Metabolomics and Physiological Methods Revealed the Effects of Drought Stress on the Quality of Broomcorn Millet during the Flowering Stage
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Determination of Agronomic Traits
2.3. Determination of Starch Content
2.3.1. Extraction and Determination of Total Starch
2.3.2. Determination of Amylose Content
2.4. Determination of Protein Components
2.5. Determination of Amino Acid Content
2.6. Determination of Medium-Long Chain Fatty Acid Content
2.7. Determination of B Vitamins Content
2.8. Determination of Anthocyanin Content
2.9. Statistical Analysis
3. Results
3.1. Impact on Agronomic Traits
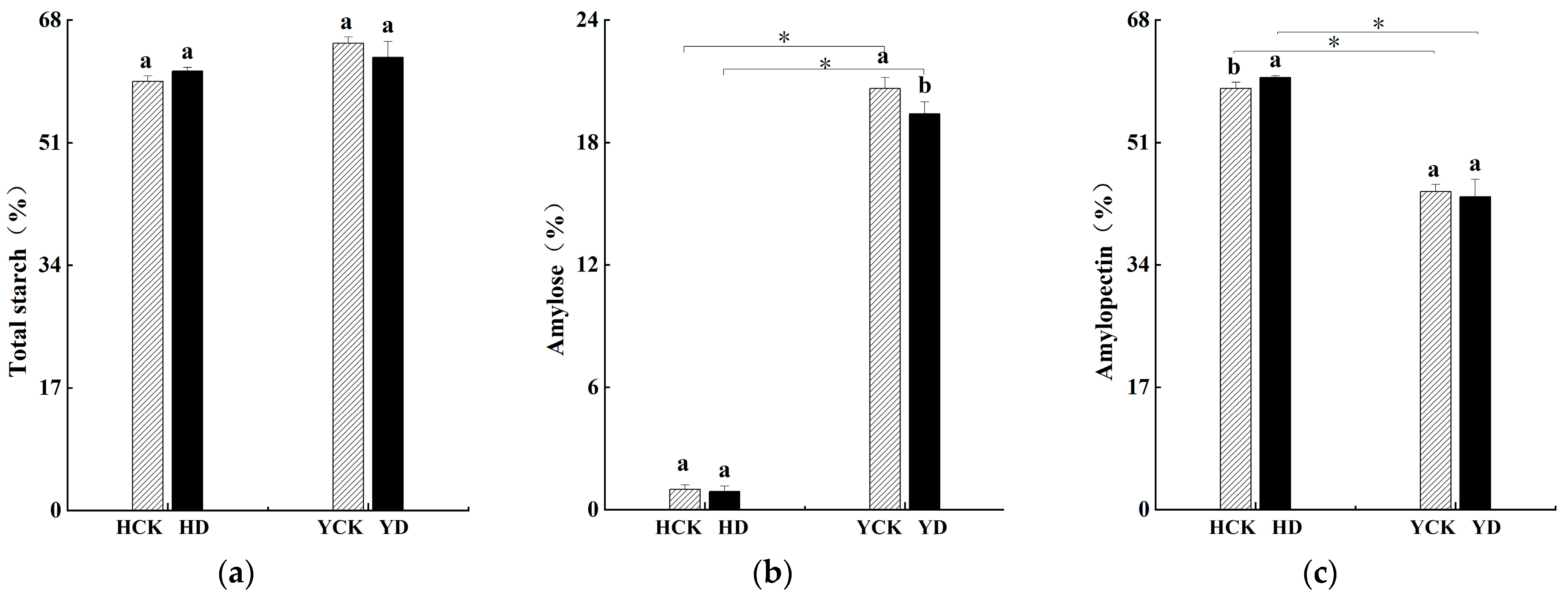
3.2. Impact on Starch Content
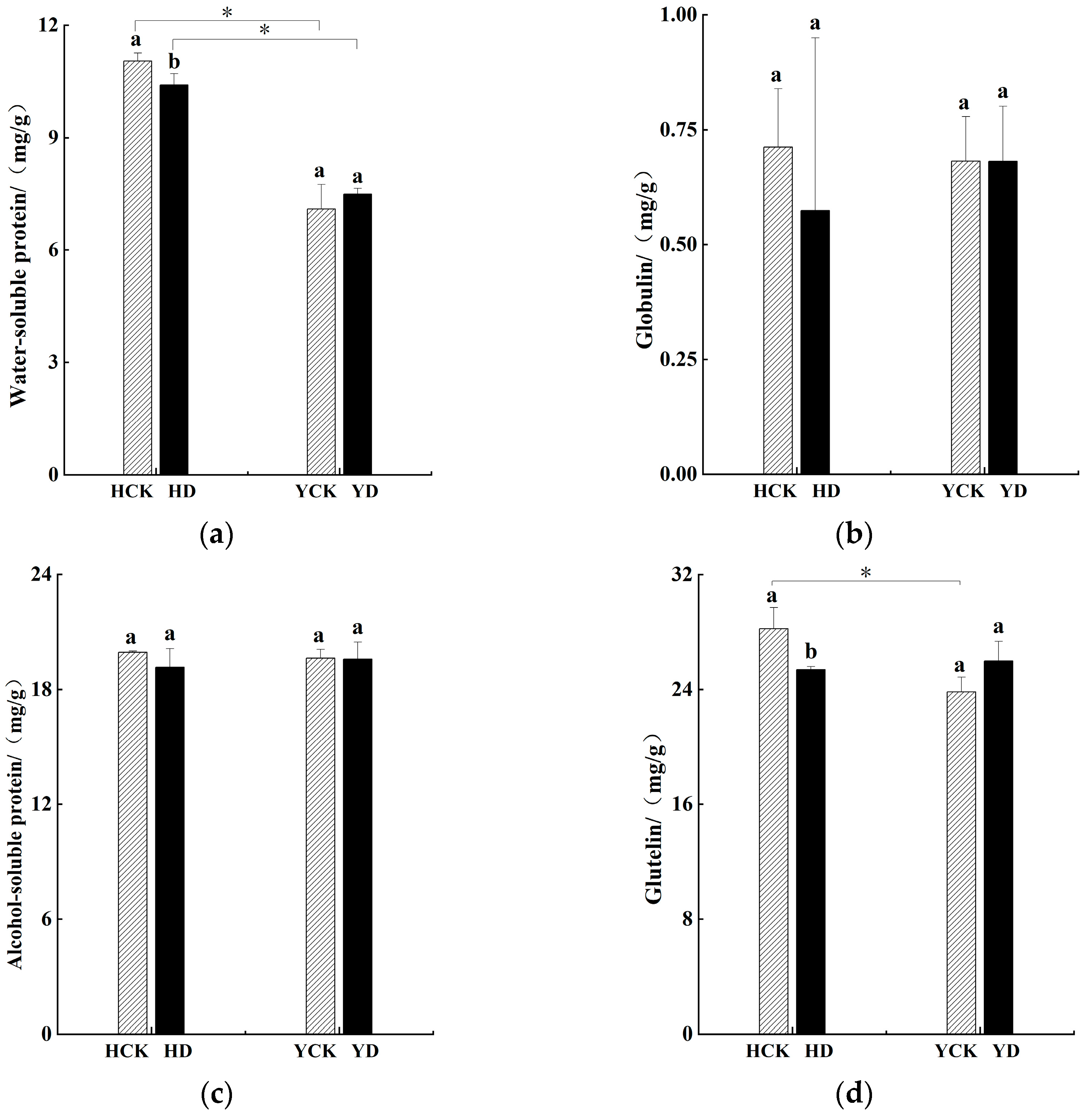
3.3. Impact on Protein Composition
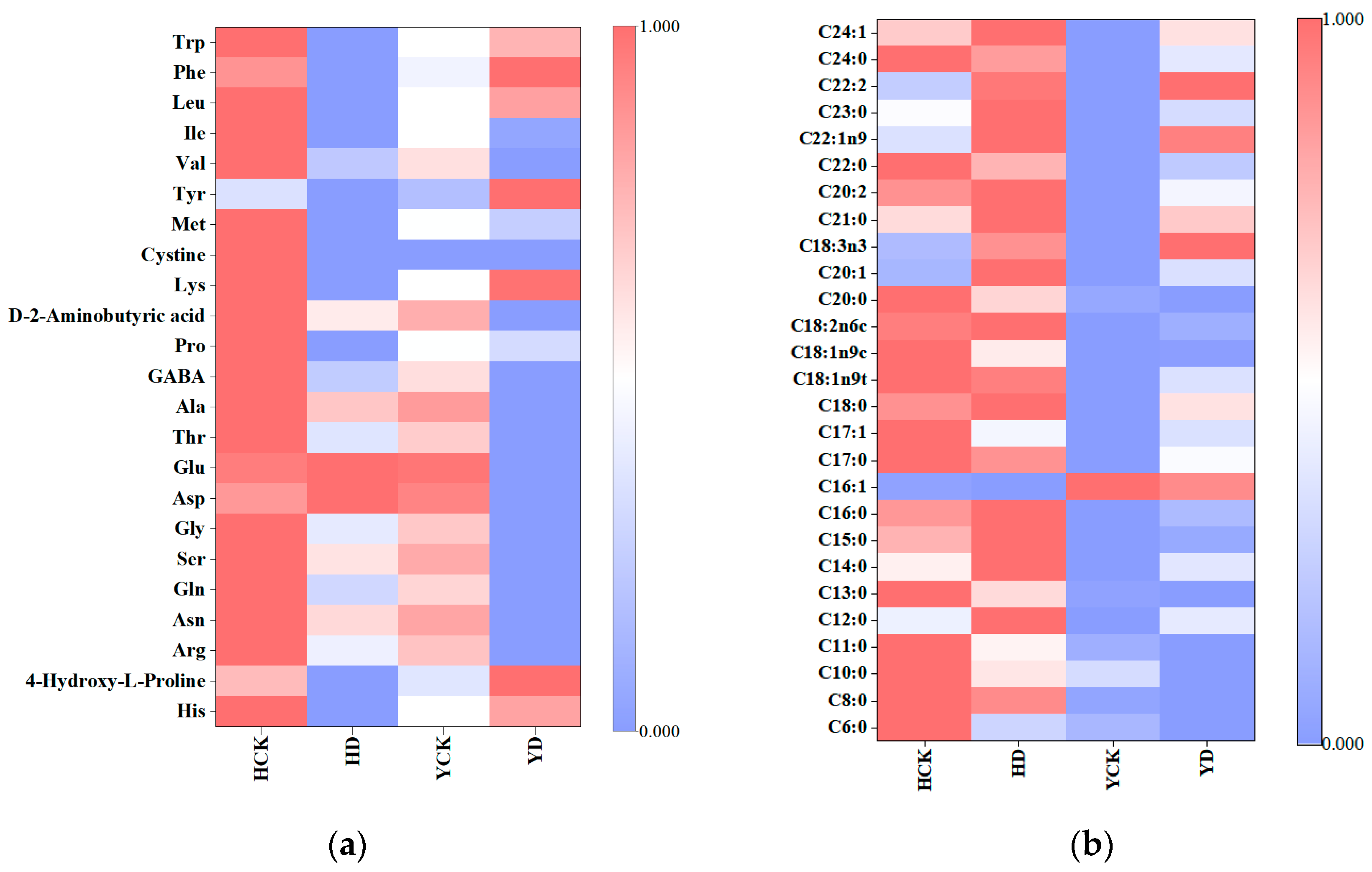
3.4. Impact on Amino Acid Composition
3.5. Impact on Medium-Longchain Fatty Acid Content
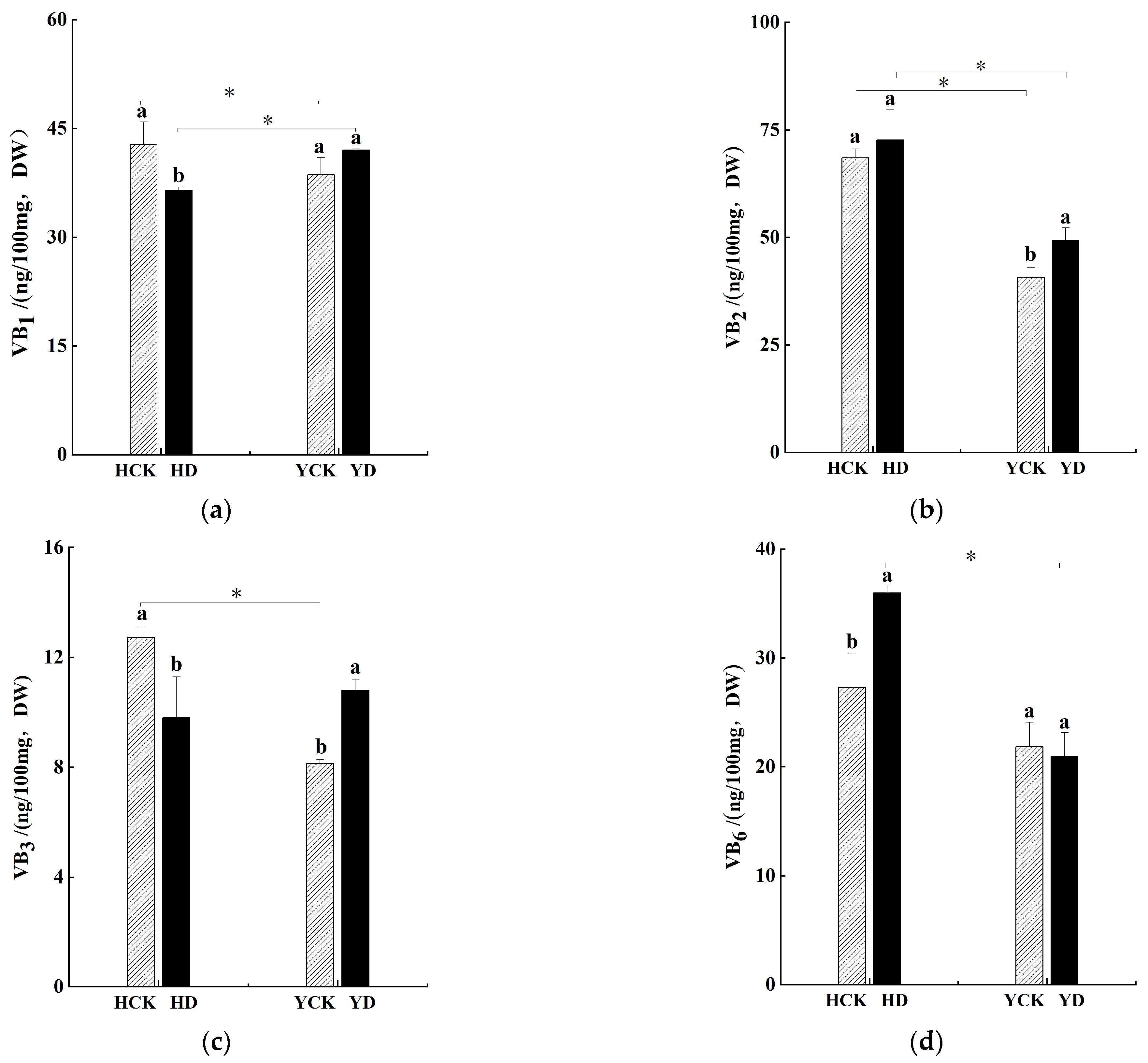
3.6. Impact on B Vitamin Content
3.7. Impact on Anthocyanin Content
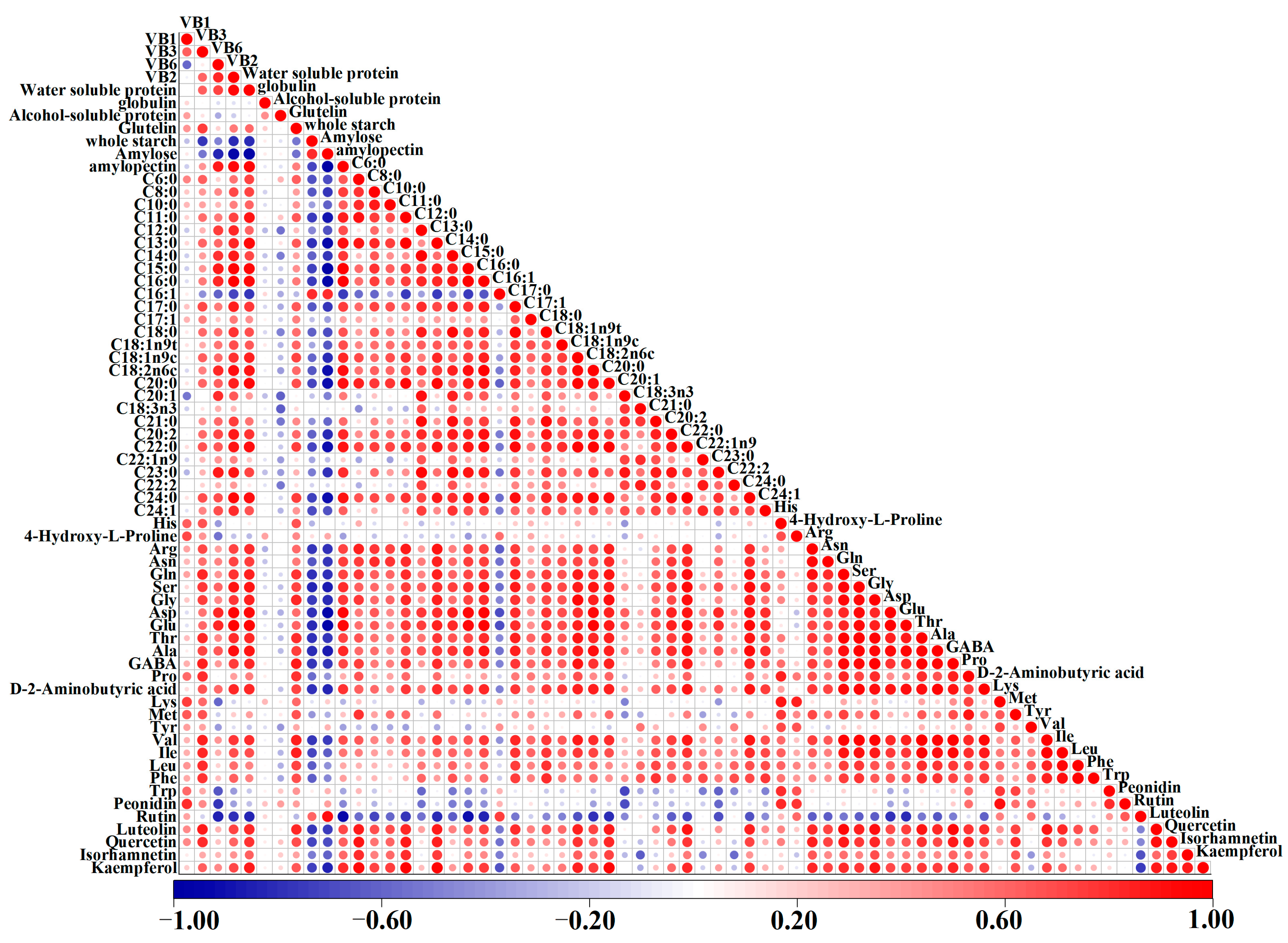
3.8. Correlation Analysis
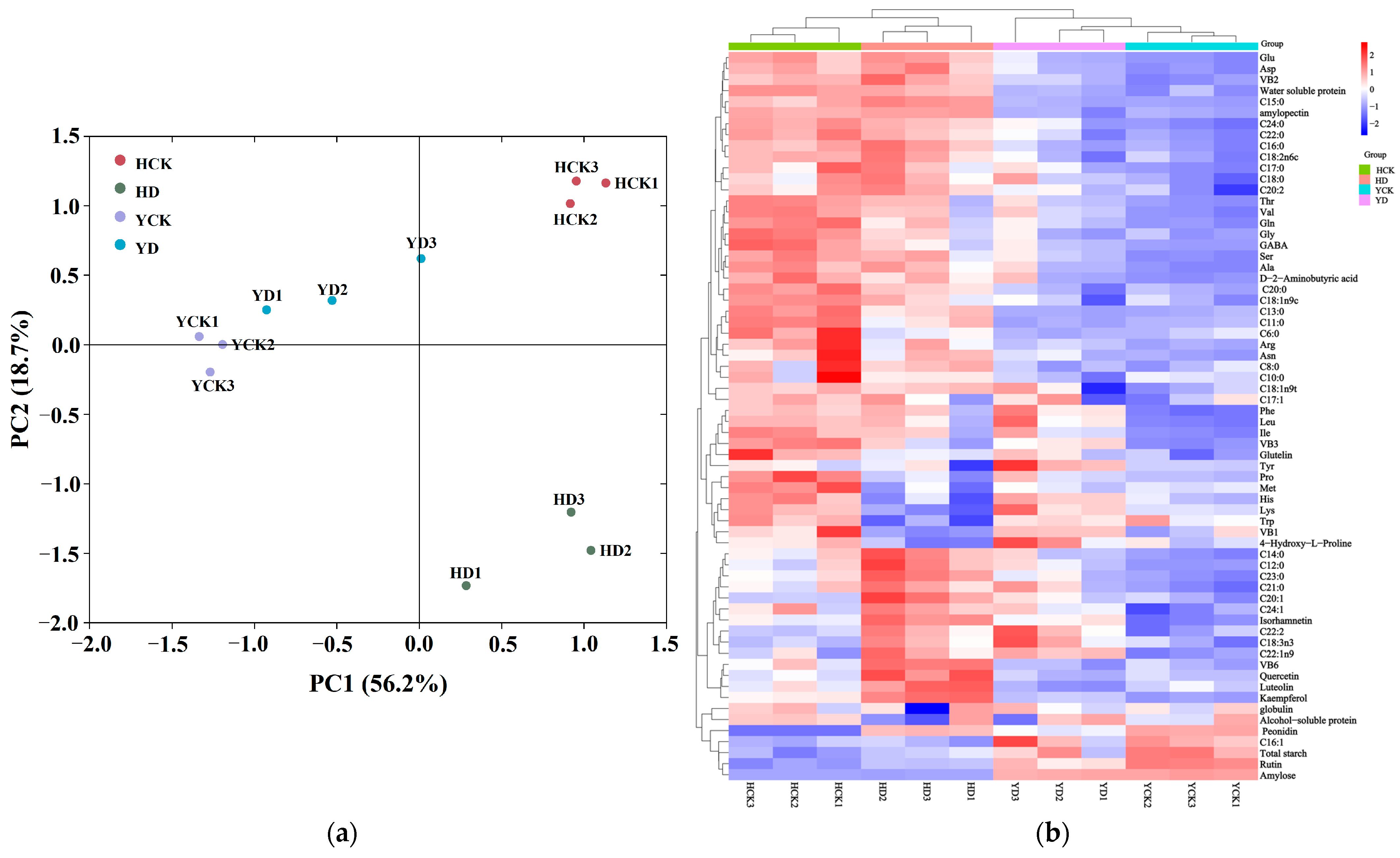
3.9. Principal Component Analysis and Hierarchical Cluster Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, Y.H.; Li, J.; Ma, H.C.; Yang, Q.H.; Liu, C.J.; Feng, B.L. Salt-tolerant Broomcorn Millet (Panicum miliaceum L.) Resists Salt Stress via Modulation of Cell Wall Biosynthesis and Na+ Balance. Land Degrad. Dev. 2021, 32, 518–532. [Google Scholar] [CrossRef]
- Chen, S.; Peng, W.; Ansah, E.O.; Xiong, F.; Wu, Y. Encoded C4 Homologue Enzymes Genes Function under Abiotic Stresses in C3 Plant. Plant Signal. Behav. 2022, 17, 2115634. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Ma, Y.; Jiang, L.; Dong, J.; Zhu, Y.; Ren, G. Chemical Composition, Antioxidant, and Antiproliferative Activities of Nine Chinese Proso Millet Varieties. Food Agric. Immunol. 2018, 29, 625–637. [Google Scholar] [CrossRef]
- Na, X.; Cao, X.; Ma, C.; Ma, S.; Xu, P.; Liu, S.; Wang, J.; Wang, H.; Chen, L.; Qiao, Z. Plant Stage, Not Drought Stress, Determines the Effect of Cultivars on Bacterial Community Diversity in the Rhizosphere of Broomcorn Millet (Panicum miliaceum L.). Front. Microbiol. 2019, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.C.; Vigil, M.F. Water Use and Environmental Parameters Influence Proso Millet Yield. Field Crops Res. 2017, 212, 34–44. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, C.; Dang, K.; Gong, X.; Feng, B. Cultivar Sensitivity of Broomcorn Millet (Panicum miliaceum L.) to Nitrogen Availability Is Associated with Differences in Photosynthetic Physiology and Nitrogen Uptake. Plant Physiol. Biochem. PPB 2022, 182, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.-K.; Duan, C.-G. Epigenetic Regulation in Plant Abiotic Stress Responses. J. Integr. Plant Biol. 2020, 62, 563–580. [Google Scholar] [CrossRef]
- Shanker, A.K.; Maheswari, M.; Yadav, S.K.; Desai, S.; Bhanu, D.; Attal, N.B.; Venkateswarlu, B. Drought Stress Responses in Crops. Funct. Integr. Genom. 2014, 14, 11–22. [Google Scholar] [CrossRef]
- Yang, Z.; Qin, F. The Battle of Crops against Drought: Genetic Dissection and Improvement. J. Integr. Plant Biol. 2023, 65, 496–525. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Qin, F. Genetic Dissection of Drought Resistance for Trait Improvement in Crops. Crop J. 2023, 11, 975–985. [Google Scholar] [CrossRef]
