The Role of Endogenous Brassinosteroids in the Mechanisms Regulating Plant Reactions to Various Abiotic Stresses
Abstract
1. Introduction
2. BR Biosynthetic Pathway and Key Genes
3. Signal Transduction Pathway of BRs
4. Responses of Endogenous BRs to Different Abiotic Stresses
4.1. Drought Stress and BRs
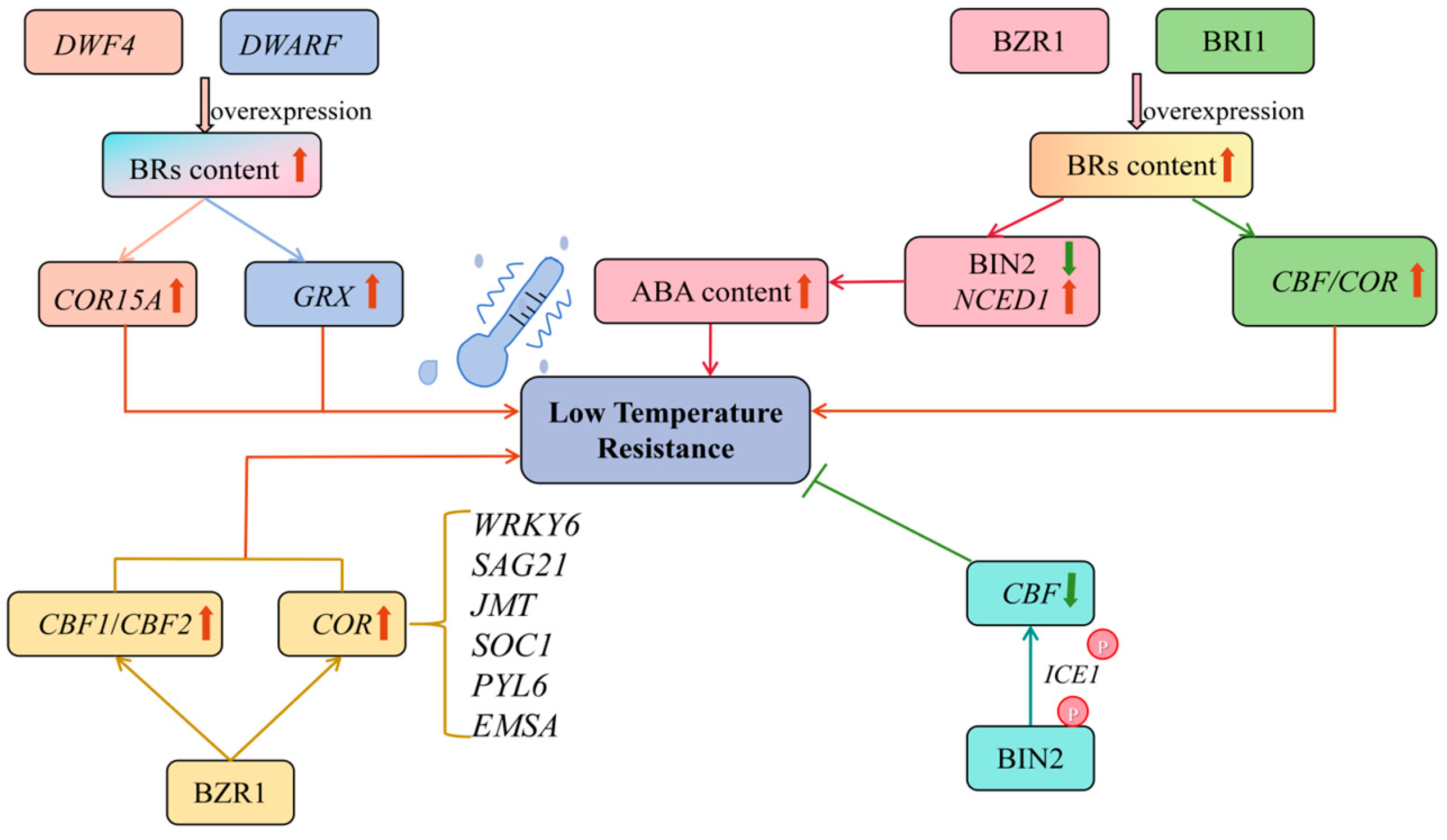
4.2. Temperature Stress and BRs
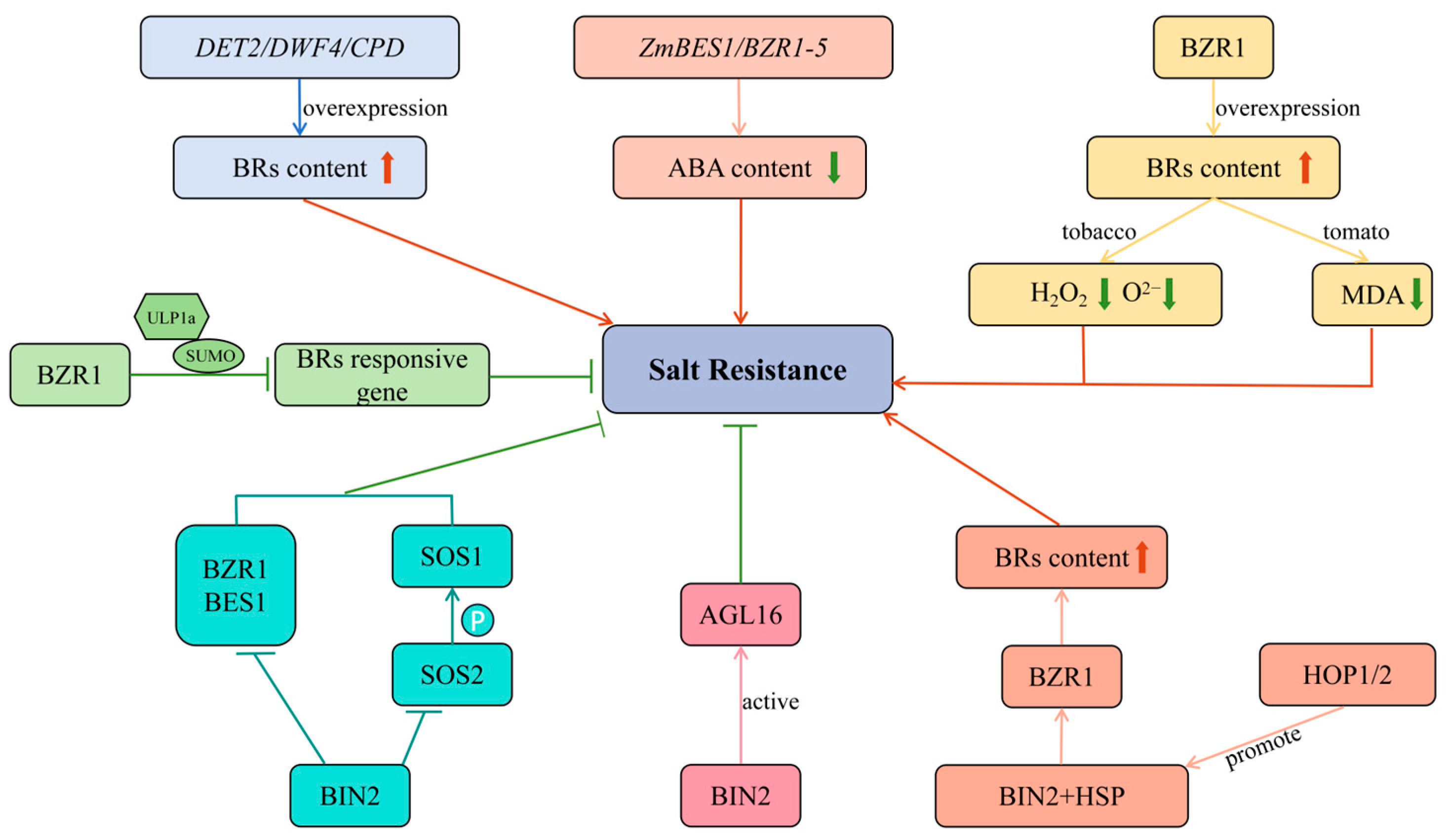
4.3. Salt Stress and BRs
4.4. Heavy Metal Stress and BRs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ response to abiotic stress: Mechanisms and strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef]
- Kang, B.; Duan, M.; Peng, B.; Li, C.; Wu, H.; Gu, W.; Gu, Q. Identification of resistance to bacterial fruit spot disease in watermelon cultivars. J. Plant Prot. 2022, 49, 547–552. [Google Scholar] [CrossRef]
- Quan, M.; Xu, J.; Yin, J.; Yang, Z.; Tan, W. Physiological mechanism of brassinolide regulation in plant response to abiotic stress. J. Plant Prot. 2023, 1, 22–31. [Google Scholar] [CrossRef]
- Singh, A.; Roychoudhury, A. Abscisic acid in plants under abiotic stress: Crosstalk with major phytohormones. Plant Cell Rep. 2023, 42, 961–974. [Google Scholar] [CrossRef]
- Liu, M.; Li, M.; Liu, K.; Sui, N. Effects of drought stress on seed germination and seedling growth of different maize varieties. J. Agric. Sci. 2015, 7, 231–240. [Google Scholar] [CrossRef]
- Bahuguna, R.N.; Jagadish, K.S.V. Temperature regulation of plant phenological development. Environ. Exp. Bot. 2015, 111, 83–90. [Google Scholar] [CrossRef]
- Shi, X.; Xue, X.; Yang, Y.; Zhang, M.; Zhang, X. Effects of 2,4-epibrassinolide on growth and physiological characteristics of peanut seedlings under low temperature stress. Chin. J. Oil Crops 2023, 45, 341–348. [Google Scholar] [CrossRef]
- Lei, Y.; Qiao, N.; Bai, Y.; Yang, Y. Effect of epibrassinolide on physiological characteristics and stress resistance genes of pepper seedlings under heavy cadmium stress. J. North China Agric. Sci. 2021, 36, 99–106. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, J.; Zhong, C.; Zhu, L.; Cao, X.; Yu, S.; Bohr, J.A.; Hu, J. Effects of salt stress on rice growth, development characteristics, and the regulating ways: A review. J. Integr. Agric. 2017, 16, 2357–2374. [Google Scholar] [CrossRef]
- Grove, M.D.; Spencer, G.F.; Rohwedder, W.K.; Mandava, N.; Cook, J.L.C. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 1979, 281, 216–217. [Google Scholar] [CrossRef]
- Mitchell, J.W.; Mandava, N.; Worley, J.F.; Plimmer, J.R.; Smith, M.V. Brassins-a new family of plant hormones from rape pollen. Nature 1970, 225, 1065–1066. [Google Scholar] [CrossRef]
- Khripach, V.; Zhabinskii, v.; Groot, A. Twenty years of brassinosteroids: Steroidal plant hormones warrant better crops for the 21st century. Ann. Bot. 2000, 86, 441–447. [Google Scholar] [CrossRef]
- Nomur, T.; Bishop, G.J. Cytochrome P450s in plant steroid hormone synthesis and metabolism. Phytochem. Rev. 2006, 5, 421–432. [Google Scholar] [CrossRef]
- Aghaee, P.; Rahmani, F. Seed priming with 24-Epibrassinolide alters growth and phenylpropanoid pathway in flax in response to water deficit. J. Agric. Sci. Technol. 2020, 22, 1039–1052. Available online: http://jast.modares.ac.ir/article-23-19677-en.html (accessed on 14 December 2023).
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) role in plant development and coping with different stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.Q.; Messias, W.F.S.; Pereira, Y.C.; Da Silva, B.R.S.; Lobato, E.M.S.G.; Alyemeni, M.N. Pretreatment with 24-epibrassinolide synergistically protects root structures and chloroplastic pigments and upregulates antioxidant enzymes and biomass in na+-stressed tomato plants. J. Plant Growth Regul. 2022, 41, 2869–2885. [Google Scholar] [CrossRef]
- Hafeez, M.B.; Zahra, N.; Zahra, K.; Raza, A.; Batool, A.; Shaukat, K.; Khan, S. Brassinosteroids: Molecular and physiological responses in plant growth and abiotic stresses. Plant Stress 2021, 2, 100029. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.H.; Lee, S.G.; An, S.; Lee, H.S.; Choi, C.K.; Kim, S.K. Foliar application of biostimulants affects physiological responses and improves heat stress tolerance in Kimchi cabbage. Hortic. Environ. Biotechnol. 2020, 61, 207. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, Y.; Irfan, A.R.; Liu, X.; Yu, Q.; Zhang, Q.; Yang, D. Exogenous brassinolide enhances the growth and cold resistance of maize (Zea mays L.) seedlings under chilling Stress. Agronomy 2020, 10, 488. [Google Scholar] [CrossRef]
- Shah, A.A.; Ahmed, S.; Yasin, N.A. 24-epibrassinolide triggers cadmium stress mitigation in Cucumis sativus through intonation of antioxidant system. S. Afr. J. Bot. 2019, 127, 349–360. [Google Scholar] [CrossRef]
