Modeling of Cotton Yield Estimation Based on Canopy Sun-Induced Chlorophyll Fluorescence
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Pilot Area
2.2. Data Acquisition
2.2.1. Canopy Sun-Induced Chlorophyll Fluorescence (SIF) Acquisition
2.2.2. Cotton Biometric Parameters and Yield Data Acquisition
- Leaf area index (LAI): The leaf area data were obtained by using an LI-3100c bench leaf area instrument (LI-COR, Inc., Lincoln, NE, USA) to measure the leaf area of all the leaves of each cotton plant. The formula for calculating LAI is as follows: m is the number of plants measured; A is the leaf area of all leaves of plant m; p is the planting density;
- 2.
- Aboveground biomass (AGB) (g·m−2): Once the measured plants were collected, the plants at the upper part of the cotyledon node were cut into pieces and packed in bags. The plants were defoliated at 105 °C for 30 min and dried at 80 °C to a constant weight. AGB is calculated by the following formula: B is the dry weight of a cotton plant and S is the area occupied by a cotton plant;
- 3.
- Seed cotton yield (kg·ha−1): Yield estimation using sample method harvesting. During the cotton fluffing period, representative sample squares (2.28 m × 1 m) were selected from each plot for yield determination; the number of plants, number of bolls per plant, and single boll weight were determined, and the weight of seed cotton was weighed in each sample square. The formula is as follows:
2.3. Data Processing
2.3.1. SIF Data Preprocessing
2.3.2. Correlation Analysis
2.3.3. Model Building Methodology
2.3.4. Evaluation of the Model
3. Results
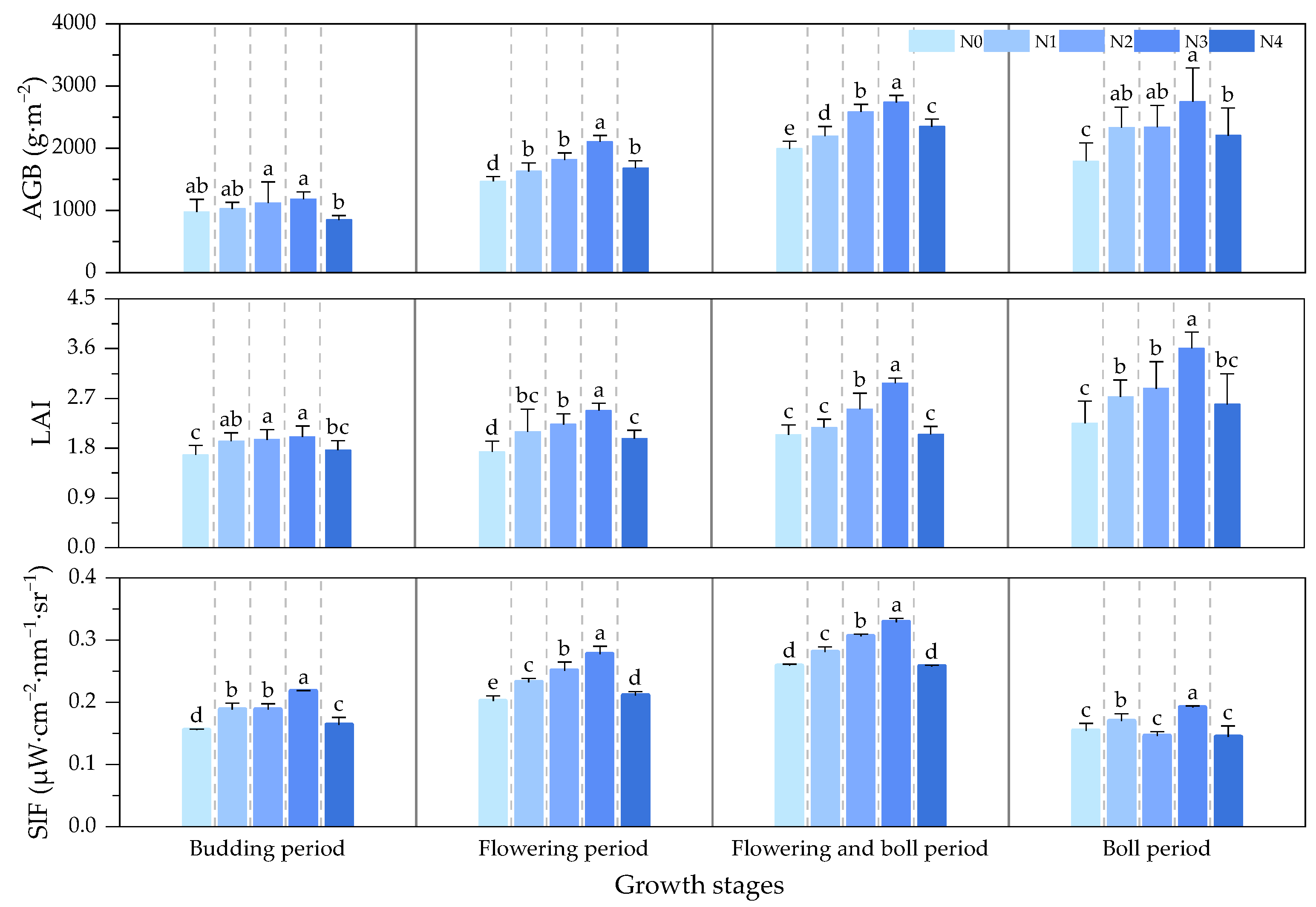
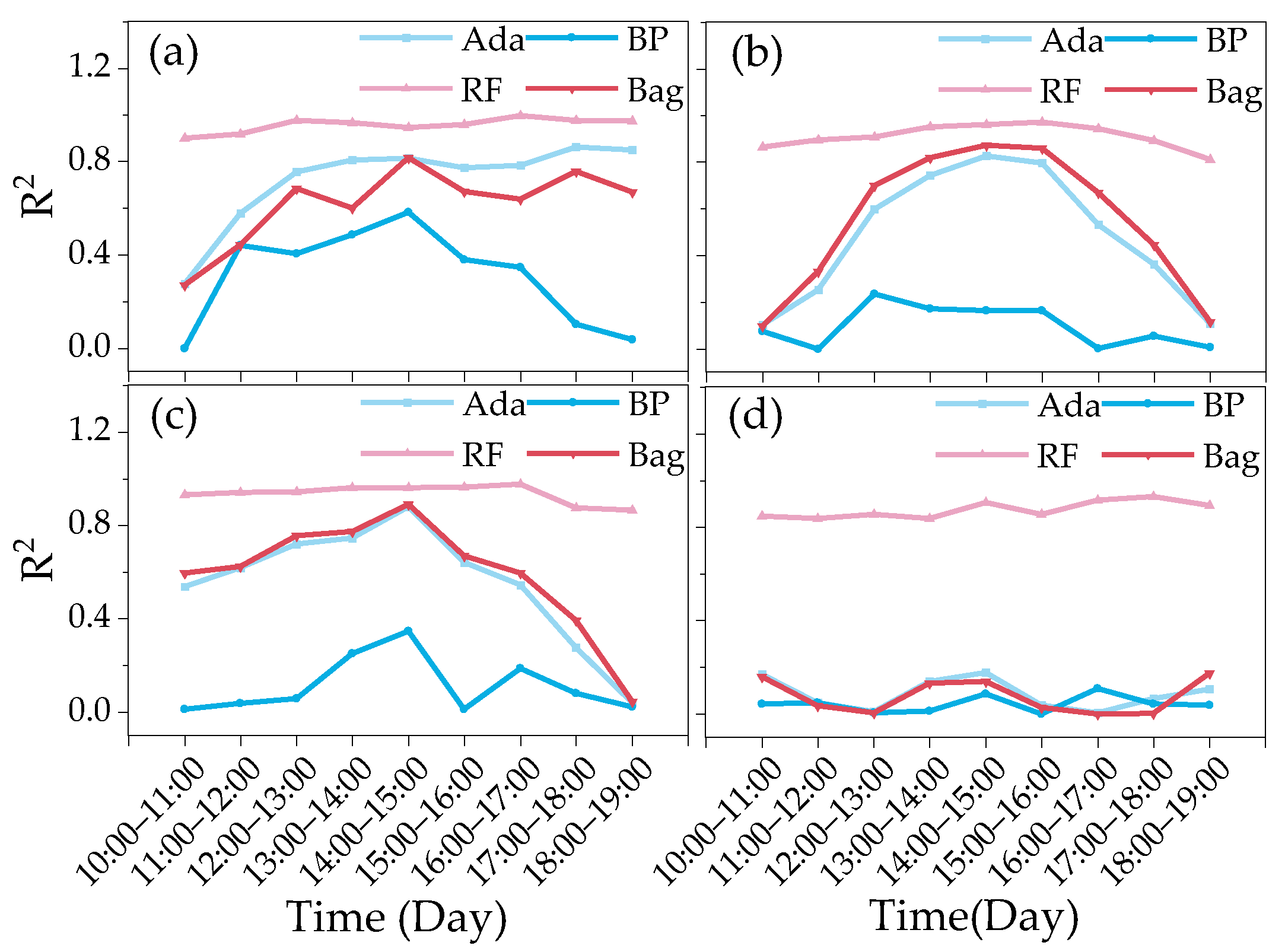
3.1. Diurnal Variation in Canopy SIF and Biometric Parameter Responses of Cotton for Different Growth Periods
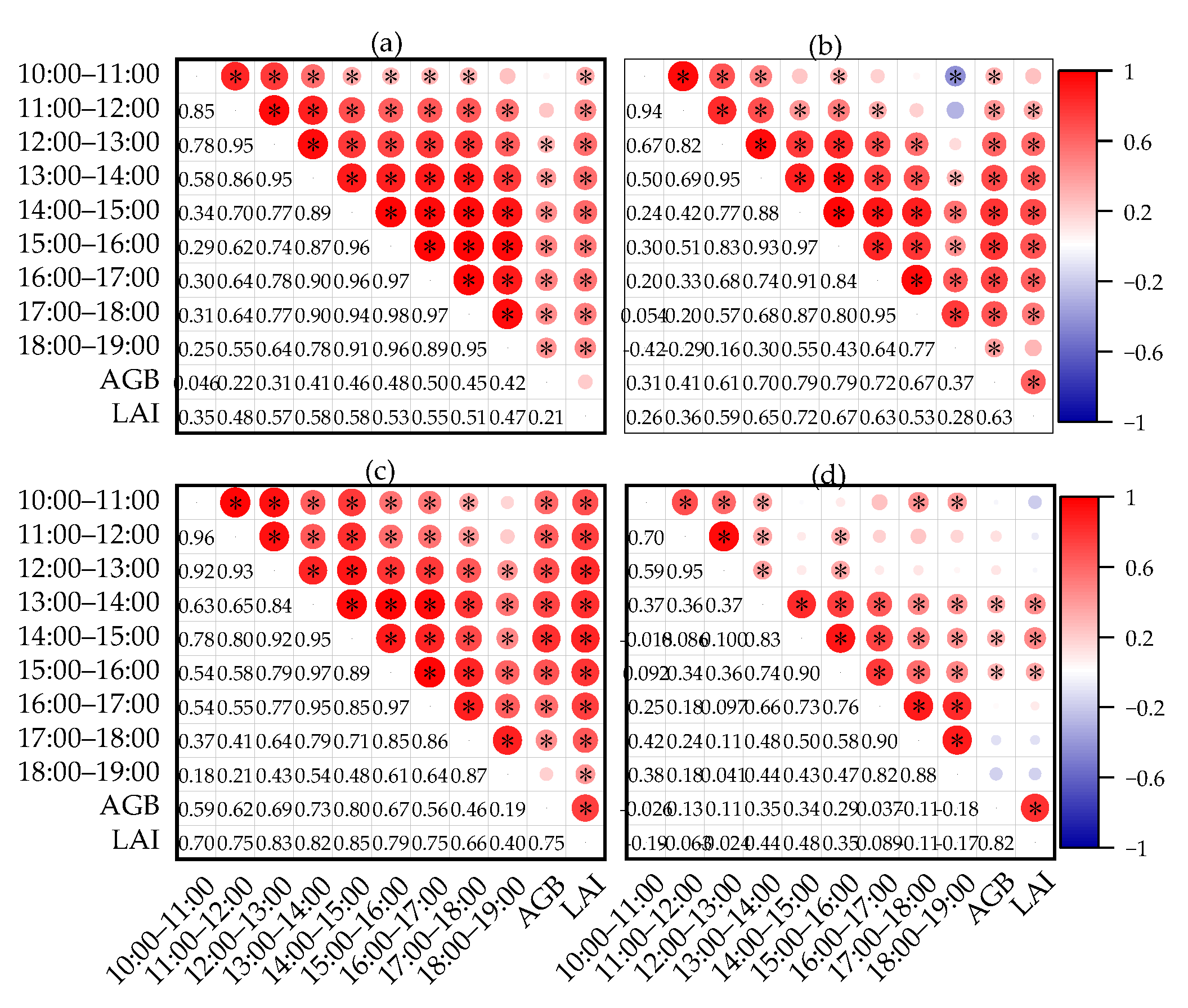
3.2. Correlation Analysis between Canopy SIF and Cotton Yield at Different Growth Periods
3.2.1. Correlation Analysis of Cotton Yield with AGB and LAI at Different Growth Periods
3.2.2. Correlation Analysis of Cotton Canopy SIF with AGB and LAI at Different Growth Periods
3.3. Construction of the Cotton Yield Estimation Model
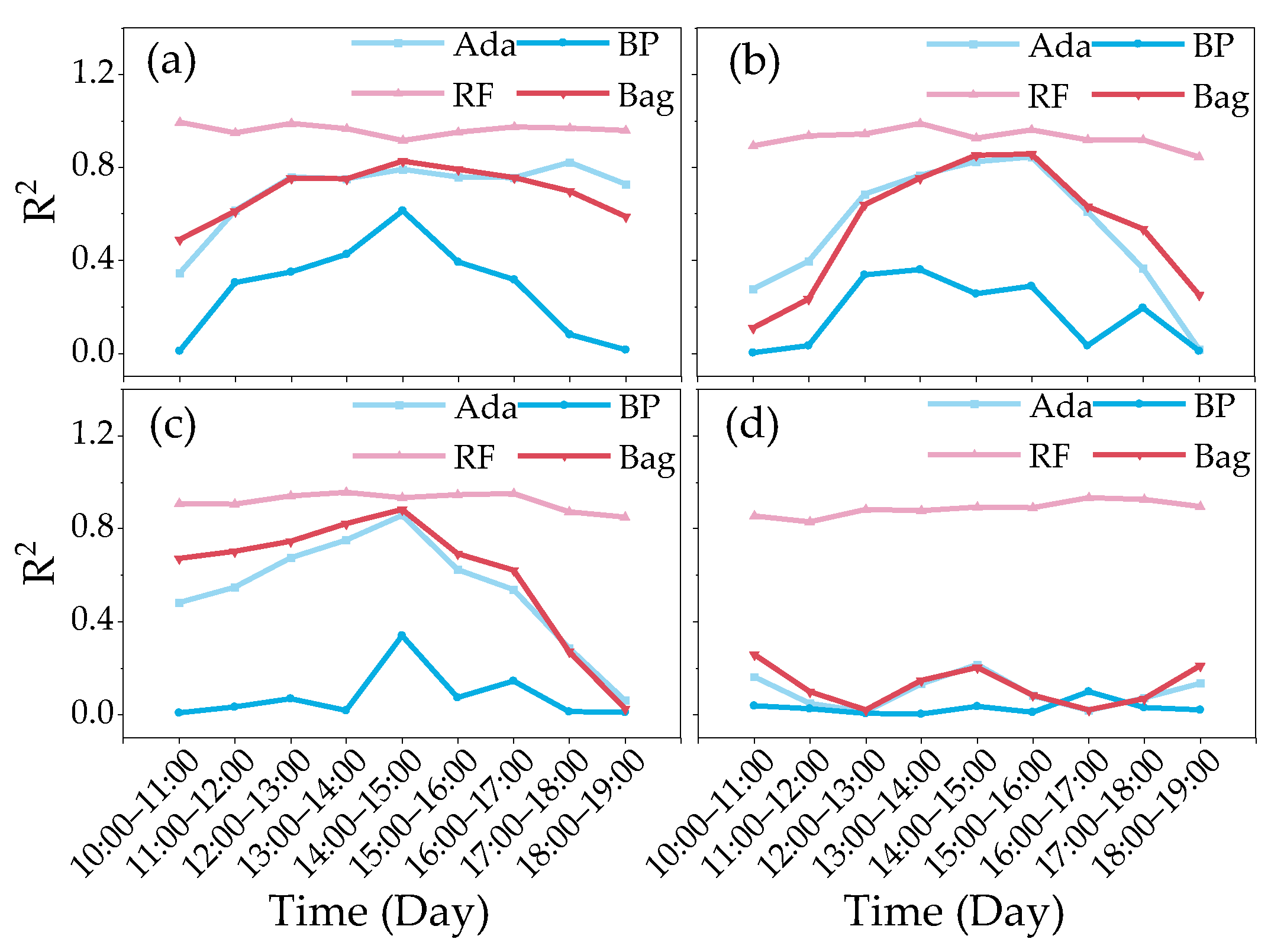
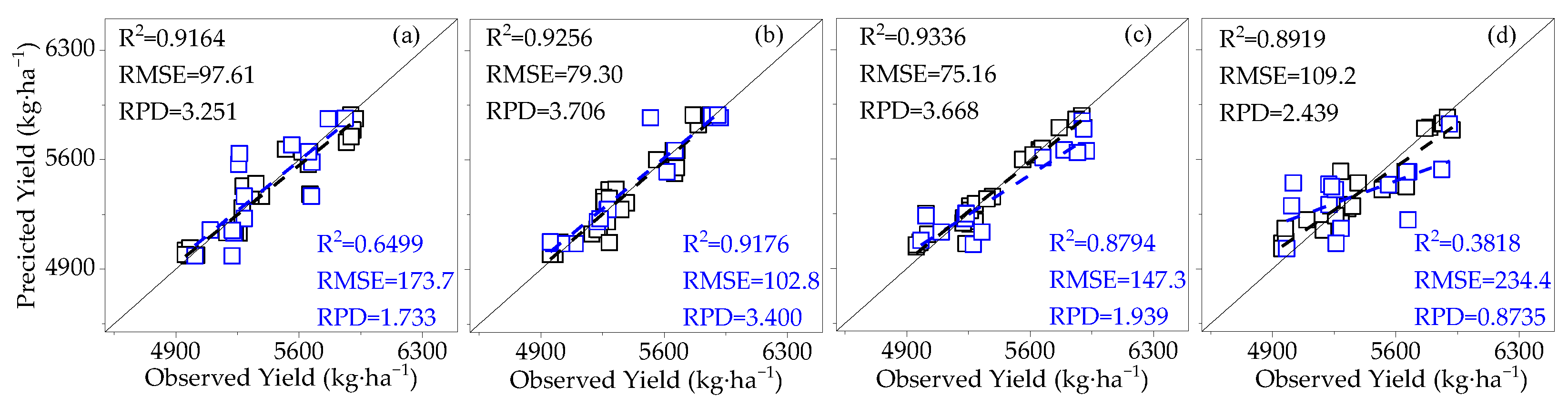
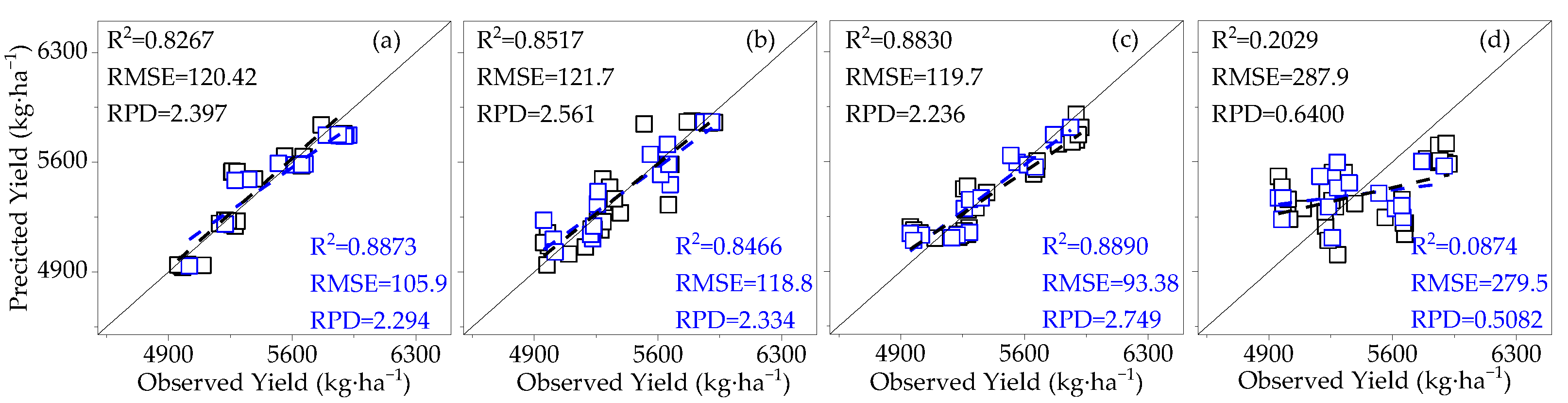
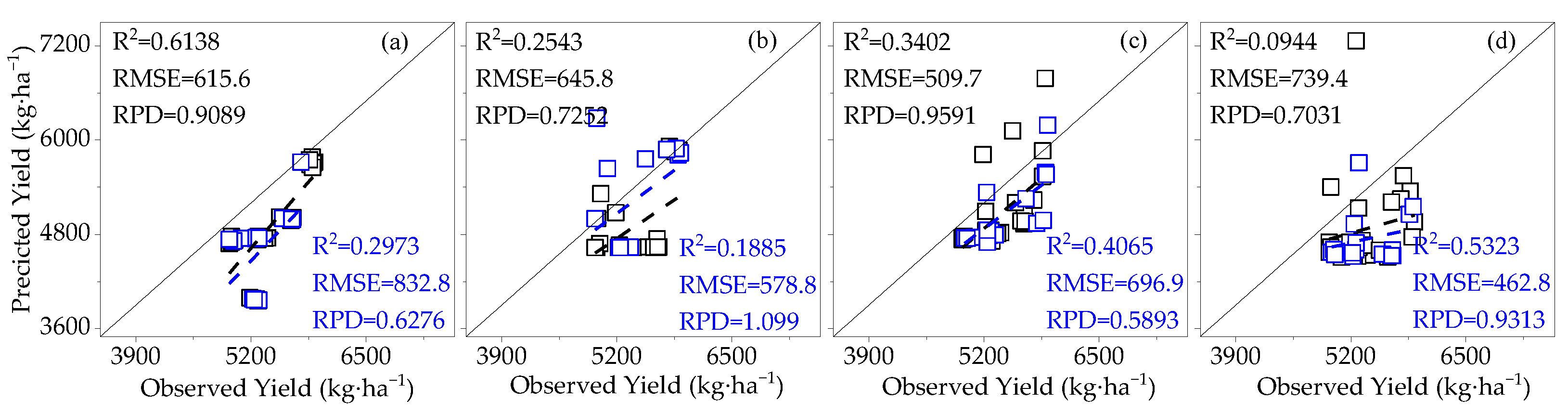
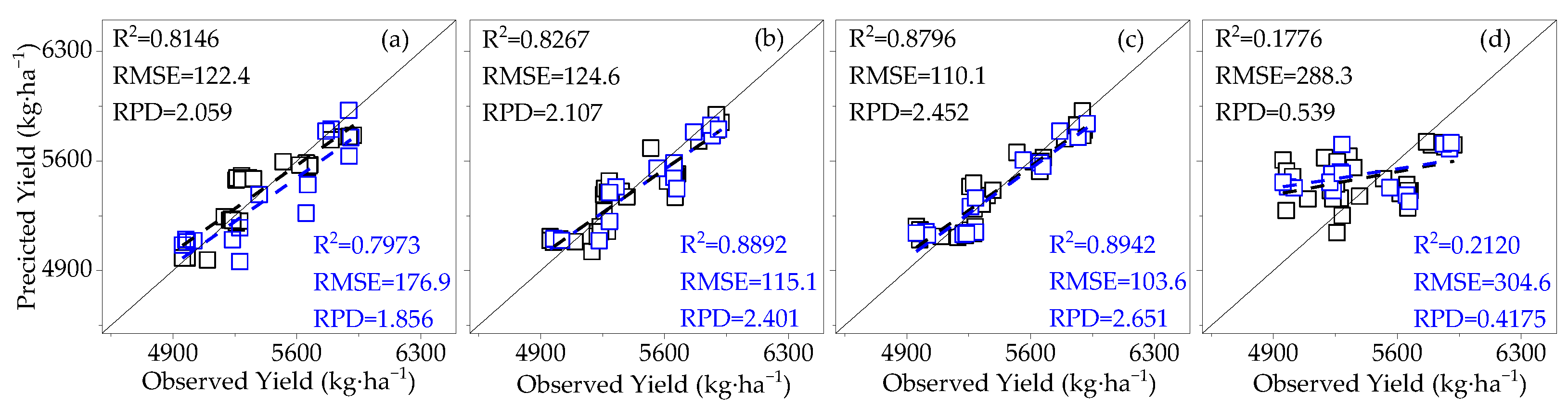
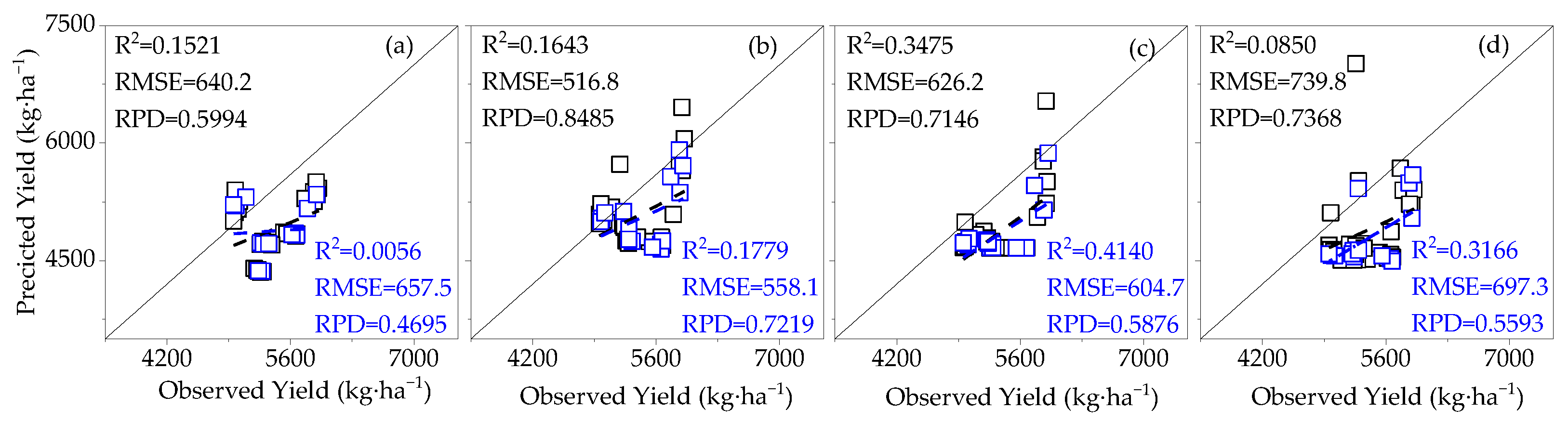
3.3.1. Construction of the Cotton Yield Estimation Model Based on Canopy SIF
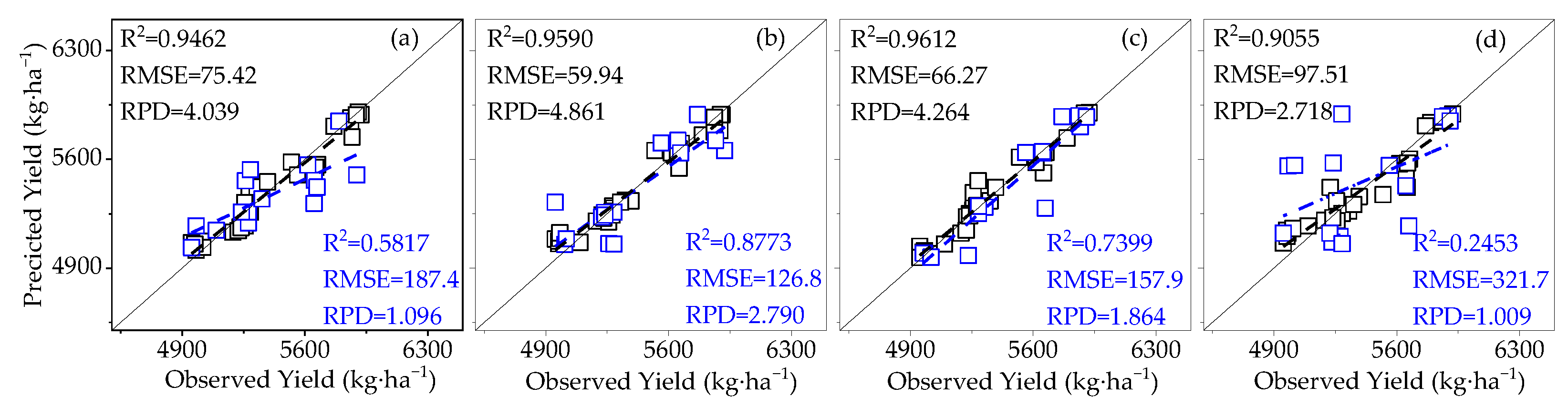
3.3.2. Construction of Cotton Yield Estimation Model Based on Canopy SIFy
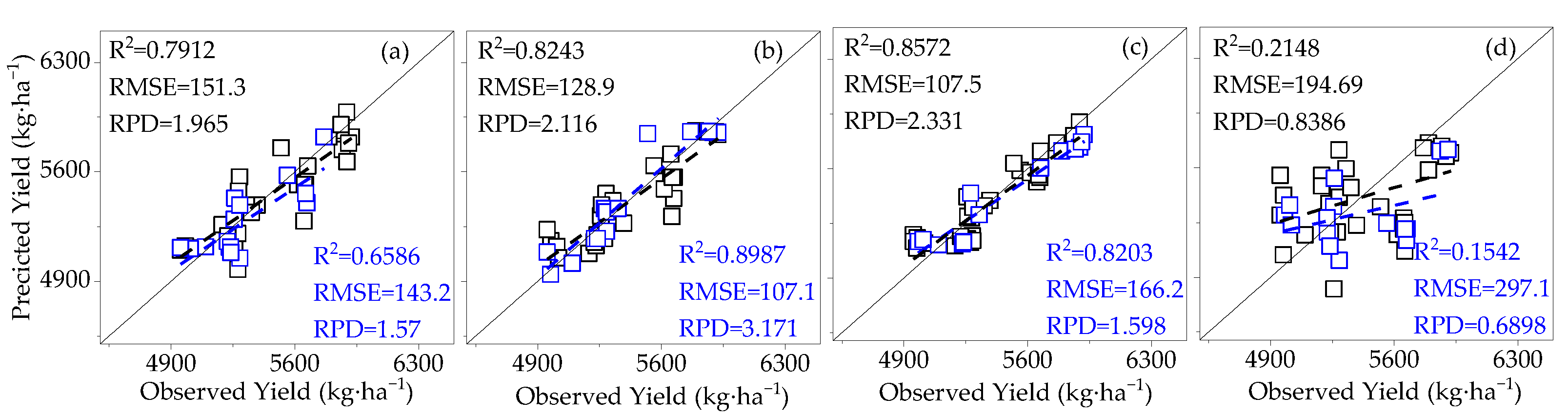
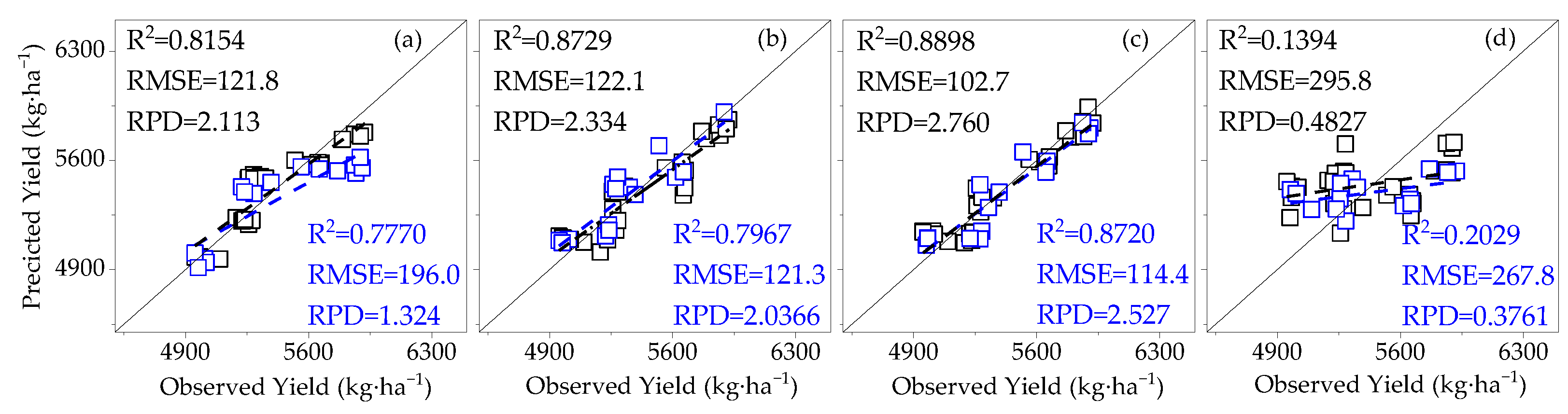
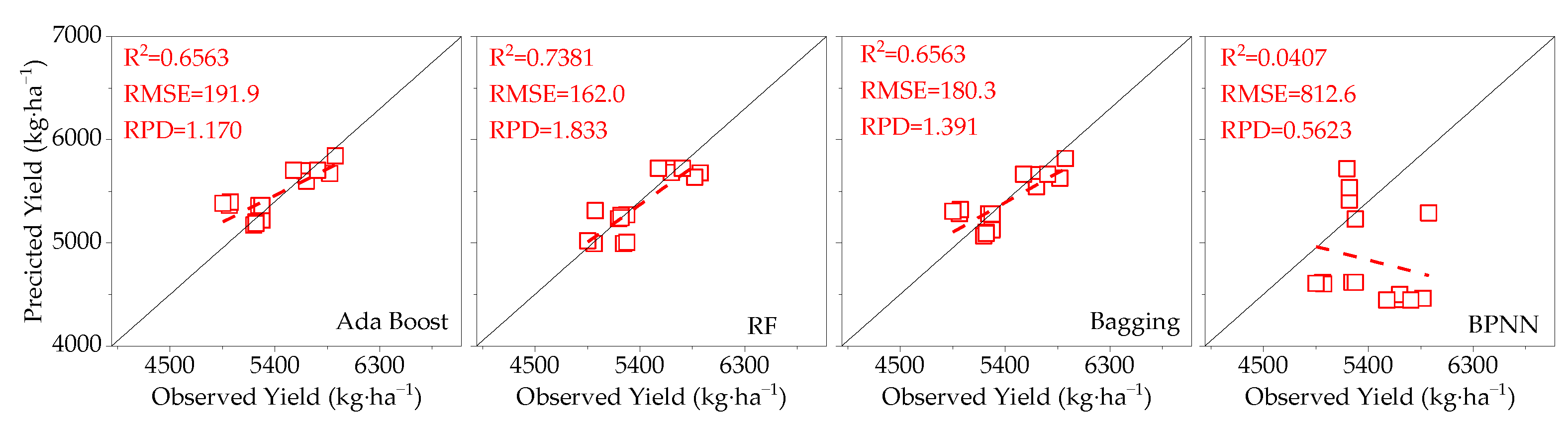
3.4. Model Validation
4. Discussion
5. Conclusions
- The effects of LAI and AGB on cotton canopy SIF and cotton yield were similar. Throughout the reproductive period of cotton, the LAI, AGB, and canopy SIF all gradually increased from the budding period to the flowering and boll period and began to decline in the boll period. The correlation coefficients of LAI and AGB with cotton yield and canopy SIF both increased from the budding period, reached a maximum during the flowering and boll period, and decreased in the boll period, all of which were significantly positively correlated. The trend of the R2 of the cotton yield model based on SIF and SIFy closely follows the pattern of LAI and AGB;
