Drought Tolerance Evaluation and Growth Response of Chinese Cabbage Seedlings to Water Deficit Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Screening for Drought Tolerance through Water Deficit Treatment of Chinese Cabbage Seedlings
2.1.1. Plant Materials
2.1.2. Water Deficit Treatment and Investigation for Drought Tolerance Seedling Test
plant number) + (3 × ‘stage 3’ plant number) + (4 × ‘stage 4’ plant number) + (5 × ‘stage 5’ plant
number)]/total number of treated plants
2.2. Water Potential and Growth of Chinese Cabbage Seedlings with Water Deficit Treatment
2.2.1. Plant Materials
2.2.2. Evaluation of Growing Media and Leaf Water Potential and Growth in Response to Water Deficit Treatment
2.3. Growth of Chinese Cabbage under Greenhouse Cultivation with Water Deficit Treatment
2.3.1. Plant Materials
2.3.2. Greenhouse Cultivation and Water Deficit Treatment
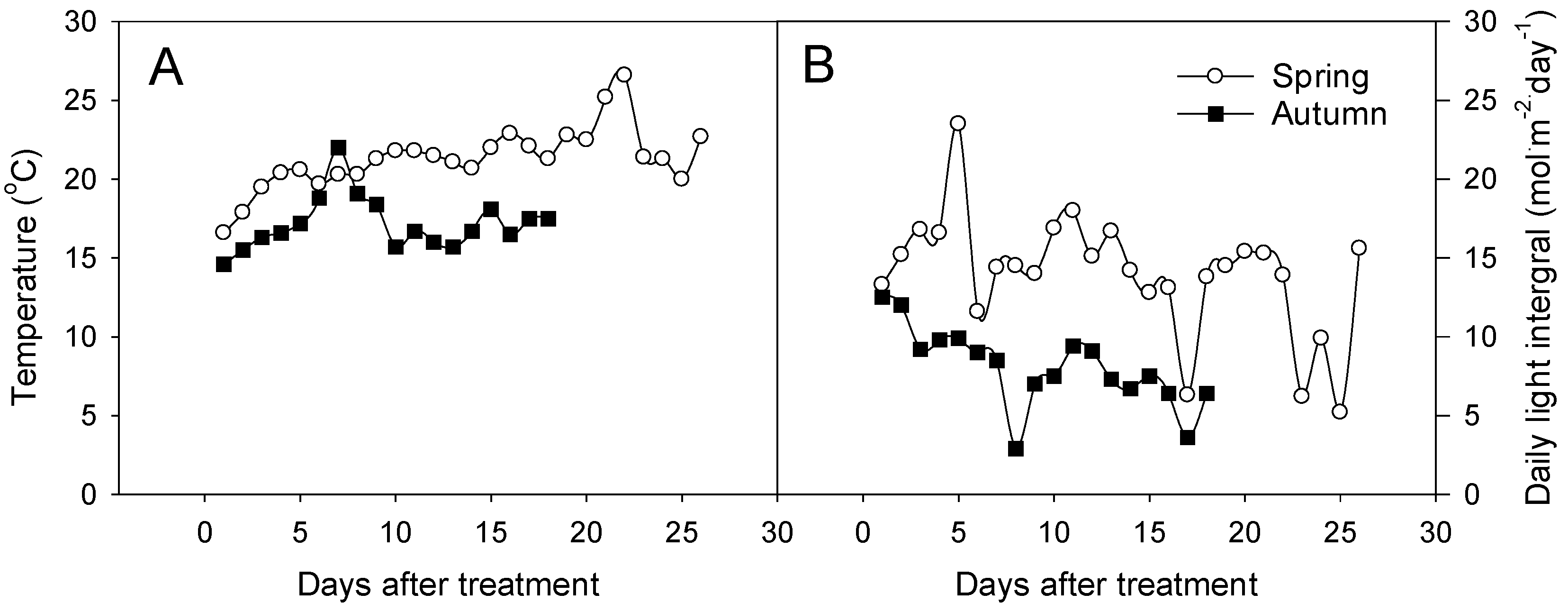
2.4. Environmental Data Measurement
2.5. Statistical Analysis
3. Results
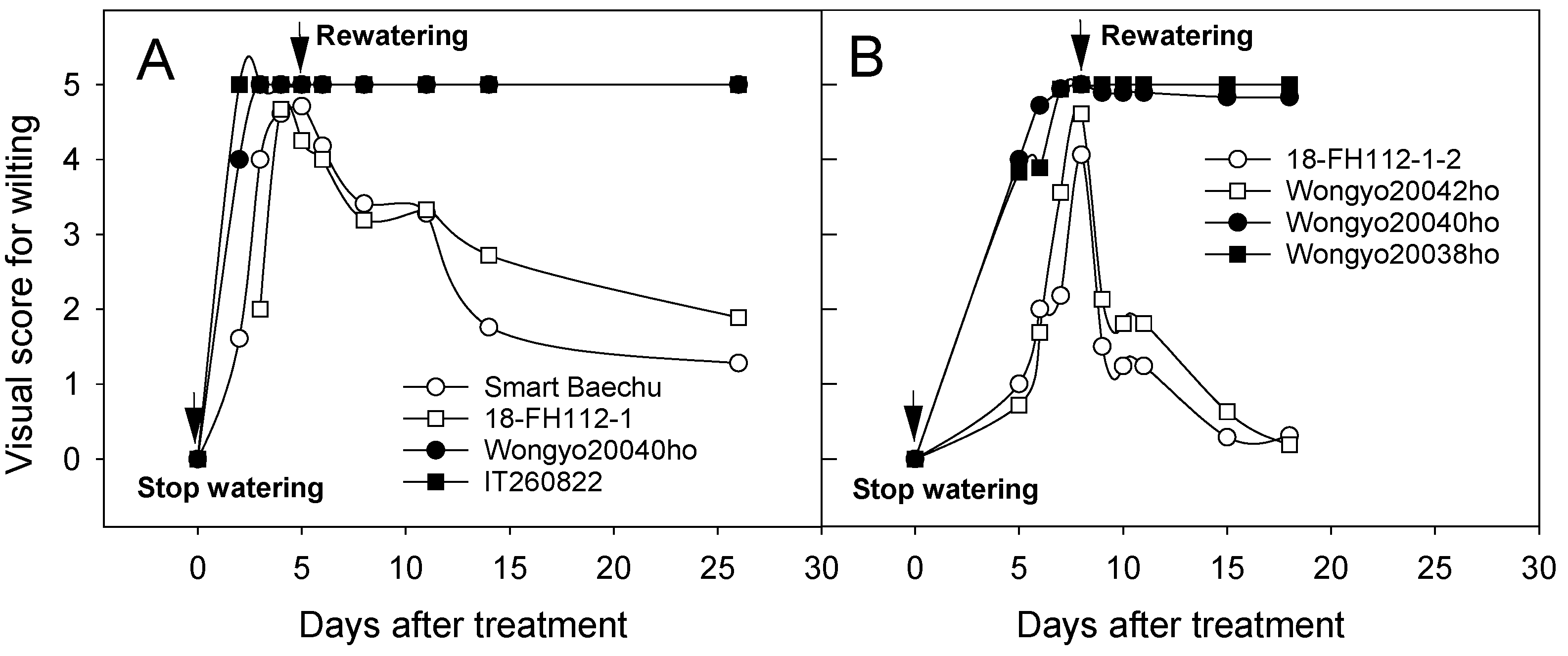
3.1. Screening for Drought Tolerance through Water Deficit Treatment of Chinese Cabbage Seedlings
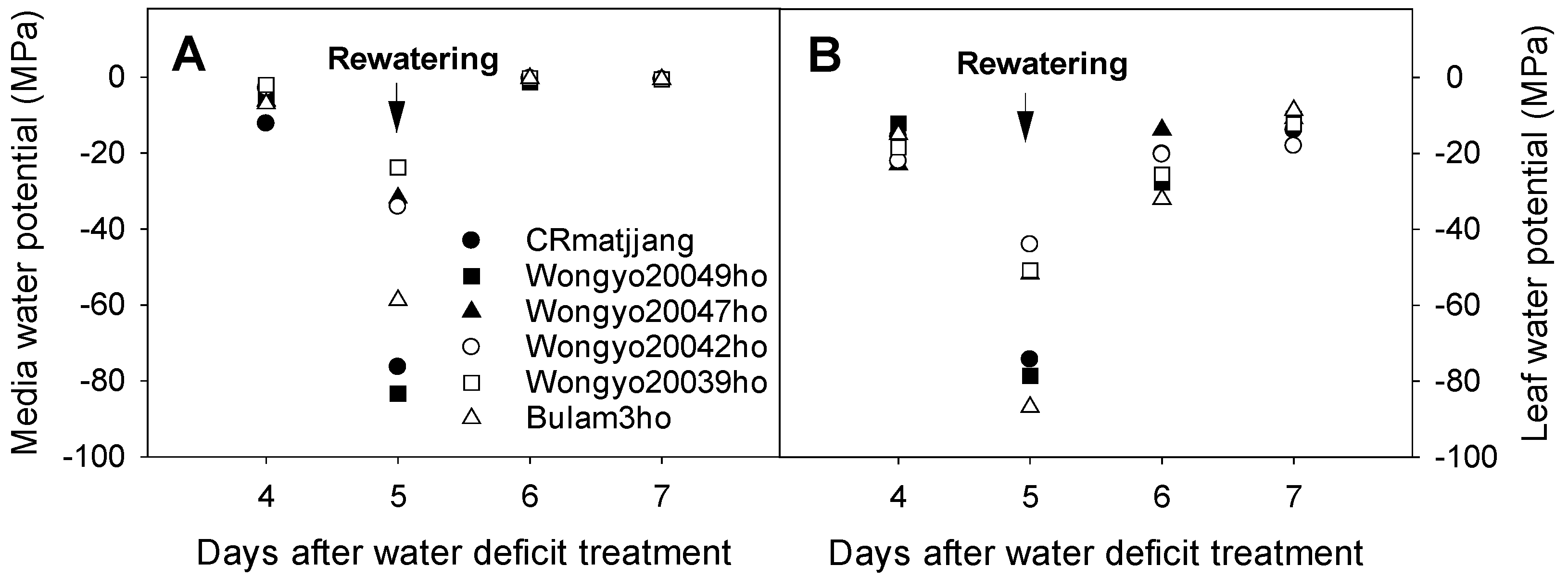
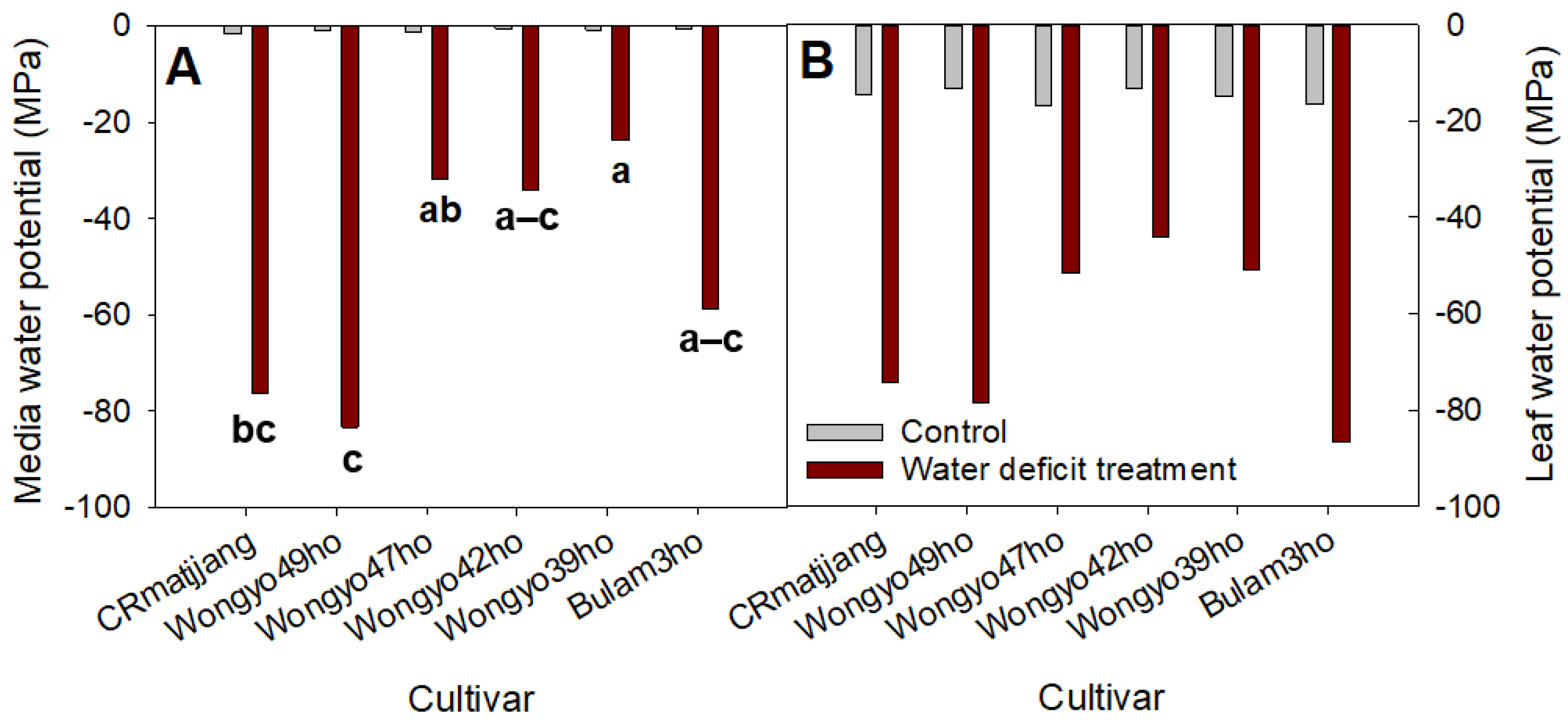
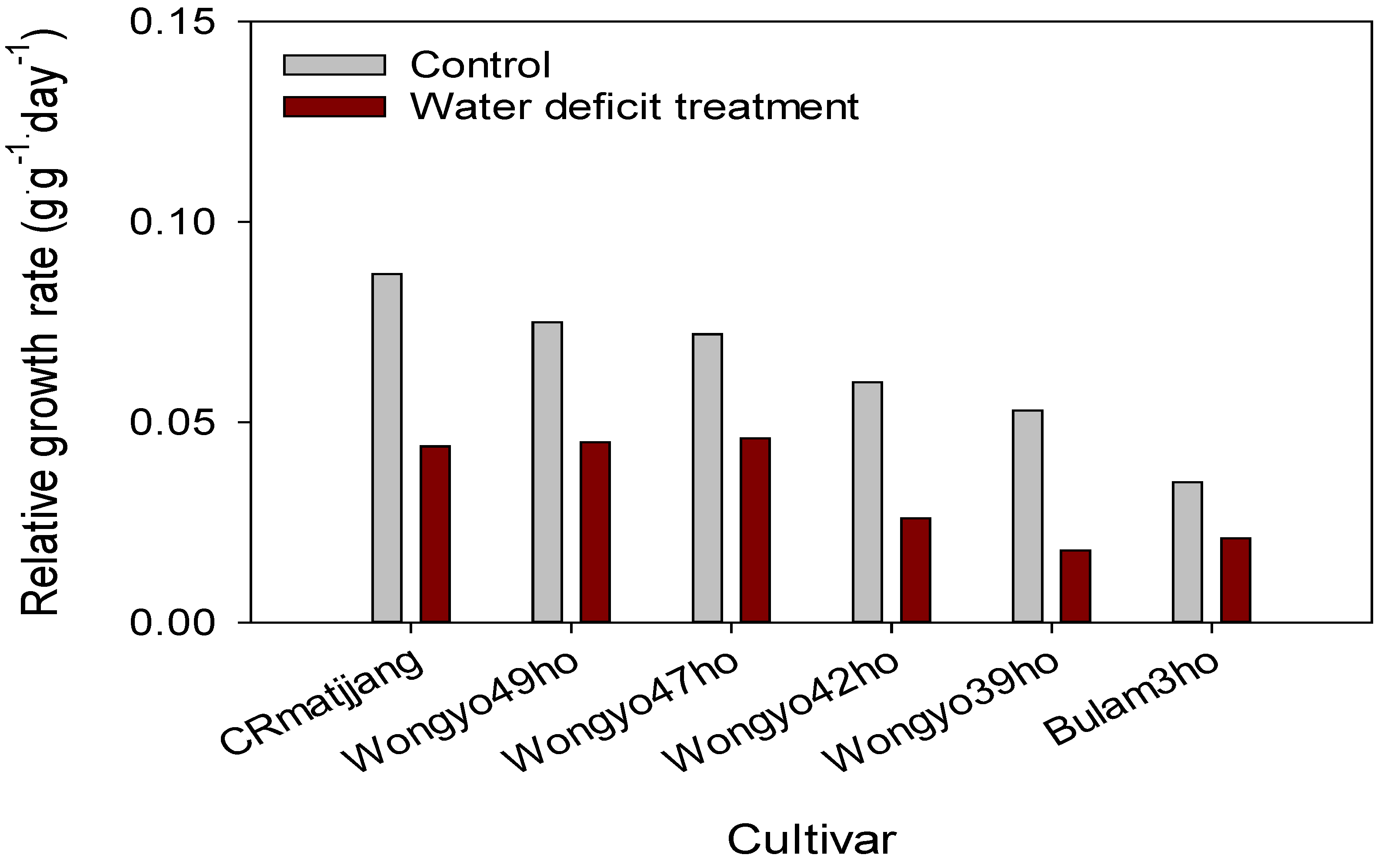
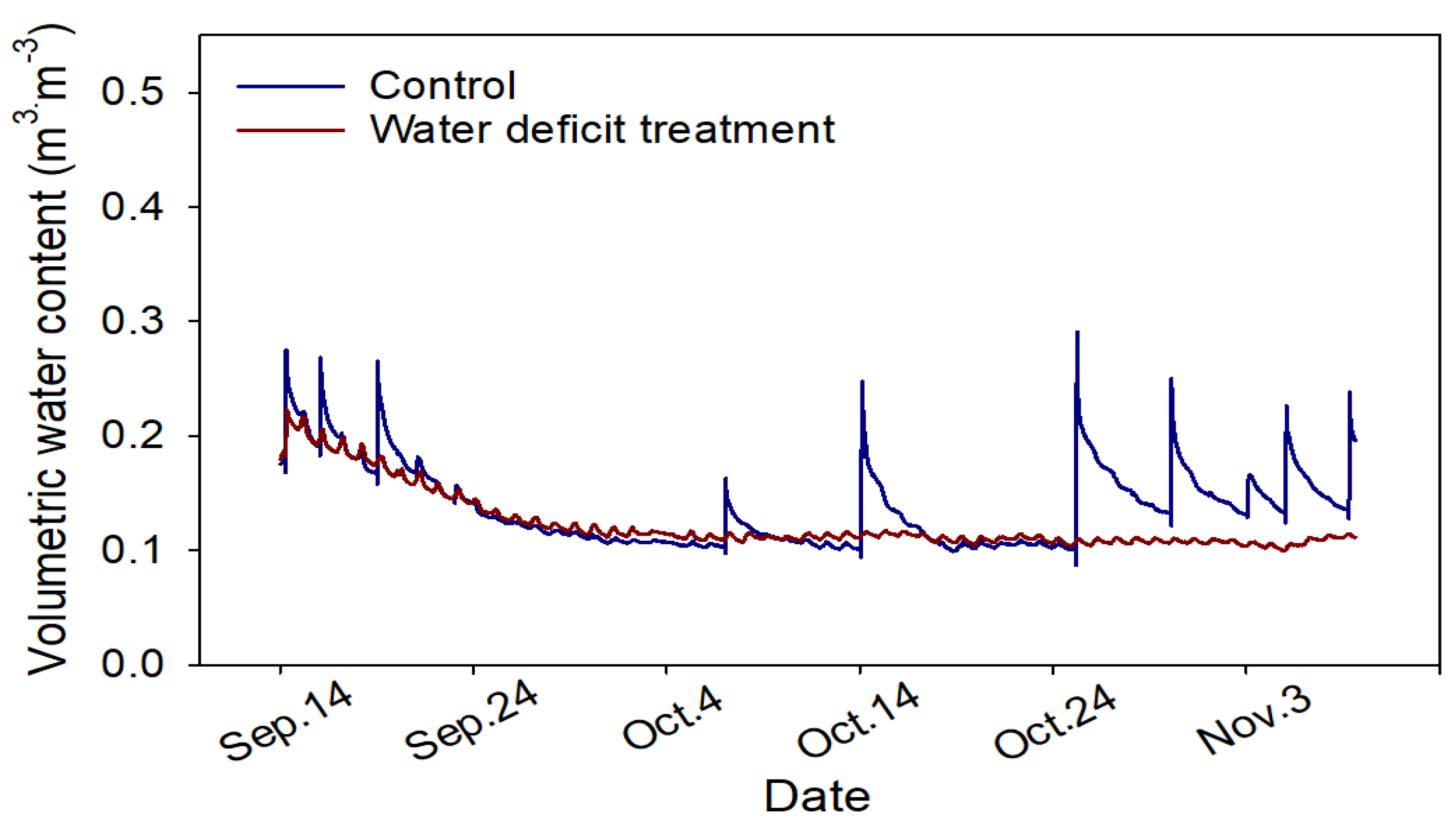
3.2. Changes in Growing Media and Leaf Water Potential and Growth Response of Chinese Cabbage Seedlings to Water Deficit Treatment
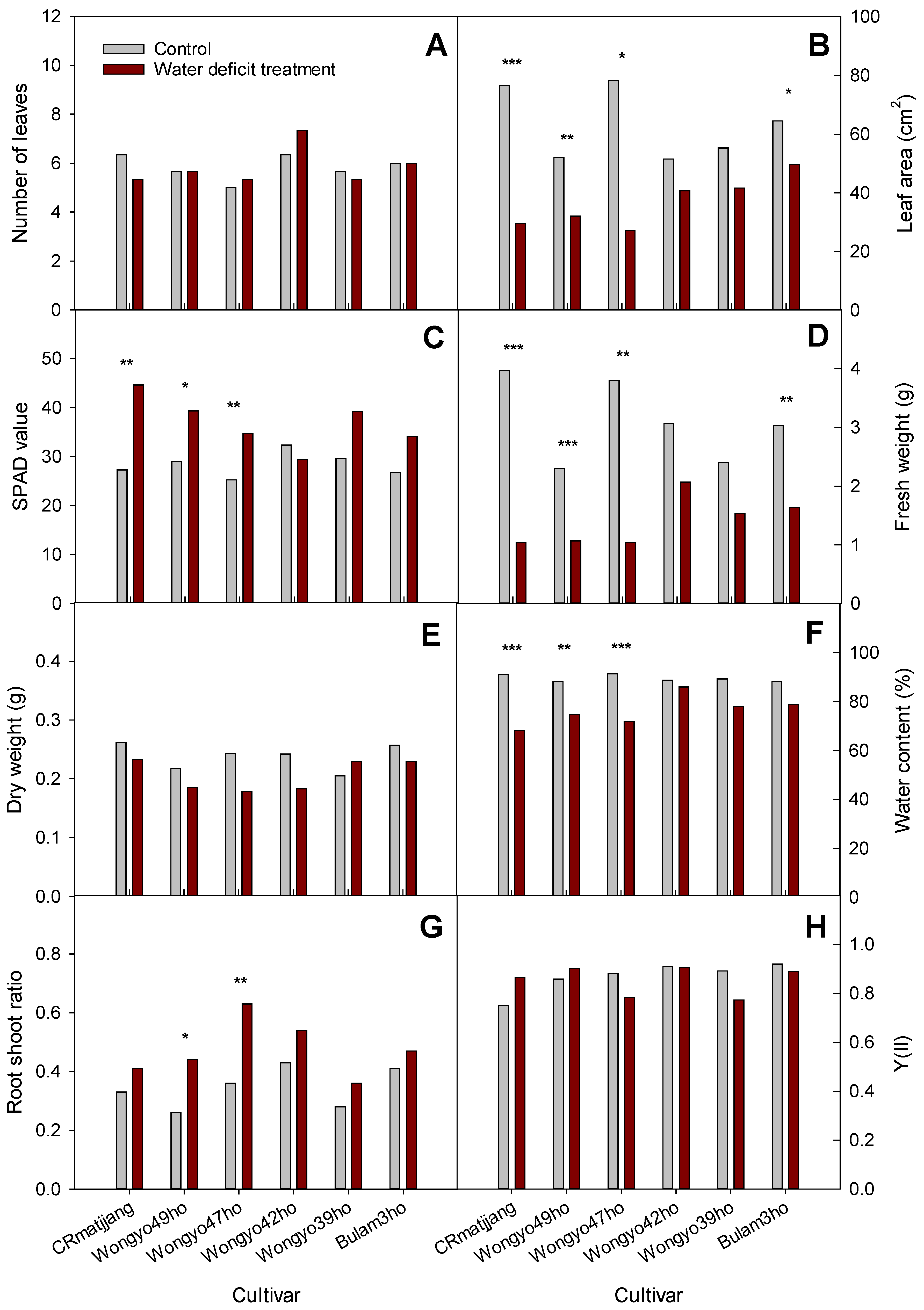
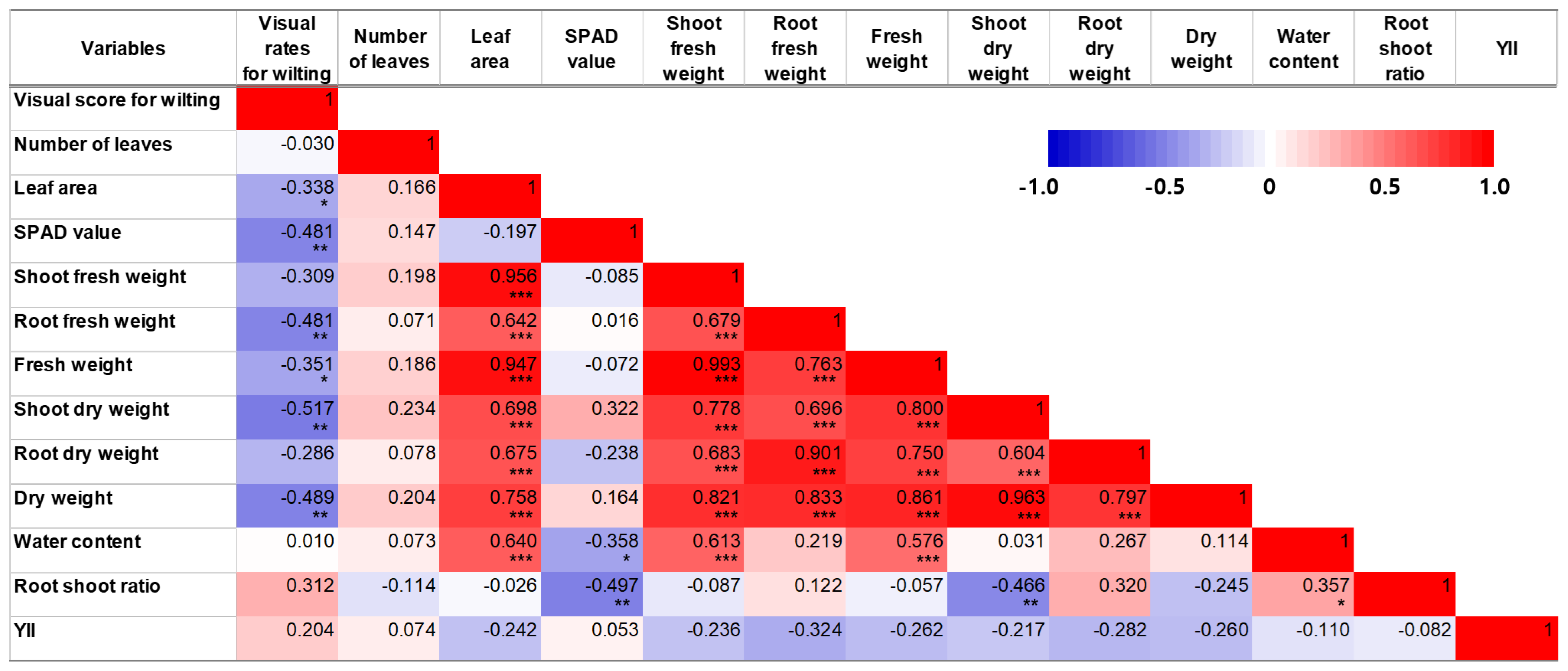
3.3. Growth of Chinese Cabbage in Greenhouse Cultivation under Water Deficit Treatment
4. Discussion
4.1. Screening for Drought Tolerance through Water Deficit Treatment of Chinese Cabbage Seedlings
4.2. Changes in Growing Media and Leaf Water Potential and Growth Response of Chinese Cabbage Seedlings to Drought Stress
4.3. Growth of Chinese Cabbage in Greenhouse Cultivation under Water Deficit Treatment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sim, H.S.; Jo, W.J.; Lee, H.J.; Moon, Y.H.; Woo, U.J.; Bin Jung, S.; Ahn, S.R.; Kim, S.K. Determination of optimal growing degree days and cultivars of Kimchi cabbage for growth and yield during spring cultivation under shading conditions. Hort. Sci. Technal. 2021, 39, 714–725. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, H.J.; Lee, H.S.; Mun, B.H.; Lee, S.G. Effect of soil water content on growth, photosynthetic rate, and stomatal conductance of Kimchi cabbage at the early growth staged after transplanting. Prot. Hort. Plant Fact. 2017, 26, 151–157. [Google Scholar] [CrossRef]
- Oh, S.J.; Moon, K.H.; Son, I.C.; Song, E.Y.; Moon, Y.E.; Koh, S.C. Growth, photosynthesis and chlorophyll fluorescence of Chinese cabbage in response to high temperature. Kor. J. Hort. Sci. Technol. 2014, 32, 318–329. [Google Scholar]
