Abstract
Cryopreservation protocols have not yet been defined for seeds of some Coffea species due to their high sensitivity to desiccation and to ultra-low temperatures, factors that lead to low seed survival rates after warming. This reduced rate occurs due to several factors that compromise cellular integrity during the steps of the process. In this study, we evaluated the effect of moisture content and the types of packaging on the cryopreservation of Coffea racemosa and Coffea liberica var dewevrei seeds. For that purpose, seeds were dried to moisture contents of 18, 20, and 25% (dry basis—db) for C. racemosa and to contents of 20, 25, 30, and 35% for C. liberica. The seeds were then packed in mesh bags, Falcon tubes, and triple-laminated aluminum foil pouches before being directly immersed in liquid nitrogen. Physiological and biochemical analyses were performed on the seeds. There was interaction between moisture content and the packaging of the seeds of the species. Drying the seeds to a 20% moisture content and packing them in triple-laminated aluminum foil pouches provided the best cryopreservation protocol among those studied, with survival rates of 79 and 8% for C. racemosa and C. liberica, respectively.
1. Introduction
Coffee, a globally significant crop, is the most important and popular beverage in the world, with an estimated 2.25 billion cups consumed daily [1]. The coffee industry is a major contributor to the global economy, with an estimated value of over USD 100 billion, and Brazil is the largest producer [2]. The Coffea spp. genus includes 130 species, which are native to the African continent, Madagascar, and the Mascarene Islands [3]. Two species are widely grown in the world: Coffea arabica L. and Coffea canephora Pierre. Some species, although they have a reduced production scale, hold high value as a source of genetic variability for breeding programs. For instance, Coffea liberica var dewevrei, originating from Liberia, Surinam, and Malaysia, exhibits resistance to leaf rust and tolerance to water deficits [4]. Similarly, Coffea racemosa, native to Mozambique [5,6], demonstrates resistance to leaf miners and tolerance to water deficits [7].
Despite the importance of coffee for world agriculture, the genetic diversity of Coffea spp. is constantly at risk from weather conditions and genetic erosion because the germplasm is maintained in plant collections in the field. Furthermore, research shows that seeds of the Coffea genus species have low longevity and do not survive when stored in conventional seed banks, where they are kept at 5 ± 2% moisture and at temperatures as low as −18 °C [8]. Thus, cryopreservation is an ideal strategy for the long-term conservation of germplasm since ultra-low temperatures reduce cell metabolism to a level that stops tissue development, allowing regeneration when ambient temperatures are restored [9,10]. Additionally, the method allows for the accessions of different species to be stored in a small space with minimal maintenance.
Seed moisture content is the most important factor in the cryopreservation process, as it is decisive for maintaining viability after freezing and thawing [11,12]. However, seeds of the Coffea species are sensitive to desiccation, and their viability decreases when cryopreserved with high levels of moisture, which results in intracellular ice formation, or with low levels of moisture, which results in physical stresses affecting cells [13]. Another factor that can also affect the effectiveness of cryopreservation protocols is the type of packaging used to expose seeds to liquid nitrogen (LN). The direct contact of the water undergoing warming in a water bath with the seeds may affect the warming rate of the cryopreserved seeds, with direct consequences for viability [14]. Processes of cooling during seed immersion and of the warming of seeds should not allow cells to pass through the temperature of ice crystal formation, which can range from −15 to −60 °C [15].
Despite their importance, advancements in cryopreservation protocols for C. racemosa and C. liberica seed species have stagnated, creating an urgent need for updated methodologies [8,16,17,18]. Considering the importance of these species for coffee breeding programs, the aim of this study was to deepen knowledge regarding the effects of seed moisture and packaging on the response of seeds when undergoing cryopreservation. Thus, we hope to contribute to establishing an effective protocol for the successful storage of C. racemosa and C. liberica var dewevrei seeds.
