Study on Spray Evaluation: The Key Role of Droplet Collectors
Abstract
1. Introduction
2. Materials and Methods
2.1. Surface Properties of Droplet Collectors
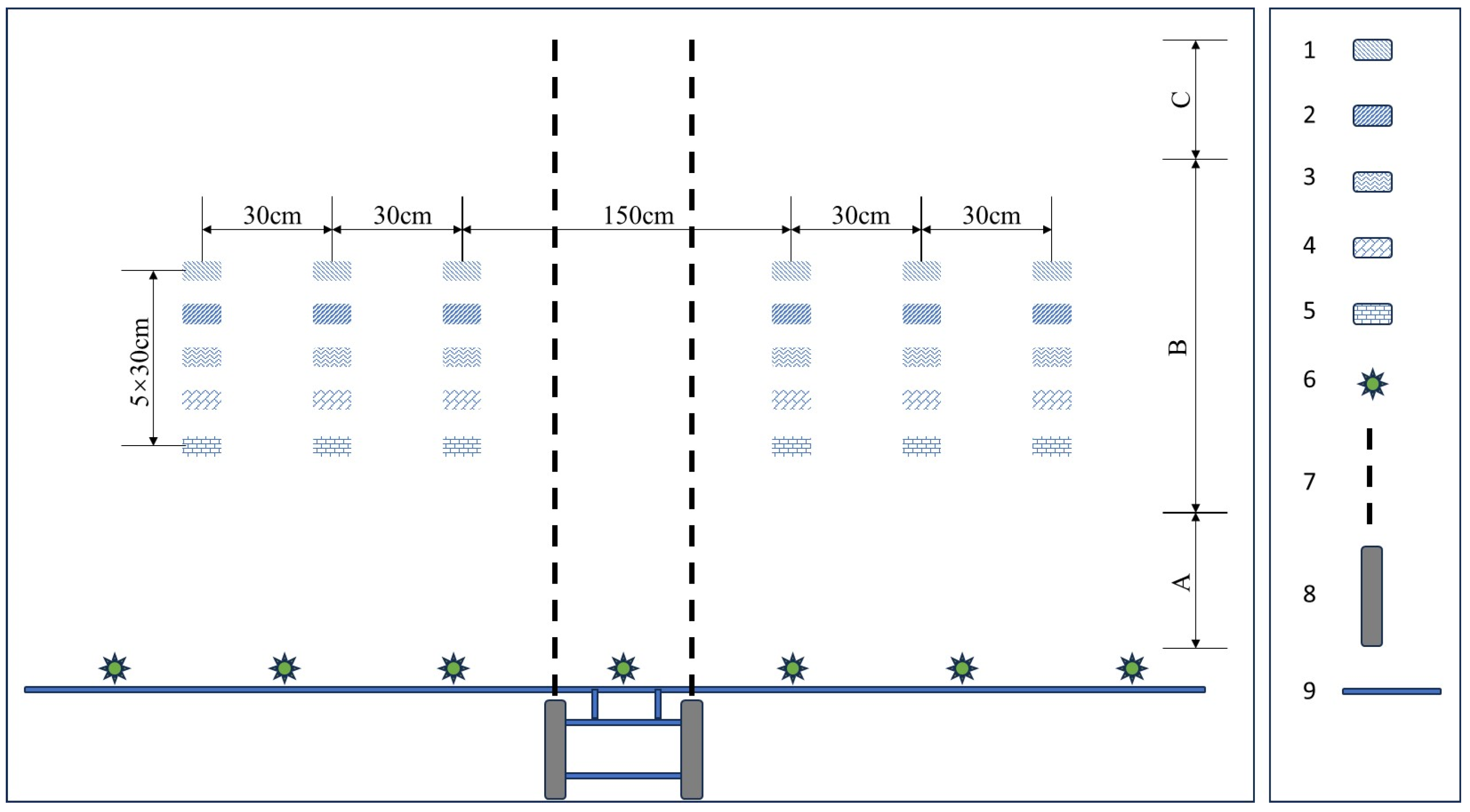
2.2. Droplet Collection Test Design and Deposition of Different Droplet Collectors
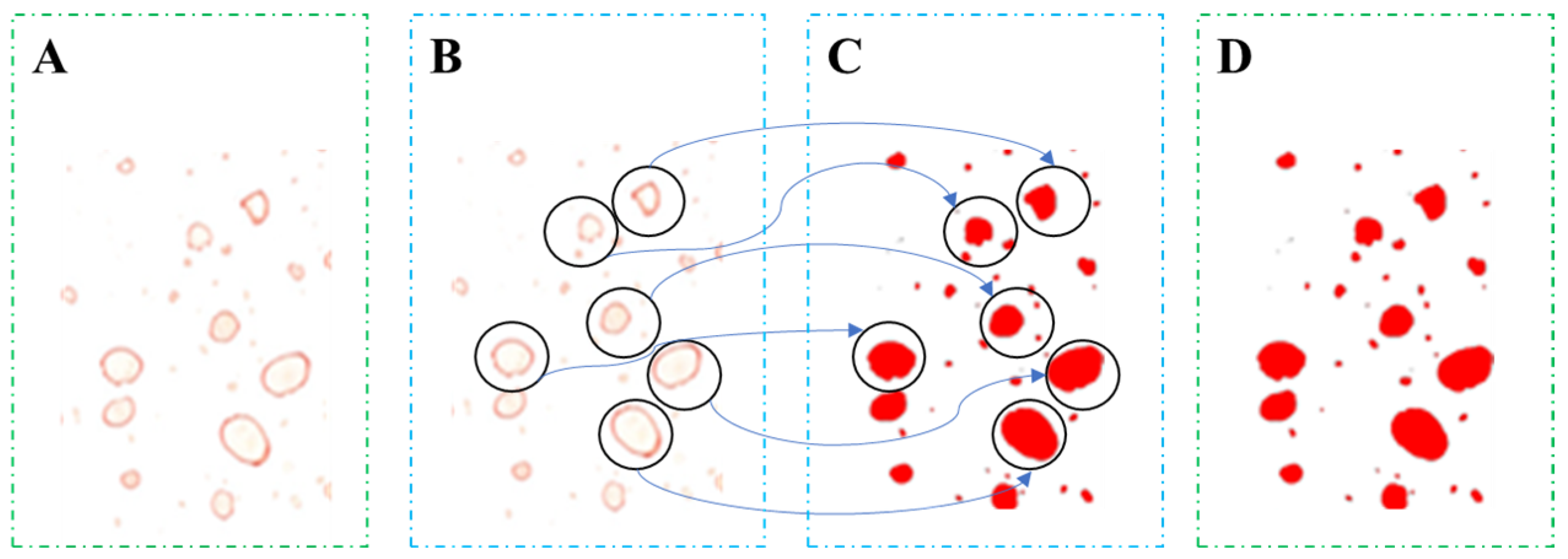
2.3. Analytical Methods
3. Result
3.1. Contact Angle and Surface Free Energy of Different Collectors
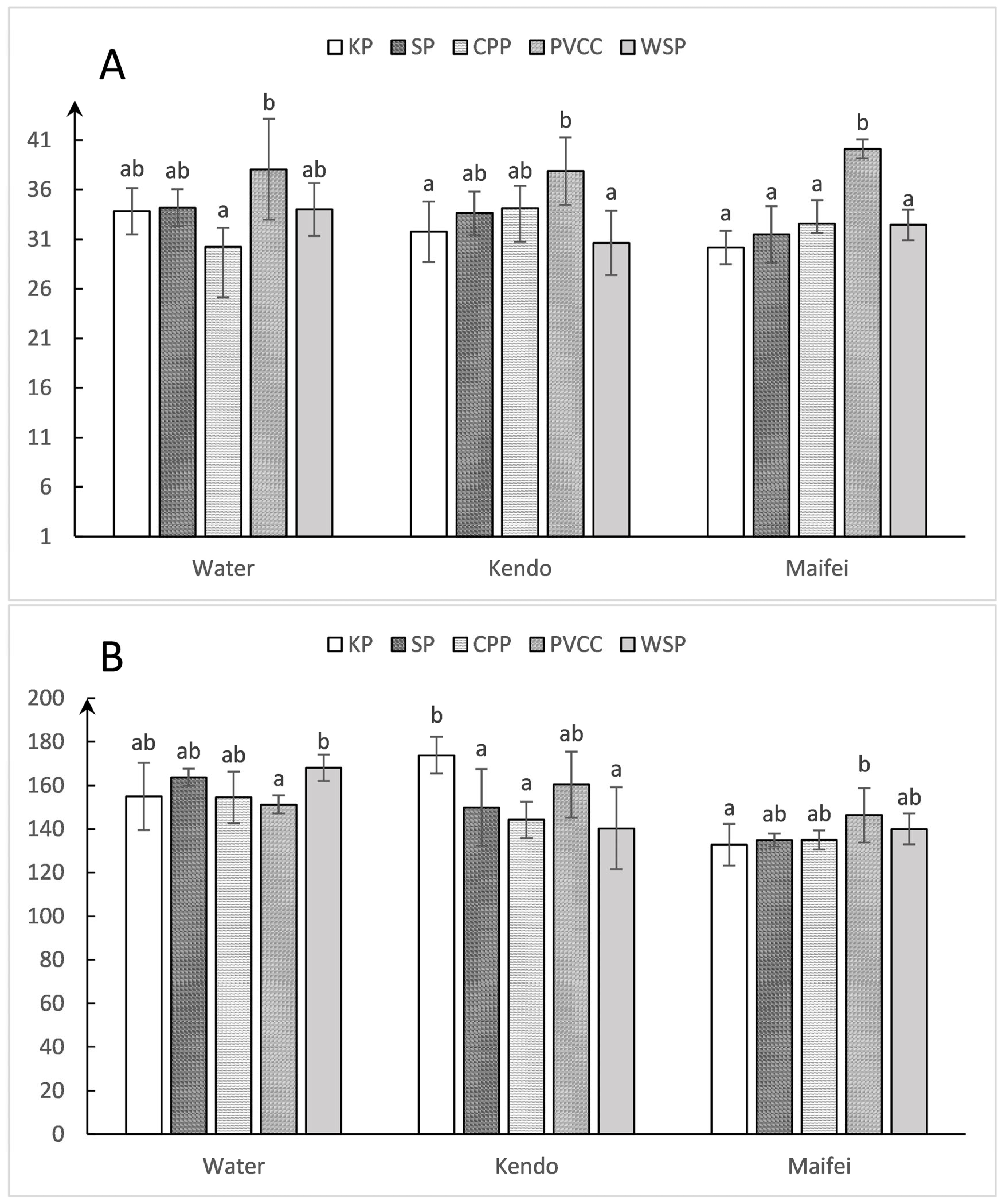
