Influence of Genotype on Antioxidant Activity and Phenolic Profile of Fennel Bulbs
Abstract
1. Introduction
2. Materials and Methods
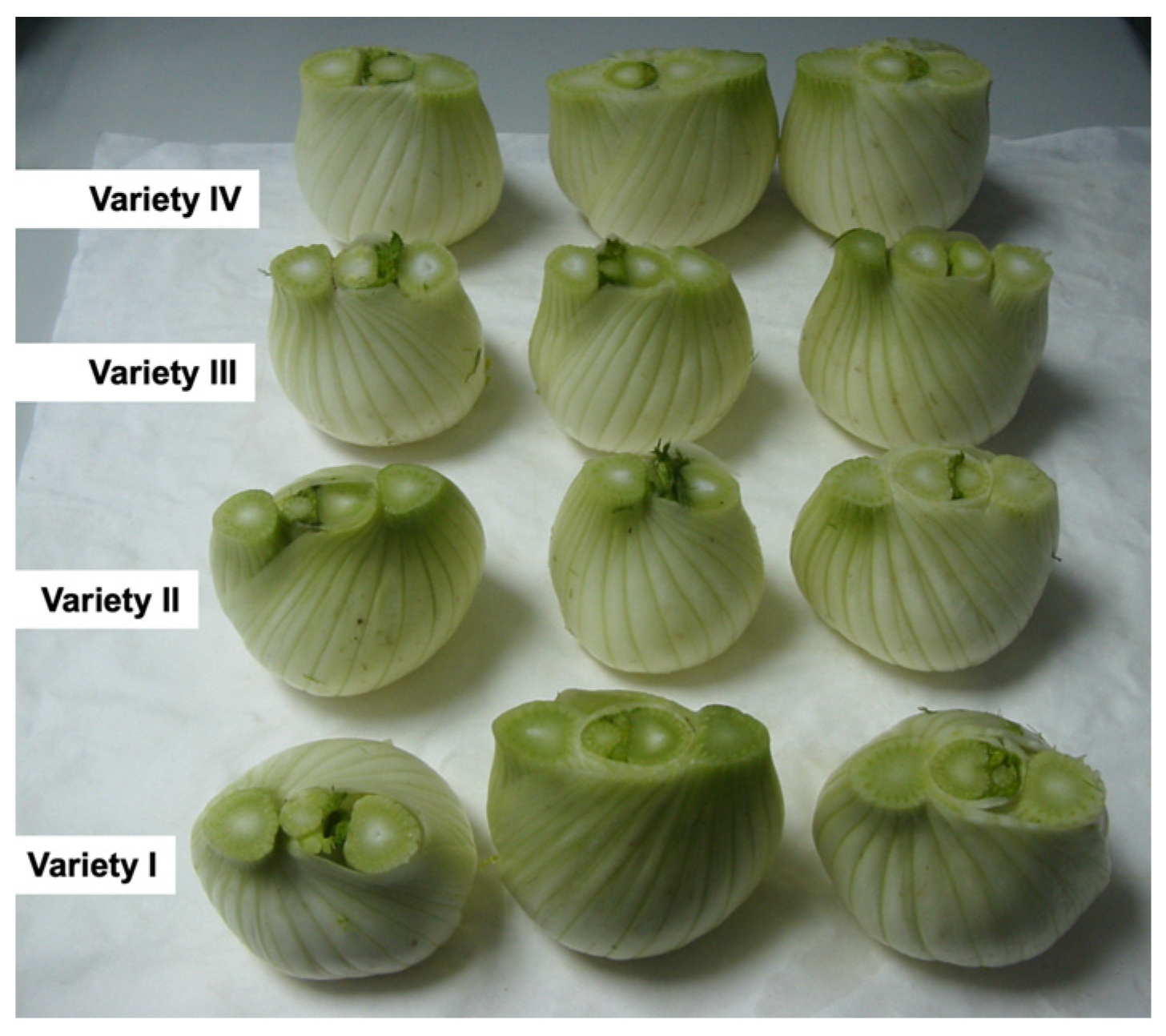
2.1. Plant Material and Extract Preparations
2.2. Preparation of Extracts
2.3. Determination of Antioxidant Properties
2.3.1. DPPH Radical-Scavenging Assay
2.3.2. Reducing Power Assay
2.3.3. Metal Chelating Activities
2.3.4. Measurement of Inhibition of Lipid Peroxidation
2.3.5. Quantification of Total Soluble Phenol Content
2.4. HPLC-MS Analysis of Phenolic Compounds
2.5. Statistical Analysis
3. Results
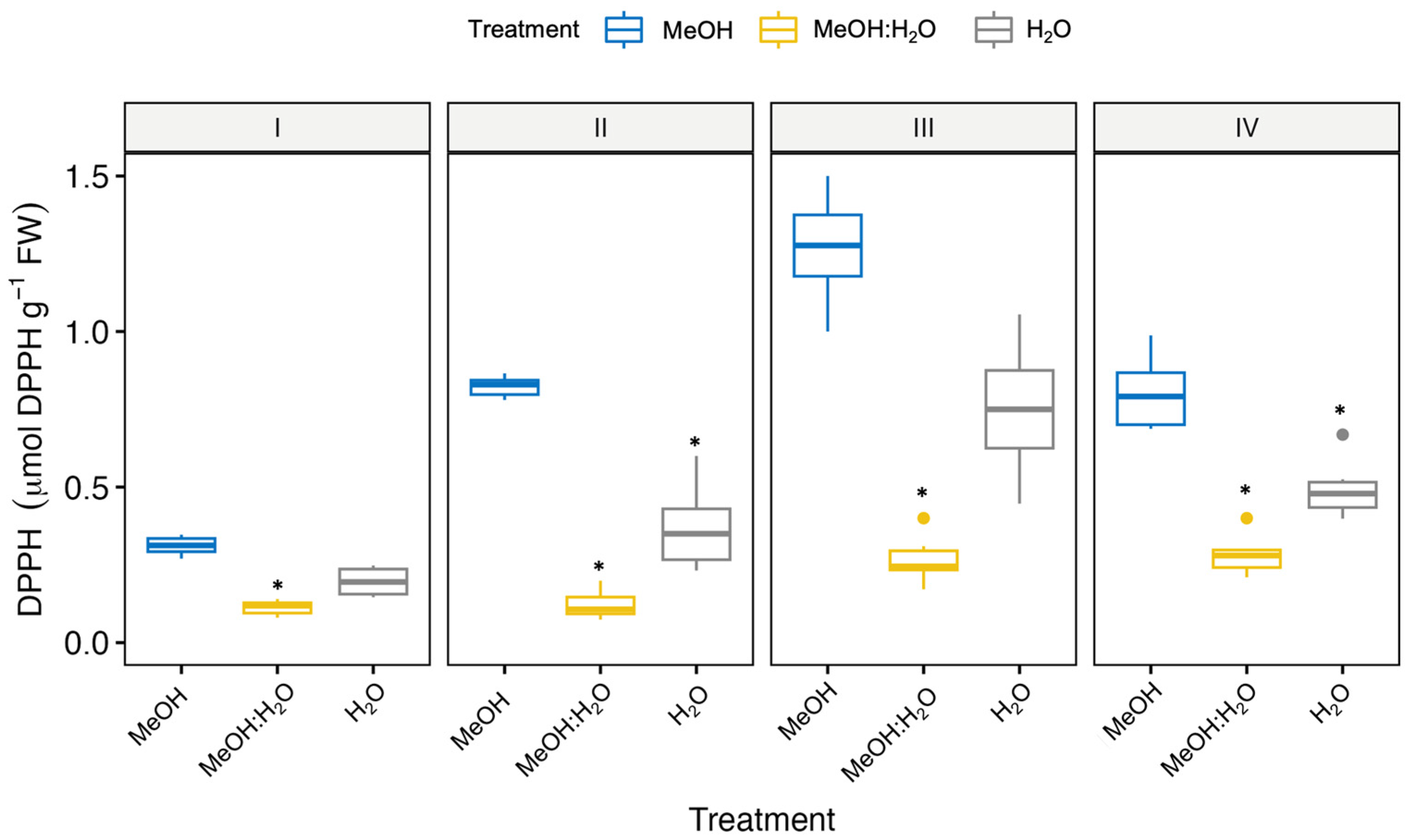
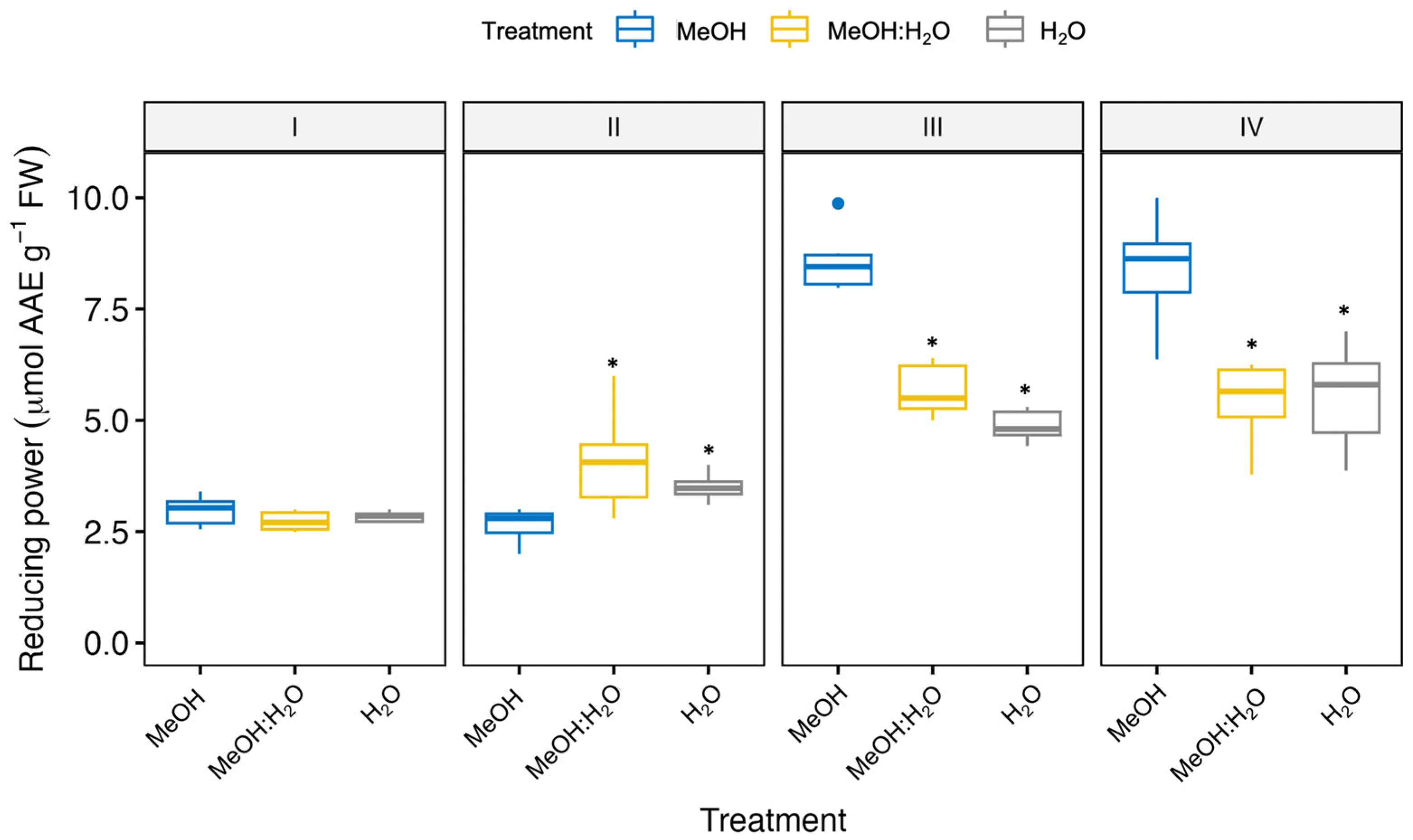
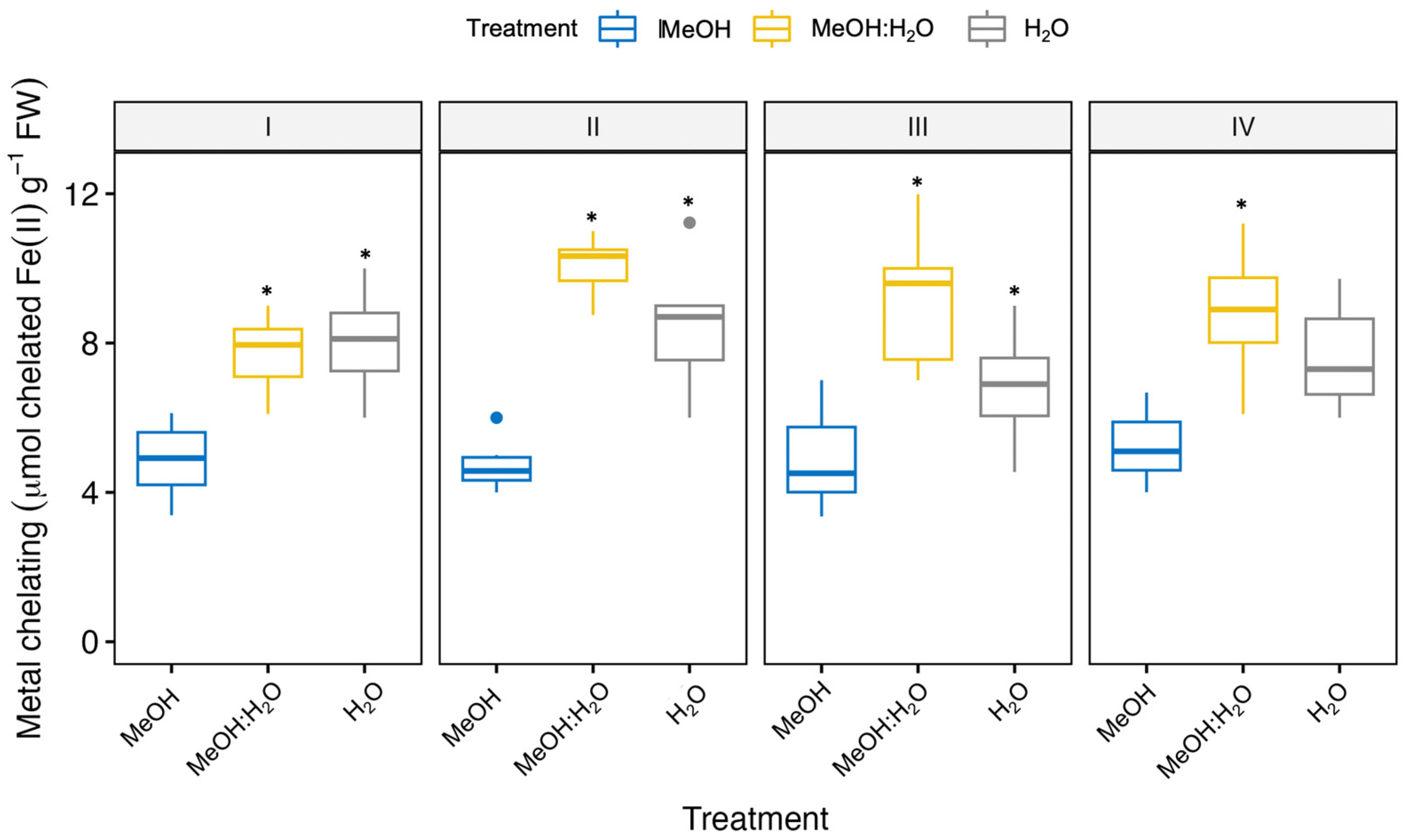
3.1. Free Radical Scavenging and Antioxidant Activities of Fennel Bulbs Using Different Extraction Solvents
3.2. Effect of Different Extraction Solvents on the Phenolic Content in Fennel Bulbs
3.3. Principal Component Analysis and Pearson’s Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical Review: Vegetables and Fruit in the Prevention of Chronic Diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and Vegetable Consumption and Mortality from All Causes, Cardiovascular Disease, and Cancer: Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef]
- Gehlich, K.H.; Beller, J.; Lange-Asschenfeldt, B.; Köcher, W.; Meinke, M.C.; Lademann, J. Consumption of Fruits and Vegetables: Improved Physical Health, Mental Health, Physical Functioning and Cognitive Health in Older Adults from 11 European Countries. Aging Ment. Health 2020, 24, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Jones, J.; Lee, G.J. Plant-Based Dietary Patterns, Plant Foods, and Age-Related Cognitive Decline. Adv. Nutr. 2019, 10, S422–S436. [Google Scholar] [CrossRef]