- Kooyers, N.J. The Evolution of Drought Escape and Avoidance in Natural Herbaceous Populations. Plant Sci. Int. J. Exp. Plant Biol. 2015, 234, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Magallanes-López, A.M.; Ammar, K.; Morales-Dorantes, A.; González-Santoyo, H.; Crossa, J.; Guzmán, C. Grain Quality Traits of Commercial Durum Wheat Varieties and Their Relationships with Drought Stress and Glutenins Composition. J. Cereal Sci. 2017, 75, 1–9. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Usman, M.; Farooq, S.; Alghamdi, S.S.; Siddique, K.H.M. Impact of Abiotic Stresses on Grain Composition and Quality in Food Legumes. J. Agric. Food Chem. 2018, 66, 8887–8897. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Yang, Q.; Luo, Y.; Gong, X.; Zhang, W.; Hu, Y.; Yang, T.; Dong, K.; Feng, B. Proteomic Changes in the Grains of Foxtail Millet (Setaria italica (L.) Beau) under Drought Stress. Span. J. Agric. Res. 2019, 17, e0802. [Google Scholar] [CrossRef]
- Guo, J.; Qu, L.; Wang, L.; Lu, W.; Lu, D. Effects of Post-Silking Drought Stress Degree on Grain Yield and Quality of Waxy Maize. J. Sci. Food Agric. 2023, 103, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Bheemanahalli, R.; Ramamoorthy, P.; Poudel, S.; Samiappan, S.; Wijewardane, N.; Reddy, K.R. Effects of Drought and Heat Stresses during Reproductive Stage on Pollen Germination, Yield, and Leaf Reflectance Properties in Maize (Zea mays L.). Plant Direct 2022, 6, e434. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhang, G. The Influence of Drought Stress on Malt Quality Traits of the Wild and Cultivated Barleys. J. Integr. Agric. 2020, 19, 2009–2015. [Google Scholar] [CrossRef]
- Chen, G.; Peng, L.; Gong, J.; Wang, J.; Wu, C.; Sui, X.; Tian, Y.; Hu, M.; Li, C.; He, X.; et al. Effects of Water Stress on Starch Synthesis and Accumulation of Two Rice Cultivars at Different Growth Stages. Front. Plant Sci. 2023, 14, 1133524. [Google Scholar] [CrossRef]
- Wang, X.; Fu, J.; Min, Z.; Zou, D.; Liu, H.; Wang, J.; Zheng, H.; Jia, Y.; Yang, L.; Xin, W.; et al. Response of Rice with Overlapping Growth Stages to Water Stress by Assimilates Accumulation and Transport and Starch Synthesis of Superior and Inferior Grains. Int. J. Mol. Sci. 2022, 23, 11157. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Interaction of Proline, Sugars, and Anthocyanins during Photosynthetic Acclimation of Arabidopsis thaliana to Drought Stress. J. Plant Physiol. 2012, 169, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant Metabolomics: An Indispensable System Biology Tool for Plant Science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Ni, S.-J.; Zhang, G.-P. Transcriptome and Metabolome Analysis Reveals Regulatory Networks and Key Genes Controlling Barley Malting Quality in Responses to Drought Stress. Plant Physiol. Biochem. PPB 2020, 152, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tiedge, K.; Li, X.; Merrill, A.T.; Davisson, D.; Chen, Y.; Yu, P.; Tantillo, D.J.; Last, R.L.; Zerbe, P. Comparative Transcriptomics and Metabolomics Reveal Specialized Metabolite Drought Stress Responses in Switchgrass (Panicum virgatum). New Phytol. 2022, 236, 1393–1408. [Google Scholar] [CrossRef]
- Ullah, S.; Khan, M.N.; Lodhi, S.S.; Ahmed, I.; Tayyab, M.; Mehmood, T.; Din, I.U.; Khan, M.; Sohail, Q.; Akram, M. Targeted Metabolomics Reveals Fatty Acid Abundance Adjustments as Playing a Crucial Role in Drought-Stress Response and Post-Drought Recovery in Wheat. Front. Genet. 2022, 13, 972696. [Google Scholar] [CrossRef]
