic patterns in intensive greenhouse tomato production systems. Arch. Agron. Soil Sci. 2020, 66, 1334–1343. [Google Scholar] [CrossRef]
- Liu, X.P.; Bi, Q.F.; Qiu, L.L.; Li, K.J.; Yang, X.R.; Lin, X.Y. Increased risk of phosphorus and metal leaching from paddy soils after excessive manure application: Insights from a mesocosm study. Sci. Total Environ. 2019, 666, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chu, C.; Zhou, D.; Sha, Z.; Wu, S. Soil nutrient status and the relation with planting area, planting age and grape varieties in urban vineyards in Shanghai. Heliyon 2019, 5, e02362. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; Holguin, G. Proposal for the division of plant growth-promoting rhizobacteria into two classifications: Biocontrol-PGPB (plant growth-promoting bacteria) and PGPB. Soil Biol. Biochem. 1998, 30, 1225–1228. [Google Scholar] [CrossRef]
- Ahmad, Z.; Wu, J.; Chen, L.; Dong, W. Isolated Bacillus subtilis strain 330-2 and its antagonistic genes identified by the removing PCR. Sci. Rep. 2017, 7, 1777. [Google Scholar] [CrossRef]
- Marques, A.; Pires, C.; Moreira, H.; Rangel, A.; Castro, P. Assessment of the plant growth promotion abilities of six bacterial isolates using Zea mays as indicator plant. Soil Biol. Biochem. 2010, 42, 1229–1235. [Google Scholar] [CrossRef]
- Pankievicz, V.; Plucani do Amaral, F.; Ane, J.M.; Stacey, G. Diazotrophic bacteria and their mechanisms to interact and benefit cereals. Mol. Plant Microbe Interact. 2021, 34, 491–498. [Google Scholar] [CrossRef]
- Matos, A.D.M.; Gomes, I.C.P.; Nietsche, S.; Xavier, A.A.; Gomes, W.S.; Neto, J.A.D.; Pereira, M.C.T. Phosphate solubilization by endophytic bacteria isolated from banana trees. Anais Acad. Bras. Cienc. 2017, 89, 2945–2954. [Google Scholar] [CrossRef] [PubMed]
- Hollensteiner, J.; Wemheuer, F.; Harting, R.; Kolarzyk, A.M.; Valeria, S.M.D.; Poehlein, A.; Brzuszkiewicz, E.B.; Nesemann, K.; Braus-Stromeyer, S.A.; Braus, G.H.; et al. Bacillus thuringiensis and Bacillus weihenstephanensis inhibit the growth of phytopathogenic Verticillium Species. Front. Microbiol. 2017, 7, 2171. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, X.; Cheng, B.S.; Ma, X.L.; Lyu, X.T.; Zhao, X.F.; Ju, Y.L.; Min, Z.; Fang, Y.L. Selection and evaluation of phosphate-solubilizing bacteria from grapevine rhizospheres for use as biofertilizers. Span. J. Agric. Res. 2017, 14, e1106. [Google Scholar] [CrossRef]
- Rolli, E.; Marasco, R.; Saderi, S.; Corretto, E.; Mapelli, F.; Cherif, A.; Borin, S.; Valenti, L.; Sorlini, C.; Daffonchio, D. Root-associated bacteria promote grapevine growth: From the laboratory to the field. Plant Soil 2017, 410, 369–382. [Google Scholar] [CrossRef]
- Kaur, J.; Pandove, G. Understanding the beneficial interaction of plant growth promoting rhizobacteria and endophytic bacteria for sustainable agriculture: A bio-revolution approach. J. Plant Nutr. 2023, 46, 3569–3597. [Google Scholar] [CrossRef]