- At different monitoring time periods, the R2 of the cotton yield estimation model based on SIF and SIFy showed a gradual increase from 10:00 to 14:00 and a gradual decrease from 15:00 to 19:00, and the optimal observation time for the cotton canopy SIF to estimate the yield was 14:00–15:00. The R2 of the cotton yield model based on SIF and SIFy increased with the course of fertility from the budding period to the flowering and boll period and decreased in the boll period, and the optimal growth period for the estimation model was the flowering and boll period;
- Compared to SIF, SIFy has a superior estimation of yield. The best yield estimation model based on the RF algorithm for canopy SIFy parameters at 14:00–15:00 during the flowering and boll period (R2 = 0.9612, RMSE = 66.27 kg·ha−1, RPD = 4.264) was followed by the model utilizing the Bagging algorithm (R2 = 0.8898) and the Ada Boost algorithm (R2 = 0.8796). Through verification, the applicability of the model is proved.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelraheem, A.; Esmaeili, N.; O’Connell, M.; Zhang, J.F. Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crops Prod. 2019, 130, 118–129. [Google Scholar] [CrossRef]
- Ashapure, A.; Jung, J.H.; Chang, A.J.; Oh, S.; Yeom, J.; Maeda, M.; Maeda, A.; Dube, N.; Landivar, J.; Hague, S.; et al. Developing a machine learning based cotton yield estimation framework using multi-temporal UAS data. ISPRS-J. Photogramm. Remote Sens. 2020, 169, 180–194. [Google Scholar] [CrossRef]
- Pazhanivelan, S.; Kumaraperumal, R.; Shanmugapriya, P.; Sudarmanian, N.S.; Sivamurugan, A.P.; Satheesh, S. Quantification of Biophysical Parameters and Economic Yield in Cotton and Rice Using Drone Technology. Agriculture 2023, 13, 1668. [Google Scholar] [CrossRef]
- Singh, J.; Gamble, A.V.; Brown, S.; Campbell, B.T.; Jenkins, J.; Koebernick, J.; Bartley, P.C.; Sanz-Saez, A. 65 years of cotton lint yield progress in the USA: Uncovering key influential yield components. Field Crops Res. 2023, 302, 10. [Google Scholar] [CrossRef]
- Ibrahim, I.A.E.; Yehia, W.M.B.; Saleh, F.H.; Lamlom, S.F.; Ghareeb, R.Y.; El-Banna, A.A.A.; Abdelsalam, N.R. Impact of Plant Spacing and Nitrogen Rates on Growth Characteristics and Yield Attributes of Egyptian Cotton (Gossypium barbadense L.). Front. Plant Sci. 2022, 13, 916734. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sanchez, J.; Li, C.; Paterson, A.H. Cotton Yield Estimation From Aerial Imagery Using Machine Learning Approaches. Front. Plant Sci. 2022, 13, 870181. [Google Scholar] [CrossRef] [PubMed]
- Mikhailenko, I.M. Estimation of Parameters of Biomass State of Sowing Spring Wheat. Remote Sens. 2022, 14, 1388. [Google Scholar] [CrossRef]
- Sishodia, R.P.; Ray, R.L.; Singh, S.K. Applications of Remote Sensing in Precision Agriculture: A Review. Remote Sens. 2020, 12, 3136. [Google Scholar] [CrossRef]
- Karthikeyan, L.; Chawla, I.; Mishra, A.K. A review of remote sensing applications in agriculture for food security: Crop growth and yield, irrigation, and crop losses. J. Hydrol. 2020, 586, 22. [Google Scholar] [CrossRef]