- Lee, S.G.; Kim, S.K.; Lee, H.J.; Choi, C.S.; Park, S.T. Impacts of climate change on the growth, morphological and physiological responses, and yield of Kimchi cabbage leaves. Hortic. Environ. Biotechnol. 2016, 57, 470–477. [Google Scholar] [CrossRef]
- Rural Development Administration (RDA). Kimchi Cabbage Cultivation (The Textbook for Farming No. 128); RDA: Jeonju, Republic of Korea, 2019. [Google Scholar]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.G.; Kim, S.K.; Park, S.T. Assessment on yield decrease of Kimchi cabbage by extreme weather conditions using physiological parameters. Korean J. Agri. For. Meteorol. 2018, 20, 127–134. [Google Scholar]
- Shawon, R.A.; Kang, B.S.; Kim, H.C.; Lee, S.G.; Kim, S.K.; Lee, H.J.; Bae, J.H.; Ku, Y.G. Changes in free amino acid, carotenoid, and proline content in Chinese cabbage (Brassica rapa subsp. pekinensis) in response to drought stress. Korean J. Plant Res. 2018, 31, 622–633. [Google Scholar]
- Shawon, R.A.; Kang, B.S.; Lee, S.G.; Kim, S.K.; Lee, H.J.; Katrich, E.; Gorinstein, S.; Ku, Y.G. Influence of drought stress on bioactive compounds, antioxidant enzymes and glucosinolate contents of Chinese cabbage (Brassica rapa). Food Chem. 2020, 308, 125657. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.R.; Kim, S.; Lee, J.H.; Shim, I.-S. Enhanced drought tolerance in Chinese cabbage (Brassica campestris L.) seedlings upon pretreatment with exogenous salicylic acid. Hort. Sci. Tech. 2019, 38, 9–20. [Google Scholar] [CrossRef]
- Yuan, J.; Shen, C.; Yuan, R.; Zhang, H.; Xiao, Y.; Wang, X.; Pan, F.; Wu, C.; Li, Q.; Yuan, J.; et al. Identification of genes related to tipburn resistance in Chinese cabbage and preliminary exploration of its molecular mechanism. BMC Plant Biol. 2021, 21, 567. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, H.J.; Lee, J.H.; An, S.; Lee, S.G. Development of crop water stress index for Kimchi cabbage precision irrigation control. Hort. Sci. Tech. 2019, 37, 490–498. [Google Scholar] [CrossRef]
- Jeong, M.; Kang, I.-K.; Kil Kim, C.; Park, K.I.; Choi, C.; Han, J.-S. Physiological responses to drought stress of transgenic Chinese cabbage expressing Arabidopsis H+-pyrophosphatase. J. Plant Biotechnol. 2013, 40, 156–162. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Shen, Y.; Yang, W.; Pan, Q.; Li, C.; Sun, Q.; Zeng, Q.; Li, B.; Zhang, L. Comparative metabolic study of two contrasting Chinese cabbage genotypes under mild and severe drought stress. Int. J. Mol. Sci. 2022, 23, 5947. [Google Scholar] [CrossRef] [PubMed]
- Susanto, U.; Rohaeni, W.R.; Sasmita, P. Selecting traits for drought tolerance screening in rice. IOP Conf. Ser. Earth Environ. Sci. 2019, 383, 012049. [Google Scholar] [CrossRef]
