Effect of Different Herbicides on Development and Productivity of Sweet White Lupine (Lupinus albus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Field Location, Plant Material, and Experiment Setting
2.2. Herbicide Treatments
2.3. Vegetation Index (VI) Datasets Collected by Manual Device and UAV
2.4. Visual Assessment of Damage Caused by Herbicides
2.5. Identification of Weed Species and Assessing the Number of Weeds per Square Meter
2.6. Harvesting and Seed-Cleaning Processes
2.7. Weather Datasets from the Deployed Meteorological Station
2.8. Rainfall and Temperature Datasets for the Months of the Growing Season
2.9. Statistical Analysis of the Datasets
3. Results
3.1. Effect of Herbicides on the Normalized Difference Vegetation Index (NDVI) Measured by GreenSeeker HCS-100 Manually
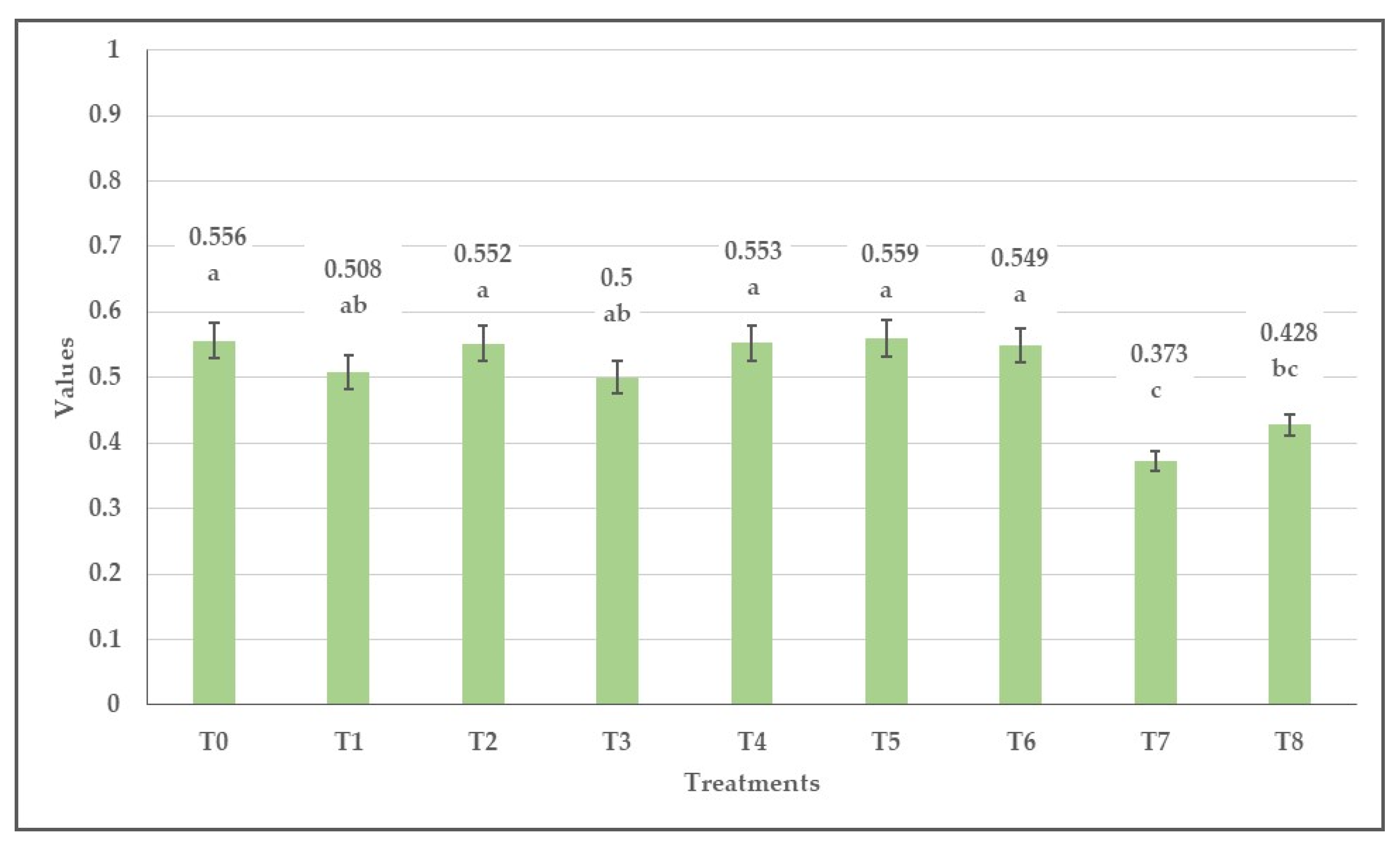
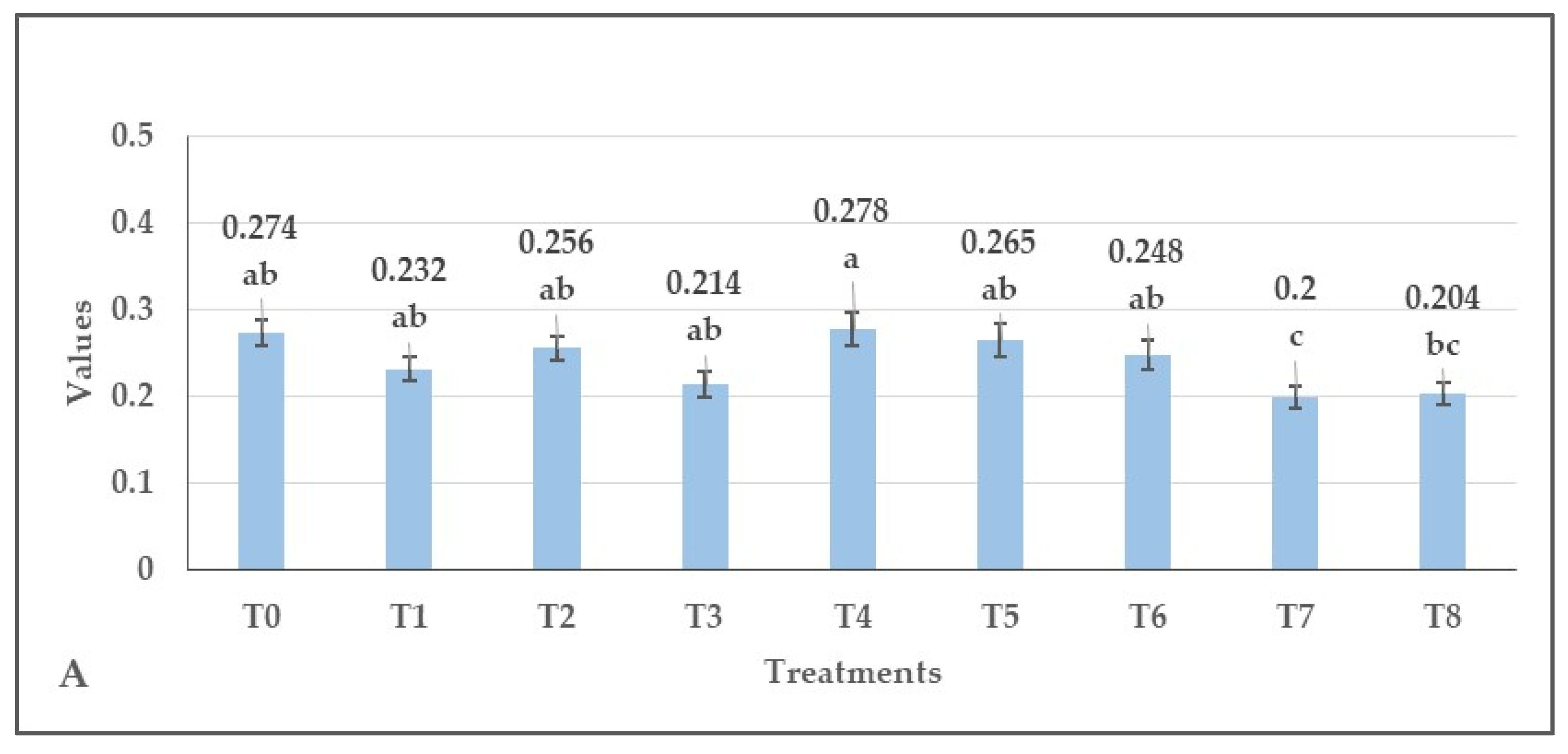
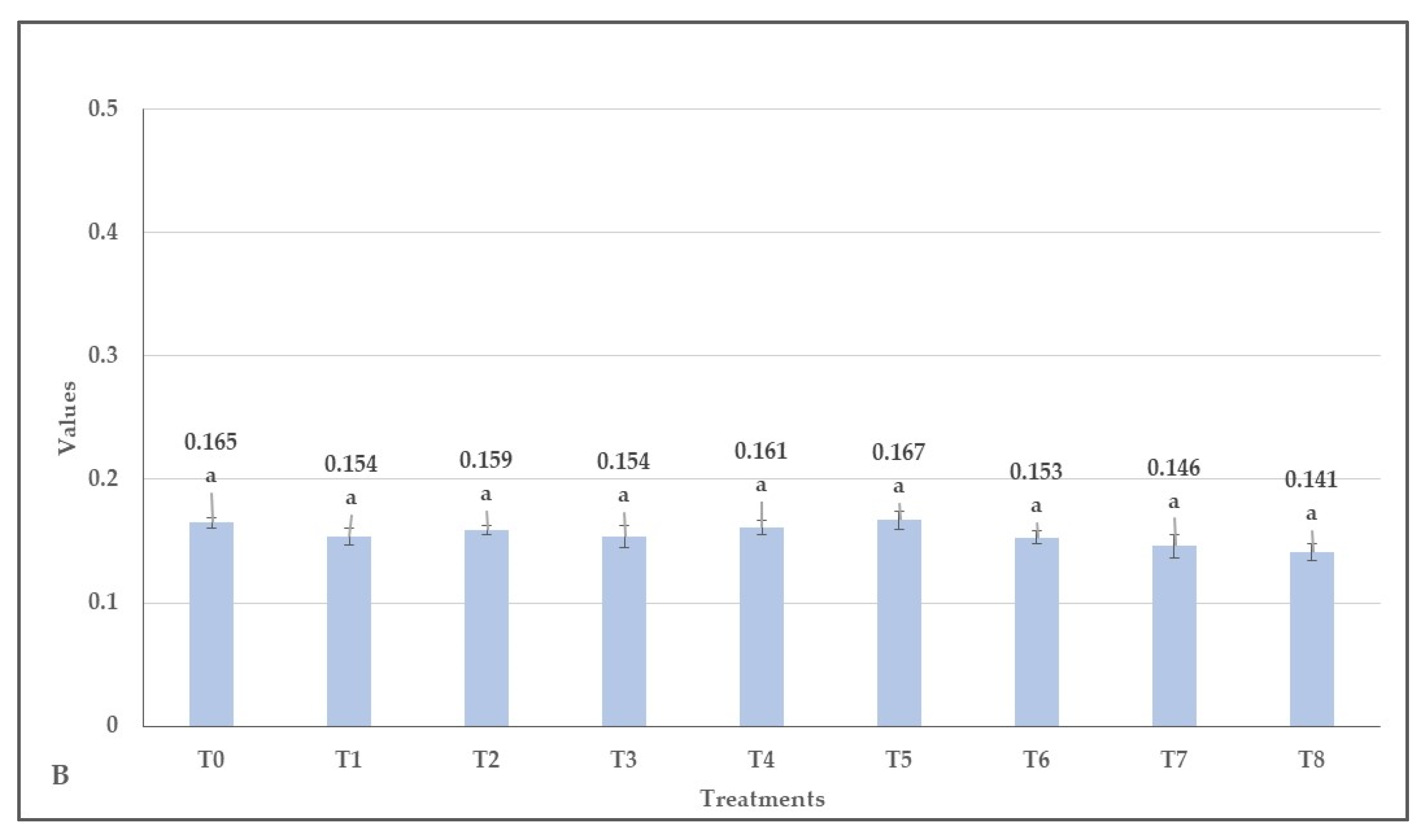
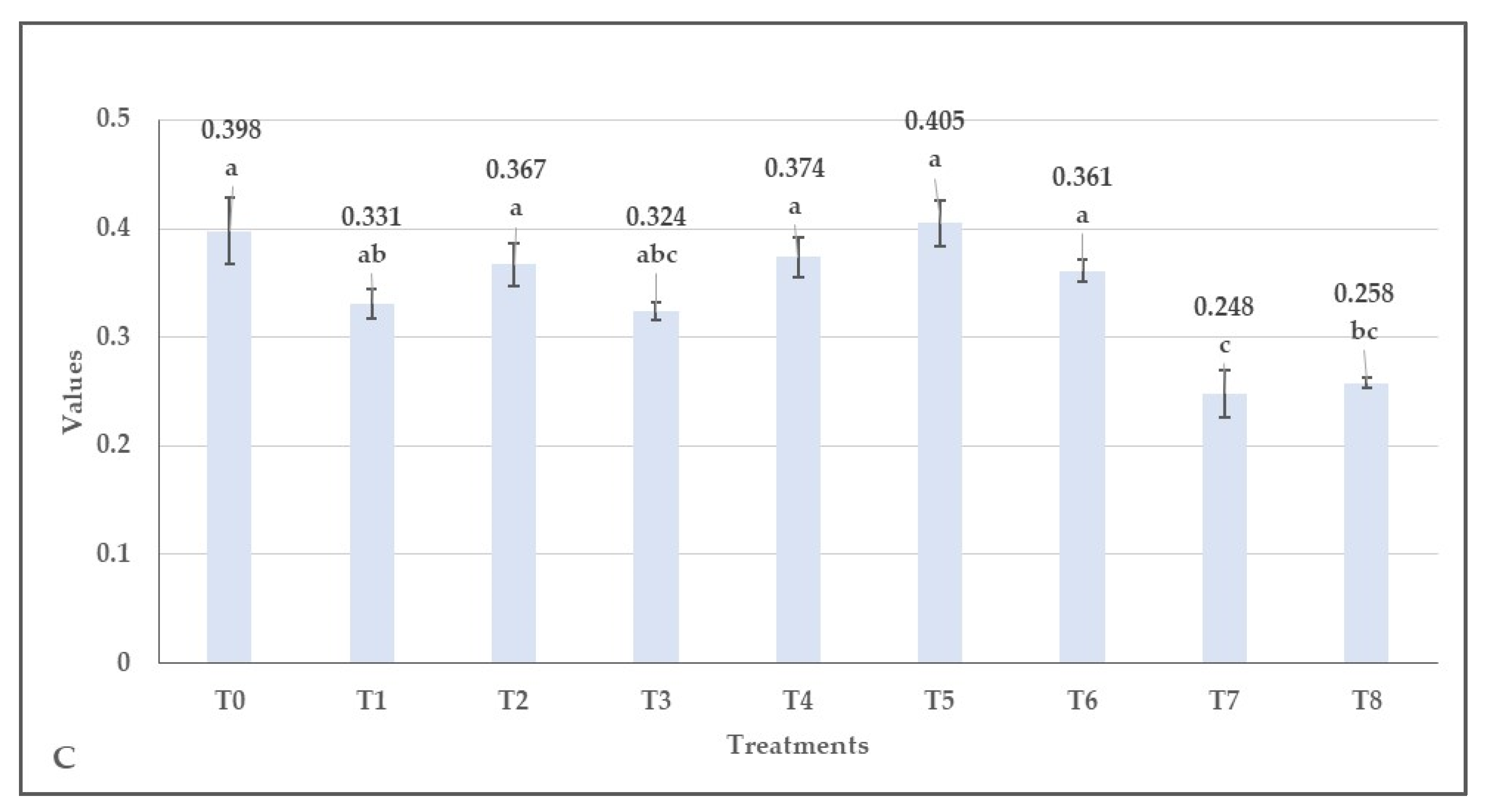
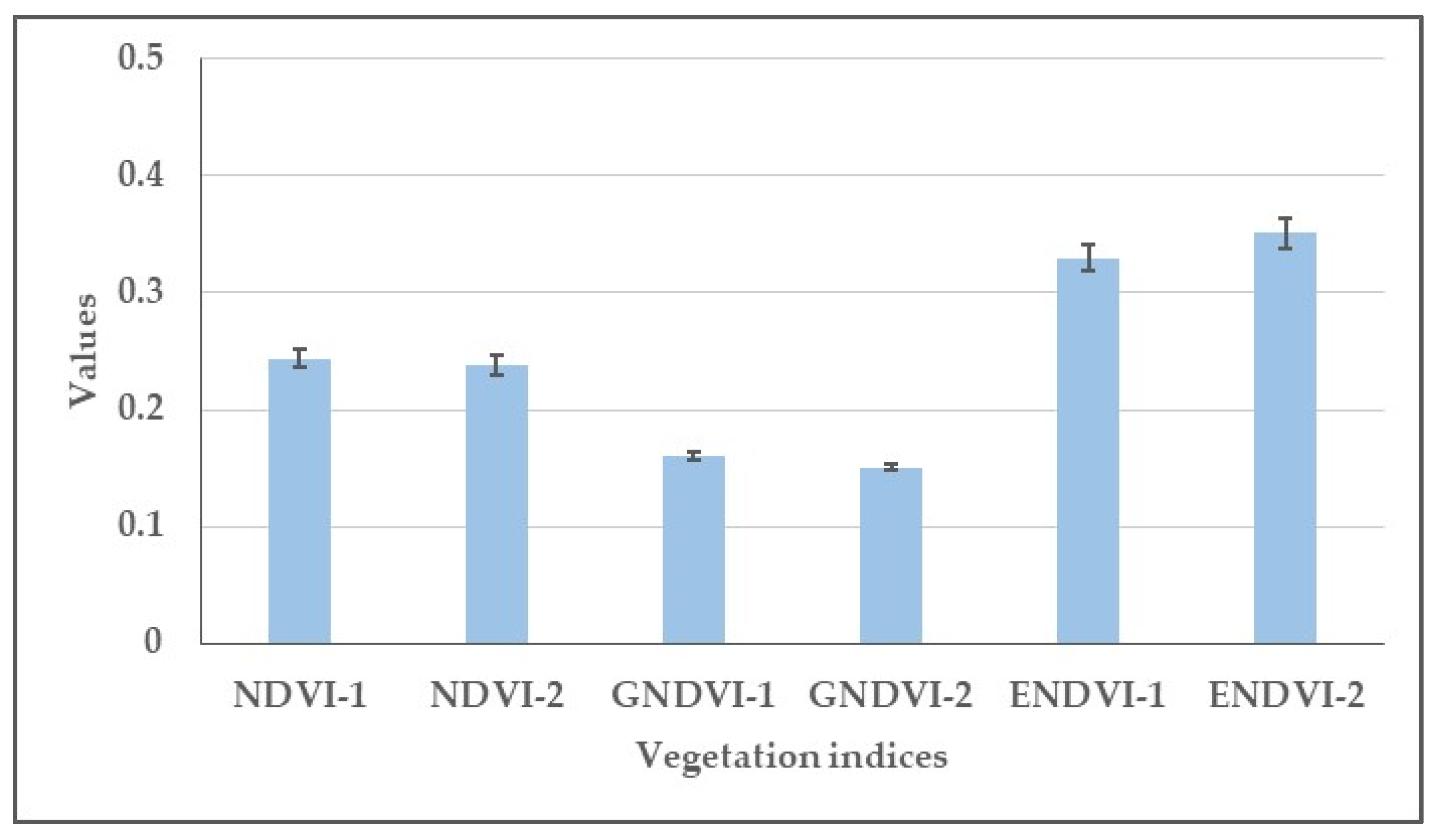
3.2. Effect of Herbicides on the Values of Normalized Difference Vegetation Index (NDVI), Green Normalized Difference Vegetation Index (GNDVI), Enhanced Normalized Difference Vegetation Index (ENDVI) Obtained from Aerially Recorded Datasets
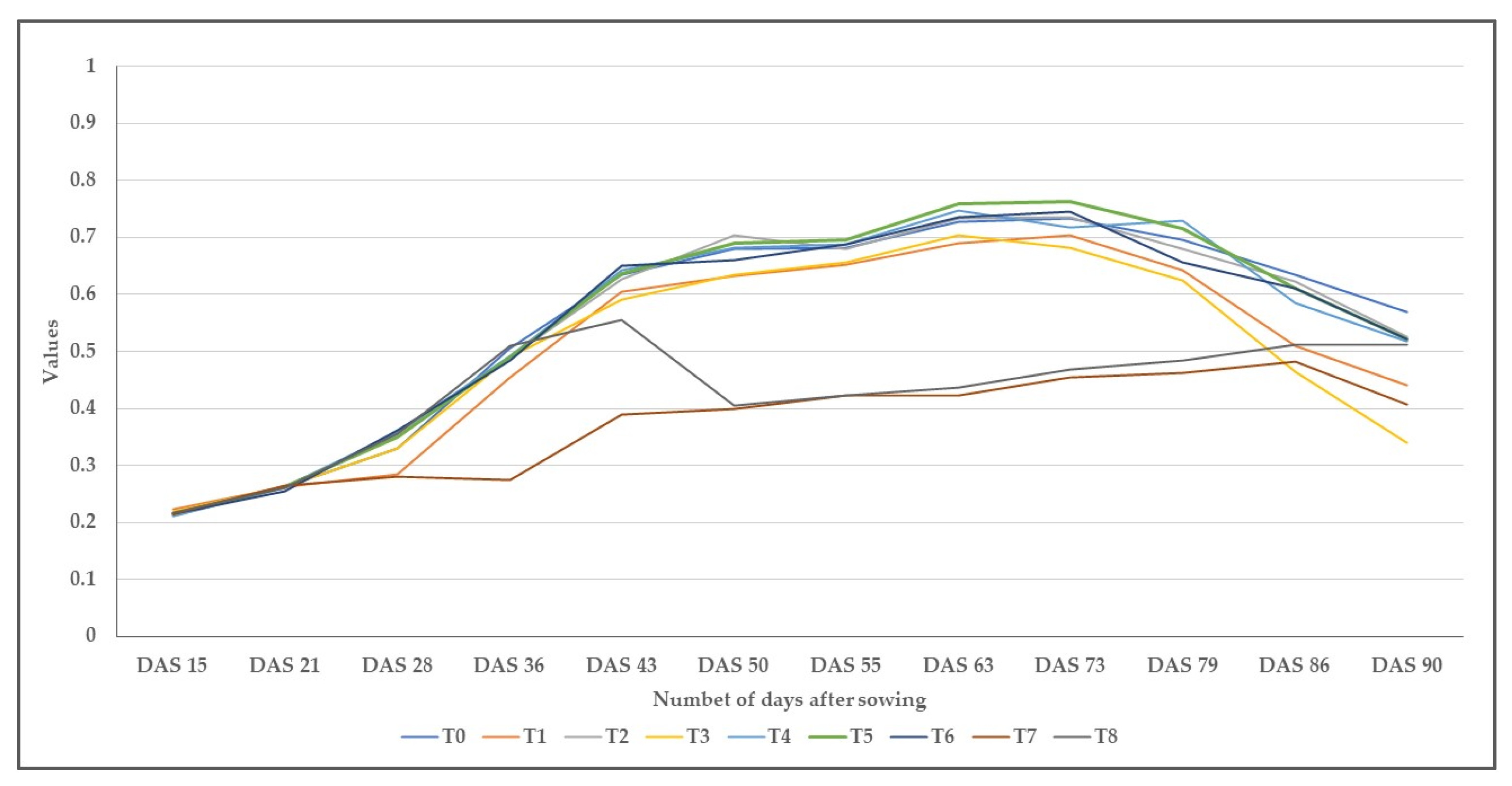
3.3. The Changes in the Normalized Difference Vegetation Indices over Time
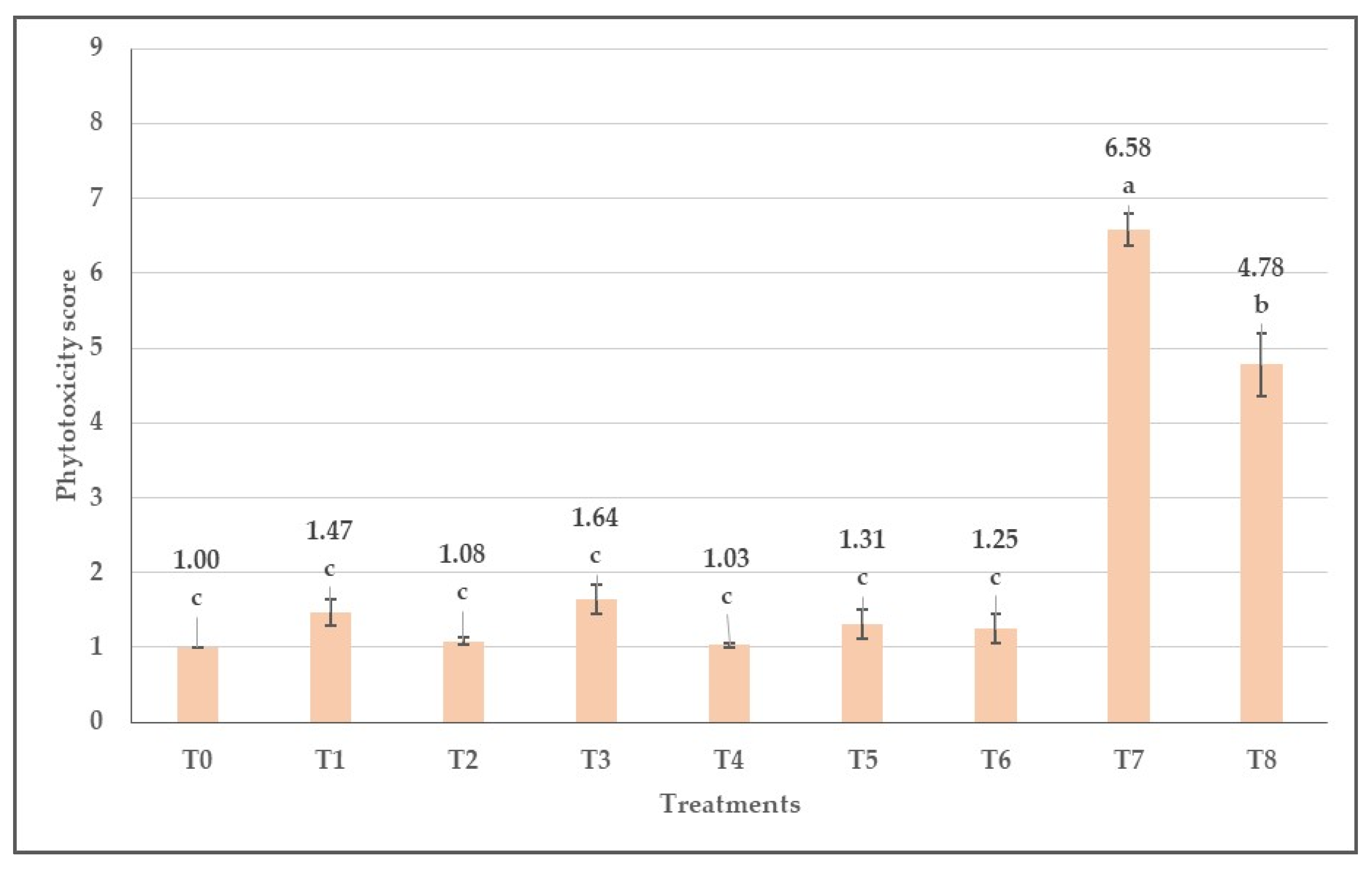
3.4. Phytotoxicity of Herbicides Evaluated Visually
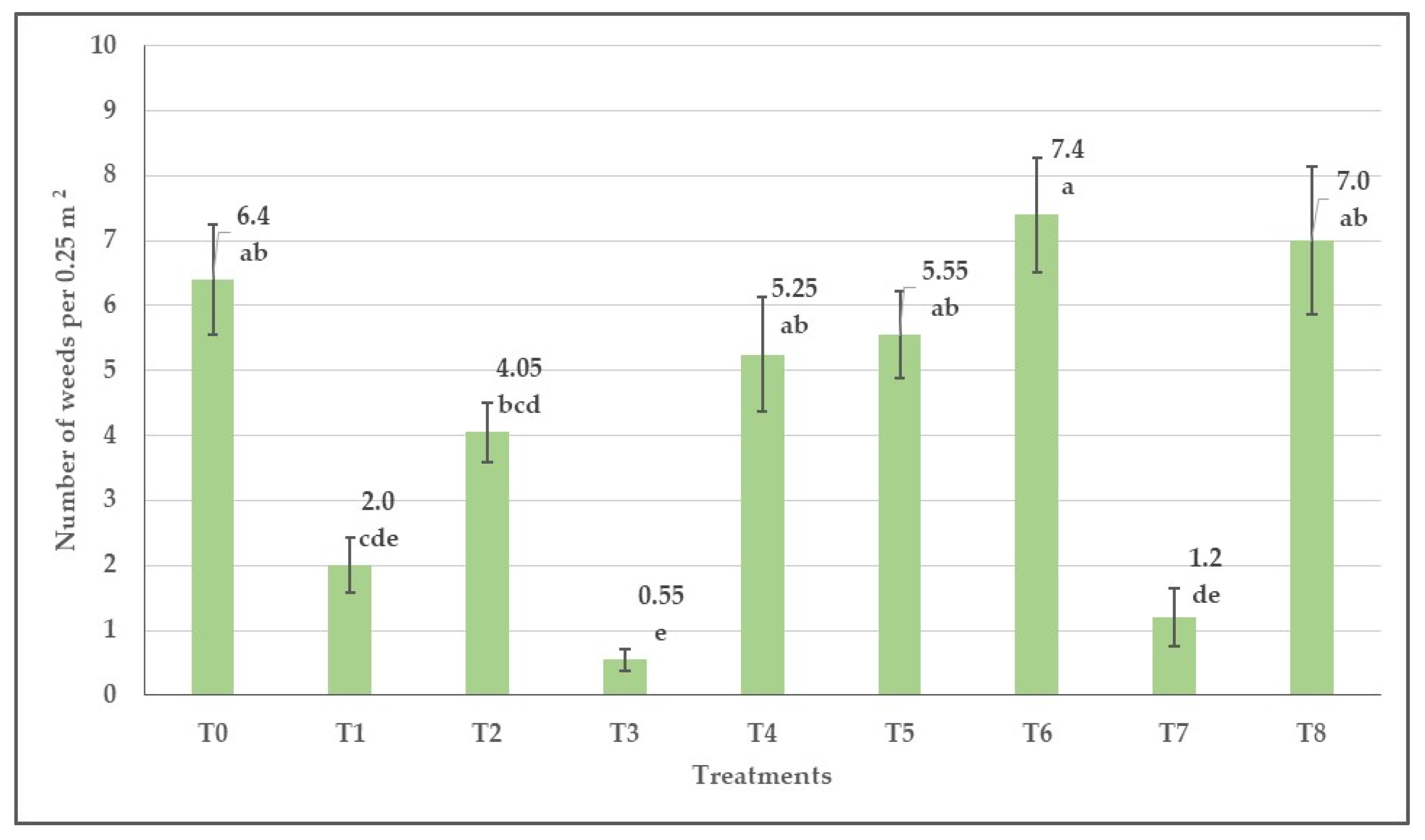
3.5. Herbicide Efficacy against Weeds
3.6. The Effect of Different Herbicides on the Yields of the White Lupine