2. Materials and Methods
2.1. Fruit Harvest and Processing
Fruit samples of the species C. racemosa and C. liberica var dewevrei were harvested from coffee plants of the Coffea Germplasm Bank of the Instituto Agronômico (IAC) in Campinas, SP, Brazil, in the 2019/2020 crop year. Fruits were harvested at the coffee berry maturation stage after being selectively chosen from the middle branches of the plants and from the middle parts of the branches. The berries were washed to remove misshapen and insect-damaged fruit, as well as impurities. After the harvest, the fruit was mechanically pulped and mucilage was removed from the seeds via fermentation in water for 48 h at an ambient temperature. After that, they were pre-dried in the shade to remove surface moisture. To ensure batch uniformity, the seeds were sieved, and the sieves with the highest proportions of retained seeds were selected. For C. racemosa, circular sieves no. 14/64 and 13/64 were used, while for C. liberica var. dewevrei, sieves no. 18/64 and 17/64 were employed. For both species, these freshly harvested seeds were used as control treatments.
Seed moisture content was determined using two replicates of ten seeds each, dried in a laboratory oven (FANEM®, São Paulo, Brazil) at 105 °C for 24 h, in accordance with the Rules for Seed Testing (RST) [19]. The germination was assessed following THE RST [19], while seed viability was evaluated using the tetrazolium test [20], as described in Section 2.2.
2.2. Seed Drying Protocol
Independent experiments were carried out for each species, and 4 replicates of 25 seeds were used for each experiment. For drying, the seeds were placed in a single layer on metal screens in transparent boxes (gerbox) that contained 80 mg of activated silica gel (NEON®, São Paulo, Brazil) below the screens. Silica gel was replaced on a daily basis during drying. The gerboxes were sealed and kept in B.O.D.-type germination chambers (EletroLab®, São Paulo, Brazil) at a constant temperature of 25 °C. Water loss during drying was monitored by continual weighing on a precision scale (0.001 g) until the seeds reached moisture contents of 18, 20, and 25% ± 2.2% dry basis (db) for C. racemosa and 20, 25, 30, and 35% ± 2.2% db for C. liberica var dewevrei. The specific moisture content was selected based on the cryopreservation protocol for the species Coffea arabica, which used a moisture content of 20% [21,22,23], and based on pre-tests carried out. Studies on seed desiccation in the genus Coffea [18] supported the choice of an 18% moisture content for the species C. racemosa, known for its higher desiccation tolerance. Additionally, a higher moisture of 25% was tested as a potential alternative for better seed viability. On the other hand, C. liberica, which is more sensitive to desiccation, higher moistures of 25%, 30%, and 35% were tested. After drying, the seeds’ moisture content and physiological quality were evaluated, as previously described.
2.3. Cryopreservation Procedures
Independent experiments were carried out for each species. Seeds, were dried with parchment, which corresponding to the endocarp of the fruit, and were placed in three types of packaging (Figure 1B): mesh-type fabric bags [21,22,23], three folded sheets of aluminum foil [24,25], and Falcon-type tubes(TPP®, Trasadingen, Switzerland). Based on the fact that the seeds of the species C. racemosa and C. liberica could be more sensitive to cryopreservation, the Falcon tubes (25 mL) were chosen because they allow greater protection of the seeds during cooling. They were then immersed directly into the cryotank containing liquid nitrogen (LN) and subjected to ultrafast cooling at approximately −200 °C/min [18].
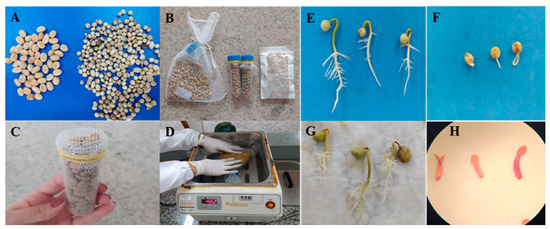
Figure 1.
The characterization of the experiment. (A) Seeds of C. liberica (left) and of C. racemosa (right); (B) types of packaging: mesh fabric bag, Falcon tube, and triple-laminated aluminum foil pouch; (C) Falcon-type tube with a mesh fabric bag to allow water to enter in the rewarming of the cryopreserved seeds; (D) the rewarming of the seeds in a water bath at 40 °C/2 min; (E) normal C. racemosa seedlings; (F) abnormal C. racemosa seedlings; (G) normal C. liberica seedlings; (H) zygotic embryos of C. liberica stained with tetrazolium salt.