- Suarez, L.A.; Robson, A.; McPhee, J.; O’Halloran, J.; van Sprang, C. Accuracy of carrot yield forecasting using proximal hyperspectral and satellite multispectral data. Precis. Agric. 2020, 21, 1304–1326. [Google Scholar] [CrossRef]
- Noda, H.M.; Muraoka, H.; Nasahara, K.N. Plant ecophysiological processes in spectral profiles: Perspective from a deciduous broadleaf forest. J. Plant Res. 2021, 134, 737–751. [Google Scholar] [CrossRef]
- Terentev, A.; Dolzhenko, V.; Fedotov, A.; Eremenko, D. Current State of Hyperspectral Remote Sensing for Early Plant Disease Detection: A Review. Sensors 2022, 22, 757. [Google Scholar] [CrossRef]
- Pokhrel, A.; Virk, S.; Snider, J.L.; Vellidis, G.; Hand, L.C.; Sintim, H.Y.; Parkash, V.; Chalise, D.P.; Lee, J.M.; Byers, C. Estimating yield-contributing physiological parameters of cotton using UAV-based imagery. Front. Plant Sci. 2023, 14, 22. [Google Scholar] [CrossRef]
- Pandya, P.; Gontia, N.K. Early crop yield prediction for agricultural drought monitoring using drought indices, remote sensing, and machine learning techniques. J. Water Clim. Change 2023, 18, 4729–4746. [Google Scholar] [CrossRef]
- Han, P.; Zhai, Y.P.; Liu, W.H.; Lin, H.R.; An, Q.S.; Zhang, Q.; Ding, S.G.; Zhang, D.W.; Pan, Z.Y.; Nie, X.H. Dissection of Hyperspectral Reflectance to Estimate Photosynthetic Characteristics in Upland Cotton (Gossypium hirsutum L.) under Different Nitrogen Fertilizer Application Based on Machine Learning Algorithms. Plants 2023, 12, 455. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.P.; Spangenberg, G.; Kant, S. CBM: An IoT Enabled LiDAR Sensor for In-Field Crop Height and Biomass Measurements. Biosensors 2022, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Sun, S.K.; Xue, J.; Gao, Z.H.; Zhao, J.F.; Yin, Y.L.; Gao, F.; Luan, X.B. Crop yield estimation based on assimilation of crop models and remote sensing data: A systematic evaluation. Agric. Syst. 2023, 210, 17. [Google Scholar] [CrossRef]
- Muruganantham, P.; Wibowo, S.; Grandhi, S.; Samrat, N.H.; Islam, N. A Systematic Literature Review on Crop Yield Prediction with Deep Learning and Remote Sensing. Remote Sens. 2022, 14, 1990. [Google Scholar] [CrossRef]
- Skakun, S.; Kalecinski, N.I.; Brown, M.G.L.; Johnson, D.M.; Vermote, E.F.; Roger, J.-C.; Franch, B. Assessing within-Field Corn and Soybean Yield Variability from WorldView-3, Planet, Sentinel-2, and Landsat 8 Satellite Imagery. Remote Sens. 2021, 13, 872. [Google Scholar] [CrossRef]
- Jia, M.; Colombo, R.; Rossini, M.; Celesti, M.; Zhu, J.; Cogliati, S.; Cheng, T.; Tian, Y.; Zhu, Y.; Cao, W.; et al. Estimation of leaf nitrogen content and photosynthetic nitrogen use efficiency in wheat using sun-induced chlorophyll fluorescence at the leaf and canopy scales. Eur. J. Agron. 2021, 122, 126192. [Google Scholar] [CrossRef]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.H.; Ahmad, H.; Li, F.B. Mechanisms Regulating the Dynamics of Photosynthesis Under Abiotic Stresses. Front. Plant Sci. 2021, 11, 25. [Google Scholar] [CrossRef]