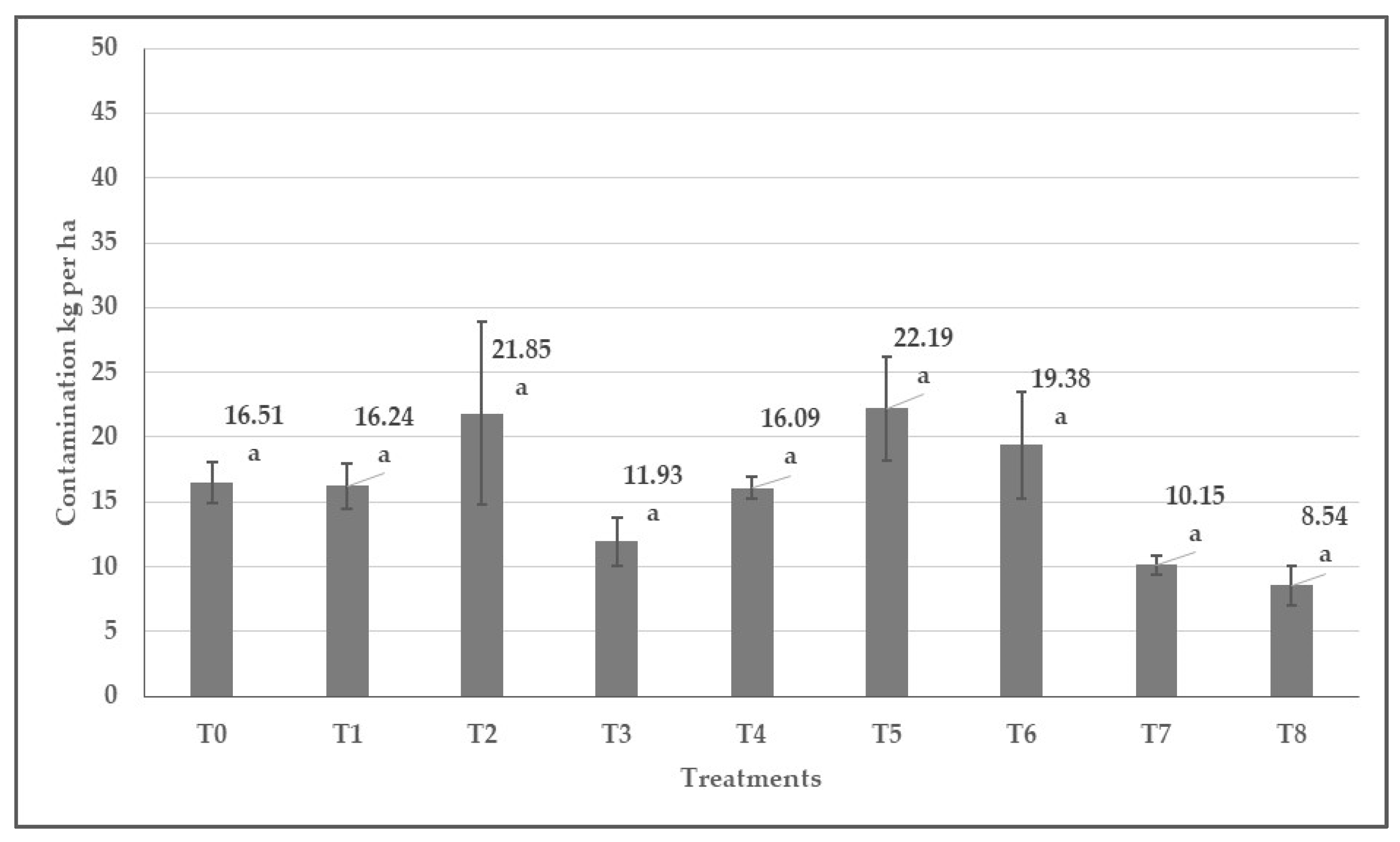
3.7. The Impact of Herbicide Treatments on the Quantity of Seed Yield Contamination
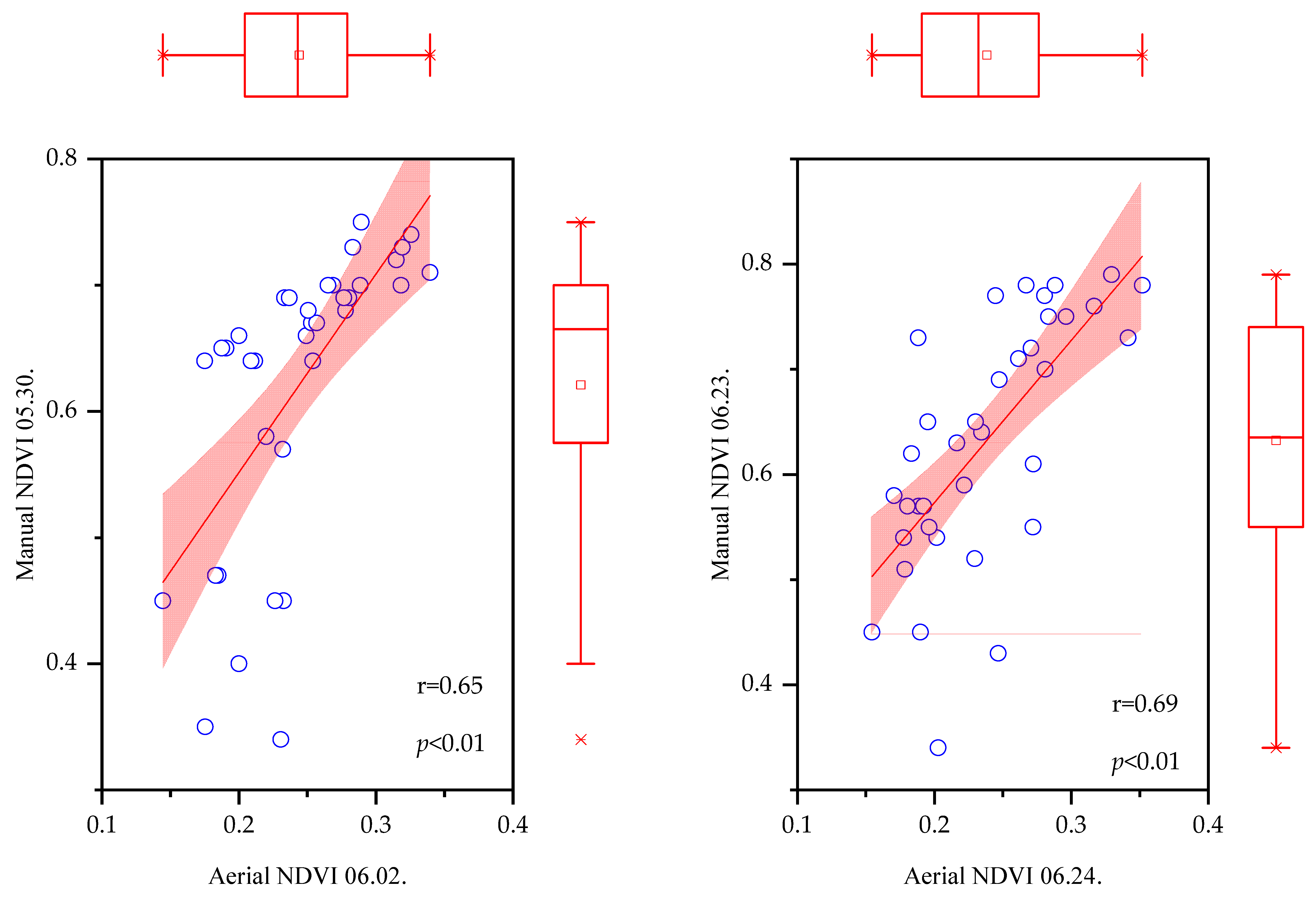
3.8. Correlation between NDVI Data Measured by Manual and Aerial Methods
3.9. Correlation Test Results at Two Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romeo, F.V.; Fabroni, S.; Ballistreri, G.; Muccilli, S.; Spina, A.; Rapisarda, P. Characterization and Antimicrobial Activity of Alkaloid Extracts from Seeds of Different Genotypes of Lupinus spp. Sustainability 2018, 10, 788. [Google Scholar] [CrossRef]
- Ishaq, A.R.; El-Nashar, H.A.S.; Younis, T.; Mangat, M.A.; Shahzadi, M.; Ul Haq, A.S.; El-Shazly, M. Genus Lupinus (Fabaceae): A review of ethnobotanical, phytochemical and biological studies. J. Pharm. Pharmacol. 2022, 74, 1700–1717. [Google Scholar] [CrossRef] [PubMed]
