Effects of Nitrogen Input and Aeration on Greenhouse Gas Emissions and Pollutants in Agricultural Drainage Ditches
Abstract
1. Introduction
2. Materials and Methods
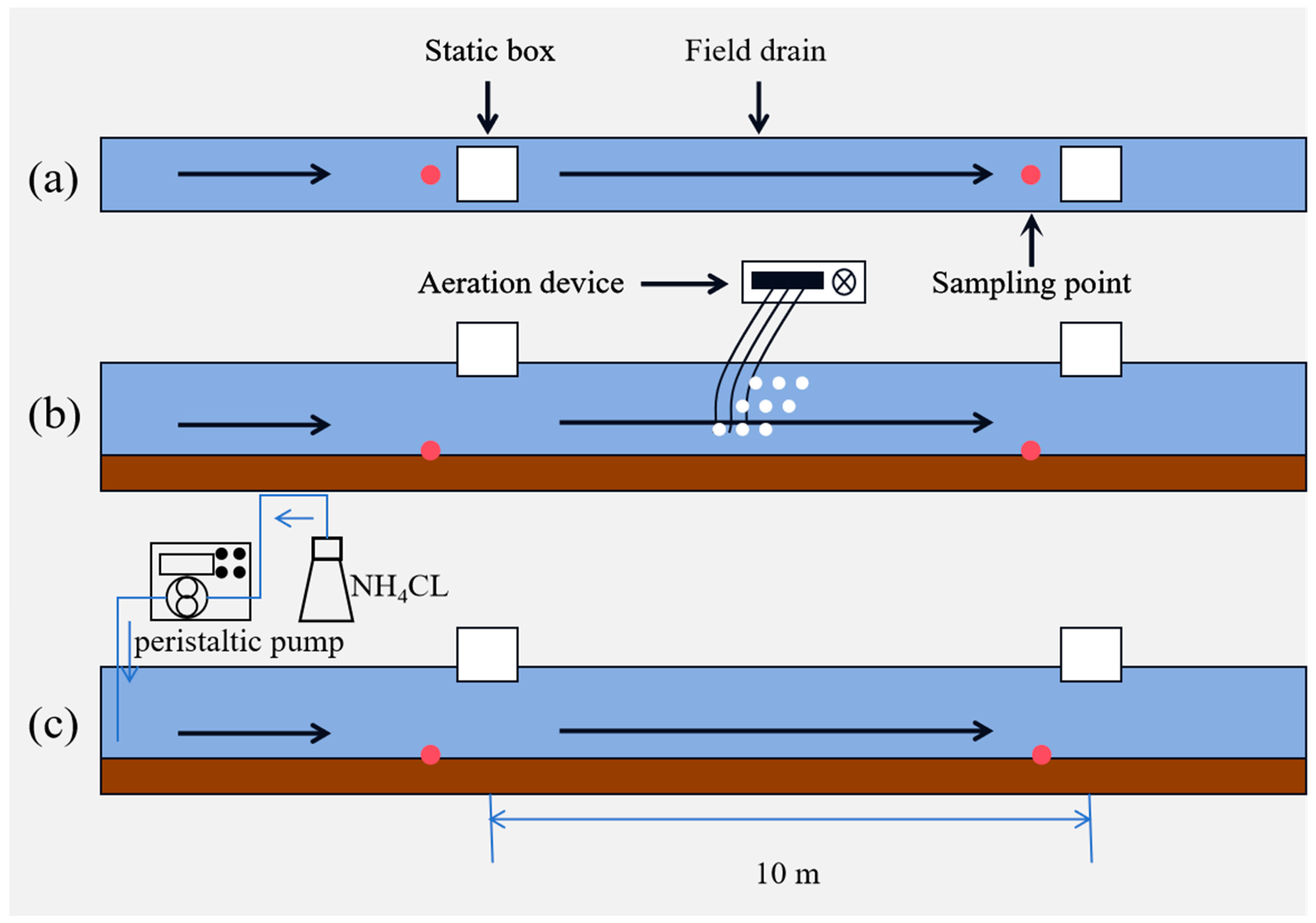
2.1. Experimental Materials and Equipment
2.2. Experimental Methods and Analytical Techniques
3. Results
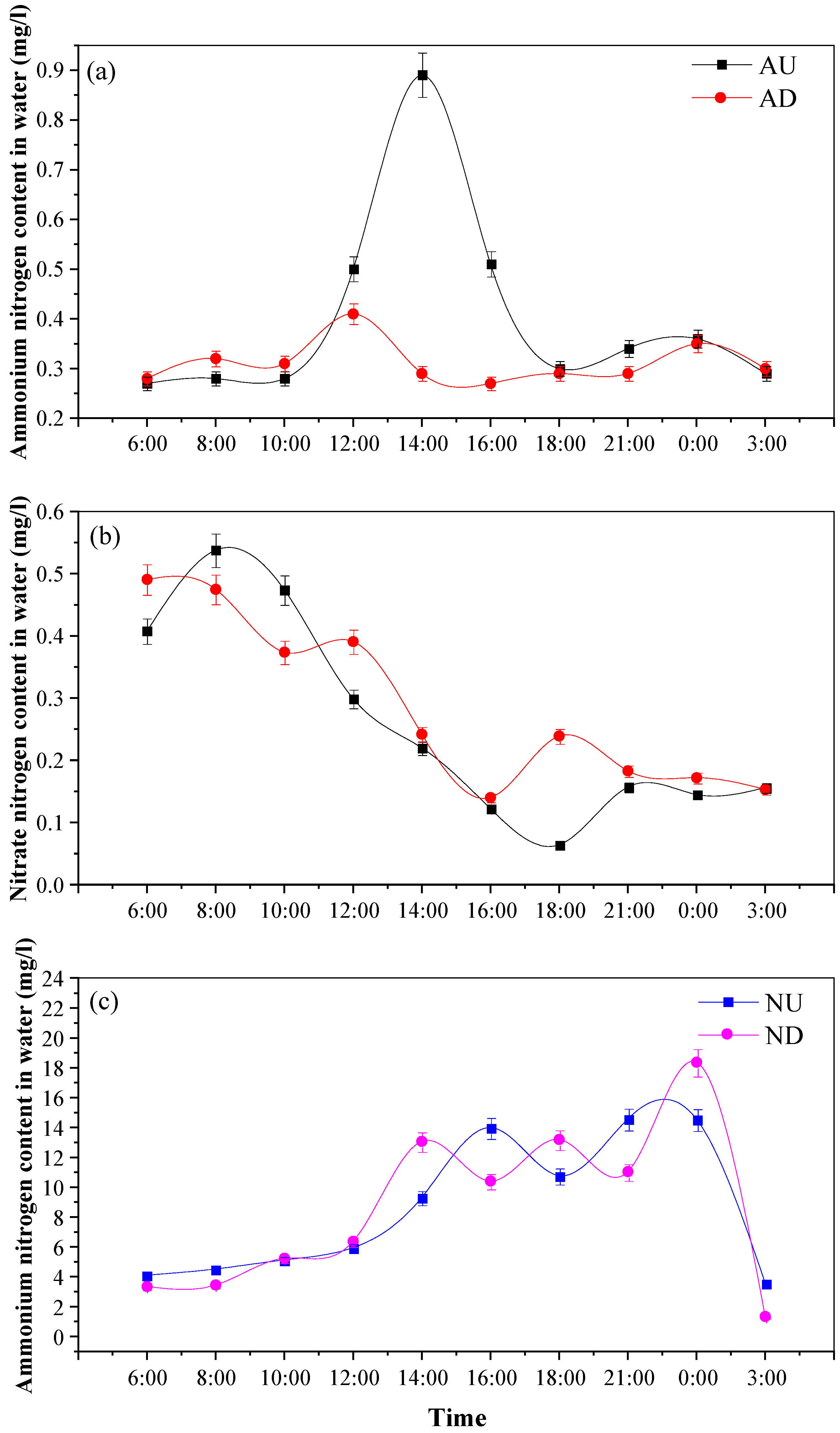
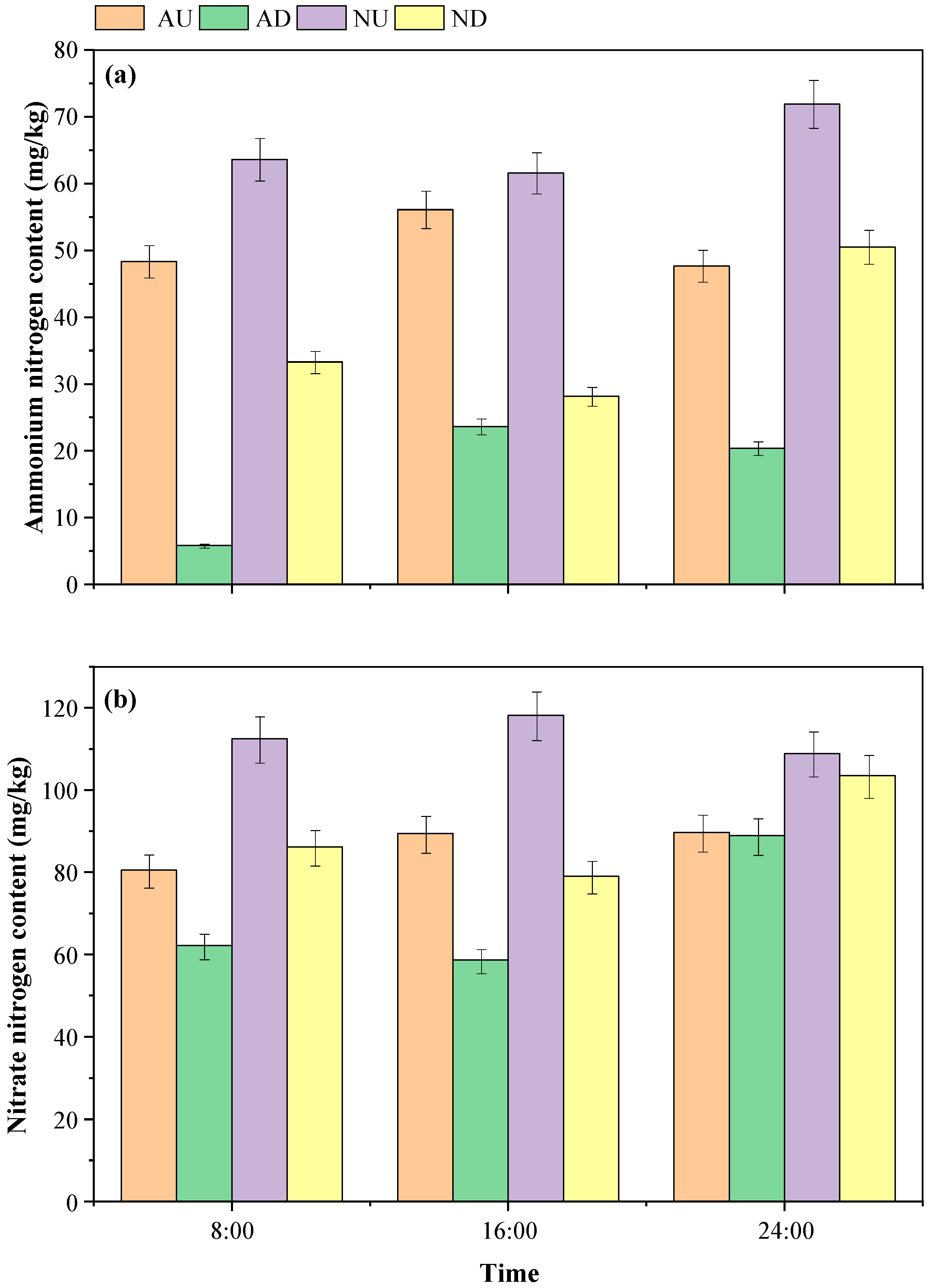
3.1. Characteristics of Nitrate Nitrogen/Ammonium Nitrogen Variation in Agricultural Drainage Ditches and Related Indicators
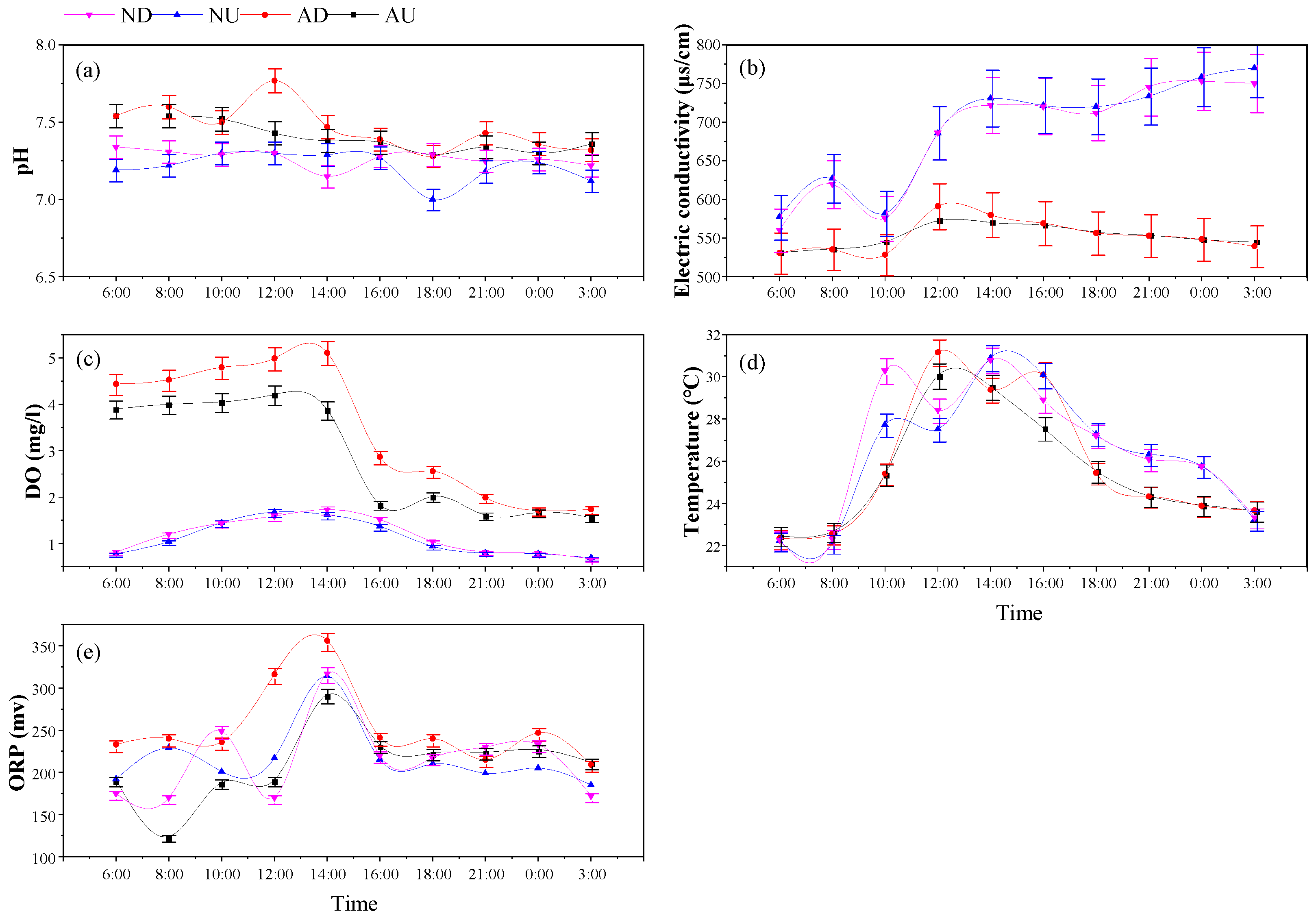
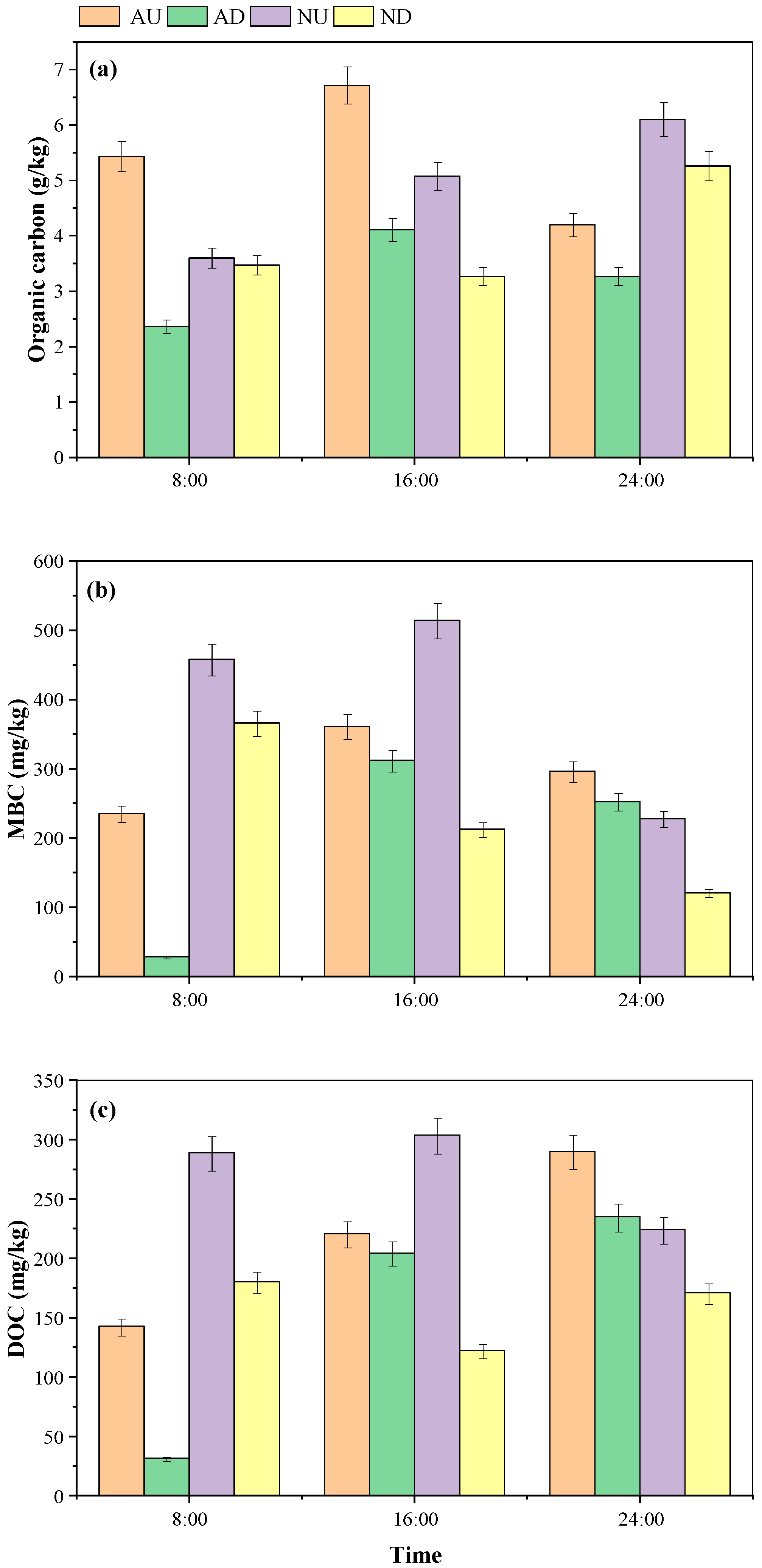
3.2. Diurnal Variation of Physicochemical Properties of Sediments in Agricultural Drainage Ditches
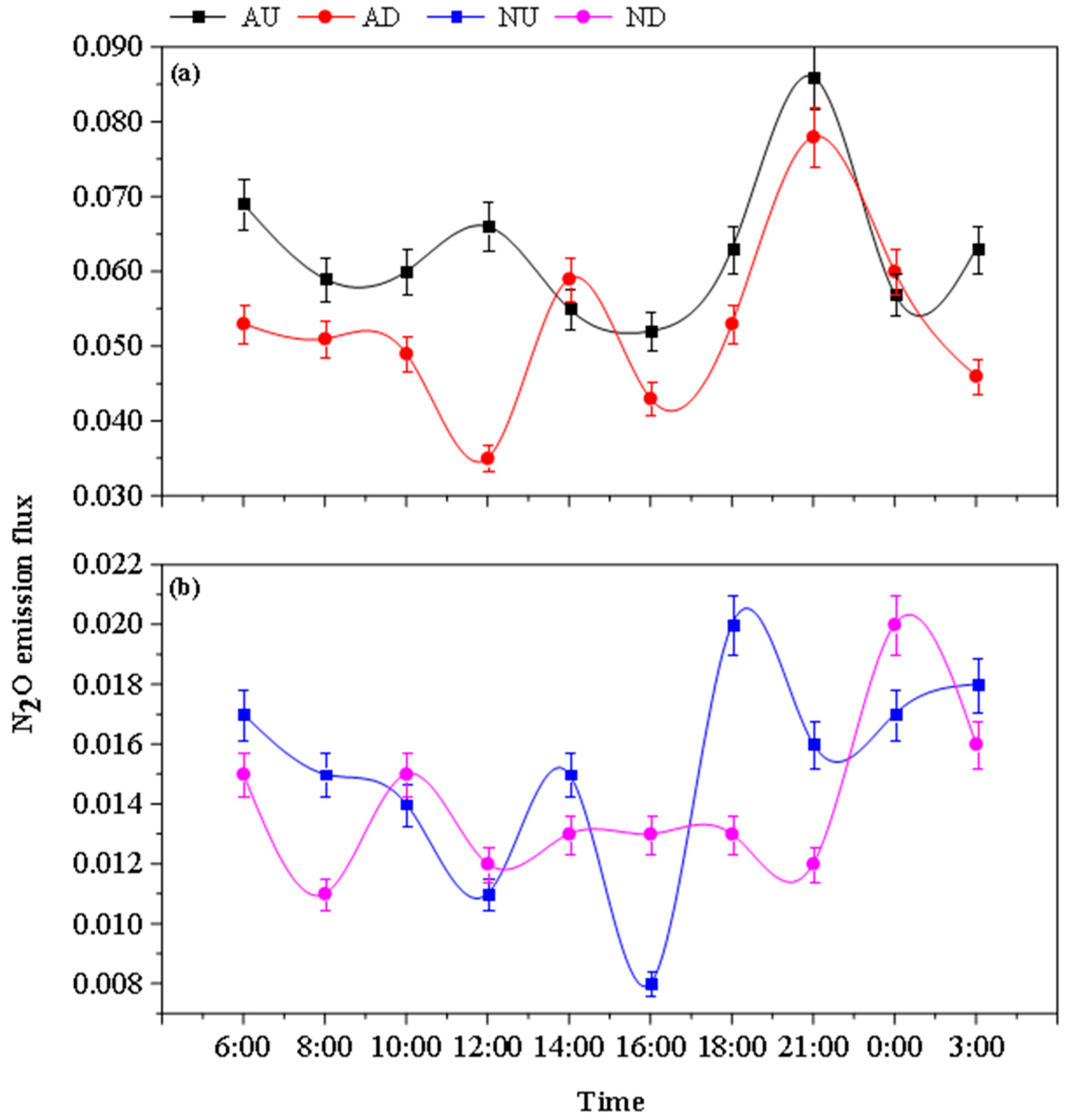
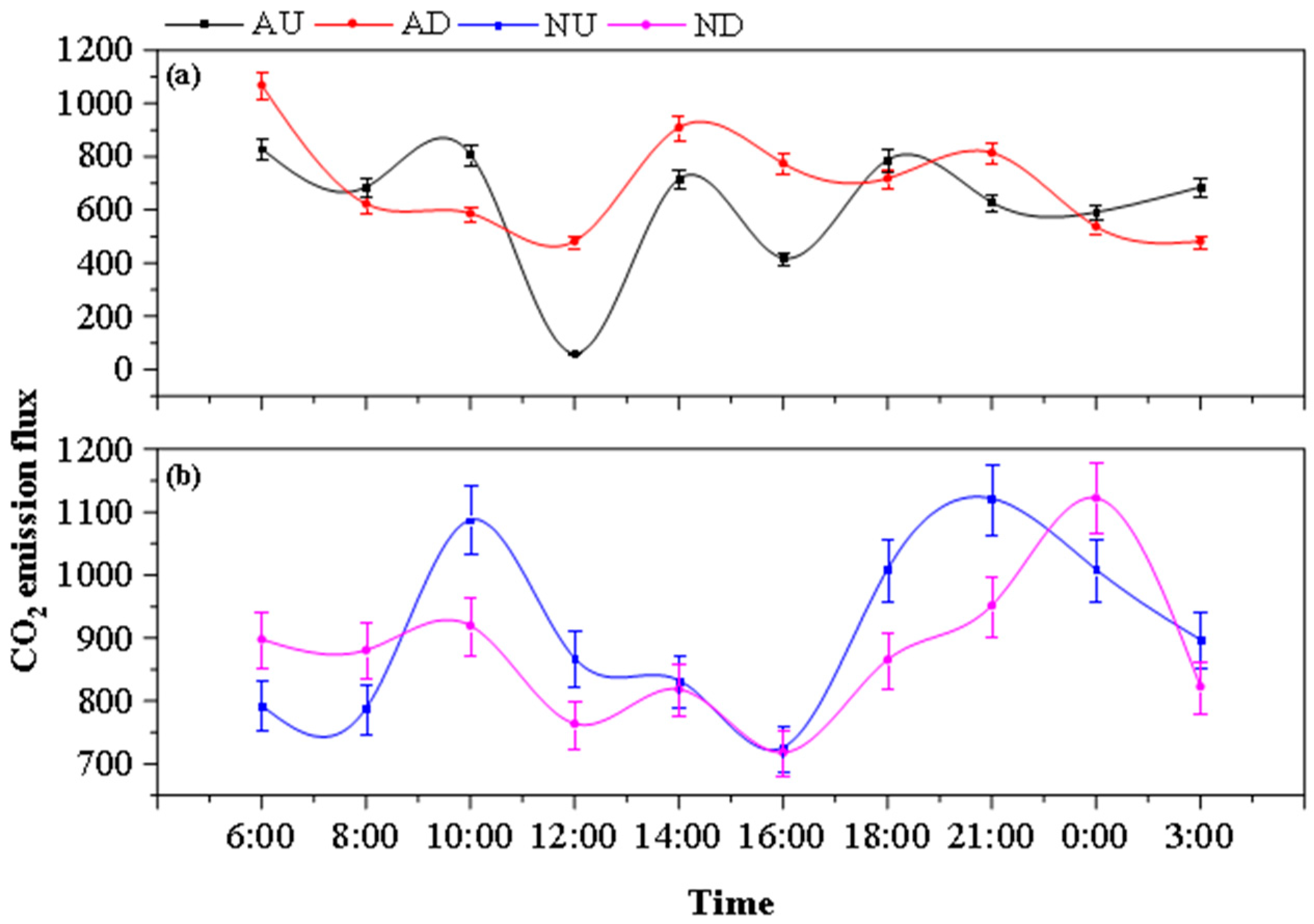
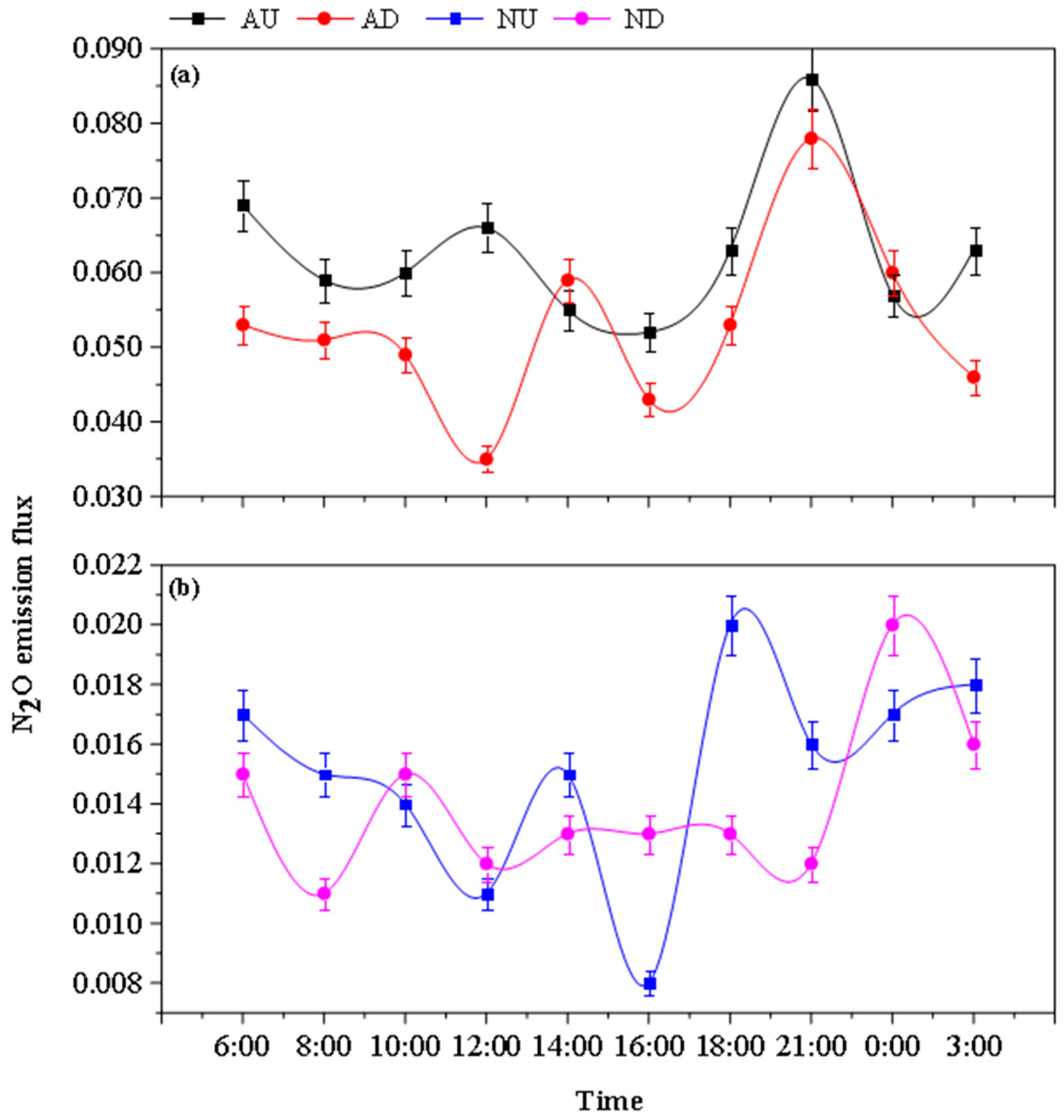
3.3. Diurnal Variation of Greenhouse Gas Emission Fluxes from Farmland Drains
4. Discussion
4.1. Effects of Aeration and Nitrogen Source Input on Physical and Chemical Properties of Drainage Water and Sediment
4.1.1. Effects of Aeration and Nitrogen Source Input on the Physical and Chemical Properties of Drainage Ditches
4.1.2. Effects of Aeration and Nitrogen Source Input on the Physical and Chemical Properties of Drainage Ditch Sediment
4.2. Effects of Aeration and Nitrogen Source Input on Greenhouse Gas Emissions in Drainage Ditches
4.2.1. Effects of Aeration and Nitrogen Source Input on N2O Emission in Drainage Ditches
4.2.2. Effects of Aeration and Nitrogen Source Input on CO2 Emissions from Drainage Ditches
4.2.3. Effects of Aeration and Nitrogen Source Input on CH4 Emissions in Drainage Ditches
4.3. Migration and Transformation of Carbon and Nitrogen in Drainage Channels and Its Impact on Greenhouse Gas Emissions
5. Conclusions
- (1)
- Aeration significantly reduced the concentration of ammoniacal nitrogen in the water and decreased the carbon and nitrogen content in the drainage ditch sediments to varying degrees. At the same time, it reduced the emissions of N2O and CH4, with a 72.39% reduction in CH4 emissions. Although there was a slight increase in CO2 emissions, the overall global warming potential was reduced by 34.02%.
- (2)
- Nitrogen input significantly raised the concentration of ammoniacal nitrogen in the water and the content of readily available nitrogen in the sediments, which had a certain suppressive effect on the emissions of N2O and CH4. However, it led to a 46.60% increase in CO2 emissions, resulting in an overall 15.24% increase in the GWP.
- (3)
- Drainage ditches play a role in reducing pollution loads, with the concentration of pollutants and the intensity of greenhouse gas emissions downstream being lower than upstream.