- Wang, H.; Li, D.; Wan, C.; Luo, Y.; Yang, Q.; Gao, X.; Feng, B. Improving the Functionality of Proso Millet Protein and Its Potential as a Functional Food Ingredient by Applying Nitrogen Fertiliser. Foods 2021, 10, 1332. [Google Scholar] [CrossRef]
- Wang, H.; Wu, E.; Ma, Q.; Zhang, H.; Feng, Y.; Yang, P.; Gao, J.; Feng, B. Comparison of the Fine Structure and Physicochemical Properties of Proso Millet (Panicum miliaceum L.) Starch from Different Ecological Regions. Int. J. Biol. Macromol. 2023, 249, 126115. [Google Scholar] [CrossRef]
- Tian, L.; Yu, S.; Zhang, L.; Yang, J.; Feng, B.; Song, Y. Effects of Mulching Types on the Yield and Water Utilization by Broomcorn Millet (Panicum miliaceum): Results of a Study in the Loess Plateau, China. J. Environ. Manag. 2023, 345, 118856. [Google Scholar] [CrossRef]
- Paul Ajithkumar, I. Influences of Natural Antioxidants, Reactive Oxygen Species and Compatible Solutes of Panicum miliaceum L. Towards Drought Stress. Cell Biochem. Biophys. 2023, 81, 141–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Li, J.; Gong, X.; Yang, P.; Gao, J.; Wang, P.; Feng, B. Comparative Analysis of Proso Millet (Panicum miliaceum L.) Leaf Transcriptomes for Insight into Drought Tolerance Mechanisms. BMC Plant Biol. 2019, 19, 397. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The Effect of Drought and Heat Stress on Reproductive Processes in Cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, J.; Hu, Y.; Wang, S.; Mao, J.; Xu, Y.; Wang, M.; Liu, S.; Qiao, Z.; Cao, X. Effects of Drought Stress during the Flowering Period on the Rhizosphere Fungal Diversity of Broomcorn Millet (Panicum miliaceum L.). Agronomy 2023, 13, 2896. [Google Scholar] [CrossRef]
- Cao, X.; Hu, Y.; Song, J.; Feng, H.; Wang, J.; Chen, L.; Wang, L.; Diao, X.; Wan, Y.; Liu, S.; et al. Transcriptome Sequencing and Metabolome Analysis Reveals the Molecular Mechanism of Drought Stress in Millet. Int. J. Mol. Sci. 2022, 23, 10792. [Google Scholar] [CrossRef]
- Deng, F.; Li, Q.; Chen, H.; Zeng, Y.; Li, B.; Zhong, X.; Wang, L.; Ren, W. Relationship between Chalkiness and the Structural and Thermal Properties of Rice Starch after Shading during Grain-Filling Stage. Carbohydr. Polym. 2021, 252, 117212. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, Y.; Wu, Y.; Zhou, W.; Yang, J.; Yuan, L.; Zhang, P.; Wang, H. The H+-Pyrophosphatase IbVP1 Regulates Carbon Flux to Influence the Starch Metabolism and Yield of Sweet Potato. Hortic. Res. 2021, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.; Antes, S.; Seilmeier, W. Quantitative Determination of Gluten Protein Types in Wheat Flour by Reversed-Phase High-Performance Liquid Chromatography. Cereal Chem. 1998, 75, 644–650. [Google Scholar] [CrossRef]
- Kovács, Z.; Simon-Sarkadi, L.; Sovány, C.; Kirsch, K.; Galiba, G.; Kocsy, G. Differential Effects of Cold Acclimation and Abscisic Acid on Free Amino Acid Composition in Wheat. Plant Sci. Int. J. Exp. Plant Biol. 2011, 180, 61–68. [Google Scholar] [CrossRef]
- Li, S.-S.; Yuan, R.-Y.; Chen, L.-G.; Wang, L.-S.; Hao, X.-H.; Wang, L.-J.; Zheng, X.-C.; Du, H. Systematic Qualitative and Quantitative Assessment of Fatty Acids in the Seeds of 60 Tree Peony (Paeonia Section Moutan DC.) Cultivars by GC-MS. Food Chem. 2015, 173, 133–140. [Google Scholar] [CrossRef]