- Belkhadir, Y.; Jaillais, Y.; Epple, P.; Balsemao-Pires, E.; Dangl, J.L.; Chory, J. Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proc. Natl. Acad. Sci. USA 2012, 109, 297–302. [Google Scholar] [CrossRef]
- Serna, M.; Hernández, F.; Coll, F.; Coll, Y.; Amorós, A. Brassinosteroid analogues effects on the yield and quality parameters of greenhouse-grown pepper (Capsicum annuum L.). Plant Growth Regul. 2012, 68, 333–342. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, X.; Yu, G.; Wang, J.; Wu, J.; Wang, M.; Yang, Y.; Shi, K.; Yu, Y.; Chen, Z.; et al. Brassinosteroids play a critical role in the regulation of pesticide metabolism in crop plants. Sci. Rep. 2015, 5, 9018. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Shen, Y.; Pu, R.; Hou, M.; Wei, Q.; Zhang, X.; Li, G.; Ren, H.; Wu, G. Brassinosteroids synthesised by CYP85A/A1 but not CYP85A2 function via a BRI1-like receptor but not via BRI1 in Picea abies. J. Exp. Bot. 2021, 72, 1748–1763. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zuo, Q.; Tu, S. Research progress on biosynthesis and metabolism of brassinolide. J. Plant Physiol. 2015, 51, 1787–1798. [Google Scholar] [CrossRef]
- Kim, T.W.; Hwang, J.Y.; Kim, Y.S.; Joo, S.H.; Chang, S.C.; Lee, J.S.; Takatsuto, S.; Kim, S.K. Arabidopsis CYP85A2, a cytochrome P450, mediates the Baeyer-Villiger oxidation of castasterone to brassinolide in brassinosteroid biosynthesis. Plant Cell 2005, 17, 2397–2412. [Google Scholar] [CrossRef]
- Zhao, B.; Li, J. Regulation of brassinosteroid biosynthesis and inactivation. J. Integr. Plant Biol. 2012, 54, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wang, L.; Ma, Q.; Wu, G. Advances in biosynthetic pathways of brassinolide. Acta Bot. Sin. 2015, 50, 768–778. [Google Scholar] [CrossRef]
- Choe, S.; Dilkes, B.P.; Fujioka, S.; Takatsuto, S.; Sakurai, A.; Feldmann, K.A. The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22alpha-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell 1998, 10, 31–243. [Google Scholar] [CrossRef]
- Kour, J.; Kohli, S.K.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P.; et al. Brassinosteroid signaling, crosstalk and, physiological functions in plants under heavy metal stress. Front. Plant Sci. 2021, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, J. Biosynthesis, metabolism and signal transduction of brassinosterol hormones. Chin. J. Plant Physiol. 2017, 53, 291–307. [Google Scholar] [CrossRef]
- Sahni, S.; Prasad, B.D.; Liu, Q.; Grbic, V.; Sharpe, A.; Singh, S.P.; Krishna, P. Overexpression of the brassinosteroid biosynthetic gene DWF4 in Brassica napus simultaneously increases seed yield and stress tolerance. Sci. Rep. 2016, 6, 28298. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Németh, K.; Koncz-Kálmán, Z.; Mathur, J.; Kauschmann, A.; Altmann, T.; Rédei, G.P.; Nagy, F.; Schell, J.; Koncz, C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 1996, 85, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Du, J.; He, X.; Zhao, S.; Liu, Y.; Lu, M. Cloning and functional analysis of poplar brassinolide synthesis gene DET2. Res. For. 2020, 33, 85–92. [Google Scholar] [CrossRef]
- Ohnishi, T.; Szatmari, A.M.; Watanabe, B.; Fujita, S.; Bancos, S.; Koncz, C.; Lafos, M.; Shibata, K.; Yokota, T.; Sakata, K.; et al. C-23 hydroxylation by Arabidopsis CYP90C1 and CYP90D1 reveals a novel shortcut in brassinosteroid biosynthesis. Plant Cel 2006, 18, 3275–3288. [Google Scholar] [CrossRef] [PubMed]
- Polko, J.K.; Pierik, R.; Zanten, M.V.; Tarkowská, D.; Strnad, M.; Voesenek, L.A.; Peeters, A.J. Ethylene promotes hyponastic growth through interaction with ROTUNDIFOLIA3/CYP90C1 in Arabidopsis. J. Exp. Bot. 2013, 64, 613–624. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Gendron, J.M.; Sun, Y.; Gampala, S.S.; Gendron, N.; Sun, C.; Wang, Z. BZR1 is a transcriptional repressor with dual roles inbrassinosteroid homeostasis and growth responses. Science 2005, 307, 1634–1638. [Google Scholar] [CrossRef]
- Sun, C.; Liu, Y.; Li, G.; Chen, Y.; Li, M.; Yang, R.; Qin, Y.; Chen, Y.; Chen, J.; Fu, Z.; et al. ZmCYP90D1 regulates maize internode development by modulating brassinosteroid-mediated cell division and growth. Crop J. 2023; in press. [Google Scholar] [CrossRef]
- Castle, J.; Szekeres, M.; Jenkins, G.; Bishop, G.J. Unique and overlapping expression patterns of Arabidopsis CYP85 genes involved in brassinosteroid C-6 oxidation. Plant Mol. Biol. 2005, 57, 129–140. [Google Scholar] [CrossRef]
- Li, X.; Guo, X.; Zhou, Y.; Shi, K.; Zhou, J.; Yu, J.; Xia, X. Overexpression of a brassinosteroid biosynthetic gene Dwarf enhances photosynthetic capacity through activation of Calvin cycle enzymes in tomato. BMC Plant Biol. 2016, 16, 33. [Google Scholar] [CrossRef]