- Kogan, F.N. Global drought watch from space. Bull. Am. Meteorol. Soc. 1997, 78, 621–636. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.G.; Shen, B.J.; Chen, H.C.; Opeña, R.T. Associations between heat tolerance, water consumption, and morphological characters in Chinese cabbage. Euphytica 1988, 39, 65–73. [Google Scholar] [CrossRef]
- Moon, J.C.; Shin, S.; Kim, H.C.; Song, K.; Kim, J.Y.; Kim, K.H.; Lee, B.M. Assessment of the candidate genes of expression markers associated with drought stress in maize seedlings. Kor. J. Breed. Sci. 2018, 50, 224–235. [Google Scholar] [CrossRef]
- De La Cruz, M.; Romao, R.L.; Escudero, A.; Maestre, F.T. Where do seedlings go? A spatio-temporal analysis of seedling mortality in a semi-arid sypsophyet. Ecography 2008, 31, 720–730. [Google Scholar] [CrossRef]
- Meeks, M.; Murray, S.C.; Hague, S.; Hays, D. Measuring maize seedling drought response in search of tolerant germplasm. Agronomy 2013, 3, 135–147. [Google Scholar] [CrossRef]
- Wang, L.; Liu, K.; Mao, S.S.; Li, Z.Y.; Lu, Y.L.; Wang, J.R.; Liu, Y.X.; Wei, Y.M.; Zheng, Y.L. Large-scale screening for Aegilops tauschii tolerant genotypes to phosphorus deficiency at seedling stage. Euphytica 2015, 204, 571–586. [Google Scholar] [CrossRef]
- Badr, A.; El-Shazly, H.H.; Tarawneh, R.A.; Börner, A. Screening for drought tolerance in Maize (Zea mays L.) Germplasm using germination and seedling traits under simulated drought conditions. Plant 2020, 9, 565. [Google Scholar] [CrossRef]
- Marchin, R.M.; Ossola, A.; Leishman, M.R.; Ellsworth, D.S. A simple method for simulating drought effects of plants. Front. Plant Sci. 2020, 10, 1715. [Google Scholar] [CrossRef]
- Poorter, H.; Fiorani, F.; Stitt, M.; Schurr, U.; Finck, A.; Gibon, Y.; Usadel, B.; Munns, R.; Atkin, O.K.; Tardieu, F.; et al. The art of growing plants for experimental purposes: A practical guide for the plant biologist. Funct. Plant Biol. 2012, 39, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Yeo, A.R.; Flowers, T.J. Nonosmotic effects of polyethylene glycols upon sodium transport and sodium-potassium selectivity by rice roots. Plant Physiol. 1984, 75, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.M.; Imran, M.; Atta, B.M.; Ashraf, M.Y.; Hameed, A.; Waqar, I.; Shafiq, M.; Hussain, K.; Naveed, M.; Aslam, M.; et al. Selection and screening of drought tolerant high yielding chickpea genotypes based on physio-biochemical indices and multi-environmental yield traits. BMC Plant Biol. 2020, 20, 171. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Chen, X.; Han, Z.; Wu, X.; Zhang, S.; Li, Q.; Nazir, M.M.; Zhang, G.; Zeng, F. Screening of worldwide barley collection for drought tolerance: The assessment of various physiological measures as the selection criteria. Front. Plant Sci. 2020, 11, 1159. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.L.; Henninger, D.L. Response of the water status of soybean to changes in soil water potentials controlled by the water pressure in microporous tubes. Plant Cell Environ. 1997, 20, 1506–1516. [Google Scholar] [CrossRef]