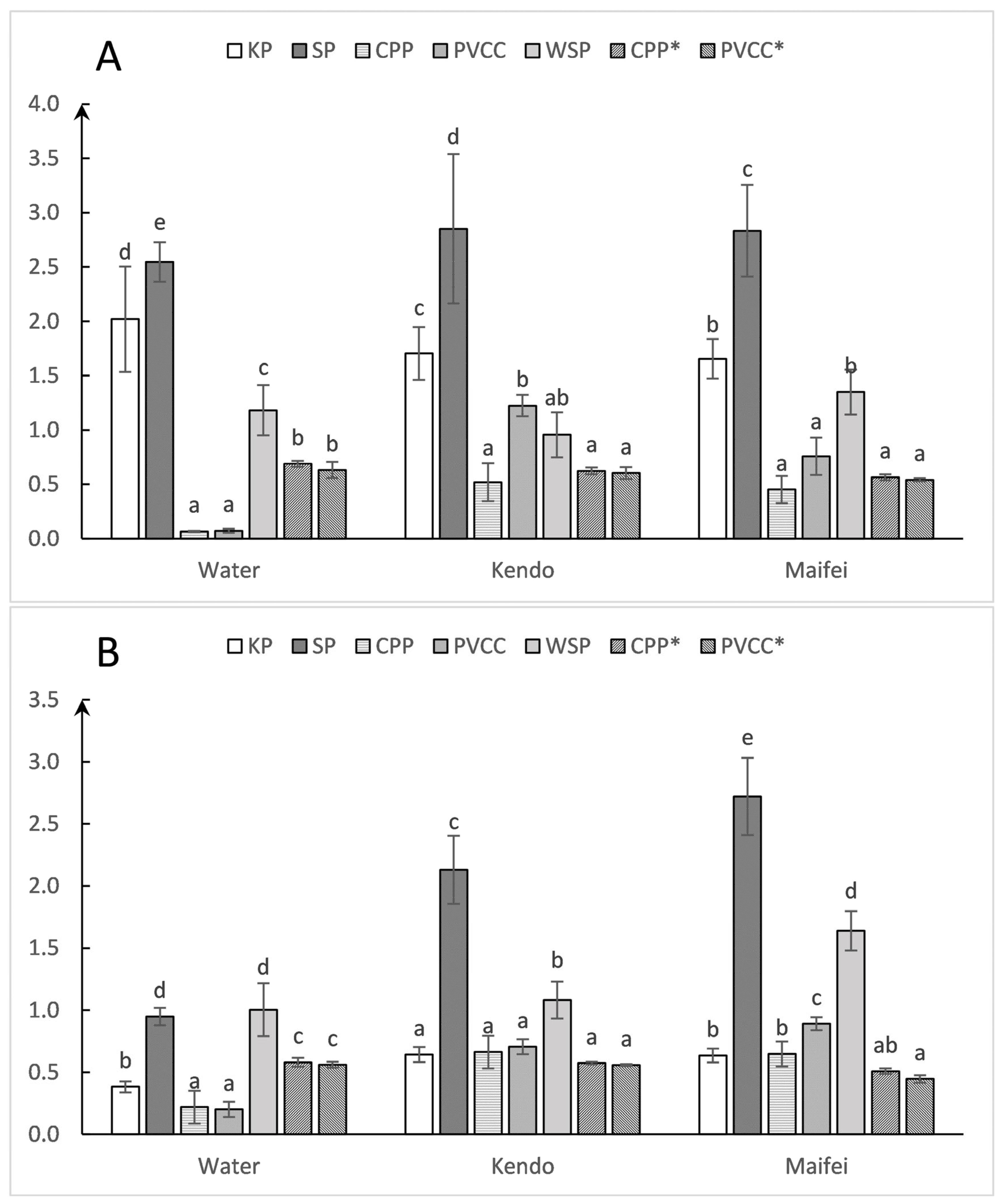
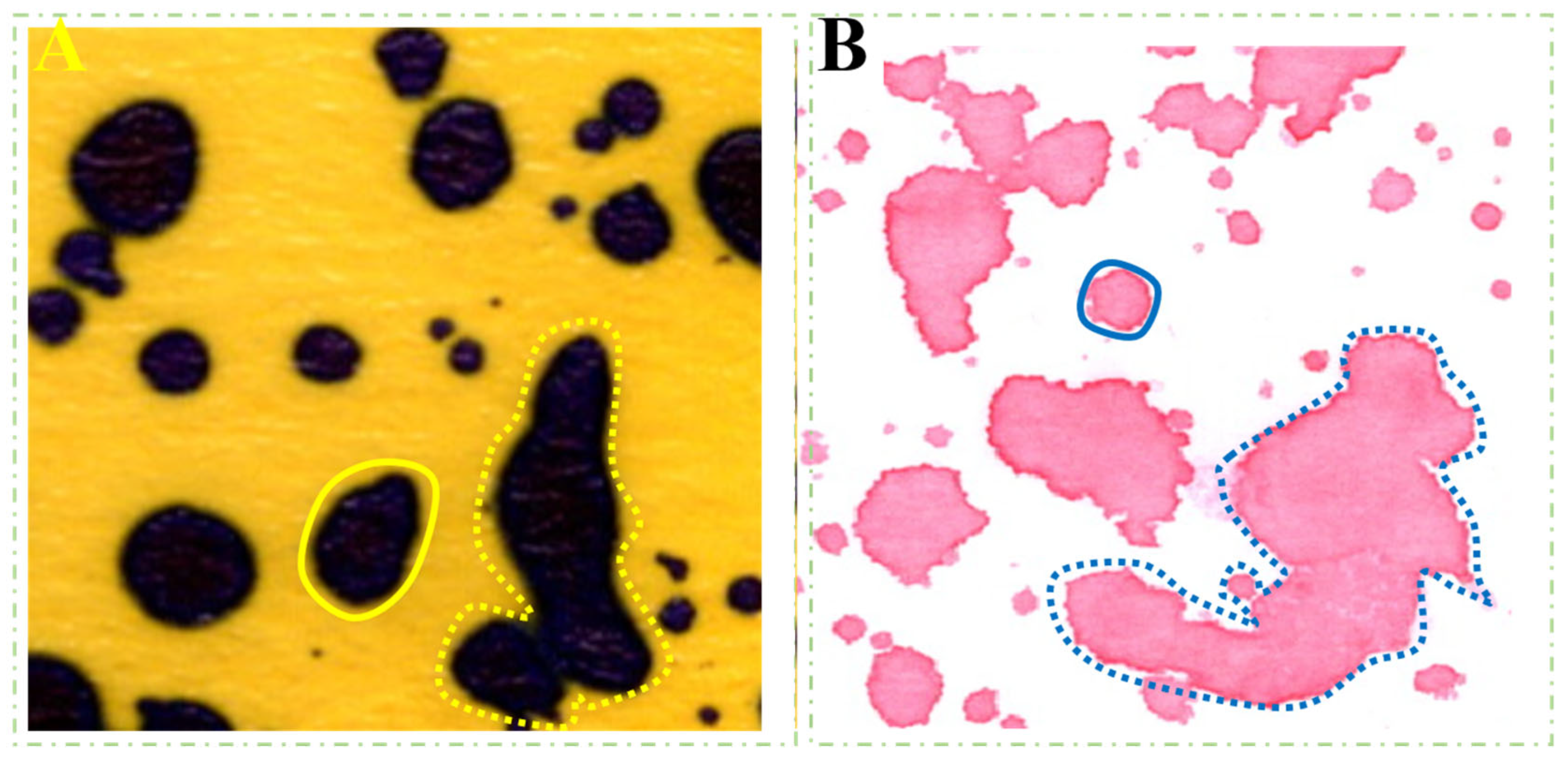
3.2. Differences in Droplet Density among Collectors
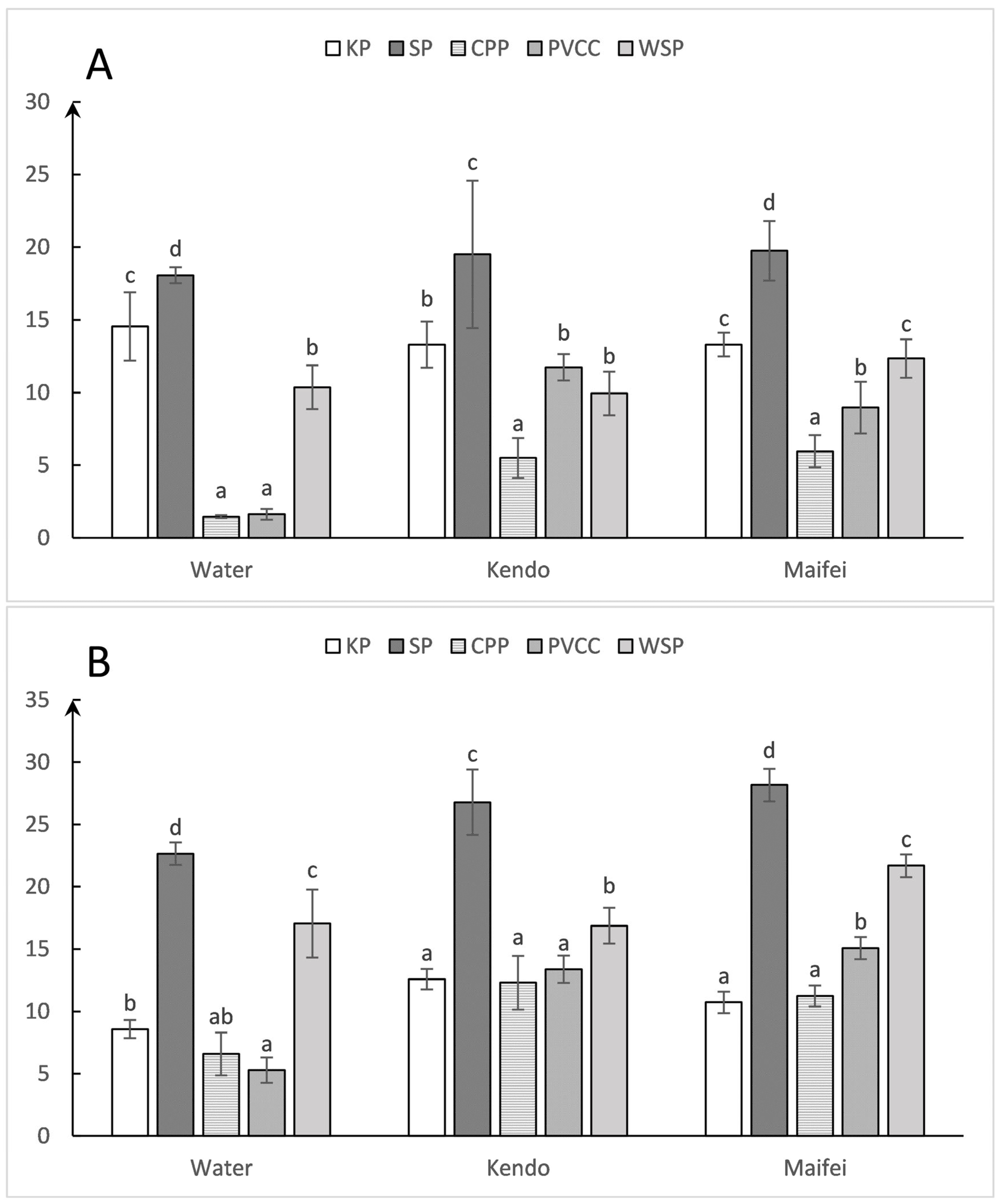
3.3. Differences in Droplet Coverage among Collectors
3.4. Differences in Droplet Deposition among Collectors
4. Discussion
4.1. Surface Free Energy
4.2. Droplet Density
4.3. Coverage
4.4. Deposition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, X. Research