- Loef, M.; Walach, H. Fruit, Vegetables and Prevention of Cognitive Decline or Dementia: A Systematic Review of Cohort Studies. J. Nutr. Health Aging 2012, 16, 626–630. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, J.; Song, D.; Deng, R.; Wei, J.; Zhang, Z. Increased Consumption of Fruit and Vegetables Is Related to a Reduced Risk of Cognitive Impairment and Dementia: Meta-Analysis. Front. Aging Neurosci. 2017, 9, 18. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. Antioxidant Phytochemicals in Fruits and Vegetables: Diet and Health Implications. HortScience 2000, 35, 588–592. [Google Scholar] [CrossRef]
- Cömert, E.D.; Mogol, B.A.; Gökmen, V. Relationship between Color and Antioxidant Capacity of Fruits and Vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Antioxidants in Fruits and Vegetables—The Millennium’s Health. Int. J. Food Sci. Technol. 2008, 36, 703–725. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Cheynier, V. Phenolic Compounds: From Plants to Foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Holvoet, S.; Mercenier, A. Dietary Polyphenols in the Prevention and Treatment of Allergic Diseases. Clin. Exp. Allergy 2011, 41, 1346–1359. [Google Scholar] [CrossRef]
- Rahaiee, S.; Assadpour, E.; Faridi Esfanjani, A.; Silva, A.S.; Jafari, S.M. Application of Nano/Microencapsulated Phenolic Compounds against Cancer. Adv. Colloid Interface Sci. 2020, 279, 102153. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant Properties of Phenolic Compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Xiong, W.; Reynolds, M.; Xu, Y. Climate Change Challenges Plant Breeding. Curr. Opin. Plant Biol. 2022, 70, 102308. [Google Scholar] [CrossRef]