- Duan, F.; Ding, J.; Lee, D.S.; Lu, X.; Feng, Y.; Song, W. Overexpression of SoCYP85A1, a spinach cytochrome p450 gene in transgenic tobacco enhances root development and drought stress tolerance. Front. Plant Sci. 2017, 8, 1909. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kota, U.; He, K.; Blackburn, K.; Li, J.; Goshe, M.B.; Huber, S.C.; Clouse, S.D.; Du, C. Sequential transphos-phorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev. Cell 2008, 15, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Wang, G.; Dong, H. Brassinolide signaling pathway and its role in inducing antiviral response in plants. J. Jiangxi Agric. Sci. 2022, 34, 41–46. [Google Scholar] [CrossRef]
- Li, S.; Zheng, H.; Lin, L.; Wang, F.; Sui, N. Roles of brassinosteroids in plant growth and abiotic stress response. Plant Growth Regul. 2021, 93, 29–38. [Google Scholar] [CrossRef]
- Vriet, C.; Russinova, E.; Reuzeau, C. From squalene to brassinolide: The steroid metabolic and signaling pathways across the plant kingdom. Mol. Plant 2013, 6, 1738–1757. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Yan, Z.; Zhu, Y.; Li, J. Regulation of the Arabidopsis GSK3-like kinase BRASSINOSTEROID-INSENSITIVE 2 through proteasome-mediated protein degradation. Mol. Plant 2008, 1, 338–346. [Google Scholar] [CrossRef]
- Li, J.; Terzaghi, W.; Gong, Y.; Li, C.; Ling, J.; Fan, Y.; Qin, N.; Gong, X.; Zhu, D.; Deng, X. Modulation of BIN2 kinase activity by HY5 controls hypocotyl elongation in the light. Nat. Commun. 2020, 11, 1592. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, T.; Wu, Z.; Wang, J.; Zhang, C.; Wang, H.; Wang, Z.; Wang, X. The intrinsically disordered protein BKI1 is essential for inhibiting BRI1 signaling in plants. Mol. Plant 2015, 8, 1675–1678. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Cao, D.; Yang, H.; Oh, E.; Bi, Y.; Zhu, S.; Wang, Z. The F-box protein KIB1 mediates Brassinosteroid-induced inactivation and degradation of GSK3-like Kinases in Arabidopsis. Mol. Cell 2017, 66, 648–657. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Halder, K.; Abdin, M.Z.; Majee, M.; Datta, A. Abiotic stress tolerance in plants: Brassinosteroids navigate competently. Int. J. Mol. Sci. 2022, 23, 14577. [Google Scholar] [CrossRef]
- Chen, E.; Yang, X.; Liu, R.; Zhang, M.; Zhang, M.; Zhou, F.; Li, D.; Hu, H.; Li, C. GhBEE3-Like gene regulated by brassinosteroids is involved in cotton drought tolerance. Front. Plant Sci. 2022, 13, 1019146. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Liu, S.; Tang, B.; Chen, J.; Xie, Z.; Nolan, T.M.; Jiang, H.; Guo, H.; Lin, H.; Li, L.; et al. RD26 mediates crosstalk between drought and brassinosteroid signalling pathways. Nat. Commun. 2017, 8, 14573. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Gao, Y.; Guo, J.; Yu, T.; Zheng, W.; Liu, Y.; Chen, J.; Xu, Z.; Ma, Y. BES/BZR transcription factor TaBZR2 positively regulates drought responses by activation of TaGST1. Plant Physiol. 2019, 180, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zuo, J.; Liu, S.; Wang, W.; Lu, Q.; Hao, X.; Fang, Z.; Liang, T.; Sun, Y.; Guo, C.; et al. BRI1 EMS SUPPRESSOR1 genes regulate abiotic stress and anther development in wheat (Triticum aestivum L.). Front. Plant Sci. 2023, 14, 1219856. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, N.; Yang, J.; Si, H. Functional analysis of potato CPD gene: A rate-limiting enzyme in brassinosteroid biosynthesis under polyethylene glycol-induced osmotic stress. Crop Sci. 2016, 56, 2675–2687. [Google Scholar] [CrossRef]
- Nie, S.; Huang, S.; Wang, S.; Mao, Y.; Liu, J.; Ma, R.; Wang, X. Enhanced brassinosteroid signaling intensity via SlBRI1 overexpression negatively regulates drought resistance in a manner opposite of that via exogenous BR application in tomato. Plant Physiol. Biochem. 2019, 138, 36–47. [Google Scholar] [CrossRef]
- Chen, J.; Nolan, T.M.; Ye, H.; Zhang, M.; Tong, H.; Xin, P.; Chu, J.; Chu, C.; Li, Z.; Yin, Y. Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Park, C.R.; Lee, K.H.; Lee, S.; Kim, C.S. BES1/BZR1 Homolog 3 (BEH3) cooperates with E3 ligase AtRZF1 to regulate dehydration and brassinosteroid responses. J. Exp. Bot. 2020, 72, 636–653. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, S.; Gao, H.; Tang, W.; Wu, X.; Zhang, B. The BR signaling pathway regulates primary root development and drought stress response by suppressing the expression of PLT1 and PLT2 in Arabidopsis thaliana. Front. Plant Sci. 2023, 14, 1187605. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, Y.; Fei, S. Down-regulation of BdBRI1, a putative brassinosteroid receptor gene produces a dwarf phenotype with enhanced drought tolerance in Brachypodium distachyon. Plant Sci. 2015, 234, 163–173. [Google Scholar] [CrossRef]
- Xie, Z.; Nolan, T.; Jiang, H.; Tang, B.; Zhang, M.; Li, Z.; Yin, Y. The AP2/ERF transcription factor TINY modulates Brassinosteroid-regulated plant growth and drought responses in Arabidopsis. Plant Cell 2019, 31, 1788–1806. [Google Scholar] [CrossRef]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef]