- Bunce, J.A.; Nasyrov, M. A new method of applying a controlled soil water stress, and its effect on the growth of cotton and soybean seedlings at ambient and elevated carbon dioxide. Environ. Exp. Bot. 2012, 77, 165–169. [Google Scholar] [CrossRef]
- Mexal, J.; Fisher, J.T.; Osteryoung, J.; Reid, C.P.P. Oxygen availability in polyethylene glycol solutions and its implications in plant-water relations. Plant Physiol. 1975, 55, 20–24. [Google Scholar] [CrossRef]
- Kumar, S.; Dwivedi, S.K.; Basu, S.; Kumar, G.; Mishra, J.; Koley, T.K.; Rao, K.; Choudhary, A.; Mondal, S.; Kumar, S.; et al. Anatomical, agro-morphological and physiological changes in rice under cumulative and stage specific drought conditions prevailed in eastern region of India. Field Crops Res. 2020, 245, 107658. [Google Scholar] [CrossRef]
- Sabouri, A.; Dadras, A.R.; Azari, M.; Kouchesfahani, A.S.; Taslimi, M.; Jalalifar, R. Screening of rice drought-tolerant lines by introducing a new composite selection index and competitive with multivariate methods. Sci. Rep. 2022, 12, 2163. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.M.; Dawood, M.F.A.; Elfarash, A.; Mohamed, E.A.; Hussein, M.Y.; Börner, A.; Sallam, A. Genetic and morpho-physiological analyses of the tolerance and recovery mechanisms in seedling stage spring wheat under drought stress. Front. Plant Sci. 2022, 13, 1010272. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.d.; Shabala, L.; Brodrib, T.J.; Zhou, M.S. Understanding the role of physiological and agronomical traits during drought recovery as a determinant of differential drought stress tolerance in barley. Agronomy 2022, 12, 2136. [Google Scholar] [CrossRef]
- Sallam, A.; Mourad, A.M.I.; Hussain, W.; Zaenziger, P.S. Genetic variation in drought tolerance at seedling stage and grain yield in low rainfall environments in wheat (Triticum aestivum L.). Euphytica 2018, 214, 169. [Google Scholar] [CrossRef]
- Macnicol, P.K.; Jacobsen, J.; Keys, M.; Stuart, I. Effects of heat and water stress on malt quality and grain parameters of Schooner barley grown in cabinets. J. Cereal Sci. 1993, 18, 61–68. [Google Scholar] [CrossRef]
- Savin, R.; Nicolas, M.E. Effects of short periods of drought and high temperature on grain growth and starch accumulation of two malting barley cultivars. Funct. Plant Biol. 1996, 23, 201–210. [Google Scholar] [CrossRef]
- Wallwork, M.A.B.; Jenner, C.F.; Logue, S.J.; Sedgley, M. Effect of high temperature during grain-filling on the structure of developing and malted barley grains. Ann. Bot. 1998, 82, 587–599. [Google Scholar] [CrossRef]