- Mir, R.R.; Rustgi, S.; Zhang, Y.-M.; Xu, C. Multi-Faceted Approaches for Breeding Nutrient-Dense, Disease-Resistant, and Climate-Resilient Crop Varieties for Food and Nutritional Security. Heredity 2022, 128, 387–390. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A Review of Its Botany, Phytochemistry, Pharmacology, Contemporary Application, and Toxicology. Biomed. Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef]
- Ibrahim, N.; Moussa, A.Y. A Comparative Volatilomic Characterization of Florence Fennel from Different Locations: Antiviral Prospects. Food Funct. 2021, 12, 1498–1515. [Google Scholar] [CrossRef]
- Chen, F.; Guo, Y.; Kang, J.; Yang, X.; Zhao, Z.; Liu, S.; Ma, Y.; Gao, W.; Luo, D. Insight into the Essential Oil Isolation from Foeniculum vulgare Mill. Fruits Using Double-Condensed Microwave-Assisted Hydrodistillation and Evaluation of Its Antioxidant, Antifungal and Cytotoxic Activity. Ind. Crops Prod. 2020, 144, 112052. [Google Scholar] [CrossRef]
- Senatore, F.; Oliviero, F.; Scandolera, E.; Taglialatela-Scafati, O.; Roscigno, G.; Zaccardelli, M.; De Falco, E. Chemical Composition, Antimicrobial and Antioxidant Activities of Anethole-Rich Oil from Leaves of Selected Varieties of Fennel [Foeniculum vulgare Mill. ssp. vulgare var. azoricum (Mill.) Thell]. Fitoterapia 2013, 90, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Ilardi, V.; Badalamenti, N.; Bruno, M. Chemical Composition of the Essential Oil from Different Vegetative Parts of Foeniculum vulgare subsp. piperitum (Ucria) Coutinho (Umbelliferae) Growing Wild in Sicily. Nat. Prod. Res. 2022, 36, 3587–3597. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and Analysis of Phenolics in Food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.D. Extraction Systems and Analytical Techniques for Food Phenolic Compounds: A Review. Foods 2022, 11, 3671. [Google Scholar] [CrossRef]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing Total Phenolics, Total Flavonoids Contents and Antioxidant Activity of Moringa oleifera Leaf Extract by the Appropriate Extraction Method. Ind. Crops Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Pérez-Tortosa, V.; López-Orenes, A.; Martínez-Pérez, A.; Ferrer, M.A.; Calderón, A.A. Antioxidant Activity and Rosmarinic Acid Changes in Salicylic Acid-Treated Thymus membranaceus Shoots. Food Chem. 2012, 130, 362–369. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Majidi, M.M.; Ehtemam, M.H.; Hughes, N. Characterization of Fennel Germplasm for Physiological Persistence and Drought Recovery: Association with Biochemical Properties. Plant Physiol. Biochem. 2023, 194, 499–512. [Google Scholar] [CrossRef]
- UNECE. UNECE Standard FFV-16 Concerning the Marketing and commercial Quality Control of Fennel (Working Party on Agricultural Quality Standards). Available online: https://unece.org/sites/default/files/2023-07/16_Fennel_e.pdf (accessed on 28 December 2023).
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, Y.; Yu, A.; Pan, B.; Pignatello, J.J. Revisiting the Phenanthroline and Ferrozine Colorimetric Methods for Quantification of Fe(II) in Fenton Reactions. Chem. Eng. J. 2020, 391, 123592. [Google Scholar] [CrossRef]
- Parejo, I.; Viladomat, F.; Bastida, J.; Codina, C. Development and Validation of a High-Performance Liquid Chromatographic Method for the Analysis of Antioxidative Phenolic Compounds in Fennel Using a Narrow Bore Reversed Phase C18 Column. Anal. Chim. Acta 2004, 512, 271–280. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Meyer, A.S. The Problems of Using One-Dimensional Methods to Evaluate Multifunctional Food and Biological Antioxidants. J. Sci. Food Agric. 2000, 80, 1925–1941. [Google Scholar] [CrossRef]