and Development of Efficient Plant Protection Equipment and Precision Spraying Technology in China: A Review. J. Plant Prot. 2022, 49, 9. [Google Scholar]
- Malneršič, A.; Dular, M.; Širok, B.; Oberti, R.; Hočevar, M. Close-Range Air-Assisted Precision Spot-Spraying for Robotic Applications: Aerodynamics and Spray Coverage Analysis. Biosyst. Eng. 2016, 146, 216–226. [Google Scholar] [CrossRef]
- Boina, D.R.; Salyani, M.; Tiwari, S.; Pelz-Stelinski, K.; Stelinski, L.L. Spray Droplet Size Affects Efficacy of Fenpropathrin against Asian Citrus Psyllid. In Proceedings of the ASTM Special Technical Publication, Tampa, FL, USA, 1–3 November 2011; Volume 1558 STP. [Google Scholar]
- Sciumbato, A.S.; Senseman, S.A.; Ross, J.; Mueller, T.C.; Chandler, J.M.; Cothren, J.T.; Kirk, I.W. Effects of 2,4-D Formulation and Quinclorac on Spray Droplet Size and Deposition 1. Weed Technol. 2005, 19, 1030–1036. [Google Scholar] [CrossRef]
- Ferguson, J.C.; Hewitt, A.J.; O’Donnell, C.C. Pressure, Droplet Size Classification, and Nozzle Arrangement Effects on Coverage and Droplet Number Density Using Air-Inclusion Dual Fan Nozzles for Pesticide Applications. Crop Prot. 2016, 89, 231–238. [Google Scholar] [CrossRef]
- ASABE S572.1; Spray Nozzle Classification by Droplet Spectra. American Society of Agricultural Engineers: St. Joseph, MI, USA, 2009.