- Cellar, N.A.; McClure, S.C.; Salvati, L.M.; Reddy, T.M. A New Sample Preparation and Separation Combination for Precise, Accurate, Rapid, and Simultaneous Determination of Vitamins B1, B2, B3, B5, B6, B7, and B9 in Infant Formula and Related Nutritionals by LC-MS/MS. Anal. Chim. Acta 2016, 934, 180–185. [Google Scholar] [CrossRef]
- Barnes, J.S.; Nguyen, H.P.; Shen, S.; Schug, K.A. General Method for Extraction of Blueberry Anthocyanins and Identification Using High Performance Liquid Chromatography-Electrospray Ionization-Ion Trap-Time of Flight-Mass Spectrometry. J. Chromatogr. A 2009, 1216, 4728–4735. [Google Scholar] [CrossRef]
- Syahariza, Z.A.; Sar, S.; Hasjim, J.; Tizzotti, M.J.; Gilbert, R.G. The Importance of Amylose and Amylopectin Fine Structures for Starch Digestibility in Cooked Rice Grains. Food Chem. 2013, 136, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, P.; Qu, Y.; Gao, X.; Liang, J.; Yang, P.; Feng, B. Comparison of Physicochemical Properties and Cooking Edibility of Waxy and Non-Waxy Proso Millet (Panicum miliaceum L.). Food Chem. 2018, 257, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tan, H.; Zhang, C.; Li, Q.; Liu, Q. Starch Biosynthesis in Cereal Endosperms: An Updated Review over the Last Decade. Plant Commun. 2021, 2, 100237. [Google Scholar] [CrossRef] [PubMed]
- Prathap, V.; Tyagi, A. Correlation between Expression and Activity of ADP Glucose Pyrophosphorylase and Starch Synthase and Their Role in Starch Accumulation during Grain Filling under Drought Stress in Rice. Plant Physiol. Biochem. PPB 2020, 157, 239–243. [Google Scholar] [CrossRef]
- Zhang, M.; He, S.; Qin, B.; Jin, X.; Wang, M.; Ren, C.; Cao, L.; Zhang, Y. Exogenous Melatonin Reduces the Inhibitory Effect of Osmotic Stress on Antioxidant Properties and Cell Ultrastructure at Germination Stage of Soybean. PLoS ONE 2020, 15, e0243537. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Poirier, Y. The Protein Acetylome and the Regulation of Metabolism. Trends Plant Sci. 2012, 17, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.J.; Nikolau, B.J.; Wurtele, E.S. Acetyl-CoA—Life at the Metabolic Nexus. Plant Sci. 2009, 176, 597–601. [Google Scholar] [CrossRef]
- Saharan, B.S.; Brar, B.; Duhan, J.S.; Kumar, R.; Marwaha, S.; Rajput, V.D.; Minkina, T. Molecular and Physiological Mechanisms to Mitigate Abiotic Stress Conditions in Plants. Life 2022, 12, 1634. [Google Scholar] [CrossRef]
- Pratelli, R.; Pilot, G. Regulation of Amino Acid Metabolic Enzymes and Transporters in Plants. J. Exp. Bot. 2014, 65, 5535–5556. [Google Scholar] [CrossRef]
- Tegeder, M. Transporters for Amino Acids in Plant Cells: Some Functions and Many Unknowns. Curr. Opin. Plant Biol. 2012, 15, 315–321. [Google Scholar] [CrossRef]
- Rentsch, D.; Schmidt, S.; Tegeder, M. Transporters for Uptake and Allocation of Organic Nitrogen Compounds in Plants. FEBS Lett. 2007, 581, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.-J. The Significance of Amino Acids and Amino Acid-Derived Molecules in Plant Responses and Adaptation to Heavy Metal Stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Amon, B.; Schulz, K.; Mehdi, B. Factors That Influence Nitrous Oxide Emissions from Agricultural Soils as Well as Their Representation in Simulation Models: A Review. Agronomy 2021, 11, 770. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Łuniewski, S.; Kaczyński, P.; Łozowicka, B. The Influence of Humic Acids and Nitrophenols on Metabolic Compounds and Pesticide Behavior in Wheat under Biotic Stress. Agronomy 2023, 13, 1378. [Google Scholar] [CrossRef]