- Mu, X.H.; Chen, Y.L. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Somkuti, P.; Bösch, H.; Feng, L.; Palmer, P.I.; Parker, R.J.; Quaife, T. A new space-borne perspective of crop productivity variations over the US Corn Belt. Agric. For. Meteorol. 2020, 281, 11. [Google Scholar] [CrossRef]
- Mohammed, G.H.; Colombo, R.; Middleton, E.M.; Rascher, U.; van der Tol, C.; Nedbal, L.; Goulas, Y.; Perez-Priego, O.; Damm, A.; Meroni, M.; et al. Remote sensing of solar-induced chlorophyll fluorescence (SIF) in vegetation: 50 years of progress. Remote Sens. Environ. 2019, 231, 111177. [Google Scholar] [CrossRef]
- Kimm, H.; Guan, K.Y.; Burroughs, C.H.; Peng, B.; Ainsworth, E.A.; Bernacchi, C.J.; Moore, C.E.; Kumagai, E.; Yang, X.; Berry, J.A.; et al. Quantifying high-temperature stress on soybean canopy photosynthesis: The unique role of sun-induced chlorophyll fluorescence. Glob. Change Biol. 2021, 27, 2403–2415. [Google Scholar] [CrossRef]
- Marrs, J.K.; Reblin, J.S.; Logan, B.A.; Allen, D.W.; Reinmann, A.B.; Bombard, D.M.; Tabachnik, D.; Hutyra, L.R. Solar-Induced Fluorescence Does Not Track Photosynthetic Carbon Assimilation Following Induced Stomatal Closure. Geophys. Res. Lett. 2020, 47, e2020GL087956. [Google Scholar] [CrossRef]
- Bacour, C.; Maignan, F.; MacBean, N.; Porcar-Castell, A.; Flexas, J.; Frankenberg, C.; Peylin, P.; Chevallier, F.; Vuichard, N.; Bastrikov, V. Improving Estimates of Gross Primary Productivity by Assimilating Solar-Induced Fluorescence Satellite Retrievals in a Terrestrial Biosphere Model Using a Process-Based SIF Model. J. Geophys. Res. Biogeosci. 2019, 26, 3281–3306. [Google Scholar] [CrossRef]
- Campbell, P.K.E.; Huemmrich, K.F.; Middleton, E.M.; Ward, L.A.; Julitta, T.; Daughtry, C.S.T.; Burkart, A.; Russ, A.L.; Kustas, W.P. Diurnal and Seasonal Variations in Chlorophyll Fluorescence Associated with Photosynthesis at Leaf and Canopy Scales. Remote Sens. 2019, 11, 488. [Google Scholar] [CrossRef]
- Zeng, Y.L.; Badgley, G.; Dechant, B.; Ryu, Y.; Chen, M.; Berry, J.A. A practical approach for estimating the escape ratio of near-infrared solar-induced chlorophyll fluorescence. Remote Sens. Environ. 2019, 232, 14. [Google Scholar] [CrossRef]
- Liu, J.G.; Huffman, T.; Qian, B.D.; Shang, J.L.; Li, Q.M.; Dong, T.F.; Davidson, A.; Jing, Q. Crop Yield Estimation in the Canadian Prairies Using Terra/MODIS-Derived Crop Metrics. IEEE J. Sel. Top. Appl. Earth Observ. Remote Sens. 2020, 13, 2685–2697. [Google Scholar] [CrossRef]
- Marshall, M.; Tu, K.; Brown, J. Optimizing a remote sensing production efficiency model for macro-scale GPP and yield estimation in agroecosystems. Remote Sens. Environ. 2018, 217, 258–271. [Google Scholar] [CrossRef]
- He, L.Y.; Magney, T.; Dutta, D.; Yin, Y.; Köhler, P.; Grossmann, K.; Stutz, J.; Dold, C.; Hatfield, J.; Guan, K.Y.; et al. From the Ground to Space: Using Solar-Induced Chlorophyll Fluorescence to Estimate Crop Productivity. Geophys. Res. Lett. 2020, 47, 12. [Google Scholar] [CrossRef]
- Sloat, L.L.; Lin, M.; Butler, E.E.; Johnson, D.; Holbrook, N.M.; Huybers, P.J.; Lee, J.E.; Mueller, N.D. Evaluating the benefits of chlorophyll fluorescence for in-season crop productivity forecasting. Remote Sens. Environ. 2021, 260, 14. [Google Scholar] [CrossRef]