- Chen, S.; Lan, Y.; Zhou, Z.; Ouyang, F.; Wang, G.; Huang, X.; Deng, X.; Cheng, S. Effect of Droplet Size Parameters on Droplet Deposition and Drift of Aerial Spraying by Using Plant Protection UAV. Agronomy 2020, 10, 195. [Google Scholar] [CrossRef]
- Yuan, H.; Ye, J.; Hu, X.; Fu, S.; Fang, Y. Numerical Simulation of Cyclone Extraction Element Based onpopulation Balance Model. Petrochem. Technol. 2017, 46, 7. [Google Scholar]
- Song, M.; Ju, J.; Luo, S.; Han, Y.; Dong, Z.; Wang, Y.; Gu, Z.; Zhang, L.; Hao, R.; Jiang, L. Controlling Liquid Splash on Superhydrophobic Surfaces by a Vesicle Surfactant. Sci. Adv. 2017, 3, e1602188. [Google Scholar] [CrossRef]
- Munjanja, B.K.; Naudé, Y.; Forbes, P.B.C. A Review of Sampling Approaches to Off-Target Pesticide Deposition. Trends Environ. Anal. Chem. 2020, 25, e00075. [Google Scholar] [CrossRef]
- Qiu, B.; Shi, C.; Wu, C.; Liu, B.; Tang, B. Analysis of Droplet Characteristics in Spray Field for Plant Protection Machinery and Reconstruction. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2005, 21, 7–10. [Google Scholar]
- Fox, R.D.; Derksen, R.C.; Cooper, J.A.; Krause, C.R.; Ozkan, H.E. Visual and Image System Measurement of Spray Deposits Using Water–Sensitive Paper. Appl. Eng. Agric. 2003, 19, 549–552. [Google Scholar] [CrossRef]
- Godyn, A.; Doruchowski, G.; Holownicki, R.; Swiechowski, W. A Method for Verification of Spray Volume Adapted to Crop Structure in Orchards. In Proceedings of the VIII Workshop on Spray Application Techniques in Fruit Growing, Barcelona, Spain, 29 June–1 July 2005. [Google Scholar]
- Jaeken, P.; Lootens, P.; Vandecasteele, P.; de Moor, M. Image Analysis of Water Sensitive Paper as a Tool for the Evaluation of Spray Distribution of Orchard Sprayers. Asp. Appl. Biol. 2000, 57, 329–342. [Google Scholar]
- Fritz, B.K.; Parker, C.; López, J.D.; Hoffman, W.C.; Schleider, P. Deposition and Droplet Sizing Characterization of a Laboratory Spray Table. Appl. Eng. Agric. 2009, 25, 175–180. [Google Scholar] [CrossRef]
- Tadić, V.; Marković, M.; Plaščak, I.; Stošić, M.; Lukinac-Čačić, J.; Vujčić, B. Impact of Technical Spraying Factors on Leaf Area Coverage in an Apple Orchard. Teh. Vjesn. 2014, 21, 1117–1124. [Google Scholar]
- Wang, J.; Luo, B.; Huo, Y.; Wang, X.; Xu, W. Influence of Adjuvant on Large-Scale Plant Protection Spray Characteristics. J. Drain. Irrig. Mach. Eng. 2019, 37, 6. [Google Scholar]
- Qin, W.; Xue, X.; Zhou, L. Effects of Spraying Parameters of Unmanned Aerial Vehicle on Droplets Deposition Distribution of Maize Canopies. Trans. Chin. Soc. Agric. Eng. 2014, 30, 7. [Google Scholar]
- Roten, R.L.; Hewitt, A.J.; Ledebuhr, M.; Thistle, H.; Connell, R.J.; Wolf, T.M.; Sankar, S.; Woodward, S.J.R. Evaluation of Spray Deposition in Potatoes Using Various Spray Delivery Systems. N. Z. Plant Prot. 2013, 66, 317–323. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Y. The elementary study of spraying droplets with filter paper ratio—Assured methods. J. Shanxi Agric. Univ. Sci. Ed. 1996, 16, 4. [Google Scholar]
- Zheng, Q.; Wu, J. Humidity Resistance Test System for Spray Droplet Density and Size Based on NO2- Color Reaction. Trans. Chin. Soc. Agric. Eng. 2015, 31, 6. [Google Scholar]
- Cunha, M.; Carvalho, C.; Marcal, A.R.S. Assessing the Ability of Image Processing Software to Analyse Spray Quality on Water-Sensitive Papers Used as Artificial Targets. Biosyst. Eng. 2012, 111, 11–23. [Google Scholar] [CrossRef]