- Gentile, C.; Di Gregorio, E.; Di Stefano, V.; Mannino, G.; Perrone, A.; Avellone, G.; Sortino, G.; Inglese, P.; Farina, V. Food Quality and Nutraceutical Value of Nine Cultivars of Mango (Mangifera indica L.) Fruits Grown in Mediterranean Subtropical Environment. Food Chem. 2019, 277, 471–479. [Google Scholar] [CrossRef]
- Imeh, U.; Khokhar, S. Distribution of Conjugated and Free Phenols in Fruits: Antioxidant Activity and Cultivar Variations. J. Agric. Food Chem. 2002, 50, 6301–6306. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.-Y.; Zhao, C.-N.; Feng, X.-L.; Xu, X.-Y.; Cao, S.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, H.-B. Comparison of Antioxidant Activities of Different Grape Varieties. Molecules 2018, 23, 2432. [Google Scholar] [CrossRef]
- Marathe, S.A.; Rajalakshmi, V.; Jamdar, S.N.; Sharma, A. Comparative Study on Antioxidant Activity of Different Varieties of Commonly Consumed Legumes in India. Food Chem. Toxicol. 2011, 49, 2005–2012. [Google Scholar] [CrossRef]
- Faudale, M.; Viladomat, F.; Bastida, J.; Poli, F.; Codina, C. Antioxidant Activity and Phenolic Composition of Wild, Edible, and Medicinal Fennel from Different Mediterranean Countries. J. Agric. Food Chem. 2008, 56, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- González, M.; González, V. Sample Preparation of Tropical and Subtropical Fruit Biowastes to Determine Antioxidant Phytochemicals. Anal. Methods 2010, 2, 1842. [Google Scholar] [CrossRef]
- Heo, B.-G.; Park, Y.-J.; Park, Y.-S.; Bae, J.-H.; Cho, J.-Y.; Park, K.; Jastrzebski, Z.; Gorinstein, S. Anticancer and Antioxidant Effects of Extracts from Different Parts of Indigo Plant. Ind. Crops Prod. 2014, 56, 9–16. [Google Scholar] [CrossRef]