- Guan, K.; Berry, J.A.; Zhang, Y.; Joiner, J.; Guanter, L.; Badgley, G.; Lobell, D.B. Improving the monitoring of crop productivity using spaceborne solar-induced fluorescence. Glob. Change Biol. 2016, 22, 716–726. [Google Scholar] [CrossRef]
- Joshi, A.; Pradhan, B.; Chakraborty, S.; Behera, M.D. Winter wheat yield prediction in the conterminous United States using solar-induced chlorophyll fluorescence data and XGBoost and random forest algorithm. Ecol. Inform. 2023, 77, 102194. [Google Scholar] [CrossRef]
- Nichol, C.J.; Drolet, G.; Porcar-Castell, A.; Wade, T.; Sabater, N.; Middleton, E.M.; MacLellan, C.; Levula, J.; Mammarella, I.; Vesala, T.; et al. Diurnal and Seasonal Solar Induced Chlorophyll Fluorescence and Photosynthesis in a Boreal Scots Pine Canopy. Remote Sens. 2019, 11, 273. [Google Scholar] [CrossRef]
- Yang, P.Q.; van der Tol, C.; Campbell, P.K.E.; Middleton, E.M. Fluorescence Correction Vegetation Index (FCVI): A physically based reflectance index to separate physiological and non-physiological information in far-red sun-induced chlorophyll fluorescence. Remote Sens. Environ. 2020, 240, 16. [Google Scholar] [CrossRef]
- Yoshida, Y.; Joiner, J.; Tucker, C.; Berry, J.; Lee, J.E.; Walker, G.; Reichle, R.; Koster, R.; Lyapustin, A.; Wang, Y. The 2010 Russian drought impact on satellite measurements of solar-induced chlorophyll fluorescence: Insights from modeling and comparisons with parameters derived from satellite reflectances. Remote Sens. Environ. 2015, 166, 163–177. [Google Scholar] [CrossRef]
- Dechant, B.; Ryu, Y.; Badgley, G.; Zeng, Y.L.; Berry, J.A.; Zhang, Y.G.; Goulas, Y.; Li, Z.H.; Zhang, Q.; Kang, M.; et al. Canopy structure explains the relationship between photosynthesis and sun-induced chlorophyll fluorescence in crops. Remote Sens. Environ. 2020, 241, 17. [Google Scholar] [CrossRef]
- Hwang, Y.; Kim, J.; Ryu, Y. Canopy structural changes explain reductions in canopy-level solar induced chlorophyll fluorescence in Prunus yedoensis seedlings under a drought stress condition. Remote Sens. Environ. 2023, 296, 15. [Google Scholar] [CrossRef]
- Wu, G.; Jiang, C.; Kimm, H.; Wang, S.; Bernacchi, C.; Moore, C.E.; Suyker, A.; Yang, X.; Magney, T.; Frankenberg, C.; et al. Difference in seasonal peak timing of soybean far-red SIF and GPP explained by canopy structure and chlorophyll content. Remote Sens. Environ. 2022, 279, 113104. [Google Scholar] [CrossRef]
- Wang, X.; Pan, S.; Pan, N.; Pan, P. Grassland productivity response to droughts in northern China monitored by satellite-based solar-induced chlorophyll fluorescence. Sci. Total Environ. 2022, 830, 154550. [Google Scholar] [CrossRef]
- Magney, T.S.; Barnes, M.L.; Yang, X. On the Covariation of Chlorophyll Fluorescence and Photosynthesis Across Scales. Geophys. Res. Lett. 2020, 47, 7. [Google Scholar] [CrossRef]
- Zhang, Y.; Guanter, L.; Berry, J.A.; van der Tol, C.; Yang, X.; Tang, J.; Zhang, F. Model-based analysis of the relationship between sun-induced chlorophyll fluorescence and gross primary production for remote sensing applications. Remote Sens. Environ. 2016, 187, 145–155. [Google Scholar] [CrossRef]