- Shi, L.N.; Lu, L.X.; Ye, J.R.; Shi, H.M. The endophytic strain ZS-3 enhances salt tolerance in Arabidopsis thaliana by regulating photosynthesis, osmotic stress, and ion homeostasis and inducing systemic tolerance. Front. Plant Sci. 2022, 13, 820837. [Google Scholar] [CrossRef] [PubMed]
- Li, P.S.; Kong, W.; Wu, X.Q. Salt tolerance mechanism of the Rhizosphere Bacterium JZ-GX1 and its effects on tomato seed germination and seedling growth. Front. Microbiol. 2021, 12, 1342. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, J.; Qingqing, K. Potassium-solubilizing activity of Bacillus aryabhattai S. K. its growth-promoting effect on Populus alba, L. Forests 2020, 11, 1348. [Google Scholar] [CrossRef]
- de Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.S.; Weber, R.P. Colorimetric estimation of indole acetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef]
- Alexander, D.B.; Zuberer, D.A. Use of Chrome Azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol. Fertil. Soils 1991, 12, 39–45. [Google Scholar] [CrossRef]
- Jiang, Y.; Tian, L.; Chen, C.-Q.; Zhang, G.-J.; Li, T.; Chen, J.-X.; Wang, X. Screening and identification of indoleacetic acid producing endophytic bacterium in Panax ginseng. Zhongguo Zhong Yao Za Zhi 2015, 40, 213–217. [Google Scholar]
- Romanazzi, G.; Mancini, V.; Feliziani, E.; Servili, A.; Endeshaw, S.; Neri, D. Impact of alternative fungicides on grape downy mildew control and vine growth and development. Plant Dis. 2016, 100, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; He, F.; Jin, W.; Huang, X.; Chen, M.; Zhang, X.; Gao, H.; Xu, C.; Ye, H.; Jia, H. Eeffect of tree shapes on growth, fruit quality and economic benefit of grape in greenhouse in Taizhou of Zhejiang. South China Fruits 2022, 51, 125–129. [Google Scholar]
- Baskan, K.S.; Tutem, E.; Akyuz, E.; Ozen, S.; Apak, R. Spectrophotometric total reducing sugars assay based on cupric reduction. Talanta 2016, 147, 162–168. [Google Scholar] [CrossRef]
- Cocco, A.; Mercenaro, L.; Muscas, E.; Mura, A.; Nieddu, G.; Lentini, A. Multiple effects of nitrogen fertilization on grape vegetative growth, berry quality and pest development in mediterranean vineyards. Horticulturae 2021, 7, 530. [Google Scholar] [CrossRef]
- Karabaghli, C.; Frey-Klett, P.; Sotta, B.; Bonnet, M.; Le Tacon, F. In vitro effects of Laccaria bicolor S238 N and Pseudomonas fluorescens strain BBc6 on rooting of de-rooted shoot hypocotyls of Norway spruce. Tree Physiol. 1998, 18, 103–111. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Maiti, T.K.; Pramanik, K.; Ghosh, S.K.; Mitra, S.; De, T.K. The role of arsenic resistant Bacillus aryabhattai MCC3374 in promotion of rice seedlings growth and alleviation of arsenic phytotoxicity. Chemosphere 2018, 213, 611–612. [Google Scholar] [CrossRef]
- Li, G.E.; Kong, W.L.; Wu, X.Q.; Ma, S.B. Phytase-producing Rahnella aquatilis JZ-GX1 promotes seed germination and growth in corn (Zea mays L.). Microorganisms 2021, 9, 1647. [Google Scholar] [CrossRef] [PubMed]
- Kour, D.; Rana, K.L.; Kaur, T.; Yadav, N.; Yadav, A.N.; Kumar, M.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Biodiversity, current developments and potential biotechnological applications of phosphorus-solubilizing and mobilizing microbes: A review. Pedosphere 2021, 31, 43–75. [Google Scholar] [CrossRef]