- Fang, J.; Zhu, W.; Tong, Y. Knock-Down the expression of brassinosteroid receptor TaBRI1 reduces photosynthesis, tolerance to high light and high temperature stresses and grain yield in wheat. Plants 2020, 9, 840. [Google Scholar] [CrossRef]
- Ibanez, C.; Delker, C.; Martinez, C.; Burstenbinder, K.; Janitza, P.; Lippmann, R.; Ludwig, W.; Sun, H.; James, G.V.; Klecker, M.; et al. Brassinosteroids dominate hormonal regulation of plant thermomorphogenesis via BZR1. Curr. Biol. 2018, 28, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; Ohme-Takagi, M.; Perata, P. APETALA2/Ethylene Responsive Factor (AP2/ERF) transcription factors: Mediators of stress responses and developmental programs. New Phytol. 2013, 199, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; He, R.; Xie, Q.; Zhao, X.; Deng, X.; He, J.; Song, L.; He, J.; Marchant, A.; Chen, X.; et al. ETHYLENE RESPONSE FACTOR 74 (ERF74) plays an essential role in controlling a respiratory burst oxidase homolog D (RbohD)-dependent mechanism in response to different stresses in Arabidopsis. New Phytol. 2017, 213, 1667–1681. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Jiang, J.; Sun, S.; Wang, X. Brassinosteroids promote thermotolerance through releasing BIN2-mediated phosphorylation and suppression of HsfA1 transcription factors in Arabidopsis. Plant Commun. 2022, 3, 100419. [Google Scholar] [CrossRef] [PubMed]
- Orvar, B.L.; Sangwan, V.; Omann, F.; Dhindsa, R.S. Early steps in cold sensing by plant cells: The role of actin cytoskeleton and membrane fluidity. Plant J. 2010, 23, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Li, H.; Ding, Y.; Shi, Y.; Song, C.; Gong, Z.; Yang, S. BRASSINOSTEROID-INSENSITIVE2 negatively regulates the stability of transcription factor ICE1 in response to cold stress in Arabidopsis. Plant Cell 2019, 31, 2682–2696. [Google Scholar] [CrossRef]
- Xia, X.; Fang, P.; Guo, X.; Qian, X.; Zhou, J.; Shi, K.; Zhou, Y.; Yu, J. Brassinosteroid-mediated apoplastic H2O2-glutaredoxin 12/14 cascade regulates antioxidant capacity in response to chilling in tomato. Plant Cell Environ. 2018, 41, 1052–1064. [Google Scholar] [CrossRef] [PubMed]
- Divi, U.K.; Krishna, P. Overexpression of the brassinosteroid biosynthetic gene AtDWF4 in Arabidopsis seeds overcomes abscisic acid-induced inhibition of germination and increases cold tolerance in transgenic seedlings. J. Plant Growth Regul. 2010, 29, 385–393. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Z.; Wu, M.; Wang, W.; Wang, Y.; Nie, S. Enhanced brassinosteroid signaling via the overexpression of SlBRI1 positively regulates the chilling stress tolerance of tomato. Plant Sci. 2022, 320, 111281. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Liu, Y.; Sang, K.; Wang, T.; Yu, J.; Zhou, Y.; Xia, X. Brassinosteroid signaling positively regulates abscisic acid biosynthesis in response to chilling stress in tomato. J. Integr. Plant Biol. 2023, 65, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, K.; Shi, Y.; Cheng, J.; Zhang, X.; Yang, S. BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis. Mol. Plant 2017, 10, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yao, X.; Lv, L.; Zhao, D. Cloning of PtDET2 gene from Populus sinense and analysis of salinity and drought tolerance in Tobacco. J. Mt. Agric. Biol. 2017, 36, 6–13. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, N.; Yang, J.; Tang, X.; Wen, Y. Functional analysis of StDWF4 gene in response to salt stress in potato. Plant Physiol. Biochem. 2018, 125, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Ou, Y.; Han, Y.; Dios, V.R.; Wang, L.; Zhang, Q.; Yao, Y. The brassinosteroid biosynthesis enzyme gene PeCPD improves plant growth and salt tolerance in Populus tomentosa. Ind. Crop. Prod. 2021, 162, 113218. [Google Scholar] [CrossRef]
- Jia, C.; Zhao, S.; Bao, T.; Zhao, P.; Peng, K.; Guo, Q.; Gao, X.; Qin, J. Tomato BZR/BES transcription factor SlBZR1 positively regulates BR signaling and salt stress tolerance in tomato and Arabidopsis. Plant Sci. 2021, 302, 110719. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Z.; Li, W.; Liu, S.; Feng, Q.; Yao, Y.; Gao, Y. Heterologous overexpression of Populus euphratica PeBZR1 gene enhanced tolerance of tobacco to salt stress. Genom. Appl. Biol. 2020, 39, 3593–3599. [Google Scholar] [CrossRef]
- Sun, F.; Yu, H.; Qu, J.; Cao, Y.; Ding, L.; Feng, W.; Khalid, M.; Li, W.; Fu, F. Maize ZmBES1/BZR1-5 decreases ABA sensitivity and confers tolerance to osmotic stress in transgenic Arabidopsis. Int. J. Mol. Sci. 2020, 21, 996. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, A.K.; Orosa-Puente, B.; Campanaro, A.; Zhang, C.; Sadanandom, A. Sumo conjugation to BZR1 enables brassinosteroid signaling to integrate environmental cues to shape plant growth. Curr. Biol. 2021, 31, 668–669. [Google Scholar] [CrossRef]