- Peng, B.; Guan, K.; Zhou, W.; Jiang, C.; Frankenberg, C.; Sun, Y.; He, L.; Kohler, P. Assessing the benefit of satellite-based Solar-Induced Chlorophyll Fluorescence in crop yield prediction. Int. J. Appl. Earth Obs. Geoinf. 2020, 90, 102126. [Google Scholar] [CrossRef]
- Condran, S.; Bewong, M.; Islam, M.Z.; Maphosa, L.; Zheng, L. Machine Learning in Precision Agriculture: A Survey on Trends, Applications and Evaluations Over Two Decades. IEEE Access 2022, 10, 73786–73803. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Wang, X.; Chen, B.; Chen, J.; Wang, J.; Huang, M.; Wang, Z.; Ma, L.; Wang, P.; et al. Exploring the superiority of solar-induced chlorophyll fluorescence data in predicting wheat yield using machine learning and deep learning methods. Comput. Electron. Agric. 2022, 192, 106612. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Leng, P.; Shang, G.-F.; Zhang, X.; Li, Z.-L. Sun-induced chlorophyll fluorescence is superior to satellite vegetation indices for predicting summer maize yield under drought conditions. Comput. Electron. Agric. 2023, 205, 107615. [Google Scholar] [CrossRef]
- Meroni, M.; Waldner, F.; Seguini, L.; Kerdiles, H.; Rembold, F. Yield forecasting with machine learning and small data: What gains for grains? Agric. For. Meteorol. 2021, 308, 13. [Google Scholar] [CrossRef]
- Liu, Z.G.; He, X.; Yang, P.Q.; Jiang, H.; Xu, S.; Zhao, H.R.; Ren, S.X.; Chen, M. Diurnal Pattern of Sun-Induced Chlorophyll Fluorescence as Reliable Indicators of Crop Water Stress. IEEE Geosci. Remote Sens. Lett. 2023, 20, 5. [Google Scholar] [CrossRef]
- Damm, A.; Erler, A.; Hillen, W.; Meroni, M.; Schaepman, M.E.; Verhoef, W.; Rascher, U. Modeling the impact of spectral sensor configurations on the FLD retrieval accuracy of sun-induced chlorophyll fluorescence. Remote Sens. Environ. 2011, 115, 1882–1892. [Google Scholar] [CrossRef]
- Daumard, F.; Goulas, Y.; Champagne, S.; Fournier, A.; Ounis, A.; Olioso, A.; Moya, I. Continuous Monitoring of Canopy Level Sun-Induced Chlorophyll Fluorescence During the Growth of a Sorghum Field. IEEE Trans. Geosci. Remote Sens. 2012, 50, 4292–4300. [Google Scholar] [CrossRef]
- Freund, Y.; Schapire, R.E. A decision-theoretic generalization of on-line learning and an application to boosting. J. Comput. Syst. Sci. 1997, 55, 119–139. [Google Scholar] [CrossRef]
- Pino-Mejias, R.; Jimenez-Gamero, M.-D.; Cubiles-de-la-Vega, M.-D.; Pascual-Acosta, A. Reduced bootstrap aggregating of learning algorithms. Pattern Recognit. Lett. 2008, 29, 265–271. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Irmak, A.; Jones, J.W.; Batchelor, W.D.; Irmak, S.; Boote, K.J.; Paz, J.O. Artificial neural network model as a data analysis tool in precision farming. Trans. ASABE 2006, 49, 2027–2037. [Google Scholar] [CrossRef]
- Grundy, P.R.; Yeates, S.J.; Bell, K.L. Cotton production during the tropical monsoon season. II—Biomass accumulation, partitioning and RUE in response to boll loss and compensation. Field Crops Res. 2020, 255, 11. [Google Scholar] [CrossRef]
- He, L.M.; Mostovoy, G. Cotton Yield Estimate Using Sentinel-2 Data and an Ecosystem Model over the Southern US. Remote Sens. 2019, 11, 2000. [Google Scholar] [CrossRef]
- Cui, T.X.; Sun, R.; Xiao, Z.Q.; Liang, Z.Y.; Wang, J. Simulating spatially distributed solar-induced chlorophyll fluorescence using a BEPS-SCOPE coupling framework. Agric. For. Meteorol. 2020, 295, 20. [Google Scholar] [CrossRef]