2.4. Rewarming Protocol
After 24 h in the cryotank, the packages were removed and immediately immersed in a water bath at 40 °C (Figure 1D) for 2 min [17]. For mesh-type fabric bags, the seeds were placed in direct contact with the water during rewarming [21,22,23]. In order to achieve the same heating speed for all treatments, in the Falcon tube, the cap was removed to allow hot water to enter the container (Figure 1C). As for the aluminum packaging, the water partially penetrated the packaging through the side openings. After reheating, the seeds were superficially dried with paper towels and their parchments were manually removed for physiological evaluation and isoenzyme analyses.
2.5. Physiological Assessments
Independent experiments were carried out for each species. Physiological quality was evaluated using the germination test, with 4 replications of 25 seeds created on sheets of germination paper. They were moistened with water in the amount of 2.5 times the weight of the dry paper [19]. The seeds were kept in a germinator at a constant temperature of 30 °C in order to evaluate the percentage of normal seedlings (NSs) at 14 days for C. racemosa and at 30 days for C. liberica var dewevrei (Figure 1E–G), working according to preliminary tests. During the germination test, the percentage of root protrusion (RP) was evaluated at 8 days for C. racemosa and at 15 days for C. liberica var dewevrei, working according to preliminary tests. At the end of the germination test, the percentages of seedlings with expanded cotyledonary leaves (SECLs) and seedling dry matter (SDM) were evaluated at 30 days after sowing C. racemose, working according to preliminary tests, and at 45 days for C. liberica var dewevrei, working according to the research of Figueiredo et al. [22]. To determine dry matter, the SECLs were placed in Kraft paper bags, placed in a forced air circulation oven at 60 °C for five days, and weighed. The results were expressed in mg per seedling.
Seed viability (SV) was determined using the tetrazolium test with 4 replications of 10 seeds soaked in distilled water for 48 h at 30 °C [20]. The embryos were removed using a scalpel and immersed in a 0.5% tetrazolium solution, in the dark, for a period of three hours at 30 °C. The results were expressed as a percentage of viable seeds, which were characterized by the location and extent of the staining brought about by reaction with tetrazolium (Figure 1H).
2.6. Isoenzyme Analyses
The seeds were ground in LN along with polyvinylpyrrolidone, and the powder was stored at −86 °C up to the time of analysis. The isoenzymes glutamic oxaloacetic transaminase (GOT), peroxidase (PO), esterase (EST), and catalase (CAT) were evaluated following the protocols proposed by Alfenas [26]. For each isoenzyme, interpretation of the results was determined based on the qualitative analysis of the presence/absence and the intensity of each band studied in polyacrylamide gel.
2.7. Experimental Design and Statistical Analyses
A completely randomized design was used throughout the study. Initially, seed moisture levels were evaluated. For C. racemosa, three moisture contents (18, 20, and 25%) and a control treatment (freshly harvested seeds) were used; for C. liberica, four moisture contents (20, 25, 30, and 35%) and a control treatment were used. After cryopreservation, a factorial scheme was used for both species. For C. racemosa, a 3 × 3 factorial arrangement was used, with three moisture contents and three types of packaging (mesh type fabric bags, Falcon-type tubes, and three folded sheets of aluminum foil). For C. liberica, a 4 × 3 factorial arrangement was used, employing the four moisture contents and the same three types of packaging mentioned above. Overall, 4 replicates of 25 seeds were used for each experiment for both species. The normality of the data was verified using the Shapiro–Wilk test, and, when necessary, data were transformed into or . Data were submitted for analysis of variance using R statistical software, version 4.3.1 [27], and averages were compared using the Scott Knott test, considering p < 0.05. Results of isoenzyme analyses were interpreted, considering the presence/absence and intensity of the bands seen on the gels analyzed for each enzyme.
3. Results and Discussion
3.1. Physiological Responses to Drying
Seed drying curves are shown in Figure 2, and the moisture contents of C. racemosa and C. liberica var dewevrei seeds after drying in silica gel are shown in Table 1 and Table 2. The initial seed moisture contents were 96 and 72% for C. racemosa and C. liberica, respectively. For C. racemosa seeds, it took 52 h to reach the lowest moisture content of 17.3%. Meanwhile, for C. liberica seeds, it took 71 h to reach 18.5% moisture content (Figure 2).