- Chaves, M.M.; Marôco, J.P.; Pereira, J.S. Understanding plant responses to drought from genes to the whole plant. Funct. Plant Biol. 2003, 36, 239–264. [Google Scholar] [CrossRef]
- Rhizhsky, I.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef]
- Chen, D.; Neumann, K.; Friedel, S.; Kilian, B.; Chen, M.; Altmann, T.; Klukas, C. Dissecting the Phenotypic Components of Crop Plant Growth and Drought Responses Based on High-Throughput Image Analysis. Plant Cell 2014, 26, 4636–4655. [Google Scholar] [CrossRef]
- Correia, P.M.P.; Westergaard, J.C.; da Silva, A.B.; Roitsch, T.; Carmo-Silva, E.; da Silva, J.M. High-throughput phenotyping of physiological traits for wheat resilience to high temperature and drought stress. J. Exp. Bot. 2022, 73, 5235–5251. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, W.; Chang, Y.; Ma, X.; Tu, H.; Xiong, F.; Jiang, N.; Feng, H.; Huang, C.; Yang, P.; et al. Genome-wide association studies of image traits reveal genetic architecture of drought resistance in rice. Mol. Plant 2018, 11, 789–805. [Google Scholar] [CrossRef]
- Vesala, T.; Sevanto, S.; Grönholm, T.; Salmon, Y.; Nikinmaa, E.; Hari, P.; Hölttä, T. Effect of leaf water potential on internal humidity and CO, dissolution: Reverse transpiration and improved water use efficiency under negative pressure. Front. Plant Sci. 2017, 8, 54. [Google Scholar] [CrossRef]
- Nonami, H. Water Relations in Plant Physiology (Japanese in Text); Yokendo: Tokyo, Japan, 2001. [Google Scholar]
- Xu, Z.; Zhou, G.; Shimizu, H. Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J. Exp. Bot. 2009, 60, 3737–3749. [Google Scholar] [CrossRef] [PubMed]
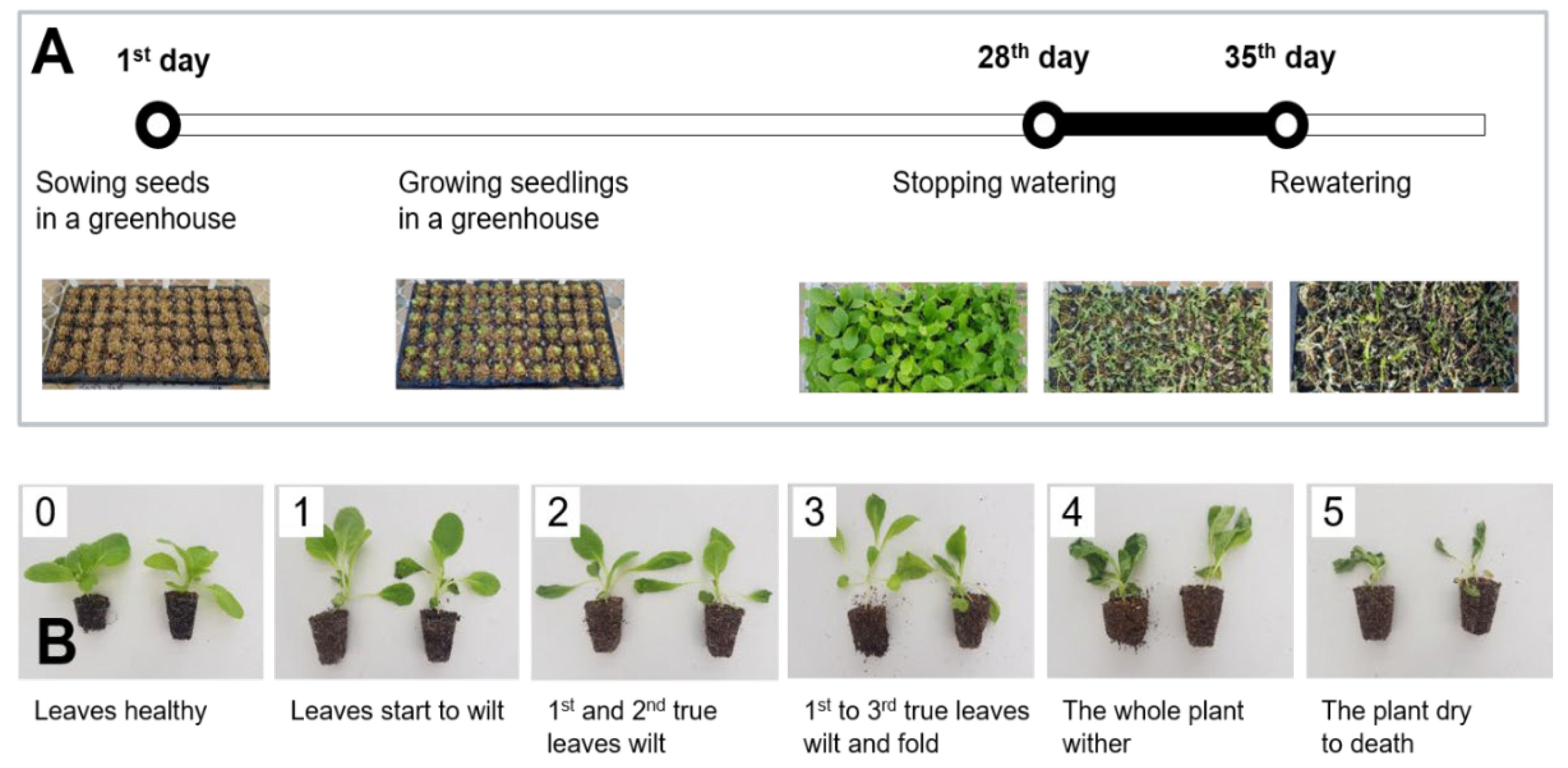
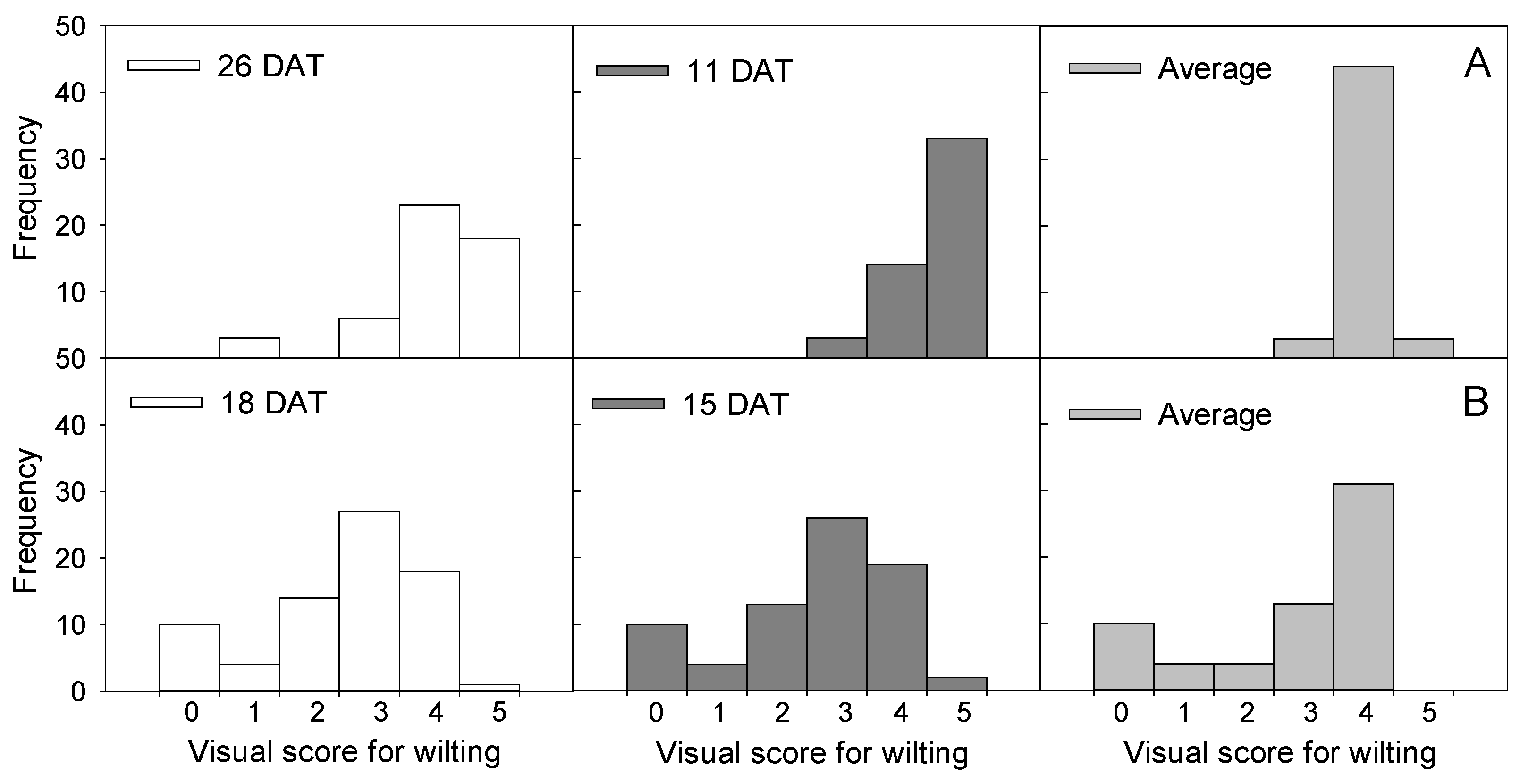


| Cultivar/Breeding Line | Company/Organization | Visual Score for Wilting | ||
|---|---|---|---|---|
| 26 DAT z | 11 DAT | Average | ||
| Smart-baechu | PPS | 1.28 | 3.28 | 3.20 |
| Ssam-ilang | Nongwoo Bio | 1.61 | 3.44 | 3.49 |
| 18-FH112-1 | NIHHS | 1.89 | 3.33 | 3.26 |
| AVRDC-KJH-1985-100390 | NAAS | 3.33 | 4.39 | 4.46 |
| Chunssamhwang 51 Cabbage | Asian seedlings | 3.72 | 4.33 | 4.06 |
| Wongyo20037ho | NIHHS | 3.77 | 4.31 | 4.11 |
| Sangjang-gun | Sakata Korea | 3.83 | 4.61 | 4.34 |
| 13-2-10 | NIHHS | 3.94 | 4.61 | 4.44 |
| 15-CDY-B3-1 | NIHHS | 3.94 | 4.22 | 4.19 |
| CRmatjjang | Farm Hannong | 4.06 | 4.44 | 4.25 |
| Dodam-baechu | The Kiban | 4.20 | 5.00 | 4.79 |
| Summer Star-baechu | Farm Hannong | 4.22 | 4.67 | 4.53 |
| 12-2-3 | NIHHS | 4.28 | 5.00 | 4.65 |
| Hwipalam | Sakata Korea | 4.28 | 4.67 | 4.32 |
| Gaeul-daejang | PPS | 4.29 | 4.89 | 4.49 |
| 18-BD85 | NIHHS | 4.33 | 4.83 | 4.56 |