- Sakai, Y.; Kobayashi, H.; Kato, T. FLiES-SIF version 1.0: Three-dimensional radiative transfer model for estimating solar induced fluorescence. Geosci. Model Dev. 2020, 13, 4041–4066. [Google Scholar] [CrossRef]
- Song, L.; Guanter, L.; Guan, K.Y.; You, L.Z.; Huete, A.; Ju, W.M.; Zhang, Y.G. Satellite sun-induced chlorophyll fluorescence detects early response of winter wheat to heat stress in the Indian Indo-Gangetic Plains. Glob. Change Biol. 2018, 24, 4023–4037. [Google Scholar] [CrossRef]
- Wang, N.; Suomalainen, J.; Bartholomeus, H.; Kooistra, L.; Masiliunas, D.; Clevers, J.G.P.W. Diurnal variation of sun-induced chlorophyll fluorescence of agricultural crops observed from a point-based spectrometer on a UAV. Int. J. Appl. Earth Obs. Geoinf. 2021, 96, 102276. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, Y.; Hu, M.; Wang, F.; Huang, H.; Zhang, J. The Links between Canopy Solar-Induced Chlorophyll Fluorescence and Gross Primary Production Responses to Meteorological Factors in the Growing Season in Deciduous Broadleaf Forest. Remote Sens. 2021, 13, 2363. [Google Scholar] [CrossRef]
- Goulas, Y.; Fournier, A.; Daumard, F.; Champagne, S.; Ounis, A.; Marloie, O.; Moya, I. Gross Primary Production of a Wheat Canopy Relates Stronger to Far Red Than to Red Solar-Induced Chlorophyll Fluorescence. Remote Sens. 2017, 9, 97. [Google Scholar] [CrossRef]
- Joiner, J.; Yoshida, Y.; Köehler, P.; Campbell, P.; Frankenberg, C.; van der Tol, C.; Yang, P.Q.; Parazoo, N.; Guanter, L.; Sun, Y. Systematic Orbital Geometry-Dependent Variations in Satellite Solar-Induced Fluorescence (SIF) Retrievals. Remote Sens. 2020, 12, 2346. [Google Scholar] [CrossRef]
- Loayza, H.; Moya, I.; Quiroz, R.; Ounis, A.; Goulas, Y. Active and passive chlorophyll fluorescence measurements at canopy level on potato crops. Evidence of similitude of diurnal cycles of apparent fluorescence yields. Photosynth. Res. 2023, 155, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Cordon, G.B.; Lagorio, M.G. Optical properties of the adaxial and abaxial faces of leaves.: Chlorophyll fluorescence, absorption and scattering coefficients. Photochem. Photobiol. Sci. 2007, 6, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.Q.; van der Tol, C.; Campbell, P.K.E.; Middleton, E.M. Unraveling the physical and physiological basis for the solar- induced chlorophyll fluorescence and photosynthesis relationship using continuous leaf and canopy measurements of a corn crop. Biogeosciences 2021, 18, 441–465. [Google Scholar] [CrossRef]
- Yang, G.; Tang, H.; Tong, J.; Nie, Y.; Zhang, X. Effect of fertilization frequency on cotton yield and biomass accumulation. Field Crops Res. 2012, 125, 161–166. [Google Scholar] [CrossRef]
- Cai, Y.P.; Guan, K.Y.; Lobell, D.; Potgieter, A.B.; Wang, S.W.; Peng, J.; Xu, T.F.; Asseng, S.; Zhang, Y.G.; You, L.Z.; et al. Integrating satellite and climate data to predict wheat yield in Australia using machine learning approaches. Agric. For. Meteorol. 2019, 274, 144–159. [Google Scholar] [CrossRef]
