Abstract
Determining the amount of heat units required to kill weed seeds is a crucial aspect for the success of weed control through soil solarization. Lab experiments were designed to determine the duration of exposure for weed seeds that is required to suppress germination at temperatures (40, 45, 50, 55, and 60 °C) in the range of those typically achieved during soil solarization in California. The species tested were annual sowthistle (Sonchus oleraceus L.), bristly oxtongue (Picris echioides L.), nettleleaf goosefoot (Chenopodium murale L.), redroot pigweed (Amaranthus retroflexus L.), common purslane (Portulaca oleracea L.), little mallow (Malva parviflora L.), and redstem filaree (Erodium cicutarium L.). Germination tests were performed to assess the germinability of the weed seeds. The germination suppression by the lab-simulated solarization temperatures differed among the species based on their seasonality. The cool-season annuals S. oleraceus and P. echioides were more susceptible to the heat treatments than the warm-season annuals P. oleracea, A. retroflexus, and C. murale. The hard-seeded weed species M. parviflora and E. cicutarium were the least susceptible to the heat treatments. The germination rates of S. oleraceus, P. echioides, and C. murale were reduced at all of the temperatures that were tested. The germination rates for A. retroflexus and M. parviflora were not affected by temperatures below 40 °C. The germination rates for P. oleracea were not affected by temperature below 45 °C and the germination of E. cicutarium was not affected by any of the temperatures that were tested. The duration (hours) of exposure and percent of germination suppression of the weed seeds were used to create thermal-time hazard models for weed seeds using logistic regression.
1. Introduction
Soil solarization is a non-chemical soil disinfestation technique which creates lethal temperatures for soil-borne organisms through the placement of a clear, thin (25–50 µm) plastic tarp over the surface of wet soil for from 4 to 8 weeks [1]. It has been effectively used for weed and pathogen management in areas with warm, sunny summers including California’s central valley [2,3,4]. However, weed seeds differ in their susceptibility to temperatures generated during solarization [5].
Models simulating the thermal tolerances of weed species to soil steaming have been documented for multiple species [6,7,8]. Steaming experiments demonstrate that exposure to 70 °C for 30 min under moist conditions kills most weed seeds [9]. Solarization, however, rarely achieves temperatures this high and generally generates temperatures between 40 and 60 °C [3]. Soil temperatures only reach these temperatures for a few hours each day and thermal death occurs slowly over time [10]. Thus, simulating solarization and steaming requires different models due to the differences in temperature and the duration of exposure.
Different weeds vary in their susceptibility to solarization. Shorter times are required to kill cool-season annuals than warm-season annuals [5]. Additionally, perennial weeds such as field bindweed (Convolvulus arvensis L.) and perennial propagules such as nutsedge (Cyperus spp.) tubers are more difficult to control with solarization than annual weeds [3,11]. For example, nutsedge tuber mortality will not occur until it is exposed to temperatures of 50 °C or higher [12]. Also, many hard-seeded weeds with thick seed coats are difficult to control with solarization, requiring longer solarization periods to achieve mortality [13,14,15].
Logistic and Weibull models simulating the responses of important weed species to solarization temperatures have been created for a few important weed species. Logistic models and thermal death thresholds have also been created for five important pathogen species [16,17]. To refine applications of solarization, models need to be developed for more species of weeds and pathogens. A greater understanding of temperature-duration mortality thresholds for weeds and pathogens could help practitioners understand and optimize the effects of solarization against weeds and pathogens.
A wide variety of weed species were chosen due to their importance to California’s agriculture and prevalence in local farmland. The selected species are annual sowthistle (Sonchus oleraceus L.), bristly oxtongue (Picris echioides L.), nettleleaf goosefoot (Chenopodium murale L.), redroot pigweed (Amaranthus retroflexus L.), common purslane (Portulaca oleracea L.), little mallow (Malva parviflora L.), and redstem filaree (Erodium cicutarium L.). The species were selected to include hard-seeded weeds, species whose seed coat prevents the uptake of water and gaseous exchange, causing physical dormancy (Baskin and Baskin, 2000), warm-season annuals, and cool-season annuals. The hard-seeded weed species M. parviflora and E. cicutarium are of particular importance to California’s agriculture as they are resistant to soil fumigation and many herbicides that are commonly used in strawberries, a major commodity in California [18,19,20]. The objectives of this study were to:
- (1)
- Determine the germination rates of important Californian weed species after exposure to solarization temperatures from 40 to 60 °C for a variety of time intervals;
- (2)
- Use the germination rates to create regression models simulating the responses of weed speeds to solarization temperatures.