- (4)
- The emissions of N2O and CO2 in the paddy field drainage ditches were significantly related to the electrical conductivity of the water and the concentration of readily available nitrogen in the water, while CH4 had a significant relationship with the ORP of the water.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, N.W.; Wu, J.H.; Zhou, X.P.; Chen, Z.H.; Lu, T. Riverine N2O production, emissions and export from a region dominated by agriculture in Southeast Asia (Jiulong River). Agric. Ecosyst. Environ. 2015, 208, 37–47. [Google Scholar] [CrossRef]
- Khan, A.; Tan, D.K.Y.; Munsif, F.; Afridi, M.Z.; Shah, F.; Wei, F.; Fahad, S.; Zhou, R.Y. Nitrogen nutrition in cotton and control strategies for greenhouse gas emissions: A review. Environ. Sci. Pollut. Res. 2017, 24, 23471–23487. [Google Scholar] [CrossRef]
- Khan, A.; Tan, D.K.Y.; Afridi, M.Z.; Luo, H.H.; Tung, S.A.; Ajab, M.; Fahad, S. Nitrogen fertility and abiotic stresses management in cotton crop: A review. Environ. Sci. Pollut. Res. 2017, 24, 14551–14566. [Google Scholar] [CrossRef]
- Shou, C.G.; Du, H.S.; Liu, X.P. Research Progress of Source and Mechanism of Agricultural Non-Point Source Pollution in China. Appl. Ecol. Environ. Res. 2019, 17, 10611–10621. [Google Scholar] [CrossRef]
- Cui, N.X.; Zhang, X.; Cai, M.; Zhou, L.; Chen, G.F.; Zou, G.Y. Roles of vegetation in nutrient removal and structuring microbial communities in different types of agricultural drainage ditches for treating farmland runoff. Ecol. Eng. 2020, 155, 105941. [Google Scholar] [CrossRef]
- Jin, B.B.; Liu, X.N.; Tan, J.Y.; Shao, X.H.; Cheng, J. Effect of Plant Buffer Zone-Antifouling Curtain Wall on Reducing Non-Point Source Pollution in Paddy Fields, China. Sustainability 2022, 14, 6044. [Google Scholar] [CrossRef]
- Rosset, T.; Gandois, L.; Le Roux, G.; Teisserenc, R.; Jimenez, P.D.; Camboulive, T.; Binet, S. Peatland Contribution to Stream Organic Carbon Exports From a Montane Watershed. J. Geophys. Res.-Biogeosci. 2019, 124, 3448–3464. [Google Scholar] [CrossRef]
- Abbasi, S.A.; Dhanuja, C.; Abbasi, T. Emission of greenhouse gases from Indian wetlands: An overview. Trop. Ecol. 2021, 62, 319–328. [Google Scholar] [CrossRef]
- Shen, W.S.; Qian, D.; Xiong, R.N.; Qiu, Z.J.; Rajasekar, A. Land use intensification significantly reduced CH4 emissions while increasing N2O emissions: Taihu Lake region, China. Agric. Ecosyst. Environ. 2022, 340, 108189. [Google Scholar] [CrossRef]
- Hu, B.B.; Wang, D.Q.; Zhou, J.; Meng, W.Q.; Li, C.W.; Sun, Z.B.; Guo, X.; Wang, Z.L. Greenhouse gases emission from the sewage draining rivers. Sci. Total Environ. 2018, 612, 1454–1462. [Google Scholar] [CrossRef]