- Gopal, P.S.M.; Bhargavi, R. Performance Evaluation of Best Feature Subsets for Crop Yield Prediction Using Machine Learning Algorithms. Appl. Artif. Intell. 2019, 33, 621–642. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Z.; Tao, F.; Zhang, L.; Luo, Y.; Zhang, J.; Han, J.; Xie, J. Integrating Multi-Source Data for Rice Yield Prediction across China using Machine Learning and Deep Learning Approaches. Agric. For. Meteorol. 2021, 297, 108275. [Google Scholar] [CrossRef]
- Han, S.; Liu, Z.; Chen, Z.; Jiang, H.; Xu, S.; Zhao, H.; Ren, S. Using High-Frequency PAR Measurements to Assess the Quality of the SIF Derived from Continuous Field Observations. Remote Sens. 2022, 14, 2083. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z.; Liu, L.; Lu, X.; Chen, J.; Du, S.; Zou, C. Modelling the influence of incident radiation on the SIF-based GPP estimation for maize. Agric. For. Meteorol. 2021, 307, 108522. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Z.; Han, S.; Chen, Z.; He, X.; Zhao, H.; Ren, S. Exploring the Sensitivity of Solar-Induced Chlorophyll Fluorescence at Different Wavelengths in Response to Drought. Remote Sens. 2023, 15, 1077. [Google Scholar] [CrossRef]



| Month | Average High Temperatures | Average Low Temperatures | Total Rainfall |
|---|---|---|---|
| April | 18 °C | 4 °C | 17.2 mm |
| May | 24 °C | 12 °C | 10.7 mm |
| June | 33 °C | 18 °C | 36.2 mm |
| July | 35 °C | 20 °C | 11.7 mm |
| August | 33 °C | 18 °C | 45.1 mm |
| September | 25 °C | 12 °C | 25.3 mm |
| October | 20 °C | 6 °C | 0.1 mm |
| Parameters | Presentation |
|---|---|
| Spectral range | 640–800 nm, HR 200–1000 nm |
| Spectral resolution | 0.39 nm, HR 1 nm |
| Spectral response | Up to 70% quantum efficiency at 780 nm, up to 50% quantum efficiency at 680 nm |
| Optical transmission mode | Bifurcated fibers, prisms, multi-channel MPM |
| Temperature control system | Temperature range 25 ± 1.00 °C, humidity level < 60% (except under extreme conditions) |
| Fiber optics | 600 µm, 1000 µm |
| Budding Period | Flowering Period | Flowering and Boll Period | Boll Period | |
|---|---|---|---|---|
| Yield and AGB | 0.420 * | 0.858 ** | 0.868 ** | 0.556 ** |
| Yield and LAI | 0.494 * | 0.691 ** | 0.819 ** | 0.690 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ding, Y.; Yao, Q.; Ma, L.; Ma, Y.; Yang, M.; Qin, S.; Xu, F.; Zhang, Z.; Gao, Z. Modeling of Cotton Yield Estimation Based on Canopy Sun-Induced Chlorophyll Fluorescence. Agronomy 2024, 14, 364. https://doi.org/10.3390/agronomy14020364
Wang H, Ding Y, Yao Q, Ma L, Ma Y, Yang M, Qin S, Xu F, Zhang Z, Gao Z. Modeling of Cotton Yield Estimation Based on Canopy Sun-Induced Chlorophyll Fluorescence. Agronomy. 2024; 14(2):364. https://doi.org/10.3390/agronomy14020364
Chicago/Turabian StyleWang, Hongyu, Yiren Ding, Qiushuang Yao, Lulu Ma, Yiru Ma, Mi Yang, Shizhe Qin, Feng Xu, Ze Zhang, and Zhe Gao. 2024. "Modeling of Cotton Yield Estimation Based on Canopy Sun-Induced Chlorophyll Fluorescence" Agronomy 14, no. 2: 364. https://doi.org/10.3390/agronomy14020364
APA StyleWang, H., Ding, Y., Yao, Q., Ma, L., Ma, Y., Yang, M., Qin, S., Xu, F., Zhang, Z., & Gao, Z. (2024). Modeling of Cotton Yield Estimation Based on Canopy Sun-Induced Chlorophyll Fluorescence. Agronomy, 14(2), 364. https://doi.org/10.3390/agronomy14020364