- Li, J.; Zhou, H.; Zhang, Y.; Li, Z.; Yang, Y.; Guo, Y. The GSK3-like kinase BIN2 is a molecular switch between the salt stress response and growth recovery in Arabidopsis thaliana. Dev. Cell 2020, 55, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Li, B.; Chen, Y.; Jing, Y.; Wang, S.; Li, W.; Gao, N.; Liao, C.; Wang, L.; Xiao, F.; et al. BRASSINOSTEROID-INSENSITIVE 2 regulates salt stress tolerance in Arabidopsis by promoting AGL16 activity. Biochem. Biophys. Res. Commun. 2023, 678, 17–23. [Google Scholar] [CrossRef]
- Zhang, K.; Duan, M.; Zhang, L.; Li, J.; Shan, L.; Zheng, L.; Liu, J. HOP1 and HOP2 are involved in salt tolerance by facilitating the brassinosteroid-related nucleo-cytoplasmic partitioning of the HSP90-BIN2 complex. Plant Cell Environ. 2022, 45, 3551–3565. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Chang, X.; Wang, X.; Li, N.; Gao, Y. Allogeneic overexpression of PeDWF4 gene in Populus euphratica enhances tobacco tolerance to abiotic stress. Genom. Appl. Biol. 2017, 36, 4242–4249. [Google Scholar] [CrossRef]
- Tian, F.; Han, C.; Chen, X.; Wu, X.; Mi, J.; Wan, X.; Liu, Q.; He, F.; Chen, L.; Yang, H.; et al. PscCYP716A1-mediated brassinolide biosynthesis increases Cadmium tolerance and enrichment in poplar. Front. Plant Sci. 2022, 13, 919682. [Google Scholar] [CrossRef] [PubMed]
- Achard, P.; Renou, J.P.; Berthome, R.; Harberd, N.P.; Genschik, P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 2008, 18, 656–660. [Google Scholar] [CrossRef]
- Niether, W.; Glawe, A.; Pfohl, K.; Adamtey, N.; Pawelzik, E. The efect of short-term vs. long-term soil moisture stress on the physiological response of three cocoa (Theobroma cacao L.) cultivars. Plant Growth Regul. 2020, 92, 295–306. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Tran, H.; Shan, L.; Kim, J.; Childs, K.; Ervin, E.H.; Frazier, T.; Zhao, B. Assessment of drought tolerance of 49 switchgrass (Panicum virgatum) genotypes using physiological and morphological parameters. Biotechnol. Biofuels 2015, 8, 152. [Google Scholar] [CrossRef]
- Meng, J.; Xu, T.; Wang, Z.; Fang, Y.; Xi, Z.; Zhang, Z. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Song, Q.; Wu, Y.; Li, S.; Jiang, C.; Chang, L.; Yang, X.; Zhang, J. Accumulation of glycine betaine in transplastomic potato plants expressing choline oxidase confers improved drought tolerance. Planta 2019, 249, 1963–1975. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.; Awad, D.; Samarah, N. Gene expression and activity of antioxidant enzymes in barley (Hordeum vulgare L.) under controlled severe drought. J. Plant Interact. 2015, 10, 109–116. [Google Scholar] [CrossRef]
- Wang, H.; Tang, J.; Liu, J.; Hu, J.; Liu, J.; Chen, Y.; Cai, Z.; Wang, X. Abscisic acid aignaling inhibits Brassinosteroid signaling through dampening the dephosphorylation of BIN2 by ABI1 and ABI2. Mol. Plant 2018, 11, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Gao, J.; Hu, F.; Guo, D.; Zhang, G.; Zhang, R.; Xue, J. Photosynthetic and osmotic regulation of maize leaves in response to drought stress. Acta Agron. Sin. 2013, 39, 530–536. [Google Scholar] [CrossRef]
- Gong, H.; Chen, K. The regulatory role of silicon on water relations, photosynthetic gas exchange, and carboxylation activities of wheat leaves in field drought conditions. Acta Physiol. Plant 2012, 34, 1589–1594. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Guo, K.; Fan, D.; Li, G.; Zheng, Y.; Rui, Y. Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ. Exp. Bot. 2011, 71, 174–183. [Google Scholar] [CrossRef]
- Felagari, K.; Bahramnejad, B.; Siosemardeh, A.; Mirzaei, K.; Lei, X.; Zhang, J. A comparison of the physiological traits and gene expression of brassinosteroids signaling under drought conditions in two chickpea cultivars. Agronomy 2023, 13, 2963. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dwivedi, S.; Bhagavatula, L.; Datta, S. Integration of light and ABA signaling pathways to combat drought stress in plants. Plant Cell Rep. 2023, 42, 829–841. [Google Scholar] [CrossRef]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef]
- Tran, L.S.; Nakashima, K.; Sakuma, Y.; Simpson, S.D.; Fujita, Y.; Maruyama, K.; Fujita, M.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 2004, 16, 2481–2498. [Google Scholar] [CrossRef] [PubMed]
- Kono, A.; Yin, Y. Updates on BES1/BZR1 regulatory networks coordinating plant growth and stress responses. Front. Plant Sci. 2020, 11, 617162. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, L.; Zola, J.; Aluru, M.; Ye, H.; Foudree, A.; Guo, H.; Anderson, S.; Aluru, S.; Liu, P.; et al. A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J. 2011, 65, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, J.; Tang, X.; Wen, Y.; Zhang, N.; Si, H. Effect of amiRNA technique on drought resistance of potato by silencing C-3 oxidase encoding gene StCPD. Acta Agron. Sin. 2018, 44, 512–521. [Google Scholar] [CrossRef]