- Hildebrandt, T.M. Synthesis versus Degradation: Directions of Amino Acid Metabolism during Arabidopsis Abiotic Stress Response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and Heat-Stress Effects on Seed Filling in Food Crops: Impacts on Functional Biochemistry, Seed Yields, and Nutritional Quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of Glycine Betaine and Proline in Improving Plant Abiotic Stress Resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Xiao, R.; Zou, Y.; Guo, X.; Li, H.; Lu, H. Fatty Acid Desaturases (FADs) Modulate Multiple Lipid Metabolism Pathways to Improve Plant Resistance. Mol. Biol. Rep. 2022, 49, 9997–10011. [Google Scholar] [CrossRef]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid Signalling in Plant Responses to Abiotic Stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef]
- Lim, G.-H.; Singhal, R.; Kachroo, A.; Kachroo, P. Fatty Acid- and Lipid-Mediated Signaling in Plant Defense. Annu. Rev. Phytopathol. 2017, 55, 505–536. [Google Scholar] [CrossRef] [PubMed]
- Ohlrogge, J.; Browse, J. Lipid Biosynthesis. Plant Cell 1995, 7, 957–970. [Google Scholar] [PubMed]
- Halford, N.G.; Curtis, T.Y.; Chen, Z.; Huang, J. Effects of Abiotic Stress and Crop Management on Cereal Grain Composition: Implications for Food Quality and Safety. J. Exp. Bot. 2015, 66, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Bargmann, B.O.; Munnik, T. The Role of Phospholipase D in Plant Stress Responses. Curr. Opin. Plant Biol. 2006, 9, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Babiychuk, E.; Müller, F.; Eubel, H.; Braun, H.-P.; Frentzen, M.; Kushnir, S. Arabidopsis Phosphatidylglycerophosphate Synthase 1 Is Essential for Chloroplast Differentiation, but Is Dispensable for Mitochondrial Function. Plant J. Cell Mol. Biol. 2003, 33, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Miret, J.A.; Munné-Bosch, S. Plant Amino Acid-Derived Vitamins: Biosynthesis and Function. Amino Acids 2014, 46, 809–824. [Google Scholar] [CrossRef]
- Kausar, A.; Zahra, N.; Zahra, H.; Hafeez, M.B.; Zafer, S.; Shahzadi, A.; Raza, A.; Djalovic, I.; Prasad, P.V. Alleviation of Drought Stress through Foliar Application of Thiamine in Two Varieties of Pea (Pisum sativum L.). Plant Signal. Behav. 2023, 18, 2186045. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.-C.; Li, Y. Molecular Mechanism of Thiamine in Mitigating Drought Stress in Chinese Wingnut (Pterocarya Stenoptera): Insights from Transcriptomics. Ecotoxicol. Environ. Saf. 2023, 263, 115307. [Google Scholar] [CrossRef]
- Deng, B.; Jin, X.; Yang, Y.; Lin, Z.; Zhang, Y. The Regulatory Role of Riboflavin in the Drought Tolerance of Tobacco Plants Depends on ROS Production. Plant Growth Regul. 2014, 72, 269–277. [Google Scholar] [CrossRef]
- Bilski, P.; Li, M.Y.; Ehrenshaft, M.; Daub, M.E.; Chignell, C.F. Vitamin B6 (Pyridoxine) and Its Derivatives Are Efficient Singlet Oxygen Quenchers and Potential Fungal Antioxidants. Photochem. Photobiol. 2000, 71, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, J.; Wang, L.; Huang, L. Effect of Abiotic Stress on the Abundance of Different Vitamin B6 Vitamers in Tobacco Plants. Plant Physiol. Biochem. PPB 2013, 66, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Tiwari, V.; Kumari, A.; Chaudhary, E.; Sharma, A.; Ali, U.; Garg, M. Protective and Defensive Role of Anthocyanins under Plant Abiotic and Biotic Stresses: An Emerging Application in Sustainable Agriculture. J. Biotechnol. 