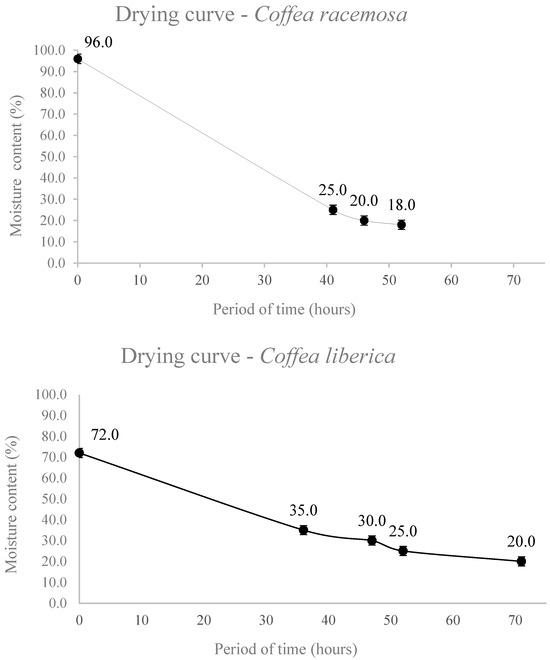
Figure 2.
Drying curve of seeds of C. racemosa and C. liberica.

Table 1.
Physiological quality of C. racemosa seeds with different moisture content levels.

Table 2.
Physiological quality of C. liberica seeds with different moisture content levels.
The physiological performance of seeds after drying is strongly influenced by the rate of water removal [25]. Desiccation-sensitive seeds can survive at lower moisture levels when dried rapidly, as was performed in the present study using silica gel. This accelerated process minimizes the time during which damage can occur, enhancing seed viability [13].
The removal of water from the seeds for the purpose of cryopreservation is a key step since the free water present in the cells can lead to the formation of ice crystals, which ruptures cell membranes. Therefore, moisture content should be ideal for cryopreservation, that is, it should not be high enough to form ice crystals nor low enough to cause mechanical damage to the cells due to the collapse of vacuoles and macromolecular structures [28]. According to Pammenter, Vertucci, and Berjak [29], the threshold of water loss that seeds or parts of seeds can tolerate without the total loss of viability may coincide with the level of intracellular water that does not freeze, something which varies among species.
The physiological quality of C. racemosa seeds and C. liberica seeds was assessed in freshly harvested seeds, as a control treatment, and after undergoing drying in silica gel. There was no significant difference in moisture content and control treatment for C. racemosa in RP and SV. The overall averages were 95 and 89%, respectively, for these characteristics. However, these high percentages were not maintained over seedling development for seeds dried to 25% or 18% moisture. At these moisture contents, lower percentages of NS, SECL, and SDM were observed compared to control treatment and seeds dried to 20%. Therefore, there was no loss of initial quality for C. racemosa seeds when dried at 20% moisture as the percentage of NS was not significantly different to that of the control treatment (Table 1).
For C. liberica seeds, there was a significant difference between all the moisture contents studied when compared to control treatment. However, this was only observed for SV (Table 2). The lowest percentages of SV were observed at moisture contents of 20% and 35%. This was significantly different from what was seen with the control treatment (Table 2). C. liberica seeds have a higher degree of recalcitrance than C. racemosa seeds [18,30], making water removal a more delicate process.
The moisture content of seeds before immersion in liquid nitrogen (LN) is one of the key factors in ensuring the preservation of seed quality. Removing water from seeds through reduction in relative humidity using silica gel has proven to be a safe method for coffee cryopreservation [17,21,22,23,24,25]. In this study, the drying process was effective, as both species across all treatments achieved NS rates exceeding 74%, a level which is considered high. However, for C. racemosa seeds, a moisture content of 20% resulted in the highest average of NS (91%) compared to moisture levels of 18% and 25% (74% and 81%, respectively; this is seen in Table 1). This trend was not observed in C. liberica seeds, where no significant differences were detected among the different moisture levels (Table 2).
The ability of plant tissues to survive below-zero (°C) temperatures depends on their tolerance not only to temperature, but also to desiccation. Many species have limits for these tolerance levels [31], which makes it necessary to know the degree of tolerance to drying along with the response of the tissues to exposure to below-zero temperatures.