| Asia-ppuli-baechu | Asian seedlings | 4.33 | 4.89 | 4.88 |
| Wongyo20042ho | NIHHS | 4.33 | 5.00 | 4.81 |
| Bulam3ho | Farm Hannong | 4.44 | 4.72 | 4.46 |
| Bom-wang-gug | Nongwoo Bio | 4.56 | 5.00 | 4.73 |
| Lyeoggang | Nongwoo Bio | 4.62 | 5.00 | 4.82 |
| Gaeul-yangban | Dana Seed | 4.67 | 5.00 | 4.64 |
| Wongyo20038ho | NIHHS | 4.67 | 5.00 | 4.96 |
| Wongyo20039ho | NIHHS | 4.72 | 5.00 | 4.86 |
| Wongyo20044ho | NIHHS | 4.72 | 4.94 | 4.85 |
| Nolang-gimchi-baechu | Dana Seed | 4.89 | 5.00 | 4.86 |
| Cheongna | Dana Seed | 4.89 | 5.00 | 4.77 |
| Cheongnam | Dana Seed | 4.89 | 5.00 | 4.75 |
| Cheongsaem-baechu | Heungnong Seed | 4.89 | 5.00 | 4.88 |
| Matnan-beta | The Kiban | 4.94 | 5.00 | 4.83 |
| Wongyo20049ho | NIHHS | 4.94 | 5.00 | 4.75 |
| 12-2-36 | NIHHS | 5.00 | 5.00 | 4.78 |
| CHN-YAAS-2010-10 | NAAS | 5.00 | 5.00 | 5.00 |
| Gyeoul-daejang | PPS | 5.00 | 5.00 | 5.00 |
| Gyeoul-wanggug | Nongwoo Bio | 5.00 | 5.00 | 5.00 |
| Geumbich-cheonglog | The Kiban | 5.00 | 5.00 | 4.84 |
| Namdogain | The Kiban | 5.00 | 5.00 | 4.89 |
| Yeong-gwang | Sakata Korea | 5.00 | 5.00 | 4.89 |
| Yeongung-sidae | The Kiban | 5.00 | 5.00 | 4.89 |
| Wongyo20040ho | NIHHS | 5.00 | 5.00 | 4.89 |
| Wongyo20047ho | NIHHS | 5.00 | 5.00 | 4.87 |
| Josaeng-wanggug | Nongwoo Bio | 5.00 | 5.00 | 4.89 |
| Jongga-bom | Nongwoo Bio | 5.00 | 5.00 | 4.89 |
| Chungkwang | Dana Seed | 5.00 | 5.00 | 4.78 |
| Chuseol | Koregon | 5.00 | 5.00 | 4.89 |
| Chupoong | Sakata Korea | 5.00 | 5.00 | 4.88 |
| Paldo-janggun | Asian seedlings | 5.00 | 5.00 | 4.89 |
| Highstar-baechu | Farm Hannong | 5.00 | 5.00 | 4.89 |
| Homerun-baechu | PPS | 5.00 | 5.00 | 4.89 |
| Cultivar/Breeding Line | Company/Organization | Visual Rates for Wilting | ||
|---|---|---|---|---|
| 18 DAT z | 15 DAT | Average | ||
| Wongyo20042ho | NIHHS | 0.19 | 0.63 | 1.91 |
| 22-DA88 | NIHHS | 0.28 | 0.28 | 2.46 |
| 18-FH112-1-2 | NIHHS | 0.31 | 0.29 | 1.53 |
| 22-DA91 | NIHHS | 0.31 | 0.31 | 2.07 |
| 22-DA72 | NIHHS | 0.50 | 0.61 | 2.81 |
| 22-DA83 | NIHHS | 0.50 | 1.06 | 2.08 |
| 18-FH112-1 | NIHHS | 0.56 | 0.56 | 2.20 |
| 18-FH112-1-1 | NIHHS | 0.56 | 0.63 | 1.64 |
| 18-FH112-1-3 | NIHHS | 0.72 | 0.63 | 1.86 |
| 22-DA84 | NIHHS | 0.83 | 0.56 | 2.09 |
| 22-DA87 | NIHHS | 1.00 | 1.00 | 2.61 |
| 22-DA82 | NIHHS | 1.39 | 1.39 | 2.70 |
| 22-DA92 | NIHHS | 1.39 | 1.78 | 2.77 |
| 22-DA90 | NIHHS | 1.89 | 2.17 | 3.13 |
| 22-DA68 | NIHHS | 2.06 | 2.28 | 3.74 |
| 22-DA85 | NIHHS | 2.11 | 2.22 | 3.43 |
| 22-DA86 | NIHHS | 2.17 | 2.39 | 3.19 |
| 22-DA89 | NIHHS | 2.24 | 3.29 | 3.79 |
| 22-DA100 | NIHHS | 2.35 | 2.47 | 3.44 |
| 22-DA95 | NIHHS | 2.39 | 2.29 | 3.59 |
| Wongyo20039ho | NIHHS | 2.50 | 2.50 | 3.73 |
| Wongyo20037ho | NIHHS | 2.56 | 2.67 | 3.34 |
| Wongyo20049ho | NIHHS | 2.56 | 2.44 | 3.81 |