- Gabur, I.; Simioniuc, D.P. Pearl lupin (Lupinus mutabilis): A neglected high protein and oil content crop. In Neglected and Underutilized Crops; Academic Press: Cambridge, MA, USA, 2023; Chapter 16; pp. 413–436. [Google Scholar]
- Spina, A.; Saletti, R.; Fabroni, S.; Natalello, A.; Cunsolo, V.; Scarangella, M.; Rapisarda, P.; Canale, M.; Muccilli, V. Multielemental, Nutritional, and Proteomic Characterization of Different Lupinus spp. Genotypes: A Source of Nutrients for Dietary Use. Molecules 2022, 27, 8771. [Google Scholar] [CrossRef]
- Pereira, A.; Ramos, F.; Sanches Silva, A. Lupin (Lupinus albus L.) Seeds: Balancing the Good and the Bad and Addressing Future Challenges. Molecules 2022, 27, 8557. [Google Scholar] [CrossRef] [PubMed]
- Khedr, T.; Juhász, A.; Singh, K.B.; Foley, R.; Nye-Wood, M.G.; Colgrave, M.L. Development and validation of a rapid and sensitive LC-MS/MS approach for alkaloid testing in different Lupinus species. J. Food Compos. Anal. 2023, 121, 105391. [Google Scholar] [CrossRef]
- Ladha, J.K.; Peoples, M.B.; Reddy, P.M.; Biswas, J.C.; Bennett, A.; Jat, M.L.; Krupnik, T.J. Biological nitrogen fixation and prospects for ecological intensification in cereal-based cropping systems. Field Crops Res. 2022, 283, 108541. [Google Scholar] [CrossRef]
- Zapata-Hernandez, I.; Rodriguez-Macias, R.; Garcia-Lopez, P.M.; Salcedo-Perez, E.; Lara-Rivera, A.H.; Zamora-Natera, J.F. Dry matter yield and nitrogen content in Lupinus spp. (Leguminosae) with potential as a green manure. Legum. Res. 2019, 42, 523–527. [Google Scholar]
- Natera, J.F.Z.; Hernández, I.Z.; Jiménez, C.E.A.; Aguilar, F.B.M.; Galdámez, J.G. Assessing the biomass yield and nitrogen fixation of Lupinus angustifolius varieties as green manure in Jalisco, Mexico. Eurasian J. Soil Sci. 2023, 12, 320–327. [Google Scholar] [CrossRef]
- Eldin, S.M.S.; Shawky, E.; Ghareeb, D.A.; El Sohafy, S.M.; Sallam, S.M. Metabolomics and Chemometrics Depict the Changes in the Chemical Profile of White Lupine (Lupinus albus L.) Bioactive Metabolites during Seed Germination. Food Chem. 2023, 418, 135967. [Google Scholar] [CrossRef]
- FAO. (Food and Agriculture Organization of the United Nation). Crops and Livestock Products. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 27 October 2023).