2. Materials and Methods
2.1. Seed Collection and Preparation
All seeds were collected from the California Polytechnic State University Organic Farm in San Luis Obispo, California (35°18′16.90″ N 120°40′19.83″ W). The cropping systems on this farm are continuous mixed vegetable rotations. Collections were made at the time of optimal mature seed production for each of the two seasonally adapted groups of species. The cool-season annuals S. oleraceus and E. cicutarium were collected in spring 2018. Picris echioides was considered here as a cool-season annual based on its growth habits in California and was collected during spring/summer 2018. The warm-season annuals, C. murale, A. retroflexus, P. oleracea, and M. parviflora were collected in summer 2018. After collection, seeds were stored in paper bags at room temperature (21 °C), except C. murale seeds, which were stored at 4 °C as cold storage increases Chenopodium spp. germination rates [21]. Seeds were separated from chaff and 15 seeds were placed in 4 cm-square seed packets made from organdy fabric [5]. Before conducting experiments, seed packets were rinsed for 5 s in deionized water then placed in a damp paper towel inside an open plastic bag overnight to imbibe moisture.
2.2. Thermal Treatments
Each temperature and time consisted of six treatment replications and three control replications. Each replication consisted of one seed packet. Seeds were tested at 5 different temperatures (40, 45, 50, 55, and 60 °C ± 1.5 °C), representing the range of temperatures frequently achieved by solarization. To conduct experiments, 400 mL mason jars were filled with 100 mL of sand (Quikrete all-purpose sand, Quikrete Cement and Concrete Products, Atlanta, GA, USA), then three seed packets were placed in each jar. Mason jars were filled to the top with sand and deionized water was added to field capacity. To fully simulate solarization conditions and minimize moisture loss, mason jars were covered with 2 mil clear, low-density polyethylene (LDPE) plastic held in place by screwing on the mason jar cap [5].
Experiments running 8 h or less were placed in a water bath (Shaker Bath 3520, Labline Instruments, Melrose Park, IL, USA). Longer treatments of 10 h or greater were placed in a thermal convection oven (Isotemp Oven 750F, Fisher Scientific, Pittsburgh, PA, USA), as water baths were unable to hold constant temperatures overnight. The temperature of sand in mason jar microcosms took 30 min to equilibrate with water bath temperature. After sand reached equilibrium with water bath the timer was started.
Sand in microcosms placed in thermal convection oven treatments took longer to equilibrate to oven temperature. To account for longer equilibration times, mason jars were placed into ovens before seed packets were inserted and allowed to equilibrate to oven temperature. Once sand in the mason jars had equilibrated with oven temperatures, seed packets were inserted to a depth of 5 cm. Insertion of seed packets caused a slight loss of temperature in mason jar microcosms. Microcosms took roughly 30 min to re-equilibrate themselves with oven temperatures after insertion of seeds. The timer was started after this 30 min time interval.
Temperatures were recorded using temperature data loggers in the oven (Thermo Recorder Tr72wf, T&D Corporation, Matsumoto, Japan and Ibutton Thermocron F5, Maxim Integrated, San Jose, CA, USA) and water bath (ECHO EM50 Datalogger and STE 50 Data Probe, Decagon Instruments, Pullman, WA, USA). Temperatures inside mason jars were monitored throughout the duration of the experiment using thermometers (ERTCO Scientific Thermometer, Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Seed Germination Tests
After treatments were completed, seed packets were immediately removed from mason jars, dipped in deionized water, and placed on paper towels to dry. Once dry, some species were scarified nondestructively between two pieces of sandpaper to overcome physical dormancy, allowing seeds to imbibe water. To overcome physical seed dormancy, M. parviflora seeds were scarified for 60 s between two pieces of 60-grit sandpaper. Erodium cicutarium was scarified for 45 s with 60-grit sandpaper. Chenopodium murale and A. retroflexus were both scarified for 10 s between two pieces of 100-grit sandpaper. Scarification protocols were determined through preliminary tests where scarification time and grit were determined based on optimal germination rates for a subsample of 100 untreated weed seeds per species. Sonchus oleraceus, P. echioides, and P. oleracea were not scarified as they germinated readily without scarification.
After scarification, seeds were placed in 100 mm × 15 mm petri dishes lined with two #4 coffee filter papers (Fresh Cup Paper Coffee Filters, 150-ct. Packs, Dollar Tree, Chesapeake, VA, USA). Petri dishes were filled with 1 mL of deionized water and placed in a growth chamber (Series 109, Percival Scientific, Perry, IA, USA) set to a 25 °C/16 h day and 15 °C/8 h night cycle. Filter paper was kept moist with deionized water and seeds were checked daily. Germination was counted after two weeks. Seeds were counted as germinated if the radicle had emerged at least 3 mm.
Erodium cicutarium and M. parviflora underwent additional scarification after a two-week germination period as scarifying once was not enough to achieve maximal germinability. After two weeks, all germinated M. parviflora and E. cicutarium seeds were removed from petri dishes. Non-germinated seeds were dried, then re-scarified for 20 s between two pieces of 60-grit sandpaper. After scarification, seeds were returned to the growth chamber under the same conditions and counted again after one week.
2.4. Verification of Germination Tests
In June 2019, non-germinated seeds underwent a one-week germination test under the same methods previously described to protect against secondary dormancy, which can occur during solarization [22].