- Lapornik, B.; Prošek, M.; Golc Wondra, A. Comparison of Extracts Prepared from Plant By-Products Using Different Solvents and Extraction Time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of Mango Byproducts: Study of the Effect of Extraction Solvent and Temperature on Their Antioxidant Properties. J. Food Sci. 2012, 77, C80–C88. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.; Lightfoot, D. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Costa-Pérez, A.; Ferrer, M.A.; Calderón, A.A. Combined Effects of Cytokinin and UV-C Light on Phenolic Pattern in Ceratonia siliqua Shoot Cultures. Agronomy 2023, 13, 621. [Google Scholar] [CrossRef]
- Dasgupta, N.; De, B. Antioxidant Activity of Some Leafy Vegetables of India: A Comparative Study. Food Chem. 2007, 101, 471–474. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Shalmashi, A.; Eliassi, A. Solubility of L-(+)-Ascorbic Acid in Water, Ethanol, Methanol, Propan-2-Ol, Acetone, Acetonitrile, Ethyl Acetate, and Tetrahydrofuran from (293 to 323) K. J. Chem. Eng. Data 2008, 53, 1332–1334. [Google Scholar] [CrossRef]
- Nada, R.; Ashmawi, A.; Mady, E.; Randhir, T.; Elateeq, A. Effect of Organic Manure and Plant Growth Promoting Microbes on Yield, Quality and Essential Oil Constituents of Fennel Bulb (Foeniculum vulgare Mill.). J. Ecol. Eng. 2022, 23, 149–164. [Google Scholar] [CrossRef]
- Sendra, J.M.; Sentandreu, E.; Navarro, J.L. Reduction Kinetics of the Free Stable Radical 2,2-Diphenyl-1-Picrylhydrazyl (DPPH•) for Determination of the Antiradical Activity of Citrus Juices. Eur. Food Res. Technol. 2006, 223, 615–624. [Google Scholar] [CrossRef]
- Shalata, A.; Neumann, P.M. Exogenous Ascorbic Acid (Vitamin C) Increases Resistance to Salt Stress and Reduces Lipid Peroxidation. J. Exp. Bot. 2001, 52, 2207–2211. [Google Scholar] [CrossRef]