- Maharjan, P.M.; Schulz, B.; Choe, S. BIN2/DWF12 antagonistically transduces brassinosteroid and auxin signals in the roots of Arabidopsis. J. Plant Biol. 2011, 54, 126–134. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukasinovic, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef]
- Jiang, H.; Du, J.; Shi, L.; Bin, J.; Yue, Y. Study on the molecular mechanism of plant high temperature stress response. Mol. Plant Breed. 2021, 19, 1022–1030. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Ruelland, E.; Zachowski, A. How plants sense temperature. Environ. Exp. Bot. 2010, 69, 225–232. [Google Scholar] [CrossRef]
- Schraudner, M.; Moeder, W.; Wiese, C.; Camp, W.V.; Inzé, D.; Langebartels, C.; Sandermann, H., Jr. Ozone-induced oxidative burst in the ozone biomonitor plant, tobacco Bel W3. Plant J. Cell Mol. Biol. 2010, 16, 235–245. [Google Scholar] [CrossRef]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Doring, P.; Vierling, E.; Scharf, K.D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Al-Whaibi, M.H. Plant heat-shock proteins: A mini review. J. King Saud Univ. Sci. 2011, 23, 139–150. [Google Scholar] [CrossRef]
- Park, C.J.; Seo, Y.S. Heat Shock Proteins: A review of the molecular chaperones for plant immunity. Plant Pathol. J. 2015, 31, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Albertos, P.; Dundar, G.; Schenk, P.; Carrera, S.; Cavelius, P.; Sieberer, T.; Poppenberger, B. Transcription factor BES1 interacts with HSFA1 to promote heat stress resistance of plants. Embo J. 2022, 41, e108664. [Google Scholar] [CrossRef] [PubMed]
- Setsungnern, A.; Munoz, P.; Perez-Llorca, M.; Muller, M.; Thiravetyan, P.; Munne-Bosch, S. A defect in BRI1-EMS-SUPPRESSOR 1 (bes1)-mediated brassinosteroid signaling increases photoinhibition and photo-oxidative stress during heat stress in Arabidopsis. Plant Sci. 2020, 296, 110470. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Qin, K.; Song, X.; Zhang, Q.; Zhou, Y.; Xia, X.; Yu, J. BZR1 transcription factor regulates heat stress tolerance through FERONIA receptor-like kinase-mediated reactive oxygen species signaling in tomato. Plant Cell Physiol. 2018, 59, 2239–2254. [Google Scholar] [CrossRef]
- Chen, X.; Xue, H.; Zhu, L.; Wang, H.; Long, H.; Zhao, J.; Meng, F.; Liu, Y.; Ye, Y.; Luo, X.; et al. ERF49 mediates brassinosteroid regulation of heat stress tolerance in Arabidopsis thaliana. BMC Biol. 2022, 20, 254. [Google Scholar] [CrossRef]
- Ren, H.; Wu, X.; Zhao, W.; Wang, Y.; Sun, D.; Gao, K.; Tang, W. Heat shock-induced accumulation of the glycogen synthase kinase 3-Like Kinase BRASSINOSTEROID INSENSITIVE 2 promotes early flowering but reduces thermotolerance in Arabidopsis. Front. Plant Sci. 2022, 13, 838062. [Google Scholar] [CrossRef]
- Juurakko, C.L.; Bredow, M.; Nakayama, T.; Imai, H.; Kawamura, Y.; Dicenzo, G.C.; Uemura, M.; Walker, V.K. The Brachypodium distachyon cold-acclimated plasma membrane proteome is primed for stress resistance. G3 Genes Genomes Genet. 2021, 11, jkab198. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Yang, S. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef]
- Guo, X.; Liu, D.; Chong, K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018, 60, 745–756. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Kazemi-Shahandashti, S.S.; Maali-Amiri, R. Global insights of protein responses to cold stress in plants: Signaling, defence, and degradation. J. Plant Physiol. 2018, 226, 123–135. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Mao, B.; Chu, C. Cold stress tolerance in rice: Physiological changes, molecular mechanism, and future prospects. Yi Chuan 2018, 40, 171–185. [Google Scholar] [CrossRef]
- Jiang, B.; Shi, Y.; Peng, Y.; Jia, Y.; Yan, Y.; Dong, X.; Li, H.; Dong, J.; Li, J.; Gong, Z.; et al. Cold-Induced CBF-PIF3 interaction enhances freezing tolerance by stabilizing the phyB thermosensor in Arabidopsis. Mol. Plant 2020, 13, 894–906. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, M.; Lee, J.H.; Lee, H.J.; Park, C.M. The unified ICE-CBF pathway provides a transcriptional feedback control of freezing tolerance during cold acclimation in Arabidopsis. Plant Mol. Biol. 2015, 89, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ding, Y.; Shi, Y.; Zhang, X.; Zhang, S.; Gong, Z.; Yang, S. MPK3- and MPK6-Mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis. Dev. Cell. 2017, 43, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Raihan, M.; Masud, A.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Xie, L. Review of Plant Adaptation Mechanism to Salt Stress. J. Plant Genet. Resour. 2022, 23, 1585–1593. [Google Scholar] [CrossRef]