Non-germinated seeds underwent tetrazolium testing to infer their viability. Ten random seeds were selected from each temperature and time combination. Seeds were placed on damp paper towels for at least 24 h to imbibe moisture. Then, seeds were placed in a 1% tetrazolium solution (w/v) for 24 h at 25 °C under dark conditions. After 24 h, seeds were removed from the solution and dried. Embryos were carefully removed from seed coat and inspected for staining under a dissecting microscope. Seed embryos that were entirely stained red were considered viable. Seeds with no red staining or irregular staining were considered non-viable. Portulaca oleracea seeds were not tested as they were too small to puncture with a needle and the seed coat did not absorb tetrazolium, making evaluation difficult.
2.5. Statistical Analysis
Models were created at all temperatures where seed germination was reduced. t-tests were conducted to compare the germination rates in the “control” with those in the “treated” dishes. When a significant effect was observed, a model was fitted for the species. Models were not created for species at temperatures that did not significantly decrease germination rates or where treatments germinated at higher rates than controls (Table 1).

Table 1.
Species and temperatures where a significant decrease in germination rate was not observed. Treatment and control germination rate are shown for experiments at the longest time tested for each specific species and temperature. p-values generated using a t-test (p ≤ 0.05).
Models were created by transforming all treatment and control data to a binary system, with yes (for germination) or no (for no germination). Logistic models were selected as they had a better fit than other common regression models used in germination studies, and they are amongst the more parsimonious models for describing such processes as seed germination. Models were created in JMP 14.0 (SAS Institute, Cary, NC, USA) using the following binomial logistic regression model:
where Gt = germination rate after time hours (h) duration of exposure to a certain temperature (t), and the fitted parameters b0 = y-intercept (log odds) and b1 = line gradient (log odds). The coefficient of determination, R2, was calculated using normalized version of pseudo R2 [23].
Gt = 1 − (1/(1 + e−b0 + b1 × h))
3. Results and Discussion
3.1. Tetrazolium Staining
Tetrazolium staining was used to determine whether the non-germinated seeds may be viable but not germinated (Table 2). The non-germinated seed viability following tetrazolium testing ranged from 0 to 8.57% across all species and temperatures. Malva parviflora had the highest percentage of viable non-germinated seeds at 8.57% at both 50 and 55 °C. Chenopodium murale also had higher numbers of viable non-germinated seeds than other species (2.8 to 7.1%). Sonchus oleraceus, P. echioides, and A. retroflexus had lower viabilities of their non-germinated seeds (0.0 to 3.3%). No models were generated for E. cicutarium, so tetrazolium testing was not conducted on this species. Overall, low viability results after tetrazolium staining indicate that the germination tests were an accurate measure for both germinability and viability. Therefore, tetrazolium tests were not incorporated into the models.

Table 2.
Percentage of non-germinated viable seeds at each temperature treatment after undergoing tetrazolium staining.
3.2. Thermal Suppression of Germinability
Erodium cicutarium was not affected significantly by heat treatments of 60 °C or lower (Table 1 and Figure 1). After 240 h of exposure at 60 °C, E. cicutarium germinated at similar rates to control tests. Malva parviflora required constant exposure for 72 h at 60 °C, 240 h at 55 °C, 340 h at 50 °C, and 672 h at 45 °C to reduce its germination rates to zero or near zero (Table 3). Malva parviflora was unaffected after 340 h of exposure to 40 °C (Table 1). Portulaca oleracea required constant exposure for 4 h at 60 °C, 68 h at 55 °C, and 168 h at 50 °C to reduce its germination rates to zero. Exposure to 45 and 40 °C had no significant negative effect on the germination of P. oleracea, which was found germinating inside the microcosms at temperatures up to 45 °C. Amaranthus retroflexus required constant exposure for 2 h at 60 °C, 6 h at 55 °C, 72 h at 50 °C, and 240 h at 45 °C to achieve zero-percent germination. Temperatures of 40 °C did not reduce A. retroflexus’ germination (Table 1).
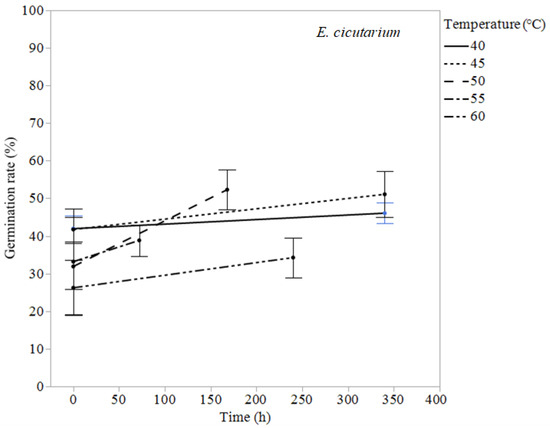
Figure 1.
Treatment and control germination rates ± standard error (%) for Erodium cicutarium are shown for experiments at the longest time tested at 40, 45, 50, 55, and 60 °C.

Table 3.
The number of hours required for 0% germination of different weed seeds at all temperatures tested.