- Torres, M.; Llamas, I.; Torres, B.; Toral, L.; Sampedro, I.; Bejar, V. Growth promotion on horticultural crops and antifungal activity of Bacillus velezensis XT1. Appl. Soil Ecol. 2020, 150, 103453. [Google Scholar] [CrossRef]
- Xiang, W.L.; Liang, H.Z.; Liu, S.; Luo, F.; Tang, J.; Li, M.Y.; Che, Z.M. Isolation and performance evaluation of halotolerant phosphate solubilizing bacteria from the rhizospheric soils of historic Dagong Brine Well in China. World J. Microbiol. Biotechnol. 2011, 27, 2629–2637. [Google Scholar] [CrossRef]
- Dinis, M.D.; Fiuza, A.; Futuro, A.; Leite, A.; Martins, D.; Figueiredo, J.; Gois, J.; Vila, M.C. Characterization of a mine legacy site: An approach for environmental management and metals recovery. Environ. Sci. Pollut. Res. 2020, 27, 10103–10114. [Google Scholar] [CrossRef]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects—A review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Li, W.; Zhang, F.; Cui, G.; Wang, Y.; Yang, J.; Cheng, H.; Liu, H.; Zhang, L. Effects of bio-organic fertilizer on soil fertility, microbial community composition, and potato growth. ScienceAsia 2021, 47, 347. [Google Scholar] [CrossRef]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, U.; Chakraborty, B.; Basnet, M. Plant growth promotion and induction of resistance in Camellia sinensis by Bacillus megaterium. J. Basic Microbiol. 2006, 46, 186–195. [Google Scholar] [CrossRef]
- Kong, W.-L.; Wu, X.-Q.; Zhao, Y.-J. Effects of Rahnella aquatilis JZ-GX1 on treat chlorosis induced by iron feficiency in Cinnamomum camphora. J. Plant Growth Regul. 2020, 39, 877–887. [Google Scholar] [CrossRef]
- Schreiner, R.P. Nutrient uptake and distribution in young Pinot noir grapevines over two seasons. Am. J. Enol. Vitic. 2016, 67, 436–448. [Google Scholar] [CrossRef]
- Yongabi, K.A.; Harris, P.L.; Lewis, D.M. Poultry faeces management with a simple low cost plastic digester. Afr. J. Biotechnol. 2009, 8, 1560–1566. [Google Scholar]
- Wei, M.; Liu, X.; He, Y.; Xu, X.; Wu, Z.; Yu, K.; Zheng, X. Biochar inoculated with Pseudomonas putida improves grape (Vitis vinifera L.) fruit quality and alters bacterial diversity. Rhizosphere 2020, 16, 100261. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant growth-promoting soil bacteria: Nitrogen fixation, phosphate solubilization, siderophore production, and other biological activities. Plants 2023, 12, 4074. [Google Scholar] [CrossRef]
- Nag, P.; Shriti, S.; Das, S. Microbiological strategies for enhancing biological nitrogen fixation in non-legumes. J. Appl. Microbiol. 2019, 129, 186–198. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moenne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dye, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Leahy, J.G.; Colwell, R.R. Microbial degradation of hydrocarbons in the environment. Microbiol. Rev. 1990, 54, 305–315. [Google Scholar] [CrossRef]