- Räsänen, T.A.; Varis, O.; Scherer, L.; Kummu, M. Greenhouse gas emissions of hydropower in the Mekong River Basin. Environ. Res. Lett. 2018, 13, 034030. [Google Scholar] [CrossRef]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 171–184. [Google Scholar] [CrossRef]
- Bastviken, D.; Tranvik, L.J.; Downing, J.A.; Crill, P.M.; Enrich-Prast, A. Freshwater Methane Emissions Offset the Continental Carbon Sink. Science 2011, 331, 50. [Google Scholar] [CrossRef]
- Butman, D.; Raymond, P.A. Significant efflux of carbon dioxide from streams and rivers in the United States. Nat. Geosci. 2011, 4, 839–842. [Google Scholar] [CrossRef]
- Neubauer, S.C. On the challenges of modeling the net radiative forcing of wetlands: Reconsidering Mitsch et al. 2013. Landsc. Ecol. 2014, 29, 571–577. [Google Scholar] [CrossRef]
- Cao, L.G.; Zhou, Z.C.; Xu, X.W.H.; Shi, F.X. Spatial and temporal variations of the greenhouse gas emissions in coastal saline wetlands in southeastern China. Environ. Sci. Pollut. Res. 2020, 27, 1118–1130. [Google Scholar] [CrossRef]
- Susilawati, H.L.; Setyanto, P.; Ariani, M.; Hervani, A.; Inubushi, K. Influence of water depth and soil amelioration on greenhouse gas emissions from peat soil columns. Soil Sci. Plant Nutr. 2016, 62, 57–68. [Google Scholar] [CrossRef][Green Version]
- Xue, D.; Chen, H.; Zhan, W.; Huang, X.Y.; He, Y.X.; Zhao, C.A.; Zhu, D.; Liu, J.L. How do water table drawdown, duration of drainage, and warming influence greenhouse gas emissions from drained peatlands of the Zoige Plateau? Land Degrad. Dev. 2021, 32, 3351–3364. [Google Scholar] [CrossRef]
- Fang, X.T.; Wang, C.; Xiao, S.Q.; Yu, K.; Zhao, J.T.; Liu, S.W.; Zou, J.W. Lower methane and nitrous oxide emissions from rice-aquaculture co-culture systems than from rice paddies in southeast China. Agric. For. Meteorol. 2023, 338, 109540. [Google Scholar] [CrossRef]
- He, H.; Li, D.D.; Pan, F.F.; Wu, Z.; Wang, F.W.; Wu, D.; Wu, S.; Yang, S.Y.; Ma, Y.H. Effects of Drainage on Greenhouse Gas Emissions and Yields of Lowland Rice-Wheat Rotation System in East China. Agronomy 2022, 12, 1932. [Google Scholar] [CrossRef]
- Li, J.K.; Liang, Z.; Gao, Z.X.; Li, Y.J. Experiment and simulation of the purification effects of multi-level series constructed wetlands on urban surface runoff. Ecol. Eng. 2016, 91, 74–84. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Roy, K.S.; Neogi, S.; Adhya, T.K.; Rao, K.S.; Manna, M.C. Effects of rice straw and nitrogen fertilization on greenhouse gas emissions and carbon storage in tropical flooded soil planted with rice. Soil Tillage Res. 2012, 124, 119–130. [Google Scholar] [CrossRef]