Chenopodium murale, S. oleraceus, and P. echioides experienced 100% germination suppression for all temperatures tested. Chenopodium murale required constant exposure for 2 h at 60 °C, 4 h at 55 °C, 72 h at 50 °C, 168 h at 45 °C, and 432 h at 40 °C to reduce its germination to zero or almost zero (Table 3). The temperature had similar effects on P. echioides and S. oleraceus, reducing their germination rates to zero in 0.25 h at 60 °C, 1–2 h at 55 °C, and 4 h at 50 °C. At 40 °C, S. oleraceus required 40 h and P. echioides required 24 h to reduce their weed germination to 0% (Table 3).
3.3. Logistic Regression Models
Logistic regression models (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7) were created for all species and temperatures that were tested, except for temperatures where seeds were not affected by the heat treatment. Additionally, no models were created for S. oleraceus and P. echioides at 60 °C, as the models were non-significant due to seed mortality occurring in 15 min. The effects of all other models were significant at a level of p < 0.0001. For all species, an increase in temperature led to decreases in the time required to reduce germination rates.
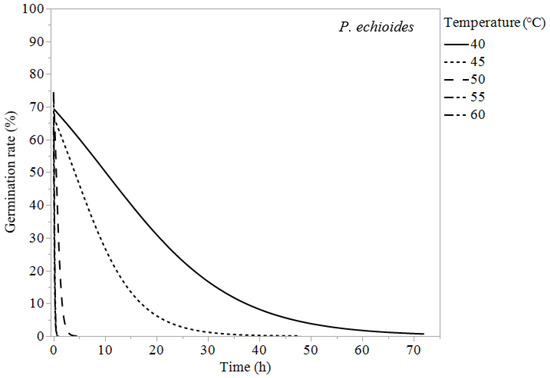
Figure 2.
Logistic regression models (Equation (1)) of Picris echioides at 40, 45, 50, and 55 °C.
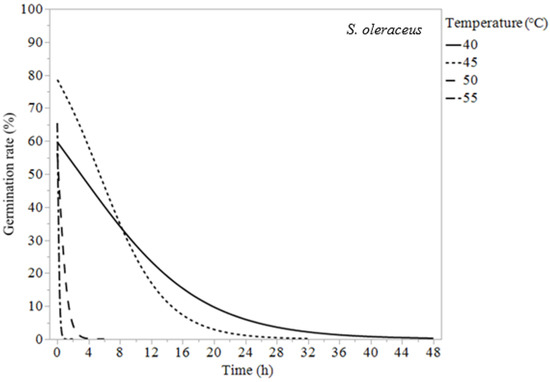
Figure 3.
Logistic regression models (Equation (1)) of Sonchus oleraceus at 40, 45, 50, and 55 °C.
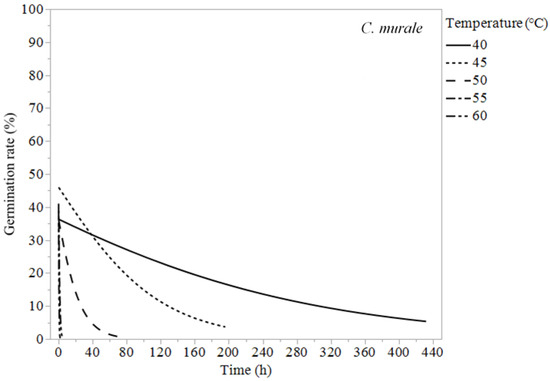
Figure 4.
Logistic regression models (Equation (1)) of Chenopodium murale at 40, 45, 50, 55, and 60 °C.
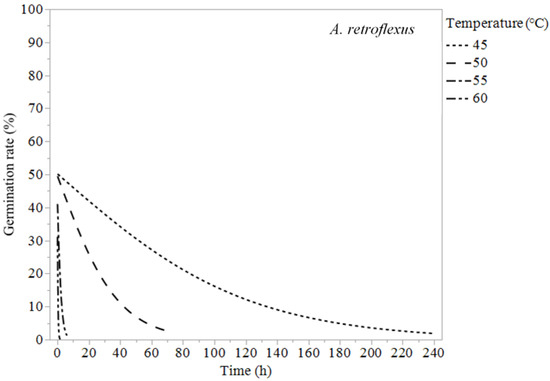
Figure 5.
Logistic regression models (Equation (1)) of Amaranthus retroflexus at 45, 50, 55, and 60 °C.
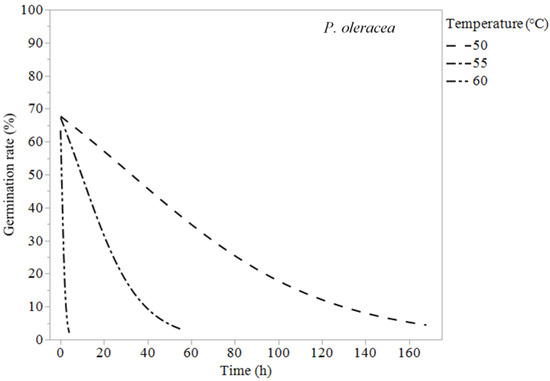
Figure 6.
Logistic regression models (Equation (1)) of Portulaca oleracea at 50, 55, and 60 °C.