2023, 361, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Abiotic Stress-Induced Anthocyanins in Plants: Their Role in Tolerance to Abiotic Stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular Localization of H2O2 in Plants. H2O2 Accumulation in Papillae and Hypersensitive Response during the Barley—Powdery Mildew Interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of Oxidative and Drought Tolerance in Arabidopsis by Overaccumulation of Antioxidant Flavonoids. Plant J. Cell Mol. Biol. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Pollastri, S.; Tattini, M. Flavonols: Old Compounds for Old Roles. Ann. Bot. 2011, 108, 1225–1233. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.-A.; Asaf, S.; Lubna; Waqas, M.; Park, J.-R.; Asif, S.; Kim, N.; Lee, I.-J.; Kim, K.-M. Drought and UV Radiation Stress Tolerance in Rice Is Improved by Overaccumulation of Non-Enzymatic Antioxidant Flavonoids. Antioxidants 2022, 11, 917. [Google Scholar] [CrossRef]
- Oksana, S.; Marek, K.; Marian, B.; Marek, Z. Cultivar-Dependent and Drought-Induced Modulation of Secondary Metabolites, Adaptative Defense in Fagopyrum esculentum L. Physiol. Mol. Biol. Plants 2023, 29, 1605–1618. [Google Scholar] [CrossRef]
| Treatment | Plant Height (cm) | 1000-Grain Weight (g) | Grain Weight per Plant (g) | Grain Area (mm2) | Grain Length-Width Ratio | Grain Diameter (mm) |
|---|---|---|---|---|---|---|
| HCK | 91.17 ± 9.28 a | 7.16 ± 0.05 a | 2.53 ± 0.24 a | 6.18 ± 1.40 a | 1.39 ± 0.02 b | 2.77 ± 0.41 a |
| HD | 70.33 ± 4.37 b | 6.35 ± 0.16 c | 1.53 ± 0.17 b | 6.12 ± 0.97 a | 1.44 ± 0.01 a | 2.77 ± 0.29 a |
| YCK | 89.17 ± 6.01 a | 6.69 ± 0.13 b | 2.37 ± 0.42 a | 4.84 ± 1.79 b | 1.27 ± 0.02 d | 2.42 ± 0.57 b |
| YD | 74.73 ± 5.01 b | 6.00 ± 0.26 d | 1.32 ± 0.18 b | 4.30 ± 2.06 b | 1.30 ± 0.03 c | 2.24 ± 0.64 b |
| Mean | 81.35 | 6.55 | 1.94 | 5.36 | 1.35 | 2.55 |
| SD | 10.87 | 0.46 | 0.59 | 1.78 | 0.07 | 0.54 |
| CV (%) | 13.36 | 7.02 | 30.45 | 33.21 | 5.19 | 21.18 |
| MS | 6698.53 | 43.09 | 4.03 | 29.39 | 1.83 | 6.55 |
| Treatment | His | 4HLP | Arg | Asn | Gln | Ser | Gly | Asp | Glu | Thr | Ala | GABA | Pro | D2A | Lys | Cys | Met | Tyr | Val | Ile | Leu | Phe | Trp |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCK | 26.88 ± 1.97 a | 0.71 ± 0.03 a | 156.49 ± 24.28 a | 439.08 ± 66.9 a | 604.95 ± 27.98 a | 100.06 ± 5.77 a | 28.51 ± 0.83 a | 203.83 ± 15.24 a | 201.05 ± 16.54 a | 23.44 ± 0.72 a | 62.66 ± 5.48 a | 33.43 ± 3.42 a | 80.35 ± 6.99 a | 0.84 ± 0.08 a | 18.54 ± 0.95 a | 0.18 ± 0.09 a | 11.22 ± 0.79 a | 12.85 ± 0.81 ab | 23.07 ± 1.09 a | 12.42 ± 0.97 a | 11.28 ± 0.19 a | 10.73 ± 0.19 a | 45.59 ± 2.64 a |
| HD | 16.92 ± 3.78 b | 0.57 ± 0.04 b | 125.35 ± 19.66 ab | 407.53 ± 32.95 ab | 455.03 ± 82.43 b | 87.69 ± 14.19 ab | 24.22 ± 1.91 b | 219.01 ± 34.48 a | 204.79 ± 16.51 a | 19.45 ± 3.7 ab | 56.18 ± 8.98 a | 21.65 ± 4.82 b | 45.97 ± 9.08 b | 0.72 ± 0.08 ab | 11.35 ± 1.4 c | 0 ± 0 b | 6.17 ± 1.7 b | 11.65 ± 2.35 b | 18.42 ± 3.25 b | 9.95 ± 2.19 a | 9.88 ± 1.61 a | 10.12 ± 1.37 a | 27.94 ± 4.5 b |
| YCK | 19.48 ± 1.62 b | 0.65 ± 0.04 ab | 91.42 ± 2.4 c | 322.68 ± 8.63 c | 285.2 ± 15.89 c | 50.5 ± 1.1 c | 20.38 ± 1.03 c | 70.84 ± 8.55 b | 97.45 ± 5.49 b | 13.79 ± 0.39 c | 28.15 ± 1.48 b | 12.23 ± 0.47 c | 45.54 ± 1.04 b | 0.41 ± 0.01 c | 14.53 ± 0.27 b | 0 ± 0 b | 7.45 ± 0.2 b | 11.89 ± 0.06 b | 12.41 ± 0.61 c | 6.58 ± 0.19 b | 7.18 ± 0.18 b | 7.43 ± 0.17 b | 41.17 ± 5.34 a |