- Rawson, A.; Hossain, M.B.; Patras, A.; Tuohy, M.; Brunton, N. Effect of Boiling and Roasting on the Polyacetylene and Polyphenol Content of Fennel (Foeniculum vulgare) Bulb. Food Res. Int. 2013, 50, 513–518. [Google Scholar] [CrossRef]
- Di Donato, P.; Taurisano, V.; Tommonaro, G.; Pasquale, V.; Jiménez, J.M.S.; de Pascual-Teresa, S.; Poli, A.; Nicolaus, B. Biological Properties of Polyphenols Extracts from Agro Industry’s Wastes. Waste Biomass Valorization 2018, 9, 1567–1578. [Google Scholar] [CrossRef]
- Barzegar, T.; Mohammadi, S.; Ghahremani, Z. Effect of Nitrogen and Potassium Fertilizer on Growth, Yield and Chemical Composition of Sweet Fennel. J. Plant Nutr. 2020, 43, 1189–1204. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic Acids and Other Cinnamates—Nature, Occurrence, Dietary Burden, Absorption and Metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic Acids: Chemistry, Biosynthesis, Occurrence, Analytical Challenges, and Bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Crescenzi, M.A.; D’Urso, G.; Piacente, S.; Montoro, P. LC-ESI/LTQOrbitrap/MS Metabolomic Analysis of Fennel Waste (Foeniculum vulgare Mill.) as a Byproduct Rich in Bioactive Compounds. Foods 2021, 10, 1893. [Google Scholar] [CrossRef]
- Liang, N.; Dupuis, J.H.; Yada, R.Y.; Kitts, D.D. Chlorogenic Acid Isomers Directly Interact with Keap 1-Nrf2 Signaling in Caco-2 Cells. Mol. Cell. Biochem. 2019, 457, 105–118. [Google Scholar] [CrossRef]
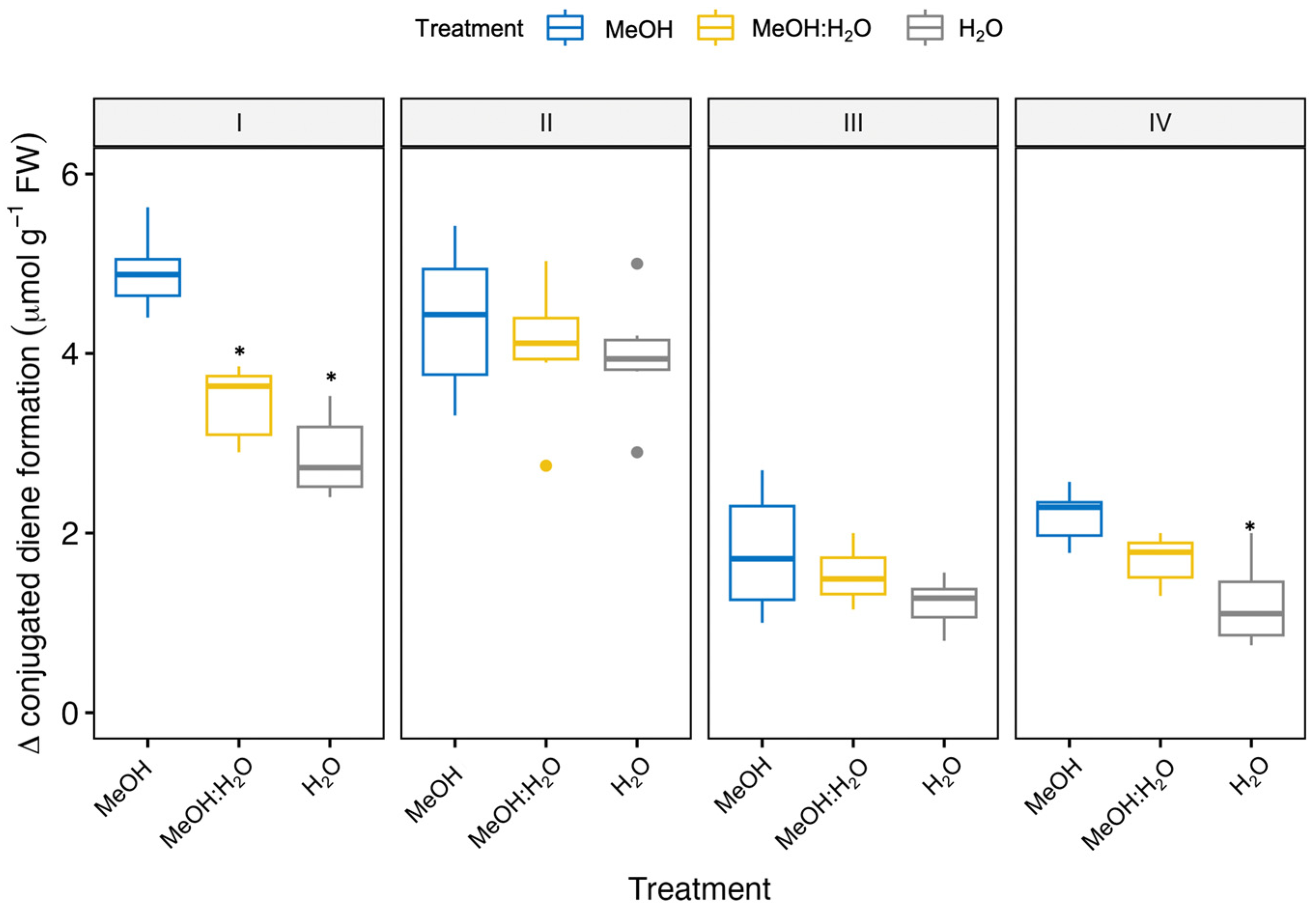
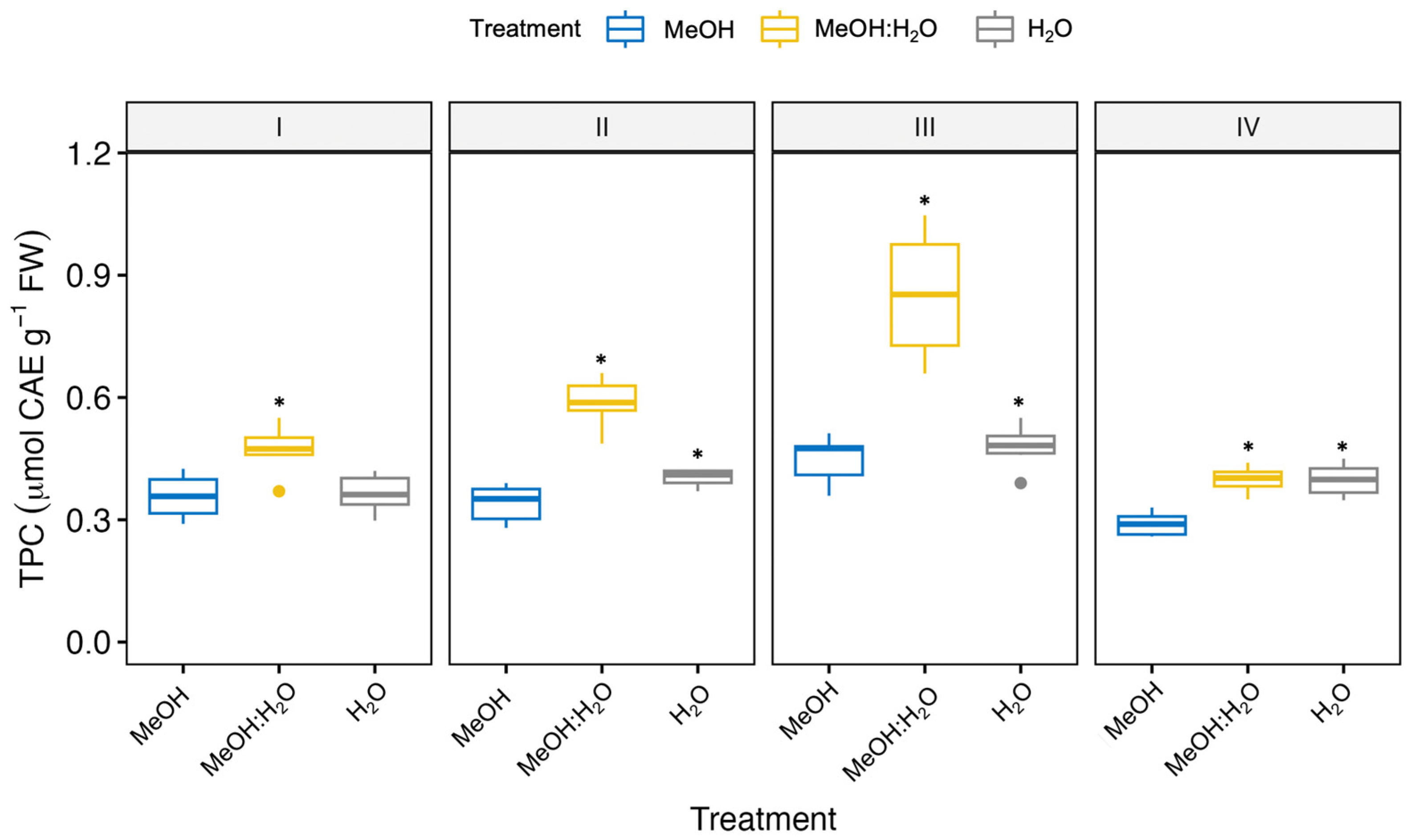
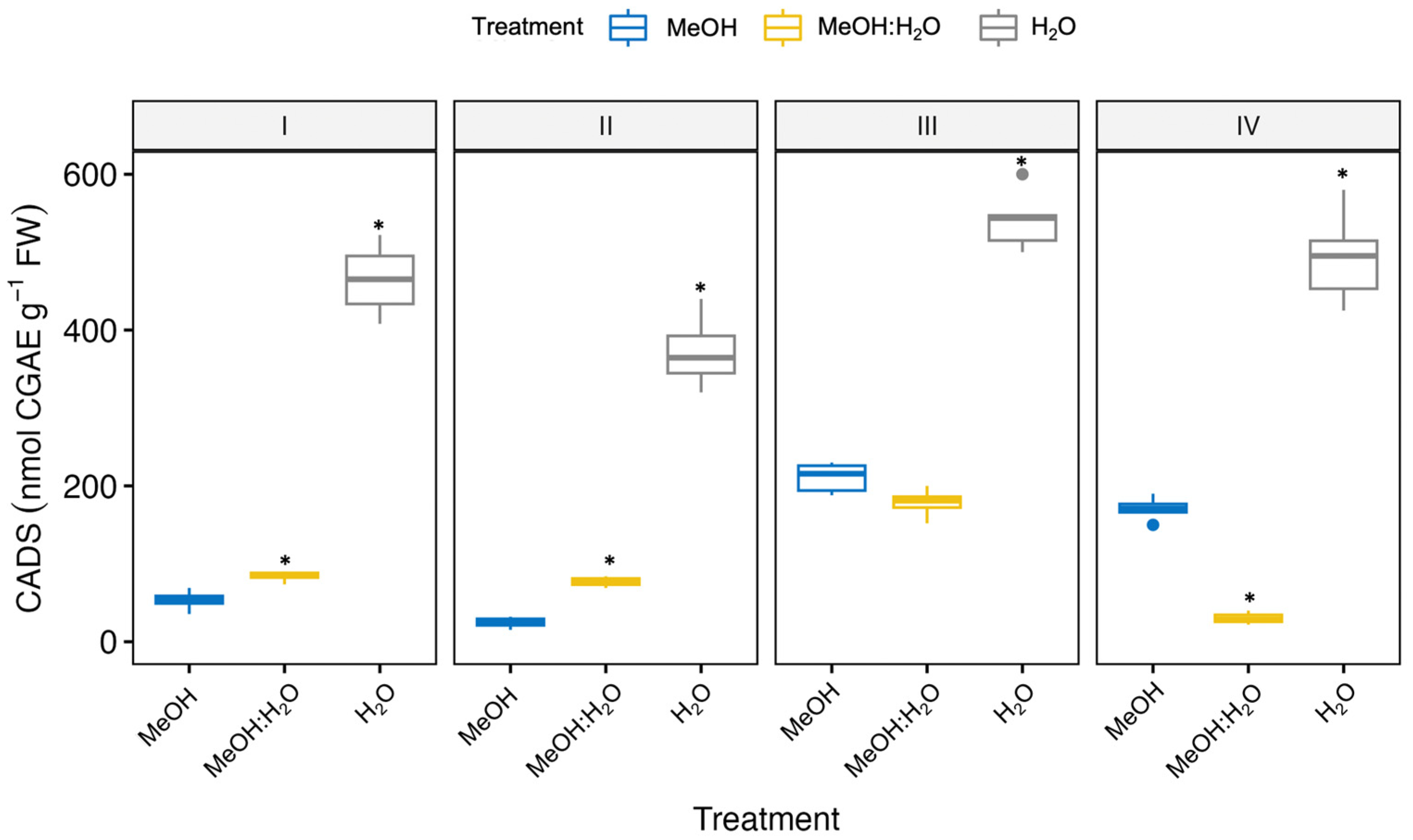
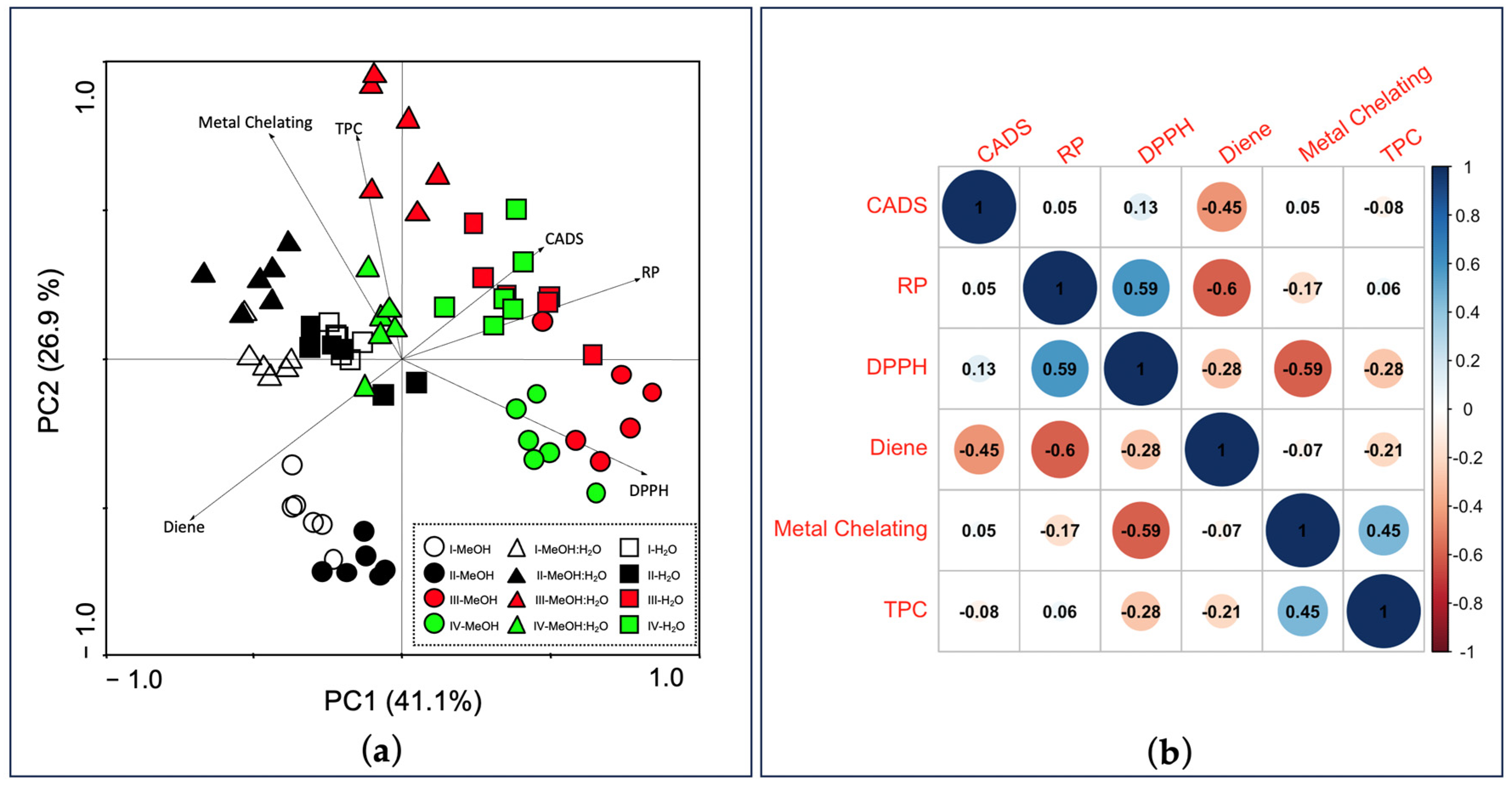
| Fennel Genotype/Solvent | CA (nmol g−1 FW) | CGA (nmol g−1 FW) | 3-CQA (nmol CGAE g−1 FW) | 1,3-diCQA (nmol CGAE g−1 FW) | 1,5-diCQA (nmol CGAE g−1 FW) | 1,4-diCQA (nmol CGAE g−1 FW) |
|---|---|---|---|---|---|---|
| I | ||||||