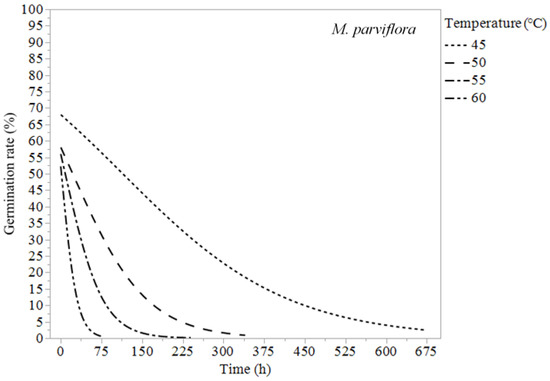
Figure 7.
Logistic regression models (Equation (1)) of Malva parviflora at 45, 50, 55, and 60 °C.
The suppression of germination in response to the solarization temperatures differed among the species based on the seasonality of the species. The cool-season species S. oleraceus and P. echioides were the most susceptible to the solarization temperatures. The germination rates for the warm-season species C. murale, A. retroflexus, and P. oleracea were more tolerant of solarization temperatures than the cool-season species, but less tolerant than the hard-seeded species. Portulaca oleracea was more tolerant than the other two warm-season species. The hard-seeded weed species, M. parviflora and E. cicutarium, were the least susceptible to the solarization temperatures.
The fitted values of b1 can be used to compare species for their sensitivity to solarization temperatures (Table 4). At 45 °C, the b1 of the cool-season species P. echioides and S. oleraceus decreased at a rate of from 10 to 14 times the b1 of C. murale and A. retroflexus, indicative of their increased sensitivity to temperature exposure. At 50 °C, the b1 of the cool-season species decreased at a rate between 27 and 84 times the b1 of all warm-season species (A. retroflexus, C. murale, and P. oleracea) and between 112 and 129 times the b1 of M. parviflora. At 55 °C, the b1 of the cool-season species decreased at a rate between 7 and 130 times the b1 of all warm-season species and between 278 to 337 times the b1 of M. parviflora. At 60 °C, the b1 of the warm-season species decreased at a rate of from 16 to 51 times the b1 of M. parviflora.

Table 4.
Parameter estimates (±SE) and significance for each parameter for the logistic regression model for equation Gt = 1 − (1/(1 + e−b0 + b1 × h)), where Gt = germination rate after time hours (h) duration of exposure to a certain temperature (t), and the fitted parameters are b0 = y-intercept (log odds) and b1 = line gradient (log odds). The coefficient of determination, R2, was calculated using normalized version of pseudo R2 [23].
The lethal dose values (LD 20, LD50, and LD90) of the models can also help to distinguish the differences in the solarization temperatures’ effects on different weed species (Table 5). At all temperatures tested (45, 50, 55, and 60 °C), M. parviflora had the longest lethal dose values (Table 5). Portulaca oleracea had the second longest lethal dose values. The exception to this is the LD20 of M. parviflora and P. oleracea at 50 °C, where there is overlap in the confidence intervals between the two species. No overlap occurred between the confidence intervals of P. oleracea and the other warm-season species at any temperature or lethal dose tested. C. murale and A. retroflexus had similar lethal dose values at all temperatures except the LD90 at 55 °C and at 40 °C, where A. retroflexus was not affected. Sonchus oleraceus and P. echioides had lower lethal dose values than all other weed species. Picris echioides was less susceptible than S. oleraceus at lower temperatures, having a longer LD50 and LD90 at 40 °C and a longer LD90 at 45 °C. However, the LD20 values were similar between P. echioides and S. oleraceus at all temperatures.

Table 5.
Time (hours) required for 20% mortality (LD20), 50% mortality (LD50), and 90% mortality (LD90) according to logistic regression models. LD20, LD50, and LD90 were calculated by finding time required to reduce germination rate at 0 h of exposure by 20%, 50%, and 90%; 95% confidence intervals calculated for each LD20, LD50, and LD90.
3.4. Application to Field Conditions
The models created in this study simplify solarization conditions and act under a multitude of assumptions. Field conditions result in diurnal temperature fluctuations, unlike the constant temperatures used to generate the current models. However, experiments on black mustard (Brassica nigra L.) that simulated solarization conditions using constant temperatures generated similar models to those using diurnal temperature fluctuations [24]. This demonstrates that constant temperature models can be used to simulate responses to solarization with similar precision despite not accounting for diurnal temperature fluctuations [24]. The advantage of diurnal fluctuating models is that they are easier to apply to field conditions [24]. The disadvantage is that these models can be complicated as they need to account for variable temperature fluctuations over time [5].
The usefulness of the logistic regression models generated in this study pertains to estimating the amount of time needed to suppress the germination of various weed species at various temperatures using solarization. By observing the cumulative number of hours that are above a certain temperature threshold, growers can estimate the amount of time needed for solarization to be effective [25]. To apply models more accurately to field conditions, temperatures that fall between the tested thresholds need to be accounted for. The methodology for accounting for temperatures between the tested thresholds has been described in other papers [5]. This can give growers a simplified heat accumulation model which can aid their decision-making regarding solarization treatments.