| YD | 25.11 ± 1.14 a | 0.76 ± 0.09 a | 102.09 ± 3.74 bc | 352.84 ± 22.77 bc | 391.56 ± 54.23 b | 69.12 ± 10.44 b | 21.45 ± 2.4 bc | 110.53 ± 25.66 b | 124.52 ± 20.37 b | 17.17 ± 2.33 bc | 40.98 ± 10.34 b | 18.01 ± 4.52 bc | 56.86 ± 10.62 b | 0.56 ± 0.18 bc | 18.48 ± 2.41 a | 0 ± 0 b | 7.43 ± 0.76 b | 15.12 ± 1.46 a | 17.1 ± 2.68 b | 10.05 ± 2.02 a | 11.05 ± 1.71 a | 10.81 ± 1.09 a | 41.5 ± 0.85 a |
| Mean | 22.10 | 0.67 | 118.84 | 380.53 | 434.18 | 76.84 | 23.64 | 151.05 | 156.95 | 18.46 | 46.99 | 21.33 | 57.18 | 0.63 | 15.73 | 0.05 | 8.07 | 12.88 | 17.75 | 9.75 | 9.85 | 9.77 | 39.05 |
| SD | 4.68 | 0.09 | 29.34 | 58.09 | 128.76 | 21.15 | 3.58 | 67.92 | 50.87 | 4.12 | 15.38 | 8.70 | 16.19 | 0.19 | 3.38 | 0.09 | 2.16 | 1.89 | 4.39 | 2.55 | 1.98 | 1.63 | 7.64 |
| CV(%) | 21.19 | 12.84 | 24.69 | 15.27 | 29.66 | 27.52 | 15.13 | 44.96 | 32.41 | 22.34 | 32.74 | 40.78 | 28.32 | 29.92 | 21.50 | 198.13 | 26.74 | 14.64 | 24.73 | 26.19 | 20.07 | 16.65 | 19.58 |
| MS | 504.79 | 0.4577 | 14745.28 | 146873.67 | 201918.47 | 6257.28 | 568.64 | 26686.76 | 26841.16 | 353.15 | 2388.56 | 515.03 | 3469.06 | 0.43 | 256.33 | 0.0082 | 68.62 | 167.72 | 329.49 | 99.45 | 99.64 | 97.41 | 1569.06 |
| Treatment | C6:0 | C8:0 | C10:0 | C11:0 | C12:0 | C13:0 | C14:0 | C15:0 | C16:0 | C16:1 | C17:0 | C17:1 | C18:0 | C18:1n9t | C18:1n9c | C18:2n6c | C20:0 | C20:1 | C18:3n3 | C21:0 | C20:2 | C22:0 | C22:1n9 | C23:0 | C22:2 | C24:0 | C24:1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCK | 0.0001 ± 0 a | 0.00023 ± 0.00006 a | 0.00027 ± 0.00006 a | 0.00010 ± 0.000002 a | 0.0022 ± 0.0001 b | 0.0001 ± 0 a | 0.01387 ± 0.0006 b | 0.00383 ± 0.00025 b | 2.62653 ± 0.05089 a | 0.02543 ± 0.00042 b | 0.00623 ± 0.00035 a | 0.00433 ± 0.00006 a | 0.74153 ± 0.0299 a | 0.00333 ± 0.00006 a | 3.29387 ± 0.01375 a | 9.69 ± 0.03932 a | 0.0879 ± 0.00292 a | 0.0515 ± 0.0002 b | 0.17607 ± 0.00235 bc | 0.0047 ± 0.0002 a | 0.00653 ± 0.00035 ab | 0.0447 ± 0.00161 a | 0.1584 ± 0.01131 b | 0.00543 ± 0.00015 b | 0.00113 ± 0.00006 ab | 0.0217 ± 0.00056 a | 0.00103 ± 0.00006 a |
| HD | 0.00007 ± 0.00006 a | 0.0002 ± 0 a | 0.0002 ± 0 b | 0.00007 ± 0.000015 b | 0.00243 ± 0.00015 a | 0.0001 ± 0 a | 0.01527 ± 0.00075 a | 0.0044 ± 0.0002 a | 2.6963 ± 0.11583 a | 0.02533 ± 0.0006 b | 0.00613 ± 0.00035 a | 0.00423 ± 0.00021 a | 0.75173 ± 0.03069 a | 0.00327 ± 0.00006 a | 3.1148 ± 0.12231 ab | 9.7377 ± 0.29777 a | 0.0798 ± 0.00171 b | 0.06153 ± 0.00275 a | 0.19117 ± 0.00726 ab | 0.00503 ± 0.00025 a | 0.0067 ± 0.0003 a | 0.0421 ± 0.00155 a | 0.17933 ± 0.01171 a | 0.0062 ± 0.0002 a | 0.0013 ± 0.0001 a | 0.02063 ± 0.0006 a | 0.00113 ± 0.00006 a |
| HCK | 0.00003 ± 0.00006 b | 0.0002 ± 0 a | 0.0002 ± 0 b | 0.00011 ± 0.000002 c | 0.002 ± 0 b | 0.00003 ± 0.00006 b | 0.0121 ± 0.0002 c | 0.00193 ± 0.00006 c | 2.1879 ± 0.0619 b | 0.0282 ± 0.0005 a | 0.00543 ± 0.00006 b | 0.00413 ± 0.00015 a | 0.66367 ± 0.0183 b | 0.003 ± 0.0001 a | 2.8734 ± 0.10135 b | 8.77 ± 0.2488 b | 0.06613 ± 0.00185 c | 0.05007 ± 0.00215 b | 0.17287 ± 0.00675c | 0.0041 ± 0.0001 b | 0.0053 ± 0.0005 c | 0.03373 ± 0.00115 b | 0.14727 ± 0.0036 b | 0.0047 ± 0.0001 c | 0.00107 ± 0.00006 b | 0.0148 ± 0.0006 c | 0.00087 ± 0.00006 b |
| HD | 0 ± 0 b | 0.0002 ± 0 a | 0.0002 ± 0 b | 0.00003 ± 0.000003 c | 0.0021 ± 0.0001 b | 0 ± 0 b | 0.01327 ± 0.00081 bc | 0.00207 ± 0.00015 c | 2.2647 ± 0.09188 b | 0.02797 ± 0.00215 a | 0.0058 ± 0.00026 ab | 0.0042 ± 0.00026 a | 0.7171 ± 0.0401 ab | 0.0031 ± 0.00036 a | 2.87643 ± 0.19585 b | 8.85627 ± 0.38993 b | 0.0649 ± 0.00536 c | 0.05397 ± 0.00265 b | 0.19353 ± 0.01118 a | 0.00473 ± 0.0004 a | 0.00593 ± 0.00031 bc | 0.0362 ± 0.00361 b | 0.17757 ± 0.00305 a | 0.00517 ± 0.00025 b | 0.0013 ± 0.0001 a | 0.01743 ± 0.00197 b | 0.00103 ± 0.00006 a |