3.2. Effects of Cryopreserved and Type of Packaging on Seeds Dried at Different Moisture Contents
The results of the physiological quality of C. racemosa seeds after cryopreservation show the significant interaction between the moisture content and type of packaging (Figure 3). At a moisture content of 18%, the cryopreserved seeds of C. racemosa showed a reduction in germination of less than 20%, regardless of the type of packaging (Figure 3B). At the same moisture content of the cryopreserved seeds of C. racemosa, regardless of the type of packaging, it was also observed that there were significant reductions of less than 50%, 20% and 100 mg for the variables of RP, SECL and SDM, respectively (Figure 3A,C,D). As for SV, there were reductions of less than 40% for the mMesh fabric bags and Falcon tubes packaging when the cryopreserved seeds of C. racemosa were dried at 18 and 25% (Figure 3D). These results suggest that there was a combination of drying at 18% moisture content with greater endosperm sensitivity to LN. The latter result was also observed by Dussert et al. [18], Coelho et al. [21,25], and Figueiredo et al. [22,23].
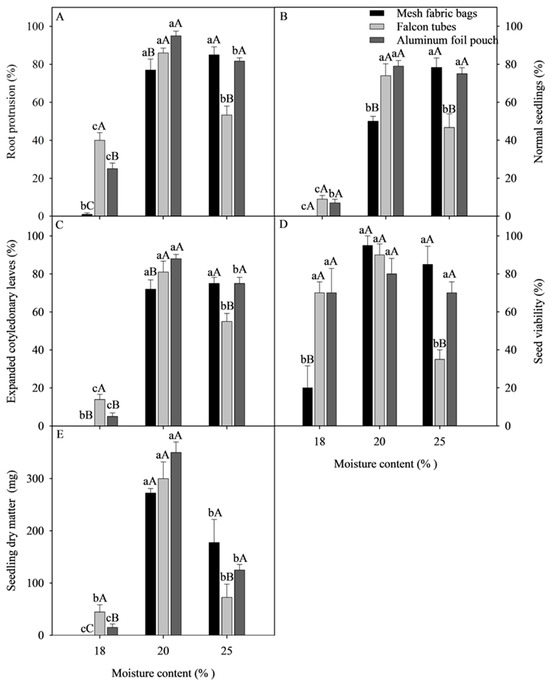
Figure 3.
The effect of the moisture content and type of packaging on the quality of cryopreserved C. racemosa seeds. (A) Root protrusion; (B) normal seedlings; (C) seedlings with expanded cotyledonary leaves; (D) seed viability; (E) seedling dry matter. Averages ± standard error (n = 4). Averages ± standard error (n = 4). Averages followed by the same lowercase letters compare seed moisture content, and capital letters compare packaging types that are not grouped together by the Scott Knott test (p < 0.05). Seedling dry matter data were transformed into √x.
When cryopreserved seeds of C. racemosa were stored in aluminum foil pouch and in Falcon tubes with 20% moisture content, the percentages of RP, NS, and SECL were higher than 70%, differing from the mesh fabric bag packaging (Figure 3A–C). For the variables of SV and SDM, there was no significant difference between the types of packaging at 20% moisture content (Figure 3D,E).
For the physiological quality of C. liberica seeds, there was a significant interaction between the factors, except for the variables SV and SECL (Figure 4). For cryopreserved seeds of C. liberica with 20% moisture in aluminum foil pouch packaging, it was observed that the means of the variables RP, NS, and SDM were 11%, 8%, and 83 mg respectively, differing significantly from the values of the other packaging (Figure 4A,B,E). For cryopreserved seeds of C. liberica with moisture contents of 30 and 35%, regardless of the type of packaging, survival was less than 2% (Figure 4B). For the variable SV, there was an isolated effect between the factors. Cryopreserved seeds of C. liberica with 20 and 25% moisture had the highest viability percentages, at 53% and 48%, respectively, with no significant difference between them (Figure 4D). For SECL, an isolated effect was observed among the factors studied, but there was no significant difference between the moisture contents and the types of packaging tested (Figure 4C).