There are other factors that occur under field conditions that are not accounted for in laboratory experiments and can contribute to differences between lab and field mortalities. For example, laboratory models do not account for the physiological weakening of seed coats which occurs at solarization temperatures in the field [15]. This can cause the germination of dormant seeds and subsequent mortality as seedlings are exposed to high temperatures in shallower layers of the soil once they germinate [14]. Higher temperatures also increase the activity of volatile allelochemicals and biofumigants which can reduce weed seed viability [26]. Incorporating organic amendments into the soil before solarizing, also known as biosolarization, can further increase the activity of volatile chemicals and organic acids, leading to increased toxicity against weed seeds [27,28]. Lastly, high temperatures can make weed seeds more susceptible to antagonistic soil organisms, especially in moist conditions [1]. As our lab experiments were conducted in semi-sterile conditions without the incorporation of organic amendments, the results obtained in this study may over- or under-estimate weed survival under various solarization conditions in the field.
Our models did not account for the differing physical and chemical properties of different soil types, which can affect heat dispersion throughout the soil and the efficacy of solarization against weed seeds [29]. The seeds were tested in coarse sand. Coarse soils were found to require slightly longer times to achieve seed mortality than finer soils at 60 °C and 70 °C, perhaps because of higher heat conduction potentials [7]. As such, shorter times may be needed to kill weed seeds in finer soils than in the sand used in this study.
Differences in susceptibility to solarization appear to be based on the thickness of the seed coats and the germination seasons of seeds. Our results are in agreement with previous findings that have shown differences between winter and summer annuals’ responses to solarization [2,5]. Differences in susceptibility to solarization between winter and summer annuals may be due to the presence of heat shock proteins which appear to be involved in the protection of seeds from heat. These proteins may be more prevalent in species that experience high temperatures during germination [30,31].
Field and laboratory studies have shown that hard-seeded weed species tend to be resistant to solarization, although that response might be variable [2,15,32]. Some hard-seeded species such as velvetleaf (Abutilon theophrasti L.) are more susceptible than many warm-season-species [10,14]. Furthermore, certain legumes such as Mediterranean sweet clover (Melilotus sulcatus L.) and Medicago spp. are completely tolerant of solarization due to their thick, insulating seed coat [13,15,33,34]. Similar trends were observed in this study, where E. cicutarium was not affected by solarization, but M. parviflora was affected after long exposure times even at lower temperatures of 45 °C.
The results for species tested in this experiment support previously collected field data, with a few contradictions. Sonchus oleraceus, A. retroflexus, and C. murale have been successfully controlled by solarization under field conditions [35]. Portulaca oleracea has been shown to be tolerant of solarization, particularly in marginal solarization conditions [13,19,36]. Models generated in this study support these field tests, as P. oleracea was unaffected at temperatures of 45 °C or below, and provide a more detailed assessment of the magnitude of sensitivity of the other species. Likewise, M. parviflora has only been partially controlled under marginal solarization conditions with maximum temperatures < 45 °C [19]. However, M. parviflora has been controlled by solarization in other studies [13]. Erodium cicutarium was found to be completely tolerant of the solarization temperatures investigated here, which disagrees with the findings of [37], who considered Erodium spp. to be susceptible to solarization. Potential reasons for this disagreement include previously mentioned factors that can increase weed mortality and that were not accounted for in the model or, alternatively, seeds surviving the solarization process but not germinating during the time period of the field experiments due to secondary dormancy or other factors. It is important to test weed seeds under actual field conditions, particularly E. cicutarium, which demonstrated contradictory results, and P. echioides, which has not been tested under field conditions.
4. Conclusions
The logistic regression models developed in this study can provide an understanding of the thermal-time requirements for the suppression of weed seed germination and provide a quick and simple tool to compare the tolerances of different weed species. In the field, these models can provide a guide to growers on whether solarization will suppress their weed populations. Further research should be directed towards validating laboratory tests under field conditions, accounting for the myriad of different environmental factors that are not assessed in laboratory experiments and testing more weed species. Furthermore, analyzing the physiological and biochemical properties of different seeds undergoing solarization could help explain differences in susceptibility between species. Additional research can look at combining weed seed thermal death models with models simulating solarization temperatures under different soil and environmental conditions to allow growers to more accurately estimate the efficacy of solarization on their fields.
Author Contributions
Conceptualization, T.M.J., S.J.S. and A.M.T.; methodology, T.M.J., S.J.S. and A.M.T.; software, T.M.J.; validation, T.M.J., S.J.S. and A.M.T.; formal analysis, T.M.J.; investigation, T.M.J.; resources, T.M.J. and A.M.T.; data curation, T.M.J.; writing—original draft preparation, T.M.J.; writing—review and editing, T.M.J., S.J.S. and A.M.T.; visualization, T.M.J.; supervision, A.M.T. and S.J.S.; project administration, A.M.T.; funding acquisition, T.M.J. and A.M.T. All authors have read and agreed to the published version of the manuscript.
Funding
Partial funding was provided for this project by the Organic Farming Research Foundation.
Data Availability Statement
The data presented in this study are available upon request.