- Tan, L.S.; Ge, Z.M.; Zhou, X.H.; Li, S.H.; Li, X.Z.; Tang, J.W. Conversion of coastal wetlands, riparian wetlands, and peatlands increases greenhouse gas emissions: A global meta-analysis. Glob. Chang. Biol. 2020, 26, 1638–1653. [Google Scholar] [CrossRef]
- Xiao, Q.T.; Hu, C.; Gu, X.H.; Zeng, Q.F.; Liu, Z.J.; Xiao, W.; Zhang, M.; Hu, Z.H.; Wang, W.; Luo, J.H.; et al. Aquaculture farm largely increase indirect nitrous oxide emission factors of lake. Agric. Ecosyst. Environ. 2023, 341, 108212. [Google Scholar] [CrossRef]
- Gogoi, B.; Baruah, K.K. Nitrous Oxide Emissions from Fields with Different Wheat and Rice Varieties. Pedosphere 2012, 22, 112–121. [Google Scholar] [CrossRef]
- Neue, H.U.; Wassmann, R.; Kludze, H.K.; Bujun, W.; Lantin, R.S. Factors and processes controlling methane emissions from rice fields. Nutr. Cycl. Agroecosystems 1997, 49, 111–117. [Google Scholar] [CrossRef]
- Fang, X.T.; Wang, C.; Zhang, T.R.; Zheng, F.W.; Zhao, J.T.; Wu, S.; Barthel, M.; Six, J.; Zou, J.W.; Liu, S.W. Ebullitive CH4 flux and its mitigation potential by aeration in freshwater aquaculture: Measurements and global data synthesis. Agric. Ecosyst. Environ. 2022, 335, 108016. [Google Scholar] [CrossRef]
- Peacock, M.; Granath, G.; Wallin, M.B.; Hogbom, L.; Futter, M.N. Significant Emissions From Forest Drainage Ditches-An Unaccounted Term in Anthropogenic Greenhouse Gas Inventories? J. Geophys. Res.-Biogeosci. 2021, 126, e2021JG006478. [Google Scholar] [CrossRef]
- Wu, J.W.; Zhang, Q.S.; Guo, C.Y.; Li, Q.K.; Hu, Y.W.; Jiang, X.M.; Zhao, Y.C.; Wang, J.; Zhao, Q. Effects of Aeration on Pollution Load and Greenhouse Gas Emissions from Agricultural Drainage Ditches. Water 2022, 14, 3783. [Google Scholar] [CrossRef]
- Muñoz-Leoz, B.; Antigüedad, I.; Garbisu, C.; Ruiz-Romera, E. Nitrogen transformations and greenhouse gas emissions from a riparian wetland soil: An undisturbed soil column study. Sci. Total Environ. 2011, 409, 763–770. [Google Scholar] [CrossRef]
- Boateng, K.K.; Obeng, G.Y.; Mensah, E. Eco-Friendly Yield and Greenhouse Gas Emissions as Affected by Fertilization Type in a Tropical Smallholder Rice System, Ghana. Sustainability 2020, 12, 10239. [Google Scholar] [CrossRef]
- Haney, R.L.; Franzluebbers, A.J.; Hons, F.M.; Zuberer, D.A. Soil C extracted with water or K2SO4: pH effect on determination of microbial biomass. Can. J. Soil Sci. 1999, 79, 529–533. [Google Scholar] [CrossRef]
- Dedkov, Y.M.; Elizarova, O.V.; Kel’ina, S.Y. Dichromate method for the determination of chemical oxygen demand. J. Anal. Chem. 2000, 55, 777–781. [Google Scholar] [CrossRef]
- Zhang, M.X.; Guo, W.Q.; Chen, Y.Y.; He, D.C.; Isaev, A.B.; Zhu, M.S. Dissolved oxygen in aeration-driven piezo-catalytic for antibiotics pollutants removal in water. Chin. Chem. Lett. 2023, 34, 108229. [Google Scholar] [CrossRef]