- Zsombik, L. Alternatív fehérjenövények: Lehetőség vagy örök ígéret? Állattenyésztés Takarmányozás 2018, 67, 225–236. [Google Scholar]
- Lucas, M.M.; Stoddard, F.L.; Annicchiarico, P.; Frías, J.; Martínez-Villaluenga, C.; Sussmann, D.; Duranti, M.; Seger, A.; Zander, P.M.; Pueyo, J.J. The future of lupin as a protein crop in Europe. Front. Plant Sci. 2015, 6, 705. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Koutroubas, S.D. Weed Competition Effects on Growth and Yield of Spring-Sown White Lupine. Horticulturae 2022, 8, 430. [Google Scholar] [CrossRef]
- Abraham, E.M.; Ganopoulos, I.; Madesis, P.; Mavromatis, A.; Mylona, P.; Nianiou-Obeidat, I.; Parissi, Z.; Polidoros, A.; Tani, E.; Vlachostergios, D. The Use of Lupin as a Source of Protein in Animal Feeding: Genomic Tools and Breeding Approaches. Int. J. Mol. Sci. 2019, 20, 851. [Google Scholar] [CrossRef] [PubMed]
- Brebaum, S.; Boland, G.J. Sweet white lupin: A potential crop for Ontario. Can. J. Plant Sci. 1995, 75, 841–849. [Google Scholar] [CrossRef]
- Borbély, F.; Lenti, I.; Kövics, G.J. Csillagfürtfajok növényvédelme. Technológia. Növényvédelem 2008, 44, 279–296. [Google Scholar]
- Seiffert, M.; Makowski, N.; Moll, A.; Naumann, S.; Oehme, H.; Schulz, H.; Wicke, H.J.; Wirsing, F. Drusch- und Hackfruchtproduktion; Veb Deutscher Landwirtschaftsverlag: Berlin, Gennany, 1981; 399p. [Google Scholar]
- Stoddard, F.L. Grain Legumes: An Overview. In Legumes in Cropping Systems; CABI: London, UK, 2017. [Google Scholar]
- Folgart, A. Evaluation of Weed Management Practices in White Lupin (Lupinus albus L.). Master’s Thesis, Auburn University, Auburn, AL, USA, 2009. [Google Scholar]
- Toit, S.A. Efficacy and phytotoxicity of simazine and terbuthylazine on lupins. S. Afr. J. Plant Soil. 2003, 20, 188–192. [Google Scholar] [CrossRef][Green Version]
- Van Der Mey, A.M. Annual lupins reports. In Reports of the Research Activities of the Oil and Protein Seed Centre; Pakendorf, K.W., Ed.; Grain Crops Institute: Potchefstroom, South Africa, 1991. [Google Scholar]
- Newman, P. Herbicide tolerance of new lupin varieties. In Crop Updates; Grains Research & Development Corporation: Canberra Australia, 1999; pp. 44–46. [Google Scholar]
- Folgart, A.; Price, A.J.; Kelton, J.A.; van Santen, E.; Wehtje, G.R. Evaulation of Herbicide Efficacy, injury, and Yield in White Lupin (Lupinus albus L.). In Herbicides; Price, A., Kelton, J., Saruniate, L., Eds.; IntechOpen: London, UK, 2015; pp. 137–153. [Google Scholar] [CrossRef]
- Halász, A. A fehérvirágú édes csillagfürt gazdasági jelentősége, termesztésének problémái. Agrártudományi Közlemények 2003, 10, 1–4. [Google Scholar]
- Kádár, A. Chemical Weed Control and Crop Control, 6th ed.; Mezőgazda Kiadó: Budapest, Hungary, 2019; 440p. [Google Scholar]
- Kousta, A.; Katsis, C.; Tsekoura, A.; Chachalis, D. Effectiveness and Selectivity of Preand Post-Emergence Herbicides for Weed Control in Grain Legumes. Plants 2024, 13, 211. [Google Scholar] [CrossRef]
- Pacanoski, Z. Weed control in newly seeded alfalfa (Medicago sativa L.) with post-emergence herbicides. Herbologia 2011, 12, 55–64. [Google Scholar]
- Matyjaszczyk, E. Protection possibilities of agricultural minor crops in the European Union: A case study of soybean, lupin and camelina. J. Plant Dis. Prot. 2020, 127, 55–61. [Google Scholar] [CrossRef]
- Haghighattalab, A.; Crain, J.; Mondal, S.; Rutkoski, R.; Singh, P.R.; Poland, J. Application of Geographically Weighted Regression to Improve Grain Yield Prediction from Unmanned Aerial System Imagery. Crop Sci. 2017, 57, 2478–2489. [Google Scholar] [CrossRef]
- Duddu, H.S.N.; Johnson, E.N.; Willenborg, C.J.; Shirtliffe, S.J. High-Throughput UAV Image-Based Method Is More Precise Than Manual Rating of Herbicide Tolerance. Plant Phenom. 2019, 2019, 6036453. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yao, H.; Zhao, F.; Reddy, K. Detection of crop herbicide injury through plant hyperspectral remote sensing of chlorophyll fluorescence. In Proceedings of the 2017 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Fort Worth, TX, USA, 23–28 July 2017; pp. 5069–5072. [Google Scholar] [CrossRef]
- Streibig, J.C.; Rasmussen, J.; Andújar, D.; Andreasen, C.; Berge, T.W.; Chachalis, D.; Dittmann, T.; Gerhards, R.; Giselsson, T.M.; Hamouz, P.; et al. Sensor-based assessment of herbicide effects. Weed Res. 2014, 54, 223–233. [Google Scholar] [CrossRef]
- Pederi, A.Y.; Cheporniuk, S.H. Unmanned Aerial Vehicles and New Technological Methods of Monitoring and Crop Protection in Precision Agriculture. In Proceedings of the IEEE 3rd International Conference Actual Problems of Unmanned Aerial Vehicles Developments (APUAVD), Kyiv, Ukraine, 13–15 October 2015; pp. 298–301. [Google Scholar]
- Vasileiou, M.; Kyrgiakos, L.S.; Kleisiari, C.; Kleftodimos, G.; Vlontzos, G.; Belhouchette, H.; Pardalos, P.M. Transforming weed management in sustainable agriculture with artificial intelligence: A systematic literature review towards weed identification and deep learning. Crop Prot. 2024, 176, 106522. [Google Scholar] [CrossRef]
- Ahmad, M.; Adnan, A.; Chehri, A. A Real-Time IoT and Image Processing based Weeds Classification System for Selective Herbicide. In Proceedings of the 2022 IEEE 95th Vehicular Technology Conference, Helsinki, Finland, 19–22 June 2022; pp. 1–5. [Google Scholar] [CrossRef]
- Singh, T.; Singh, M. Hand Held Device for Detection of Pesticides using NDVI. Int. J. Comput. Appl. 2016, 154, 29–35. [Google Scholar] [CrossRef]
- Csutoras, C.; Giran, L.; Hudak, O.; Racz, L. Development and evaluation of potential functional food biscuits made from White Lupin. Prog. Agric. Eng. Sci. 2021, 17, 89–100. [Google Scholar] [CrossRef]
- Marsujitullah, M.; Kaligis, D.A.; Manggau, F.X. Health Analysis of Rice Plants Based on the Normalized Difference Vegetation Index (NDVI) Value in Image of Unmanned Aircraft (Case Study of Merauke—Papua Selatan). Eng. Technol. J. 2023, 8, 1986–1991. [Google Scholar] [CrossRef]
- Dancza, I. Herbicide Testing Methodology; Department of Plant and Soil Protection of the Ministry of Agriculture and Rural Development: Budapest, Hungary, 2004; 245p. [Google Scholar]
- Juhász, C.; Hadházy, Á.; Abido, W.; Pál, V.; Zsombik, L. Impact of some herbicides on the growth and the yield of common vetch (Vicia sativa L.). Agron. Res. 2023, 21, 135–155. [Google Scholar] [CrossRef]