| Gyeouldaejang | PPS | 2.65 | 2.94 | 3.90 |
| 15-CDY-B3-1-1 | NIHHS | 2.83 | 2.83 | 4.04 |
| 15-CDY-B3-1-2 | NIHHS | 2.83 | 2.83 | 3.91 |
| Wongyo20047ho | NIHHS | 2.88 | 3.59 | 4.06 |
| 22-DA93 | NIHHS | 3.06 | 3.22 | 4.06 |
| Wongyo20044ho | NIHHS | 3.11 | 3.00 | 3.74 |
| 22-DA94 | NIHHS | 3.19 | 3.44 | 3.93 |
| 22-DA101 | NIHHS | 3.29 | 3.29 | 3.95 |
| 22-DA64 | NIHHS | 3.39 | 4.00 | 4.26 |
| 22-DA61 | NIHHS | 3.67 | 3.78 | 4.37 |
| Hwipalam | NIHHS | 3.67 | 3.94 | 4.02 |
| Ssamilang | Nongwoo Bio | 3.67 | 3.83 | 4.35 |
| 22-DA96 | NIHHS | 3.72 | 3.83 | 4.21 |
| 22-DA97 | NIHHS | 3.72 | 4.11 | 4.30 |
| 15-CDY-B3-1-3 | NIHHS | 3.83 | 3.94 | 4.19 |
| Wongyo20040ho | NIHHS | 3.83 | 4.33 | 4.36 |
| 22DA99 | NIHHS | 3.89 | 3.17 | 4.10 |
| 22DA63 | NIHHS | 4.00 | 3.67 | 4.33 |
| Gyeoulwanggug | Nongwoo Bio | 4.00 | 4.22 | 4.42 |
| Smartbaechu | PPS | 4.00 | 4.00 | 4.27 |
| Asiappulibaechu | Asian seedlings | 4.22 | 4.50 | 4.40 |
| Wongyo20038ho | NIHHS | 4.22 | 4.44 | 4.52 |
| 18-BD85-1 | NIHHS | 4.33 | 4.39 | 4.52 |
| 22DA69 | NIHHS | 4.33 | 4.44 | 4.48 |
| 18-BD85-2 | NIHHS | 4.44 | 4.50 | 4.61 |
| 22-DA67 | NIHHS | 4.44 | 4.72 | 4.60 |
| 22-DA54 | NIHHS | 4.56 | 4.61 | 4.61 |
| 22-DA73 | NIHHS | 4.56 | 4.67 | 4.64 |
| Bulam3ho | Farm Hannong | 4.56 | 4.56 | 4.43 |
| 22-DA62 | NIHHS | 4.67 | 4.67 | 4.64 |
| 22-DA70 | NIHHS | 4.67 | 4.78 | 4.68 |
| 22-DA71 | NIHHS | 4.67 | 4.83 | 4.67 |
| 22-DA98 | NIHHS | 4.72 | 4.67 | 4.56 |
| 22-DA65 | NIHHS | 4.78 | 5.00 | 4.66 |
| CRmatjjang | Farm Hannong | 4.79 | 4.78 | 4.52 |
| 22-DA66 | NIHHS | 5.00 | 5.00 | 4.75 |
| Cultivar (A) | Treatment (B) | Net Assimilation Rate (μmol∙m−2∙s−1) | Stomatal Conductance (mmol∙m−2∙s−1) | Transpiration Rate (mmol∙m−2∙s−1) |
|---|---|---|---|---|
| Gyeoul-daejang | WDT | 7.7 ± 0.18 z | 192.0 ± 47.0 | 2.3 ± 0.5 |
| Cont. | 8.2 ± 0.64 | 262.8 ± 73.5 | 3.1 ± 0.6 | |
| Gyeoul-wanggug | WDT | 8.3 ± 0.23 | 282.5 ± 2.6 | 3.1 ± 0.1 |
| Cont. | 8.0 ± 0.34 | 252.4 ± 40.8 | 3.0 ± 0.4 | |
| CRmatjjang | WDT | 8.6 ± 0.33 | 310.9 ± 24.1 | 3.5 ± 0.2 |
| Cont. | 8.4 ± 0.40 | 311.4 ± 10.2 | 3.3 ± 0.2 | |
| Hwipalam | WDT | 8.6 ± 0.36 | 318.9 ± 32.9 | 3.6 ± 0.3 |
| Cont. | 8.3 ± 0.44 | 309.7 ± 76.5 | 3.4 ± 0.7 | |
| Bulam3ho | WDT | 7.8 ± 0.18 | 271.8 ± 38.6 | 3.2 ± 0.4 |
| Cont. | 9.0 ± 0.19 | 286.7 ± 22.6 | 3.3 ± 0.1 | |
| Smart-baechu | WDT | 7.7 ± 0.34 | 221.4 ± 24.0 | 2.6 ± 0.3 |
| Cont. | 8.0 ± 0.63 | 233.7 ± 30.6 | 2.9 ± 0.4 | |
| Ssam-ilang | WDT | 9.0 ± 0.29 | 367.7 ± 10.1 | 3.8 ± 0.1 |
| Cont. | 8.7 ± 0.12 | 364.1 ± 29.4 | 3.9 ± 0.2 | |
| Asia-ppuli-baechu | WDT | 8.0 ± 0.86 | 303.4 ± 11.9 | 3.4 ± 0.0 |
| Cont. | 6.5 ± 0.93 | 234.7 ± 52.1 | 2.9 ± 0.5 | |
| F value | ||||
| A | 0.0006 | <0.0001 | <0.0001 | |
| B | 0.7051 | 0.8858 | 0.6547 | |
| A × B | 0.0110 | 0.1810 | 0.1223 |