- Liu, J.; Gao, H.; Wang, X.; Zheng, Q.; Wang, C.; Wang, X.; Wang, Q. Effects of 24-epibrassinolide on plant growth, osmotic regulation and ion homeostasis of salt-stressed canola. Plant Biol. 2014, 16, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Hu, T.; Zhang, X.; Pang, H.; Fu, J. Exogenous glycine betaine ameliorates the adverse effect of salt stress on Perennial ryegrass. J. Am. Soc. Hortic. Sci. 2012, 137, 38–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, S.; Li, Y.; Gao, T.; Yang, Y. Osmotic regulation and chlorophyll fluorescence characteristics in leaves of wheat seedlings under different salt stresses. Ying Yong Sheng Tai Xue Bao 2021, 32, 4381–4390. [Google Scholar] [CrossRef]
- Shohan, M.; Sinha, S.; Nabila, F.H.; Dastidar, S.G.; Seraj, Z.I. HKT1;5 transporter gene expression and association of amino acid substitutions with salt tolerance across rice genotypes. Front. Plant Sci. 2019, 10, 1420. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Liu, Y.; Zhang, X.; Zhao, B.; Childs, K.L. Analysis of salt-induced physiological and proline changes in 46 switchgrass (Panicum virgatum) lines indicates multiple response modes. Plant Physiol. Biochem. 2016, 105, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Liu, L.; Li, Y.; Wang, Q.; Dai, J.; Wang, R. Long-term effects of untreated wastewater on soil bacterial communities. Sci. Total Environ. 2019, 646, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Tang, Z.; Song, J.; Huang, X.; Wang, P. Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol. Plant 2022, 15, 27–44. [Google Scholar] [CrossRef]
- Bhatti, S.S.; Sambyal, V.; Nagpal, A.K. Analysis of genotoxicity of agricultural soils and metal (Fe, Mn, and Zn) accumulation in crops. Int. J. Environ. Res. 2018, 12, 439–449. [Google Scholar] [CrossRef]
- Xie, L.; Hao, P.; Cheng, Y.; Ahmed, I.M.; Cao, F. Effect of combined application of lead, cadmium, chromium and copper on grain, leaf and stem heavy metal contents at different growth stages in rice. Ecotox. Environ. Saf. 2018, 162, 71–76. [Google Scholar] [CrossRef]
- Sinha, V.; Pakshirajan, K.; Chaturvedi, R. Chromium tolerance, bioaccumulation and localization in plants: An overview. J. Environ. Manag. 2018, 206, 715–730. [Google Scholar] [CrossRef]
- Shen, Y.; He, F.; Zhu, J.; Zhang, H.; Wang, J.; Wang, H.; Zhan, X. Proton-coupled cotransporter involves phenanthrene xylem loading in roots. Sci. Total Environ. 2021, 773, 145637. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Tang, L.; Peng, Y.; Qian, M.; Guo, Y.; Rui, H.; Zhang, F.; Hu, Z.; Chen, Y.; et al. The root iron transporter 1 governs cadmium uptake in Vicia sativa roots. J. Hazard. Mater. 2020, 398, 122873. [Google Scholar] [CrossRef]
- Li, X.; Ziadi, N.; Bélanger, G.; Cai, Z.; Xu, H. Cadmium accumulation in wheat grain as affected by mineral N fertilizer and soil characteristics. Can. J. Soil Sci. 2011, 91, 521–531. [Google Scholar] [CrossRef]
- Abdelgawad, H.; Zinta, G.; Hamed, B.A.; Selim, S.; Beemster, G.; Hozzein, W.N.; Wadaan, M.; Asard, H.; Abuelsoud, W. Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ. Pollut. 2020, 258, 113705. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Ghorbanpour, M.; Kariman, K. Physiological and antioxidative responses of medicinal plants exposed to heavy metals stress. Plant Gene 2017, 11, 247–254. [Google Scholar] [CrossRef]
- Javed, M.T.; Akram, M.S.; Tanwir, K.; Javed, C.H.; Ali, Q.; Stoltz, E.; Lindberg, S. Cadmium spiked soil modulates root organic acids exudation and ionic contents of two differentially Cd tolerant maize (Zea mays L.) cultivars. Ecotox. Environ. Safe. 2017, 141, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Duan, S.; Zhang, X.; Wang, Y.; Wang, T. Research advances on plant root exudates in response to heavy metal stress. Fujian J. Agric. Sci. 2021, 36, 1506–1514. [Google Scholar] [CrossRef]
- Planas-Riverola, A.; Gupta, A.; Betegon-Putze, I.; Bosch, N.; Ibanes, M.; Cano-Delgado, A.I. Brassinosteroid signaling in plant development and adaptation to stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Chu, C. Functional specificities of brassinosteroid and potential utilization for crop improvement. Trends Plant Sci. 2018, 23, 1016–1028. [Google Scholar] [CrossRef]
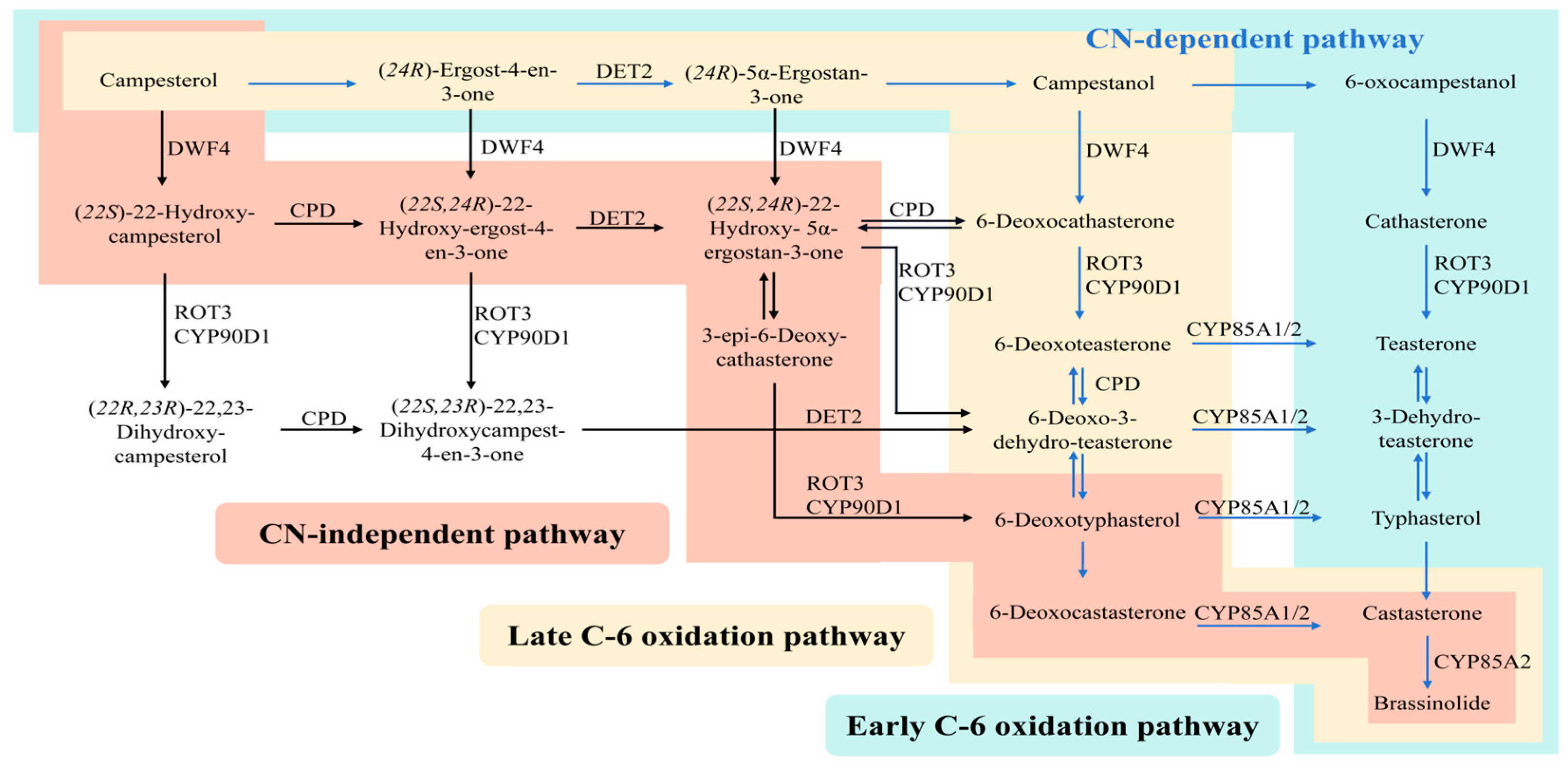

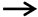 represents a promoted function;
represents a promoted function;  represents an inhibited function, same as below.
represents an inhibited function, same as below.
 represents a promoted function;
represents a promoted function;  represents an inhibited function, same as below.
represents an inhibited function, same as below.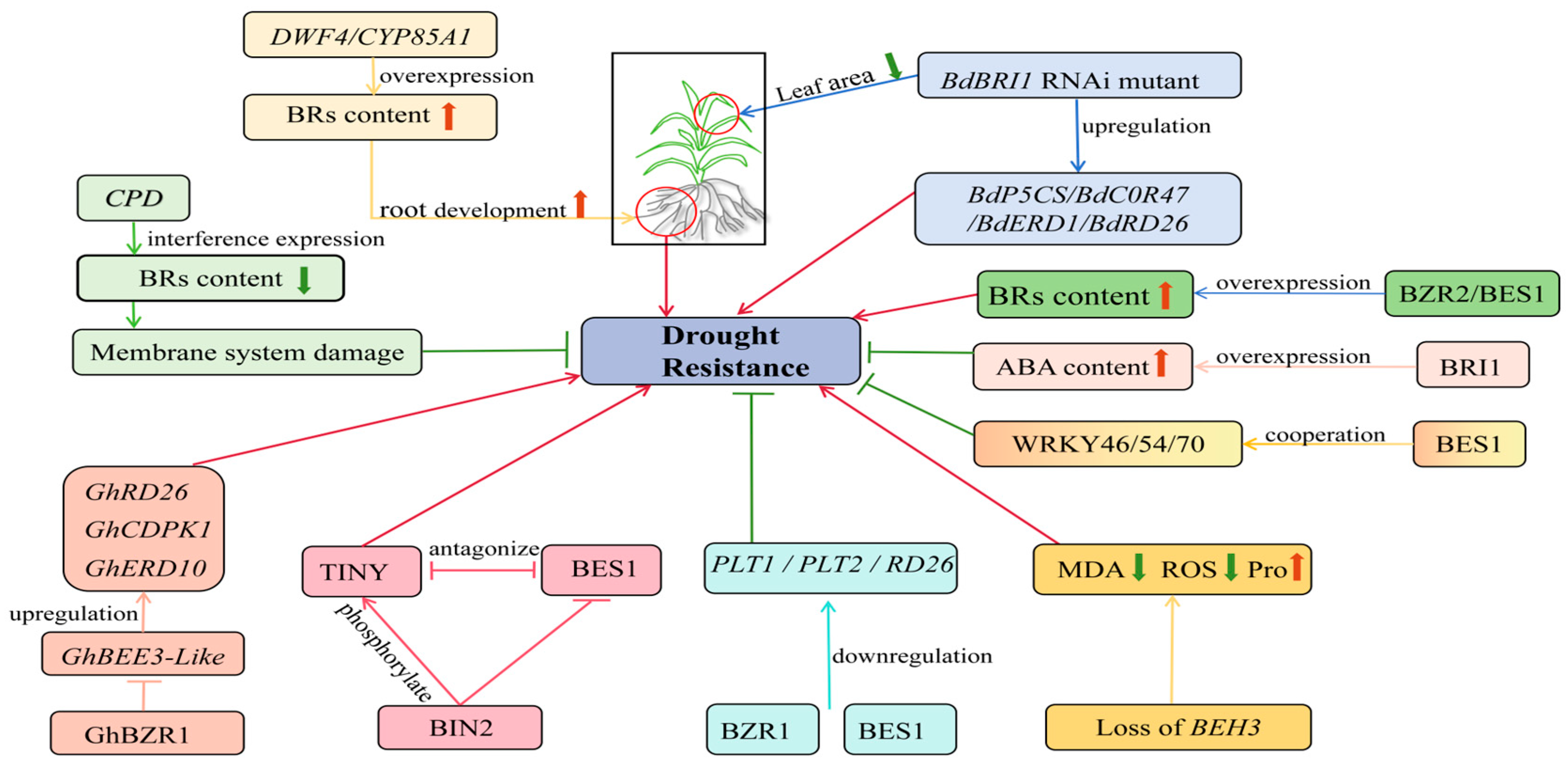

| Abiotic Stresses | Biological Function | Plant Specie | Reference |
|---|---|---|---|