- Pinke, G. Effect of Abiotic and Management Factors on the Species Composition of Arable Weeds in Hungary. Ph.D. Thesis, Széchenyi István University, Mosonmagyaróvár, Hungary, 2017; pp. 1–22. [Google Scholar]
- Novák, R.; Dancza, I.; Szentey, L.; Karamán, J. The weed vegetation of Hungary’s arable fields. In Fifth National Arable Field Weed Survey (2007–2008); Novák, R., Ed.; FVM: Budapest, Hungary, 2009. [Google Scholar]
- Kutasy, E. Beech species. 212–222. In Alternative Plants; Pepó, P., Ed.; Mezőgazda Publishing House: Budapest, Hungary, 2019; 259p. [Google Scholar]
- Vasudevan, D.A.K.; Bhuvaneswari, N.S. Precision farming using unmanned aerial and ground vehicles. In Proceedings of the 2016 IEEE Technological Innovations in ICT for Agriculture and Rural Development (TIAR), Chennai, India, 15–16 July 2016; pp. 146–150. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Dewitte, K.; Latré, J.; Haesaert, G. Possibilities of chemical weed control in Lupinus albus and Lupinus luteus-screening of herbicides. Commun. Agric. Appl. Biol. Sci. 2006, 3, 743–751. [Google Scholar]
- Penner, D.; Leep, R.H.; Roggenbucb, F.C.; Lempke, J.R. Herbicide efficacy and tolerance in sweet white lupin. Weed Technol. 1993, 7, 42–46. [Google Scholar] [CrossRef]
- Knott, C.M. Tolerance of autumn-sown determinate lupins (Lupinus albus) to herbicides. Test Agrochem. Cultiv. 1996, 17, 52–53. [Google Scholar] [CrossRef]
- Ivany, A.J.; Mccully, V.K. Evaluation of Herbicides for Sweet White Lupin (Lupinus albus). Weed Technol. 1994, 8, 819–823. [Google Scholar] [CrossRef]
- Cruz, A.B.D.S.; Albuquerque, J.D.A.A.D.; Rocha, P.R.R.; Souza, L.T.D.; Cruz, D.L.D.S.; Soares, M.B.B.; Castro, T.S.; Santos, T.S.D.; Silva, E.S.D. Effect of the use of pre- and post-emergence herbicides on nodulation and production of cowpea (Vigna unguiculata L.) in the Amazonian savannah. Agron. Colomb. 2020, 38, 280–286. [Google Scholar] [CrossRef]
- Singh, K.D.; Geddes, C.M.; Johnson, E.; Tidemann, B.; Shirtliffe, S.; Wang, H.; Natarajan, M.; Jaster, A.; Zuidhof, J.; Turkington, T.K. Proximal and remote sensing based imaging technology to quantify herbicide responses in field crops. Authorea 2022. [Google Scholar] [CrossRef]
- Kurbanov, R.K.; Zakharova, N.I. Application of vegetation indexes to assess the condition of crops. Agric. Mach. Technol. 2020, 14, 4. [Google Scholar] [CrossRef]
- Duan, T.; Chapman, S.C.; Guo, Y.; Zheng, B. Dynamic monitoring of NDVI in wheat agronomy and breeding trials using an unmanned aerial vehicle. Field Crops Res. 2017, 210, 71–80. [Google Scholar] [CrossRef]
- Rodriguez, J.E.; Alonso-Jimenez, C.; Alfonso, T.M.A.; Benito, R.M.; Díaz-Ambrona, C.G.H. Comparison of NDVI fields obtained from different remote sensors. In Proceedings of the European Geosciences Union General Assembly, Vienna, Austria, 7–12 April 2013; p. 14153. [Google Scholar]
- Fan, X.; Liu, Y. A comparison of NDVI intercalibration methods. Int. J. Remote Sens. 2017, 38, 5273–5290. [Google Scholar] [CrossRef]
- Anchal, S.; Bahuguna, S.; Priti; Pal, P.K.; Kumar, D.; Murthy, P.V.S.; Kumar, A. Non-destructive method of biomass and nitrogen (N) level estimation in Stevia rebaudiana using various multispectral indices. Geocarto Int. 2022, 37, 6409–6421. [Google Scholar] [CrossRef]




| Soil Characters | Quantitative Indicators |
|---|---|
| pH (KCl) | 7.46 |
| Plasticity index by Arany | 26 |
| Water-soluble salt (m/m) % | <0.02 |
| Carbonated lime content (m/m) % | 0.844 |
| Humus content (m/m) % | 1.11 |
| Phosphorus pentoxide mg kg−1 | 440 |
| Potassium oxide mg kg−1 | 252 |
| Active Substances | Time of Application | Chemical Name | Doses | Codes of the Treatments |
|---|---|---|---|---|
| Control | - | - | T0 | |
| Flumioxazin [24] | Pre-emergence | N-(7-fluoro-3,4-dihydro-3-oxo-4-prop-2-ynyl-2H-1,4-benzoxazin-6-yl)cyclohex-1-ene-1,2-dicarboxamide | 0.06 kg ha−1 | T1 |
| Pendimethalin [25] | Pre-emergence | 3,4-Dimethyl-2,6-dinitro-N-(pentan-3-yl)aniline | 5.0 L ha−1 | T2 |
| Dimethenamid-P [26] | Pre-emergence | (S)-2-chloro-N-(2,4-dimethyl-3-thienyl)-N-(2-methoxy-1-methylethyl)acetamide | 1.4 L ha−1 | T3 |
| Pethoxamid [26] | Pre-emergence | 2-chloro-N-(2-ethoxyethyl)-N-(2-methyl-1-phenylprop-1-enyl)acetamide | 2.0 L ha−1 | T4 |
| Clomazone [27] | Pre-emergence | 2-(2-chlorobenzyl)-4,4-dimethyl-1,2-oxazolidin-3-one | 0.2 L ha−1 | T5 |
| Metobromuron [16] | Pre-emergence | 3-(4-bromophenyl)-1-methoxy-1-methylurea | 3.0 L ha−1 | T6 |
| Metribuzin [23] | Pre-emergence | 4-amino-6-tert-butyl-3-methylsulfanyl-1,2,4-triazin-5-one | 0.55 L ha−1 | T7 |
| Imazamox [28] | Post-emergence | 2-[(RS)-4-isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl]-5-methoxymethylnicotinic acid | 1.0 L ha−1 | T8 |
| Crop Year 2022 | |||||
|---|---|---|---|---|---|
| March | April | May | June | July | |
| Temperature (°C) | 4.8 | 9.18 | 17.4 | 22.2 | 23.4 |
| Precipitation (mm) | 21.9 | 42.1 | 3.9 | 21.9 | 35.4 |
| Seed Yields | Manual-NDVI Data Sets | Aerial-NDVI | Phytotoxicity | Number of Weeds | Contamination | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seed yields | — | |||||||||||
| Manual-NDVI data sets | 0.676 | *** | — | |||||||||
| Aerial-NDVI | 0.536 | *** | 0.834 | *** | — | |||||||
| Phytotoxicity | −0.471 | ** | −0.615 | *** | −0.427 | ** | — | |||||
| Number of weeds | −0.106 | 0.282 | 0.205 | −0.377 | * | — | ||||||
| Contamination | 0.419 | * | 0.567 | *** | 0.446 | ** | −0.607 | *** | 0.16 | — | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhász, C.; Mendler-Drienyovszki, N.; Magyar-Tábori, K.; Radócz, L.; Zsombik, L. Effect of Different Herbicides on Development and Productivity of Sweet White Lupine (Lupinus albus L.). Agronomy 2024, 14, 488. https://doi.org/10.3390/agronomy14030488
Juhász C, Mendler-Drienyovszki N, Magyar-Tábori K, Radócz L, Zsombik L. Effect of Different Herbicides on Development and Productivity of Sweet White Lupine (Lupinus albus L.). Agronomy. 2024; 14(3):488. https://doi.org/10.3390/agronomy14030488
Chicago/Turabian StyleJuhász, Csaba, Nóra Mendler-Drienyovszki, Katalin Magyar-Tábori, László Radócz, and László Zsombik. 2024. "Effect of Different Herbicides on Development and Productivity of Sweet White Lupine (Lupinus albus L.)" Agronomy 14, no. 3: 488. https://doi.org/10.3390/agronomy14030488
APA StyleJuhász, C., Mendler-Drienyovszki, N., Magyar-Tábori, K., Radócz, L., & Zsombik, L. (2024). Effect of Different Herbicides on Development and Productivity of Sweet White Lupine (Lupinus albus L.). Agronomy, 14(3), 488. https://doi.org/10.3390/agronomy14030488