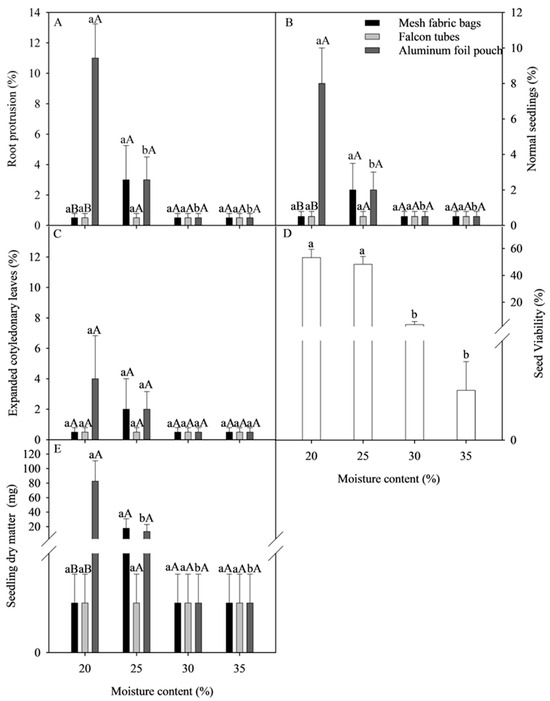
Figure 4.
The effect of moisture content and the type of packaging on the quality of cryopreserved C. liberica var. dewevrei seeds. (A) Root protrusion; (B) normal seedlings; (C) seedlings with expanded cotyledonary leaves; (D) seed viability; (E) seedling dry matter. Averages ± standard error (n = 4). Averages followed by the same lowercase letters compare seed moisture content, and capital letters compare packaging types that are not grouped together by the Scott Knott test (p < 0.05).
The survival rate of seeds cryopreserved at 20% moisture and wrapped in aluminum foil pouch was 8%, and although this was a low value, this was seen using the protocol in which the seeds still survived (Figure 4B). Although these results represented progress compared to other studies in which C. liberica seeds did not survive [18], this value was still low compared to those obtained with other species, such as C. arabica and C. racemosa.
The analysis of the physiological quality of the two species in our study made it clear that seeds of C. racemosa have greater tolerance to cryopreservation than seeds of C. liberica var. dewevrei. This result corroborates results obtained by Eira et al. [30], who studied coffee seed physiology and observed that the degree of tolerance to dehydration varies among Coffea species, with C. racemosa being the most tolerant species, in contrast with C. liberica, which is the most sensitive.
The differences among species of the Coffea genus can be attributed to various factors, as described by Chevalier [32]. As illustrated in Figure 1A, C. racemosa seeds exhibit a semi-globular or slightly elongated shape. The dimensions are smaller than those of C. liberica var dewevrei, whose seeds have a plano-convex shape. The rounded shape of C. racemosa, combined with their smaller size and larger exposed surface area, enhances their contact with LN and accelerates their cooling rate. These distinctive traits may account for the differences observed in their responses to cryopreservation.
Additionally, these differences may also be related to phylogenetic relationships and the habitat of origin of the species and/or the duration of the fruit development and maturation cycles [30]. According to the authors, species from drier regions, such as C. racemosa, C. arabica, C. canephora, and C. congensis, have a higher degree of tolerance to desiccation than C. liberica var. liberica or var. dewevrei seeds, which are native to wetter regions. Regarding the fruit development and maturation period, C. racemosa, the species most tolerant to desiccation, requires only 90 days, whereas C. liberica var. dewevrei, the least tolerant species, only reaches maturity at 360 days [33].
Dussert et al. [18] studied the response of Coffea species to cryopreservation with variation in the cooling rate of the seeds, and they differentiated species into groups according to post-freezing survival. The first group, which included C. liberica, exhibited no germination after immersion in LN. In contrast, another group, represented by C. racemosa, demonstrated high seed survival rates after cooling [18]. These findings align with those of the present study, which observed similar patterns. According to Davis et al. [34], C. racemosa originates from seasonally dry forests, while C. liberica is native to the tropical regions of West and Central Africa. This difference in habitat may explain the contrasting survival rates observed.