- Ramadan, M.A. Inoculum size as a factor limiting success of inoculation for biodegradation. Appl. Environ. Microbiol. 1990, 56, 1392–1396. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Kloepper, J.W. Plant-microbes interactions in enhanced fertilizer-use efficiency. Appl. Microbiol. Biotechnol. 2009, 85, 1–12. [Google Scholar] [CrossRef]
- Mabood, F.; Zhou, X.M.; Smith, D.L. Microbial signaling and plant growth promotion. Can. J. Plant Sci. 2014, 94, 1051–1063. [Google Scholar] [CrossRef]
- Qian, T.; Ye, J.R. The mechanism of dissolving inorganic phosphorus by Bacillus megaterium ZS-3 and its growth promotion of Cinnamomum camphora. Biotechnol. Bull. 2020, 36, 45–52. [Google Scholar]
- Shi, H.M.; Lu, L.X.; Ye, J.R.; Shi, L.N. Effects of two Bacillus velezensis microbial inoculants on the growth and rhizosphere soil environment of Prunus davidiana. Int. J. Mol. Sci. 2022, 23, 13639. [Google Scholar] [CrossRef]
- Asghar, I.; Ahmed, M.; Farooq, M.A.; Ishtiaq, M.; Arshad, M.; Akram, M.; Umair, A.; Alrefaei, A.F.; Jat, B.M.Y.; Naeem, A. Characterizing indigenous plant growth promoting bacteria and their synergistic effects with organic and chemical fertilizers on wheat (Triticum aestivum). Front. Plant Sci. 2023, 14, 1232271. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Clement, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Ji, S.H.; Kim, J.S.; Lee, C.H.; Seo, H.S.; Chun, S.C.; Oh, J.; Choi, E.H.; Park, G. Enhancement of vitality and activity of a plant growth-promoting bacteria (PGPB) by atmospheric pressure non-thermal plasma. Sci. Rep. 2019, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.H.; Yan, B.H.; Awasthi, M.K.; Zhang, J.C.; Huang, H.L.; Zhang, L.H.; Luo, L. Accelerated humification and alteration of microbial communities by distillers’ grains addition during rice straw composting. Bioresour. Technol. 2021, 342, 125937. [Google Scholar] [CrossRef]
- da Silva, M.S.R.d.A.; dos Santos, B.d.M.S.; da Silva, C.S.R.d.A.; da Silva, C.S.R.d.A.; Antunes, L.F.d.S.; dos Santos, R.M.; Santos, C.H.B.; Rigobelo, E.C. Humic substances in combination with plant growth-promoting bacteria as an alternative for sustainable agriculture. Front. Microbiol. 2021, 12, 719653. [Google Scholar] [CrossRef]
- Gong, Z.; Gao, X.; Huang, Y.; Li, R.; Wang, D.; Shen, Q. Research on continuous application of bio-organic fertilizer for improving greenhouse cucumber yield and quality. J. Nanjing Agric. Univ. 2016, 39, 777–783. [Google Scholar]
- Muhammad, S.; Sanden, B.L.; Lampinen, B.D.; Smart, D.R.; Saa, S.; Shackel, K.A.; Brown, P.H. Nutrient storage in the perennial organs of deciduous trees and remobilization in spring—A study in almond (Prunus dulcis) (Mill.) D. A. Webb. Front. Plant Sci. 2020, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Lori, M.; Symnaczik, S.; Mader, P.; De Deyn, G.; Gattinger, A. Organic farming enhances soil microbial abundance and activity-A meta-analysis and meta-regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.Z.; Chang, S.X.; Shen, Y.Y.; Chen, G.; Liu, Y.; Yao, B.; Xue, J.M.; Li, Y. Grass cover increases soil microbial abundance and diversity and extracellular enzyme activities in orchards: A synthesis across China. Appl. Soil Ecol. 2023, 182, 104720. [Google Scholar] [CrossRef]