- Lipiński, A.J.; Lipiński, S. Binarizing Water Sensitive Papers—How to Assess the Coverage Area Properly? Crop Prot. 2020, 127, 104949. [Google Scholar] [CrossRef]
- Chen, X.; Ru, Y.; Liu, B. Analysis and Comparison of Test Methods for Droplet Size and Deposition. J. Agric. Mech. Res. 2021, 43, 5. [Google Scholar]
- Zisman, W.A. Relation of the Equilibrium Contact Angle to Liquid and Solid Constitution; ACS Publications: Washington, DC, USA, 1964. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Rabel, W. Einige Aspekte Der Benetzungstheorie Und Ihre Anwendung Auf Die Untersuchung Und Veränderung Der Oberflächeneigenschaften von Polymeren. Farbe Lack 1971, 77, 997–1005. [Google Scholar]
- Young, T. An Essay on the Cohesion of Fluids. Abstr. Pap. Print. Philos. Trans. R. Soc. 1832, 1, 171–172. [Google Scholar] [CrossRef]
- Qiu, Z.; Yuan, H.; Lou, S.; Ji, M.; Yu, J.; Song, X. The Research of Water Soluble Dyes of Allura Red and Ponceau-G as Tracers for Determing Pesticide Spray Distribution. Agrochemicals 2007, 46, 323. [Google Scholar]
- Zhu, H.; Salyani, M.; Fox, R.D. A Portable Scanning System for Evaluation of Spray Deposit Distribution. Comput. Electron. Agric. 2011, 76, 38–43. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Sharma, P.K.; Hanumantha Rao, K. Analysis of Different Approaches for Evaluation of Surface Energy of Microbial Cells by Contact Angle Goniometry. Adv. Colloid Interface Sci. 2002, 98, 341–463. [Google Scholar] [CrossRef] [PubMed]
- Toraman, M.C. Effects of Leaf Surface Energy on Pesticidal Performance. Tarim Bilim. Derg. 2019, 25, 174–180. [Google Scholar] [CrossRef]
- Gu, Z.; Xu, X.; Han, L. Study on the Method of Measure of the Critical Surface Tension of Plants. Mod. Agrochem. 2002, 2, 20–22. [Google Scholar]
- Hou, C.; Tang, Y.; Luo, S.; Lin, J.; He, Y.; Zhuang, J.; Huang, W. Optimization of Control Parameters of Droplet Density in Citrus Trees Using Uavs and the Taguchi Method. Int. J. Agric. Biol. Eng. 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Meng, Y.; Lan, Y.; Mei, G.; Guo, Y.; Song, J.; Wang, Z. Effect of Aerial Spray Adjuvant Applying on the Efficiency of Small Unmanned Aerial Vehicle on Wheat Aphids Control. Int. J. Agric. Biol. Eng. 2018, 11, 46–53. [Google Scholar] [CrossRef]
- Qi, P.; Zhang, L.; Wang, Z.; Han, H.; Müller, J.; Li, T.; Wang, C.; Huang, Z.; He, M.; Liu, Y.; et al. Effect of Operational Parameters of Unmanned Aerial Vehicle (UAV) on Droplet Deposition in Trellised Pear Orchard. Drones 2023, 7, 57. [Google Scholar] [CrossRef]
- Cunha, J.P.A.R.; Farnese, A.C.; Olivet, J.J. Computer Programs for Analysis of Droplets Sprayed on Water Sensitive Papers. Planta Daninha 2013, 31, 715–720. [Google Scholar] [CrossRef]
- Turner, C.R.; Huntington, K.A. The Use of a Water Sensitive Dye for the Detection and Assessment of Small Spray Droplets. J. Agric. Eng. Res. 1970, 15, 385–387. [Google Scholar] [CrossRef]
- Kang, J.; Liu, G.; Guo, G. Image Preprocessing and Droplet Parameter Measurement Of-Sensitive Paper Based on ImagePy. Sci. Technol. Eng. 2021, 21, 10. [Google Scholar]
- Grella, M.; Gioelli, F.; Marucco, P.; Zwertvaegher, I.; Mozzanini, E.; Mylonas, N.; Nuyttens, D.; Balsari, P. Field Assessment of a Pulse Width Modulation (PWM) Spray System Applying Different Spray Volumes: Duty Cycle and Forward Speed Effects on Vines Spray Coverage. Precis. Agric. 2022, 23, 219–252. [Google Scholar] [CrossRef]