Despite the high sensitivity of C. liberica seeds, the zygotic embryos extracted from ripe fruit show tolerance to cryopreservation [16,18,35]. In the present study, the use of the tetrazolium test also showed the higher survival rates of embryos extracted from seeds after immersion in LN. This result can be attributed to the greater sensitivity of the endosperms compared to the embryos, which was also observed in other studies [21,23,25]. The search for cryopreservation protocols for whole seeds is of great interest, considering that this is a simpler and more economical method. It also requires less time to obtain seedlings and, after that, plantlets that are appropriate for planting [36].
Several protocols have been tested in recent years to improve the cryopreservation of coffee seeds. For the C. arabica L. species, some authors achieved success in the technique, with high germination percentages after the immersion of the seeds with 20% moisture content (db) in LN [21,22,23]. For C. canephora, Coelho et al. [24,25] studied different cryopreservation methodologies, achieving a 43% survival rate for seeds dried to 25% db using silica gel and then directly immersed in LN. In the present study, C. racemosa, considered one of the species most tolerant to desiccation and to ultra-low temperatures within the genus, also showed 79% NS. There was a high survival rate for seeds dried to 20% db and packed into an aluminum foil pouch before direct immersion in LN. According to Dussert et al. [18], 52% of the C. racemosa seeds previously immersed in LN developed into NS after desiccation to 0.28 g H2O per g (dw). When moisture content was reduced to 0.20 g H2O per g (dw), there was only 12% germination, and no seedlings were produced at lower moisture contents.
For both species studied, drying seeds to 20% moisture content followed by direct immersion in LN in aluminum foil pouches, was the best protocol among those studied, resulting in a 79.0% survival rate for C. racemosa and an 8% survival rate for C. liberica. Although studies related to the effect of packages on cryopreservation of coffee seeds are rare, our results show that seed viability can be affected by the type of packaging used to preserve seed quality in LN.
3.3. Isoenzyme Profiles in Cryopreserved Seeds
The results observed in the physiological evaluations of the cryopreserved seeds were as follows: For C. racemosa, all the protocols analyzed resulted in viable seed results and isoenzyme analyses were therefore carried out on all of them (Figure 5). For C. liberica, these analyses were restricted to seeds with moisture contents of 20% and 25%, the packaging of mesh-type fabric bags, and three folded sheets of aluminum foil that exhibited some survival (Figure 4 and Figure 6). These were compared with the control treatment and dried seeds with different moisture contents.
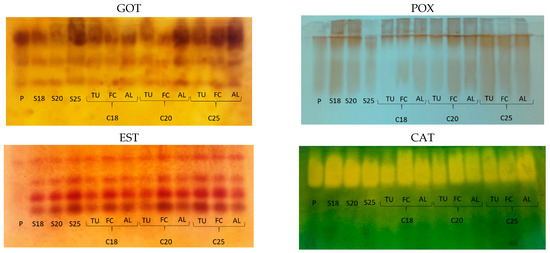
Figure 5.
Profiles observed for glutamate oxaloacetate transaminase (GOT), peroxidase (POX), esterase (EST), and catalase (CAT) in Coffea racemosa seeds under different cryopreservation protocols and moisture contents: (P) the control treatment; (S) the effect of drying to moisture content levels of 18, 20, and 25%; (C) the effect of cryopreservation of the seeds at different moisture content levels and storage in different types of packaging; (TU) mesh fabric bags; (FC) Falcon tubes; (AL) three folded sheets of aluminum foil.
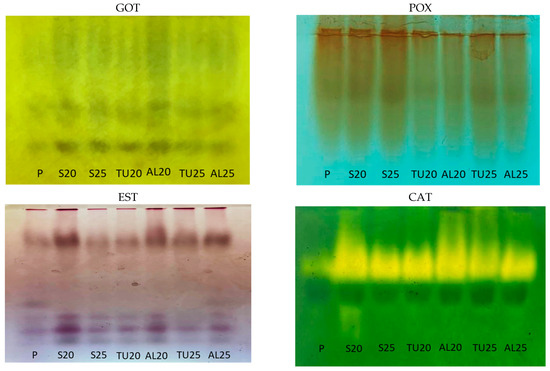
Figure 6.