| 2019 Spring | 2019 Autumn | 2020 Spring | 2020 Autumn | |
|---|---|---|---|---|
| YH-18 | 1.8 × 109 CFU/mL | 1.6 × 109 CFU/mL | 1.4 × 109 CFU/mL | 1.9 × 109 CFU/mL |
| YH-20 | 1.5 × 109 CFU/mL | 1.4 × 109 CFU/mL | 1.9 × 109 CFU/mL | 1.5 × 109 CFU/mL |
| ZS-3 | 1.3 × 109 CFU/mL | 1.3 × 109 CFU/mL | 1.7 × 109 CFU/mL | 1.6 × 109 CFU/mL |
| SK1-7 | 1.3 × 109 CFU/mL | 1.6 × 109 CFU/mL | 1.2 × 109 CFU/mL | 1.7 × 109 CFU/mL |
| JZ-GX1 | 1.6 × 109 CFU/mL | 1.8 × 109 CFU/mL | 1.3 × 109 CFU/mL | 1.5 × 109 CFU/mL |
| First Year | Second Year | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Microbial Inoculant Type | Fertilization in Autumn of 2019/hm2 | Inoculation in Spring of 2020/hm2 | Fertilization in Autumn of 2020/hm2 | Inoculation in Spring of 2021/hm2 | |||
| Cow Dung Organic Fertilizer/ton | Distillers’ Grain Organic Fertilizer | Microbial Inoculation/L | Microbial Inoculation/L | Cow Dung Organic Fertilizer/ton | Microbial Inoculation/L | Microbial Inoculation/L | ||
| CK1 | / | 15 | / | 0 | 0 | 15 | 0 | 0 |
| T1 | YH-18 | 15 | / | 150 | 150 | 15 | 150 | 150 |
| T2 | YH-20 | 15 | / | 150 | 150 | 15 | 150 | 150 |
| T3 | ZS-3 | 15 | / | 150 | 150 | 15 | 150 | 150 |
| T4 | SK1-7 | 15 | / | 150 | 150 | 15 | 150 | 150 |
| T5 | JZ-GX1 | 15 | / | 150 | 150 | 15 | 150 | 150 |
| CK2 | / | / | 15 | 0 | 0 | / | / | / |
| D1 | YH-18 | / | 15 | 150 | 150 | / | / | / |
| D2 | YH-20 | / | 15 | 150 | 150 | / | / | / |
| D3 | ZS-3 | / | 15 | 150 | 150 | / | / | / |
| D4 | SK1-7 | / | 15 | 150 | 150 | / | / | |
| D5 | JZ-GX1 | / | 15 | 150 | 150 | / | / | / |
| Salt Tolerance | Alkali Resistance | Nitrogen-Fixing Ability | |
|---|---|---|---|
| YH-18 | 9% | 10 | + |
| YH-20 | 9% | 9 | + |
| ZS-3 | 9% | 11 | + |
| SK1-7 | 6% | 12 | + |
| JZ-GX1 | 6% | 11 | + |
| Treatment | SPAD Value | Leaf Area | Leaf Thickness |
|---|---|---|---|
| CK1 | 37.37 ± 3.31 a | 540.31 ± 97.25 a | 1.12 ± 0.21 a |
| T1 | 36.23 ± 3.24 a | 623.17 ± 98.17 b | 1.30 ± 0.22 a |
| T2 | 37.52 ± 5.26 a | 648.93 ± 110.62 b | 1.14 ± 0.25 a |
| T3 | 37.97 ± 3.51 ab | 610.04 ± 82.57 b | 1.17 ± 0.15 a |
| T4 | 37.53 ± 4.05 a | 627.73 ± 87.57 b | 1.31 ± 0.20 a |
| T5 | 38.18 ± 3.3 ab | 605.64 ± 98.74 b | 1.19 ± 0.19 a |
| CK2 | 40.67 ± 4.85 b B | 595.76 ± 77.61 b A | 1.11 ± 0.21 a A |
| D1 | 37.78 ± 2.94 A | 608.96 ± 77.47 A | 1.24 ± 0.27 A |
| D2 | 35.86 ± 3.81 A | 615.96 ± 92.97 A | 1.21 ± 0.20 A |
| D3 | 38.48 ± 3.79 AB | 603.81 ± 76.43 A | 1.22 ± 0.20 A |
| D4 | 37.38 ± 3.78 A | 615.66 ± 77.99 A | 1.24 ± 0.34 A |
| D5 | 38.33 ± 4.04 AB | 580.81 ± 76.63 A | 1.18 ± 0.16 A |
| Treatment | Cluster Biomass/g | The Cross Diameter of Grape Cluster/cm | The Longitudinal Diameter of Grape Cluster/cm | Berry Biomass/g | TSS/% |
|---|---|---|---|---|---|
| CK1 | 439.18 ± 66.19 a | 9.82 ± 0.96 a | 17.67 ± 0.87 a | 9.64 ± 1.62 a | 15.62 ± 1.48 ab |
| T1 | 730.30 ± 81.39 c | 11.50 ± 0.29 bc | 19.31 ± 1.05 b | 12.51 ± 1.97 de | 16.00 ± 1.08 b |
| T2 | 715.90 ± 86.47 c | 11.88 ± 1.91 bc | 21.01 ± 1.64 c | 12.89 ± 0.99 e | 15.39 ± 1.25 ab |