- Kim, D.J.; Ahn, D.H.; Lee, D.I. Effects of free ammonia and dissolved oxygen on nitrification and nitrite accumulation in a biofilm airlift reactor. Korean J. Chem. Eng. 2005, 22, 85–90. [Google Scholar] [CrossRef]
- Bock, E.; Schmidt, I.; Stuven, R.; Zart, D. Nitrogen Loss Caused by Denitrifying Nitrosomonas Cells Using Ammonium or Hydrogen as Electron-Donors and Nitrite as Electron-Acceptor. Arch. Microbiol. 1995, 163, 16–20. [Google Scholar] [CrossRef]
- Miao, Y.Y.; Zhang, L.; Li, B.K.; Zhang, Q.; Wang, S.M.; Peng, Y.Z. Enhancing ammonium oxidizing bacteria activity was key to single-stage partial nitrification-anammox system treating low-strength sewage under intermittent aeration condition. Bioresour. Technol. 2017, 231, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. The nitrogen cascade. Bioscience 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Austin, D.; Vazquez-Burney, R.; Dyke, G.; King, T. Nitrification and total nitrogen removal in a super-oxygenated wetland. Sci. Total Environ. 2019, 652, 307–313. [Google Scholar] [CrossRef]
- Hu, B.; Lu, J.Y.; Qin, Y.X.; Zhou, M.; Tan, Y.; Wu, P.; Zhao, J.Q. A critical review of heterotrophic nitrification and aerobic denitrification process: Influencing factors and mechanisms. J. Water Process Eng. 2023, 54, 103995. [Google Scholar] [CrossRef]
- Wang, H.B.; Li, Y.; Cheng, G.X.; Wu, W.; Zhang, Y.H.; Li, X.Y. Electrochemical Investigation of Corrosion of Mild Steel in NH4Cl Solution. Int. J. Electrochem. Sci. 2018, 13, 5268–5283. [Google Scholar] [CrossRef]
- Hanson, B.; May, D. Effect of subsurface drip irrigation on processing tomato yield, water table depth, soil salinity, and profitability. Agric. Water Manag. 2004, 68, 1–17. [Google Scholar] [CrossRef]
- Tang, X.Q.; Huang, S.L.; Scholz, M. Nutrient Removal in Wetlands During Intermittent Artificial Aeration. Environ. Eng. Sci. 2008, 25, 1279–1290. [Google Scholar] [CrossRef]
- Simek, M. Nitrification in soil—Terminology and methodology (review). Rostl. Vyrob. 2000, 46, 385–395. [Google Scholar]
- Wareham, D.G.; Hall, K.J.; Mavinic, D.S. Real-Time Control of Waste-Water Treatment Systems Using ORP. Water Sci. Technol. 1993, 28, 273–282. [Google Scholar] [CrossRef]
- El-Naggar, M.M. Effects of Cl−, NO3− and SO42− anions on the anodic behavior of carbon steel in deaerated 0.50 M NaHCO3 solutions. Appl. Surf. Sci. 2006, 252, 6179–6194. [Google Scholar] [CrossRef]