Acknowledgments
The authors would like to thank all the undergraduate students who helped with completing the experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stapleton, J.J.; DeVay, J.E. Soil Solarization: A Non-Chemical Approach for Management of Plant Pathogens and Pests. Crop Prot. 1986, 5, 190–198. [Google Scholar] [CrossRef]
- Elmore, C.L.; Stapleton, J.J.; Bell, C.E. Soil Solarization: A Nonpesticidal Method for Controlling Diseases, Nematodes, and Weeds; FAO: Rome, Italy, 1997. [Google Scholar]
- Stapleton, J.J.; Wilen, C.A.; Molinar, R.H. Soil Solarization for Gardens and Landscapes; Taylor & Francis Ltd.: Abington, UK, 2008. [Google Scholar]
- Stapleton, J.J.; Molinar, R.H.; Lynn-Patterson, K.; McFeeters, S.K.; Shrestha, A. Methyl Bromide Alternatives… Soil Solarization Provides Weed Control for Limited-Resource and Organic Growers in Warmer Climates. Calif. Agric. 2008, 59, 84–89. [Google Scholar] [CrossRef]
- Dahlquist, R.M.; Prather, T.S.; Stapleton, J.J. Time and Temperature Requirements for Weed Seed Thermal Death. Weed Sci. 2007, 55, 619–625. [Google Scholar] [CrossRef]
- Hoyle, J.A.; Mcelroy, J.S. Relationship between Temperature and Heat Duration on Large Crabgrass (Digitaria sanguinalis), Virginia Buttonweed (Diodia virginiana), and Cock’s-Comb Kyllinga (Kyllinga squamulata) Seed Mortality. Weed Technol. 2012, 26, 800–806. [Google Scholar] [CrossRef]
- Melander, B.; Kristensen, J.K. Soil Steaming Effects on Weed Seedling Emergence under the Influence of Soil Type, Soil Moisture, Soil Structure and Heat Duration. Ann. Appl. Biol. 2011, 158, 194–203. [Google Scholar] [CrossRef]
- Melander, B.; Jørgensen, M.H. Soil Steaming to Reduce Intrarow Weed Seedling Emergence. Weed Res. 2005, 45, 202–211. [Google Scholar] [CrossRef]
- Van Loenen, M.C.A.; Turbett, Y.; Mullins, C.E.; Feilden, N.E.H.; Wilson, M.J.; Leifert, C.; Seel, W.E. Low Temperature—Short Duration Steaming of Soil Kills Soil-Borne Pathogens, Nematode Pests and Weeds. Eur. J. Plant Pathol. 2003, 109, 993–1002. [Google Scholar] [CrossRef]
- Horowitz, M.; Taylorson, R.B. Effect of High Temperatures on Imbibition, Germination, and Thermal Death of Velvetleaf (Abutilon threophrasti) Seeds. Can. J. Bot. 1983, 61, 2269–2276. [Google Scholar] [CrossRef]
- Elmore, C.L.; Roncoroni, J.A.; Giraud, D.D. Perennial Weeds Respond to Control by Soil Solarization. Calif. Agric. 1993, 47, 19–22. [Google Scholar] [CrossRef]
- Webster, T.M. High Temperatures and Durations of Exposure Reduce Nutsedge (Cyperus spp.) Tuber Viability. Weed Sci. 2003, 51, 1010–1015. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Al-Hamadi, F. Assessment of the Differential Response of Weeds to Soil Solarization by Two Methods. Weed Biol. Manag. 2009, 9, 72–78. [Google Scholar] [CrossRef]
- Egley, G. High-Temperature Effects on Germination and Survival of Weed Seeds in Soil. Weed Sci. 1990, 38, 429–435. [Google Scholar] [CrossRef]
- Rubin, B.; Benjamin, A. Solar Heating of the Soil: Involvement of Environmental Factors in the Weed Control Process. Weed Sci. 1984, 32, 138–142. [Google Scholar] [CrossRef]
- Pullman, G.S.; DeVay, J.E.; Garber, R.H. Soil Solarization and Thermal Death: A Logarithmic Relationship between Time and Temperature for Four Soilborne Plant Pathogens. Phytopathology 1981, 71, 959–964. [Google Scholar] [CrossRef]
- Yildiz, A.; Benlioǧlu, S.; Boz, Ö.; Benlioǧlu, K. Use of Different Plastics for Soil Solarization in Strawberry Growth and Time-Temperature Relationships for the Control of Macrophomina phaseolina and Weeds. Phytoparasitica 2010, 38, 463–473. [Google Scholar] [CrossRef]
- Fennimore, S.A. Weed control as part of soil disinfestation with fumigants and nonfumigants. In Proceedings of the 2012 CWSS Proceedings; California Weed Science Society: West Sacramento, CA, USA, 2012; pp. 118–123. [Google Scholar]
- Samtani, J.B.; Gilbert, C.; Ben Weber, J.; Subbarao, K.V.; Goodhue, R.E.; Fennimore, S.A. Effect of Steam and Solarization Treatments on Pest Control, Strawberry Yield, and Economic Returns Relative to Methyl Bromide Fumigation. HortScience 2012, 47, 64–70. [Google Scholar] [CrossRef]