- Shan, C.; Wang, G.; Wang, H.; Xie, Y.; Wang, H.; Wang, S.; Chen, S.; Lan, Y. Effects of Droplet Size and Spray Volume Parameters on Droplet Deposition of Wheat Herbicide Application by Using Uav. Int. J. Agric. Biol. Eng. 2021, 14, 74–81. [Google Scholar] [CrossRef]
- Vermant, J. Fluid Mechanics: When Shape Matters. Nature 2011, 476, 286–287. [Google Scholar] [CrossRef]
- Mampallil, D.; Eral, H.B. A Review on Suppression and Utilization of the Coffee-Ring Effect. Adv. Colloid Interface Sci. 2018, 252, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xu, X.; Wang, X.; Gong, C.; Zhang, Y.; Ruan, Y.; Yang, D.; Yang, X. Effects of Different Auxiliaries, Spray Nozzles and Spray Pressures of Self-Propelled Boom Sprayer on Spray Effect in Paddy Field. Chin. J. Pestic. Sci. 2021, 23, 587–596. [Google Scholar]
- Wu, Q.; Hua, D.; Zheng, X.; Zhang, K.; Zhang, Z.; Wan, Y.; Zhou, X.; Zhang, Y. Assessing Pesticide Residue and Spray Deposition in Greenhouse Eggplant Canopies to Improve Residue Analysis. J. Agric. Food Chem. 2020, 68, 11920–11927. [Google Scholar] [CrossRef]
- Hong, S.W.; Zhao, L.; Zhu, H. CFD Simulation of Pesticide Spray from Air-Assisted Sprayers in an Apple Orchard: Tree Deposition and off-Target Losses. Atmos. Environ. 2018, 175, 109–119. [Google Scholar] [CrossRef]
- Salyani, M.; Zhu, H.; Sweeb, R.D.; Pai, N. Assessment of Spray Distribution with Water-Sensitive Paper. Agric. Eng. Int. CIGR J. 2013, 15, 101–111. [Google Scholar]
| Collector | Material | γ (mN m−1) | Contact Angle (°) | cosθ |
|---|---|---|---|---|
| SP | DMSO | 39.82 | 36.74 ± 2.34 | 0.80 |
| Deionized Water | 71.34 | 72.48 ± 2.55 | 0.30 | |
| CPP | DMSO | 39.82 | 32.57 ± 1.10 | 0.85 |
| Deionized Water | 71.34 | 84.97 ± 0.30 | 0.088 | |
| KP | DMSO | 39.82 | 14.94 ± 2.69 | 0.97 |
| Deionized Water | 71.34 | 52.13 ± 1.46 | 0.61 | |
| PVCC | DMSO | 39.82 | 32.30 ± 1.83 | 0.86 |
| Deionized Water | 71.34 | 85.59 ± 5.28 | 0.077 | |
| WSP | DMSO | 39.82 | 26.24 ± 0.19 | 0.90 |
| Deionized Water | 71.34 | 65.82 ± 1.88 | 0.41 |
| Collectors | cosθ-γ | Surface Free Energy (mN m−1) | |
|---|---|---|---|
| Zisman | OWRK | ||
| SP | y = −0.0159x + 1.4334 | 27.26 | 35.11 |
| CPP | y = −0.024x + 1.7968 | 33.2 | 33.81 |
| KP | y = −0.0114x + 1.4271 | 37.46 | 48.38 |
| PVCC | y = −0.0244x + 1.816 | 33.44 | 33.9 |
| WSP | y = −0.0171x + 1.5986 | 35.01 | 39.95 |
| Plant Species | Minimum | Maximum | Average |
|---|---|---|---|
| Eggplant | 44.6 | 52.6 | 48.6 |
| Tomato | 32.4 | 35.5 | 34.0 |
| Luffa cylindrica | 39 | 43.4 | 41.2 |
| Chinese cabbage | 31.5 | 36.2 | 33.9 |
| Cabbage | 32.8 | 36.8 | 34.8 |
| Spinach | 41.3 | 46.4 | 43.9 |
| Wheat | 30.6 | 33.8 | 32.3 |
| Rice | 36.3 | 39 | 37.7 |
| Peanut | 29.8 | 32.6 | 31.2 |
| Soybean | 38.6 | 43.8 | 41.2 |
| Grape | 32.2 | 36.6 | 34.4 |
| Peach | 28.6 | 33.5 | 31.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Qi, P.; Han, L.; He, X. Study on Spray Evaluation: The Key Role of Droplet Collectors. Agronomy 2024, 14, 305. https://doi.org/10.3390/agronomy14020305
He M, Qi P, Han L, He X. Study on Spray Evaluation: The Key Role of Droplet Collectors. Agronomy. 2024; 14(2):305. https://doi.org/10.3390/agronomy14020305
Chicago/Turabian StyleHe, Miao, Peng Qi, Leng Han, and Xiongkui He. 2024. "Study on Spray Evaluation: The Key Role of Droplet Collectors" Agronomy 14, no. 2: 305. https://doi.org/10.3390/agronomy14020305
APA StyleHe, M., Qi, P., Han, L., & He, X. (2024). Study on Spray Evaluation: The Key Role of Droplet Collectors. Agronomy, 14(2), 305. https://doi.org/10.3390/agronomy14020305