- Qian, Z.Z.; Zhuang, S.Y.; Gao, J.S.; Tang, L.Z. Can aeration improve bamboo soil fertility of soil below bamboo and fungal diversity under mulching conditions? Land Degrad. Dev. 2022, 33, 2353–2365. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Jia, H.Z.; Zhao, H.R.; Zhang, R.; Zhang, C.; Zhu, K.C.; Guo, X.T.; Wang, T.C.; Zhu, L.Y. Oxygen Limitation Accelerates Regeneration of Active Sites on a MnO2 Surface: Promoting Transformation of Organic Matter and Carbon Preservation. Environ. Sci. Technol. 2022, 56, 9806–9815. [Google Scholar] [CrossRef]
- Liu, W.X.; Jiang, L.; Hu, S.J.; Li, L.H.; Liu, L.L.; Wan, S.Q. Decoupling of soil microbes and plants with increasing anthropogenic nitrogen inputs in a temperate steppe. Soil Biol. Biochem. 2014, 72, 116–122. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Du, X.Q.; Feng, J.; Fang, M.; Ye, X.Y. Sources, Influencing Factors, and Pollution Process of Inorganic Nitrogen in Shallow Groundwater of a Typical Agricultural Area in Northeast China. Water 2020, 12, 3292. [Google Scholar] [CrossRef]
- Ma, J.; Ma, E.D.; Xu, H.; Yagi, K.; Cai, Z.C. Wheat straw management affects CH4 and N2O emissions from rice fields. Soil Biol. Biochem. 2009, 41, 1022–1028. [Google Scholar] [CrossRef]
- Gaihre, Y.K.; Wassmann, R.; Tirol-Padre, A.; Villegas-Pangga, G.; Aquino, E.; Kimball, B.A. Seasonal assessment of greenhouse gas emissions from irrigated lowland rice fields under infrared warming. Agric. Ecosyst. Environ. 2014, 184, 88–100. [Google Scholar] [CrossRef]
- Simek, M.; Jisova, L.; Hopkins, D.W. What is the so-called optimum pH for denitrification in soil? Soil Biol. Biochem. 2002, 34, 1227–1234. [Google Scholar] [CrossRef]
- Carreira, C.; Nunes, R.F.; Mestre, O.; Moura, I.; Pauleta, S.R. The effect of pH on Marinobacter hydrocarbonoclasticus denitrification pathway and nitrous oxide reductase. J. Biol. Inorg. Chem. 2020, 25, 927–940. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, L.F.; Wang, J.S.; Tang, H.Q.; Qu, Z.; Zhu, T.B. Effects of warming and nitrogen input on soil N2O emission from Qinghai-Tibetan Plateau: A synthesis. Agric. For. Meteorol. 2022, 326, 109167. [Google Scholar] [CrossRef]
- Yu, H.Y.; Wang, T.Y.; Huang, Q.; Song, K.F.; Zhang, G.B.; Ma, J.; Xu, H. Effects of elevated CO2 concentration on CH4 and N2O emissions from paddy fields: A meta-analysis. Sci. China-Earth Sci. 2022, 65, 96–106. [Google Scholar] [CrossRef]
- Maltais-Landry, G.; Maranger, R.; Brisson, J.; Chazarenc, F. Greenhouse gas production and efficiency of planted and artificially aerated constructed wetlands. Environ. Pollut. 2009, 157, 748–754. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Almeida, F.; Rocha, F.A.; Ferreira, A. Improving CO2 mass transfer in microalgal cultures using an oscillatory flow reactor with smooth periodic constrictions. J. Environ. Chem. Eng. 2021, 9, 106505. [Google Scholar] [CrossRef]