| Mean | 0.0001 | 0.0002 | 0.0002 | 0.0001 | 0.0022 | 0.0001 | 0.0136 | 0.0031 | 2.4439 | 0.0267 | 0.0059 | 0.0042 | 0.7185 | 0.0032 | 3.0396 | 9.2635 | 0.0747 | 0.0543 | 0.1834 | 0.0046 | 0.0061 | 0.0392 | 0.1656 | 0.0054 | 0.0012 | 0.0186 | 0.0010 |
| SD | 3.9 × 10−5 | 2.13 × 10−5 | 2.38 × 10−5 | 3.05 × 10−5 | 0.0002 | 3.43 × 10−5 | 0.0013 | 0.0011 | 0.2413 | 0.0017 | 0.0004 | 0.0002 | 0.0442 | 0.0002 | 0.2135 | 0.5271 | 0.0104 | 0.0050 | 0.0114 | 0.0004 | 0.0007 | 0.0050 | 0.0158 | 0.0006 | 0.0001 | 0.0030 | 0.0001 |
| CV(%) | 56.7717 | 9.8470 | 10.1683 | 50.1538 | 8.8026 | 45.5362 | 9.5474 | 37.1328 | 9.8755 | 6.3884 | 6.8631 | 3.9569 | 6.1476 | 6.6552 | 7.0231 | 5.6905 | 13.9513 | 9.1746 | 6.2362 | 9.0647 | 11.1081 | 12.6505 | 9.5213 | 10.7867 | 9.5092 | 16.1188 | 10.2804 |
| MS | 9.05 × 10−9 | 1.28 × 10−7 | 1.58 × 10−7 | 7.42 × 10−9 | 1.25 × 10−5 | 1.08 × 10−8 | 0.000482 | 1.79 × 10−5 | 15.59484 | 0.002307 | 9.34 × 10−5 | 5.24 × 10−5 | 1.398412 | 2.79 × 10−5 | 25.78318 | 239.843 | 0.014416 | 0.007978 | 0.093493 | 5.51 × 10−5 | 9.38 × 10−5 | 0.00383 | 0.070986 | 7.36 × 10−5 | 3.78 × 10−6 | 0.000791 | 2.54 × 10−6 |
| Treatment | Peonidin (ng/mL) | Rutin (ng/mL) | Luteolin (ng/mL) | Quercetin (ng/mL) | Isorhamnetin (ng/mL) | Kaempferol (ng/mL) |
|---|---|---|---|---|---|---|
| HCK | 0.11 ± 0.004 d | 3.42 ± 0.157 d | 0.98 ± 0.081 b | 0.75 ± 0.038 b | 0.81 ± 0.048 b | 1.06 ± 0.036 b |
| HD | 0.63 ± 0.012 b | 3.87 ± 0.026 c | 1.31 ± 0.086 a | 1.13 ± 0.088 a | 1.18 ± 0.074 a | 1.59 ± 0.065 a |
| YCK | 0.69 ± 0.011 a | 5.69 ± 0.070 a | 0.91 ± 0.059 b | 0.68 ± 0.041 b | 0.48 ± 0.050 c | 0.58 ± 0.040 d |
| YD | 0.43 ± 0.035 c | 4.88 ± 0.275 b | 0.76 ± 0.053 c | 0.64 ± 0.046 b | 0.87 ± 0.062 b | 0.76 ± 0.029 c |
| Mean | 0.47 | 4.46 | 0.99 | 0.80 | 0.83 | 1.00 |
| SD | 0.24 | 0.93 | 0.22 | 0.21 | 0.26 | 0.40 |
| CV(%) | 50.63 | 20.90 | 21.88 | 26.14 | 31.67 | 40.37 |
| MS | 0.27 | 20.68 | 1.02 | 0.68 | 0.76 | 1.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Liu, Y.; Mao, J.; Xu, Y.; Wang, M.; Hu, Y.; Wang, S.; Liu, S.; Qiao, Z.; Cao, X. Metabolomics and Physiological Methods Revealed the Effects of Drought Stress on the Quality of Broomcorn Millet during the Flowering Stage. Agronomy 2024, 14, 236. https://doi.org/10.3390/agronomy14020236
Ren J, Liu Y, Mao J, Xu Y, Wang M, Hu Y, Wang S, Liu S, Qiao Z, Cao X. Metabolomics and Physiological Methods Revealed the Effects of Drought Stress on the Quality of Broomcorn Millet during the Flowering Stage. Agronomy. 2024; 14(2):236. https://doi.org/10.3390/agronomy14020236
Chicago/Turabian StyleRen, Jiangling, Yuhan Liu, Jiao Mao, Yuanmeng Xu, Mengyao Wang, Yulu Hu, Shu Wang, Sichen Liu, Zhijun Qiao, and Xiaoning Cao. 2024. "Metabolomics and Physiological Methods Revealed the Effects of Drought Stress on the Quality of Broomcorn Millet during the Flowering Stage" Agronomy 14, no. 2: 236. https://doi.org/10.3390/agronomy14020236
APA StyleRen, J., Liu, Y., Mao, J., Xu, Y., Wang, M., Hu, Y., Wang, S., Liu, S., Qiao, Z., & Cao, X. (2024). Metabolomics and Physiological Methods Revealed the Effects of Drought Stress on the Quality of Broomcorn Millet during the Flowering Stage. Agronomy, 14(2), 236. https://doi.org/10.3390/agronomy14020236