| Cultivar (A) | Treatment (B) | Fresh Mass (kg) | Number of Leaves | |||
|---|---|---|---|---|---|---|
| Shoot | Head | Inner z | Outer y | Total | ||
| Gyeoul-daejang | WDT | 6.4 ± 0.6 (83) x | 2.7 ± 0.4 (81) | 52 ± 6 (111) | 24 ± 4 (106) | 76 ± 5 (109) |
| Cont. | 7.7 ± 1.3 | 3.4 ± 0.8 | 47 ± 4 | 23 ± 5 | 70 ± 3 | |
| Gyeoul-wanggug | WDT | 7.6 ± 1.6 (87) | 3.1 ± 1.3 (86) | 44 ± 2 (90) | 23 ± 4 (87) | 67 ± 5 (89) |
| Cont. | 8.7 ± 1.6 | 3.7 ± 1.6 | 49 ± 5 | 26 ± 5 | 75 ± 8 | |
| CRmatjjang | WDT | 6.7 ± 0.6 (96) | 3.0 ± 0.6 (93) | 59 ± 5 (89) | 27 ± 6 (89) | 86 ± 10 (89) |
| Cont. | 7.0 ± 1.2 | 3.2 ± 1.2 | 66 ± 8 | 30 ± 9 | 96 ± 11 | |
| Hwipalam | WDT | 7.4 ± 1.9 (79) | 2.6 ± 0.5 (73) | 67 ± 6 (99) | 32 ± 6 (82) | 99 ± 9 (93) |
| Cont. | 9.4 ± 3.1 | 3.5 ± 1.0 | 68 ± 6 | 39 ± 8 | 107 ± 8 | |
| Bulam3ho | WDT | 6.9 ± 0.9 (97) | 3.0 ± 0.4 (111) | 67 ± 6 (92) | 25 ± 4 (74) | 91 ± 8 (86) |
| Cont. | 7.1 ± 0.1 | 2.7 ± 0.6 | 72 ± 10 | 33 ± 4 | 106 ± 12 | |
| Smart-baechu | WDT | 5.9 ± 1.1 (94) | 3.1 ± 0.5 (92) | 65 ± 3 (98) | 19 ± 3 (83) | 84 ± 4 (94) |
| Cont. | 6.3 ± 1.5 | 3.4 ± 0.8 | 66 ± 7 | 23 ± 1 | 89 ± 7 | |
| Ssam-ilang | WDT | 6.2 ± 0.8 (84) | 3.7 ± 0.7 (88) | 77 ± 10 (100) | 22 ± 2 (74) | 100 ± 10 (93) |
| Cont. | 7.3 ± 1.4 | 4.2 ± 1.2 | 76 ± 9 | 30 ± 4 | 108 ± 6 | |
| Asia-ppuli-baechu | WDT | 3.8 ± 1.1 (102) | 0.8 ± 0.2 (72) | - | - | - |
| Cont. | 3.8 ± 1.7 | 1.1 ± 0.3 | - | - | - | |
| F value | ||||||
| A | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| B | 0.0472 | 0.0266 | 0.1379 | 0.0003 | 0.0006 | |
| A × B | 0.8279 | 0.6962 | 0.4189 | 0.3482 | 0.1123 | |
| Treatment (B) | ||||||
| WDT | 6.4b (88) | 2.8b (86) | 61a (94) | 24b (83) | 75b (88) | |
| Cont. | 7.2a | 3.2a | 65a | 29a | 85a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, Y.; Kim, J.; Lee, J.; Lee, S.; Jung, H.; Park, G.-H. Drought Tolerance Evaluation and Growth Response of Chinese Cabbage Seedlings to Water Deficit Treatment. Agronomy 2024, 14, 279. https://doi.org/10.3390/agronomy14020279
Jang Y, Kim J, Lee J, Lee S, Jung H, Park G-H. Drought Tolerance Evaluation and Growth Response of Chinese Cabbage Seedlings to Water Deficit Treatment. Agronomy. 2024; 14(2):279. https://doi.org/10.3390/agronomy14020279
Chicago/Turabian StyleJang, Yoonah, Jinhee Kim, Junho Lee, Sangdeok Lee, Hwahyen Jung, and Gyu-Hyeon Park. 2024. "Drought Tolerance Evaluation and Growth Response of Chinese Cabbage Seedlings to Water Deficit Treatment" Agronomy 14, no. 2: 279. https://doi.org/10.3390/agronomy14020279
APA StyleJang, Y., Kim, J., Lee, J., Lee, S., Jung, H., & Park, G.-H. (2024). Drought Tolerance Evaluation and Growth Response of Chinese Cabbage Seedlings to Water Deficit Treatment. Agronomy, 14(2), 279. https://doi.org/10.3390/agronomy14020279