- Daims, H.; Lücker, S.; Wagner, M. A New Perspective on Microbes Formerly Known as Nitrite-Oxidizing Bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef]
- Xu, Q.; Dai, L.X.; Shang, Z.Y.; Zhou, Y.; Li, J.Y.; Dou, Z.; Yuan, X.C.; Gao, H. Application of controlled-release urea to maintain rice yield and mitigate greenhouse gas emissions of rice-crayfish coculture field. Agric. Ecosyst. Environ. 2023, 344, 108312. [Google Scholar] [CrossRef]
- Cha-Un, N.; Chidthaisong, A.; Yagi, K.; Sud, S.; Towprayoon, S. Greenhouse gas emissions, soil carbon sequestration and crop yields in a rain-fed rice field with crop rotation management. Agric. Ecosyst. Environ. 2017, 237, 109–120. [Google Scholar] [CrossRef]
- Schnürer, A.; Nordberg, Å. Ammonia, a selective agent for methane production by syntrophic acetate oxidation at mesophilic temperature. Water Sci. Technol. 2008, 57, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Shangguan, X.; Pollard, D.; Barron, E.J. Simulating methane emission from a Chinese rice field as influenced by fertilizer and water level. Hydrol. Process. 2003, 17, 3485–3501. [Google Scholar] [CrossRef]
- Boon, P.I.; Mitchell, A. Methanogenesis in the Sediments of an Australian Fresh-Water Wetland—Comparison with Aerobic Decay, and Factors Controlling Methanogenesis. Fems Microbiol. Ecol. 1995, 18, 175–190. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wu, D.F.; Mao, X.M.; Hou, J.M.; Wang, L.; Han, Y.P. Estimating soil ammonium adsorption using pedotransfer functions in an irrigation district of the North China Plain. Pedosphere 2021, 31, 157–171. [Google Scholar] [CrossRef]
- Zeglin, L.H. Stream microbial diversity in response to environmental changes: Review and synthesis of existing research. Front. Microbiol. 2015, 6, 454. [Google Scholar] [CrossRef]
- Battin, T.J.; Kaplan, L.A.; Findlay, S.; Hopkinson, C.S.; Marti, E.; Packman, A.I.; Newbold, J.D.; Sabater, F. Biophysical controls on organic carbon fluxes in fluvial networks. Nat. Geosci. 2008, 1, 95–100. [Google Scholar] [CrossRef]
- Haag, D.; Kaupenjohann, M. Landscape fate of nitrate fluxes and emissions in Central Europe—A critical review of concepts, data, and models for transport and retention. Agric. Ecosyst. Environ. 2001, 86, 1–21. [Google Scholar] [CrossRef]
| Type of Gas | Emission Flux (mg/m2·d) | GWP (mg/m2·d) | ||||||
|---|---|---|---|---|---|---|---|---|
| AU | AD | NU | ND | AU | AD | NU | ND | |
| N2O | 1.53 | 1.29 | 0.37 | 0.34 | 456.54 | 384.72 | 110.71 | 101.92 |
| CO2 | 15,142.45 | 16,720.54 | 22,198.96 | 21,304.75 | 15,142.45 | 16,720.54 | 22,198.96 | 21,304.75 |