| T3 | 762.66 ± 18.11 c | 11.70 ± 1.11 bc | 20.61 ± 0.96 bc | 11.43 ± 2.00 bc | 15.19 ± 0.97 a |
| T4 | 587.20 ± 67.70 b | 12.38 ± 1.07 c | 19.75 ± 1.84 bc | 11.85 ± 1.84 cd | 15.42 ± 0.99 ab |
| T5 | 665.10 ± 120.47 bc | 11.17 ± 0.87 bc | 20.00 ± 1.63 bc | 10.56 ± 1.34 b | 14.98 ± 0.79 a |
| CK2 | 611.40 ± 112.90 b A | 10.20 ± 0.75 ab A | 19.20 ± 1.28 b AB | 11.64 ± 1.90 cd AB | 15.48 ± 1.13 ab AB |
| D1 | 618.50 ± 93.90 A | 11.65 ± 1.06 B | 20.76 ± 1.55 AB | 12.09 ± 1.43 AB | 15.90 ± 1.40 BC |
| D2 | 630.22 ± 81.14 A | 12.20 ± 0.59 B | 20.95 ± 1.92 B | 11.72 ± 1.41 AB | 15.79 ± 0.97 BC |
| D3 | 601.78 ± 109.55 A | 11.85 ± 1.20 B | 19.00 ± 2.57 A | 11.47 ± 1.79 AB | 16.35 ± 0.75 C |
| D4 | 623.10 ± 92.02 A | 12.00 ± 1.08 B | 19.83 ± 1.17 AB | 12.01 ± 2.18 AB | 15.09 ± 1.12 A |
| D5 | 581.20 ± 141.90 A | 12.20 ± 1.18 B | 19.95 ± 2.15 AB | 12.46 ± 2.02 B | 15.59 ± 0.90 AB |
| Treatment | Shoot Internode Length/cm | Stem–Pith Ratio | Roundness of Primary Shoots/% | Shoot Internode Thickness/mm |
|---|---|---|---|---|
| CK1 | 9.12 ± 1.03 ab | 4.58 ± 0.36 a | 91.04 ± 4.73 a | 9.37 ± 3.74 a |
| T1 | 10.81 ± 1.57 c | 5.29 ± 0.37 cd | 92.61 ± 3.89 ab | 9.72 ± 3.41 ab |
| T2 | 9.82 ± 2.11 abc | 5.14 ± 0.35 bcd | 93.78 ± 5.23 ab | 9.75 ± 3.43 ab |
| T3 | 9.43 ± 1.43 ab | 5.42 ± 0.41 d | 93.79 ± 4.52 ab | 9.57 ± 2.97 ab |
| T4 | 9.98 ± 1.69 bc | 5.44 ± 0.25 d | 93.9 ± 4.10 ab | 10.01 ± 3.83 b |
| T5 | 9.62 ± 1.88 ab | 4.93 ± 0.38 bc | 94.61 ± 3.59 b | 9.66 ± 3.67 ab |
| CK2 | 9.06 ± 1.32 ab B | 4.65 ± 0.28 ab BC | 95.27 ± 3.28 b A | 9.46 ± 3.96 a A |
| D1 | 10.31 ± 1.60 C | 4.32 ± 0.24 AB | 95.28 ± 3.72 A | 9.42 ± 3.68 A |
| D2 | 9.61 ± 1.66 BC | 4.16 ± 0.25 A | 94.72 ± 4.10 A | 9.86 ± 4.16 AB |
| D3 | 9.26 ± 1.56 BC | 4.73 ± 0.31 BC | 94.01 ± 4.27 A | 9.50 ± 3.36 A |
| D4 | 9.80 ± 2.26 BC | 4.85 ± 0.35 C | 93.42 ± 5.38 A | 9.89 ± 4.13 AB |
| D5 | 8.76 ± 1.56 AB | 5.11 ± 0.41 C | 92.97 ± 7.21 A | 10.06 ± 3.83 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Zhu, X.; Lu, L.; Ye, J. Effect of Microbial Inoculants Endowed with Multifarious Plant Growth-Promoting Traits on Grape Growth and Fruit Quality under Organic Fertilization Scenarios. Agronomy 2024, 14, 491. https://doi.org/10.3390/agronomy14030491
Shi H, Zhu X, Lu L, Ye J. Effect of Microbial Inoculants Endowed with Multifarious Plant Growth-Promoting Traits on Grape Growth and Fruit Quality under Organic Fertilization Scenarios. Agronomy. 2024; 14(3):491. https://doi.org/10.3390/agronomy14030491
Chicago/Turabian StyleShi, Huimin, Xiaoxia Zhu, Lanxiang Lu, and Jianren Ye. 2024. "Effect of Microbial Inoculants Endowed with Multifarious Plant Growth-Promoting Traits on Grape Growth and Fruit Quality under Organic Fertilization Scenarios" Agronomy 14, no. 3: 491. https://doi.org/10.3390/agronomy14030491
APA StyleShi, H., Zhu, X., Lu, L., & Ye, J. (2024). Effect of Microbial Inoculants Endowed with Multifarious Plant Growth-Promoting Traits on Grape Growth and Fruit Quality under Organic Fertilization Scenarios. Agronomy, 14(3), 491. https://doi.org/10.3390/agronomy14030491