- Wu, J.Y.; Dastgheib, F. Effects of Various Herbicides and Surfactants on Mallow (Malva Spp.). In Proceedings of the 18th Asian-Pacific Weed Science Society Conference; Asian-Pacific Weed Science Society: Beijing, China, 2001; pp. 589–593. [Google Scholar]
- Moravcova, L.; Dostalek, J. Contribution to the Biology of Germination of Four Species of Chenopodium album Agg. under Different Condition. Folia Geobot. Phytotaxon. 1989, 24, 431–439. [Google Scholar] [CrossRef]
- Mauromicale, G.; Lo Monaco, A.; Longo, A.M.G.; Restuccia, A. Soil Solarization, a Nonchemical Method to Control Branched Broomrape (Orobanche Ramosa) and Improve the Yield of Greenhouse Tomato. Weed Sci. 2005, 53, 877–883. [Google Scholar] [CrossRef]
- Nagelkerke, N.J.D. A Note on a General Definition of the Coefficient of Determination. Biometrika 1991, 78, 691–692. [Google Scholar] [CrossRef]
- Dahlquist-Willard, R.M.; Marshall, M.N.; Betts, S.L.; Tuell-Todd, C.C.; VanderGheynst, J.S.; Stapleton, J.J. Development and Validation of a Weibull–Arrhenius Model to Predict Thermal Inactivation of Black Mustard (Brassica nigra) Seeds under Fluctuating Temperature Regimens. Biosyst. Eng. 2016, 151, 350–360. [Google Scholar] [CrossRef]
- Peachey, A.R.E.; Pinkerton, J.N.; Ivors, K.L.; Miller, M.L.; Moore, L.W. Effect of Soil Solarization, over Crops, and Metham on Field Emergence and Survival of Buried Annual Bluegrass (Poa annua). Weed Technol. 2001, 15, 81–88. [Google Scholar] [CrossRef]
- Gamliel, A.; Austerweil, M.; Kritzman, G. Non-Chemical Approach to Soilborne Pest Management—Organic Amendments. Crop Prot. 2000, 19, 847–853. [Google Scholar] [CrossRef]
- Gamliel, A.; Stapleton, J.J. Effect of Chicken Compost or Ammonium Phosphate and Solarization on Pathogen Control, Rhizosphere Microorganisms, and Lettuce Growth. Plant Dis. 1993, 77, 886–891. [Google Scholar] [CrossRef]
- Simmons, C.W.; Higgins, B.; Staley, S.; Joh, L.D.; Simmons, B.A.; Singer, S.W.; Stapleton, J.J.; VanderGheynst, J.S. The Role of Organic Matter Amendment Level on Soil Heating, Organic Acid Accumulation, and Development of Bacterial Communities in Solarized Soil. Appl. Soil Ecol. 2016, 106, 37–46. [Google Scholar] [CrossRef]
- Marshall, M.N.; Rumsey, T.R.; Stapleton, J.J.; Vandergheynst, J.S. A Predictive Model for Soil Temperature during Solarization and Model Validation at Two California Field Sites. Trans. ASABE 2013, 56, 117–133. [Google Scholar] [CrossRef]
- Medina, C.; Cardemil, L. Prosopis chilensis Is a Plant Highly Tolerant to Heat Shock. Plant Cell Environ. 1993, 16, 305–310. [Google Scholar] [CrossRef]
- Coca, M.A.; Almoguera, C.; Jordano, J. Expression of Sunflower Low-Molecular-Weight Heat-Shock Proteins during Embryogenesis and Persistence after Germination: Localization and Possible Functional Implications. Plant Mol. Biol. 1994, 25, 479–492. [Google Scholar] [CrossRef]
- Horowitz, M.; Regev, Y.; Herzlinger, G. Solarization for Weed Control. Weed Sci. 1983, 31, 170–179. [Google Scholar] [CrossRef]
- Candido, V.; D’addabbo, T.; Miccolis, V.; Castronuovo, D. Weed Control and Yield Response of Soil Solarization with Different Plastic Films in Lettuce. Sci. Hortic. 2011, 130, 491–497. [Google Scholar] [CrossRef]
- Powles, S.B.; Charman, N.; Poole, F. Solar Heating (Solarization) of the Soil Surface: Effect on Weed Control, and Yield of Phaseolus vulgaris. Plant Prot. Q. 1988, 3, 31–35. [Google Scholar]
- Stapleton, J.J. Fumigation and Solarization Practice in Plasticulture Systems. HortTechnology 1996, 6, 189–192. [Google Scholar] [CrossRef]
- DeVay, J.E.; Stapleton, J.J.; Elmore, C.L. Soil solarization. In Proceedings of the First International Conference on Soil Solarization, Amman, Jordan, 19–25 February 1990; FAO: Rome, Italy, 1991. [Google Scholar]
- Porter, I.J.; Merriman, P.R. Effects of Solarization of Soil on Nematode and Fungal Pathogens at Two Sites in Victoria. Soil Biol. Biochem. 1983, 15, 39–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).