| MeOH | nd | 6.27 ± 0.60 h | nd | nd | nd | nd |
| MeOH:H2O | nd | 1.17 ± 0.13 i | nd | nd | nd | nd |
| H2O | nd | 66.58 ± 0.88 d | 9.01 ± 0.43 d | 4.27 ± 0.12 b | nd | 17.38 ± 1.07 d |
| II | ||||||
| MeOH | nd | 13.16 ± 1.27 g | nd | nd | nd | nd |
| MeOH:H2O | nd | nd | nd | nd | nd | nd |
| H2O | 8.15 ± 0.15 a | 232.42 ± 3.99 b | 23.76 ± 0.15 b | 5.02 ± 0.30 b | 4.87 ± 0.74 b | 23.31 ± 0.88 c |
| III | ||||||
| MeOH | nd | 47.43 ± 4.48 f | nd | nd | nd | 4.54 ± 0.52 e |
| MeOH:H2O | nd | nd | nd | nd | nd | nd |
| H2O | nd | 117.07 ± 2.08 c | 13.75 ± 0.88 c | nd | 3.66 ± 0.63 b | 21.57 ± 1.26 c |
| IV | ||||||
| MeOH | 4.06 ± 0.15 b | 96.58 ± 2.32 c | nd | nd | nd | 32.72 ± 1.19 b |
| MeOH:H2O | nd | 5.00 ± 0.15 h | nd | nd | nd | nd |
| H2O | 8.10 ± 0.30 a | 315.36 ± 5.17 a | 30.89 ± 0.30 a | 19.74 ± 0.22 a | 15.58 ± 0.19 a | 78.68 ± 1.38 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio, A.; López-Orenes, A.; Ferrer, M.A.; Calderón, A.A. Influence of Genotype on Antioxidant Activity and Phenolic Profile of Fennel Bulbs. Agronomy 2024, 14, 484. https://doi.org/10.3390/agronomy14030484
Rubio A, López-Orenes A, Ferrer MA, Calderón AA. Influence of Genotype on Antioxidant Activity and Phenolic Profile of Fennel Bulbs. Agronomy. 2024; 14(3):484. https://doi.org/10.3390/agronomy14030484
Chicago/Turabian StyleRubio, Alfonso, Antonio López-Orenes, María A. Ferrer, and Antonio A. Calderón. 2024. "Influence of Genotype on Antioxidant Activity and Phenolic Profile of Fennel Bulbs" Agronomy 14, no. 3: 484. https://doi.org/10.3390/agronomy14030484
APA StyleRubio, A., López-Orenes, A., Ferrer, M. A., & Calderón, A. A. (2024). Influence of Genotype on Antioxidant Activity and Phenolic Profile of Fennel Bulbs. Agronomy, 14(3), 484. https://doi.org/10.3390/agronomy14030484