| Drought stress | GhBZR1 inhibited GhBEE3-Like gene expression and improved drought resistance | Gossypium hirsutum L. | [52] |
| BZR1 and BES1 inhibited RD26 expression and drought tolerance | Arabidopsis thaliana | [53] | |
| Overexpression of TaBZR2 and TaBES1 increased drought resistance | Triticum aestivum L. | [54,55] | |
| Under the drought, overexpression of the DWF4 increased the yield | Brassica napus | [33] | |
| Interference expression of the StCPD reduced resistance to drought stress | Solanum tuberosum | [56] | |
| Overexpression of the CYP85A1 enhanced drought resistance | Nicotiana tabacum L. | [42] | |
| The SlBRI1 gene expression negatively regulated drought resistance | Solanum lycopersicum L. | [57] | |
| BES1 interacts with WRKY46, 54 and 70 to negatively regulated drought tolerance | Arabidopsis thaliana | [58] | |
| Loss of the BEH3 gene in Arabidopsis improved drought resistance | Arabidopsis thaliana | [59] | |
| BZR1/BES1 inhibited the transcriptional activity of PLTs and negatively regulated drought resistance | Arabidopsis thaliana | [60] | |
| Down-regulation of the BdBRI1 gene increased drought resistance | Brachypodium distachyon | [61] | |
| The TINY interacted with and antagonized the BES1 to improve drought resistance | Arabidopsis thaliana | [62] | |
| Temperature stress | BES1 and HSFA1 interacted to enhance heat resistance | Arabidopsis thaliana | [63] |
| The bes1 mutant increased sensitivity to high temperature | Arabidopsis thaliana | [64] | |
| The TaBRI1 knock-down mutant reduced tolerance to high-temperature stress | Triticum aestivum L. | [65] | |
| The BZR1 positively regulated heat stress | Solanum lycopersicum L. Arabidopsis thaliana | [66,67] | |
| The BIN2 inhibited heat resistance by activating ABI5 and HSFA1S | Arabidopsis thaliana | [68,69] | |
| The BIN2 inhibited cold tolerance by down-regulating CBF expression | Arabidopsis thaliana | [70] | |
| Overexpression of the DWRF enhanced the expression of GRX to improve cold tolerance | Solanum lycopersicum L. | [71] | |
| Overexpression of DWF4 enhanced the expression of COR15A to improve cold tolerance | Arabidopsis thaliana | [72] | |
| Overexpression of the SlBRI1 increased plant tolerance to low temperature stress | Solanum lycopersicum L. | [73] | |
| The BZR1 positively regulated low temperature stress | Solanum lycopersicum L. Arabidopsis thaliana | [74,75] | |
| Salt stress | Overexpression of the DET2 increased salt tolerance | Nicotiana tabacum L. | [76] |
| Overexpression of the StDWF4 enhanced salt tolerance | Solanum tuberosum | [77] | |
| Overexpression of the PeCPD improved plant growth and salt tolerance | Populus tomentosa | [78] | |
| Overexpression of BZR1 increased salt tolerance | Nicotiana tabacum L. Solanum lycopersicum L. | [79,80] | |
| ZmBES1/BZR1-5 improved salt tolerance | Arabidopsis thaliana | [81] | |
| Reduction in salt tolerance by deSUMOylation of BZR1 | Arabidopsis thaliana | [82] | |
| BIN2 and the SOS3/ SCaBP8-SOS2 module interacted to coordinate salt stress | Arabidopsis thaliana | [83] | |
| The BIN2 negatively regulated salt stress | Arabidopsis thaliana | [84] | |
| HOP enhanced salt tolerance by promoting BIN2-HSP complex accumulation. | Arabidopsis thaliana | [85] | |
| Heavy metal stress | Overexpression of the DWF4 improved the resistance of CuSO4 stress | Nicotiana tabacum L. | [86] |
| Overexpression of the PscCYP716A1 improved the accumulation and transport capacity of Cd | Populus | [87] | |
| DELLA protein interacted with the BRZ1 to enhance plant resistance to heavy metals | Arabidopsis thaliana | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, R.; Li, C.; Liu, Z.; Zhou, X.; Chen, S.; Zhang, D.; Luo, J.; Tang, W.; Wang, C.; Wu, J.; et al. The Role of Endogenous Brassinosteroids in the Mechanisms Regulating Plant Reactions to Various Abiotic Stresses. Agronomy 2024, 14, 356. https://doi.org/10.3390/agronomy14020356
Miao R, Li C, Liu Z, Zhou X, Chen S, Zhang D, Luo J, Tang W, Wang C, Wu J, et al. The Role of Endogenous Brassinosteroids in the Mechanisms Regulating Plant Reactions to Various Abiotic Stresses. Agronomy. 2024; 14(2):356. https://doi.org/10.3390/agronomy14020356
Chicago/Turabian StyleMiao, Rong, Caijuan Li, Ziliang Liu, Xiangyan Zhou, Sijin Chen, Dan Zhang, Jiaqi Luo, Wenhui Tang, Cuiling Wang, Jiling Wu, and et al. 2024. "The Role of Endogenous Brassinosteroids in the Mechanisms Regulating Plant Reactions to Various Abiotic Stresses" Agronomy 14, no. 2: 356. https://doi.org/10.3390/agronomy14020356
APA StyleMiao, R., Li, C., Liu, Z., Zhou, X., Chen, S., Zhang, D., Luo, J., Tang, W., Wang, C., Wu, J., & Chen, Z. (2024). The Role of Endogenous Brassinosteroids in the Mechanisms Regulating Plant Reactions to Various Abiotic Stresses. Agronomy, 14(2), 356. https://doi.org/10.3390/agronomy14020356