| CH4 | 719.14 | 201.95 | 655.44 | 547.03 | 17,978.50 | 5048.63 | 16,385.88 | 13,675.75 |
| The total | 33,577.49 | 22,153.88 | 38,695.54 | 35,082.42 | ||||
| Physicochemical Properties of Soil and Water | Index | N2O Emission | CO2 Emission | CH4 Emission |
|---|---|---|---|---|
| Soil organic carbon content (g/kg) | Pearson correlation | 0.088 | −0.065 | 0.940 ### |
| Significance | 0.912 | 0.935 | 0.060 | |
| Soil ammonium nitrogen content (mg/kg) | Pearson correlation | −0.371 # | 0.417 # | 0.893 ### |
| Significance | 0.629 | 0.583 | 0.107 | |
| Soil nitrate nitrogen content (mg/kg) | Pearson correlation | −0.640 ## | 0.727 ## | 0.377 # |
| Significance | 0.360 | 0.273 | 0.623 | |
| Soil MCB content (mg/kg) | Pearson correlation | −0.354 # | 0.433# | 0.726 ## |
| Significance | 0.646 | 0.567 | 0.274 | |
| Soil DOC content (mg/kg) | Pearson correlation | −0.197 | 0.287 | 0.676 ## |
| Significance | 0.803 | 0.713 | 0.324 | |
| Conductivity of water (μs/cm) | Pearson correlation | −0.986 *### | 0.980 *### | 0.355# |
| Significance | 0.014 | 0.020 | 0.645 | |
| Dissolved oxygen in water (mg/L) | Pearson correlation | 0.933 ### | −0.919 ### | −0.535 ## |
| Significance | 0.067 | 0.081 | 0.465 | |
| ORP (mv) | Pearson correlation | 0.316 # | −0.283 | −0.969 *### |
| Significance | 0.684 | 0.717 | ·0.031 | |
| Nitrate nitrogen content in water (mg/L) | Pearson correlation | 0.973 *### | −0.961 *### | −0.417 # |
| Significance | 0.027 | 0.039 | 0.583 | |
| Ammonium nitrogen content in water (mg/L) | Pearson correlation | −0.986 *### | 0.976 *### | 0.361 # |
| Significance | 0.014 | 0.024 | 0.639 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Wu, J.; Guo, C.; Wang, J.; Zhao, Y.; Li, Q.; Hu, Y. Effects of Nitrogen Input and Aeration on Greenhouse Gas Emissions and Pollutants in Agricultural Drainage Ditches. Agronomy 2024, 14, 235. https://doi.org/10.3390/agronomy14020235
Zhang Q, Wu J, Guo C, Wang J, Zhao Y, Li Q, Hu Y. Effects of Nitrogen Input and Aeration on Greenhouse Gas Emissions and Pollutants in Agricultural Drainage Ditches. Agronomy. 2024; 14(2):235. https://doi.org/10.3390/agronomy14020235
Chicago/Turabian StyleZhang, Qisen, Jingwei Wu, Chenyao Guo, Jing Wang, Yanchao Zhao, Qiangkun Li, and Yawei Hu. 2024. "Effects of Nitrogen Input and Aeration on Greenhouse Gas Emissions and Pollutants in Agricultural Drainage Ditches" Agronomy 14, no. 2: 235. https://doi.org/10.3390/agronomy14020235
APA StyleZhang, Q., Wu, J., Guo, C., Wang, J., Zhao, Y., Li, Q., & Hu, Y. (2024). Effects of Nitrogen Input and Aeration on Greenhouse Gas Emissions and Pollutants in Agricultural Drainage Ditches. Agronomy, 14(2), 235. https://doi.org/10.3390/agronomy14020235