Profiles observed for glutamate oxaloacetate transaminase (GOT), peroxidase (POX), esterase (EST), and catalase (CAT) in Coffea liberica seeds under different cryopreservation protocols: (P) the control treatment; (S20) the effect of drying to a moisture content level of 20%; (S25) the effect of drying to a moisture content level of 25%. The effect of cryopreservation on seeds stored in mesh fabric (TU) with moisture content levels of 20% and 25%, represented by TU20 and TU25. The effect of cryopreservation on the seeds stored in three folded sheets of aluminum foil (AL) with moisture content levels of 20% and 25%, represented by AL20 and AL25.
Glutamate oxaloacetate transaminase (GOT) is an important enzyme for germination, and it is present throughout the plant development cycle. It is involved in transamination during the removal of N from amino acids and in the formation of alpha-keto acid groups for the citric acid cycle and gluconeogenesis [37]. In C. racemosa, GOT exhibited three well-defined isoforms, with variations in bands intensity in the different treatments (Figure 5). Seeds with a moisture content of 25% had a GOT profile similar to that of the control treatment with a moisture content of 96%. In contrast, the intensity of the bands of this enzyme decreased when the seed moisture content values were 20% and 18% (Figure 5). Cryopreservation did not alter GOT activity in seeds with 25% moisture content stored in Falcon tubes and in three folded sheets of aluminum foil. It led to an increase in GOT activity in seeds with 20% moisture content in three folded sheets of aluminum foil (Figure 5). Seeds with 18% moisture content that presented lower NS, SECL and SDM values compared to control treatment and those with 20% moisture content (Table 1) did not show any change in the GOT profile after cryopreservation, regardless of the packaging used.
Thus, GOT activity was not related to the physiological quality of cryopreserved seeds, which was high for seeds with 20% moisture content packaged in mesh or Falcon tubes. These protocols showed the same patterns observed for GOT in seeds with 18% moisture content after cryopreservation. The differences observed in the gel seem to be related to the higher moisture content in control treatment and those with 25% moisture content, cryopreserved or not, and to the way the seeds are packaged, with aluminum foil packaging appearing to favor GOT activity in seeds with 20% moisture content.
Unlike C. racemosa, in the GOT profile obtained for the C. liberica seeds, only two isoforms appeared in the polyacrylamide gel (Figure 6). In C. racemosa, GOT was more active in seeds with higher moisture contents. In C. liberica, the control treatment and the sampled cryopreserved with 25% moisture content showed the lowest activity (Table 1). Drying the seeds at 20% and 25% moisture content, just as was conducted for those cryopreserved with 20% moisture content, produced similar GOT profiles, only with more activity. It is important to emphasize that the cryopreservation of C. liberica resulted in viable results in the tetrazolium test, which was conducted with embryos, at over 50% viability. However, the seeds had very low germination and vigor indices (Figure 4).
Peroxidases are enzymes that also are present throughout the plant life cycle. They belong to a large multigene family with a large number of isoforms. With two possible catalytic cycles, the peroxidative and the hydroxylating cycles, peroxidases can generate free radicals, such as reactive oxygen species, and be involved in the polymerization of cell wall compounds [38]. POX also catalyzes cross-linking between phenolic groups (tyrosine, phenylalanine, ferulic acid) in cell wall proteins, pectins, and other wall polymers [38].
The drying of C. racemosa seeds at a 25% moisture content showed the same pattern as the control treatment. An increase in POX expression was observed when the seeds were dried at 18% and 20%. After cryopreservation, this pattern was only maintained in seeds with 20% moisture content, which were stored in Falcon tubes and in three folded sheets of aluminum foil. In contrast, seeds cryopreserved at 18% moisture content showed a reduction in POX expression, which was also observed for seeds at 20% moisture content cryopreserved in mesh packaging (Figure 5).
The drying process is known to increase stress in seeds, and excessive amounts of ROS cause cell damage by the oxidation of biomolecules such as lipids and proteins [39]. In addition, Passardi et al. [38] indicate that physical stresses can induce the expression of POX to trigger tissue repair mechanisms. The damage caused by LN to drier C. racemosa seeds with 18% and 20% moisture content, especially those kept in the mesh fabric packaging and those that came into direct contact with the LN, seems to have had a negative impact on POX activity, especially in relation to its cell wall-related function. This is shown by a drastic reduction in the germination of these cryopreserved seeds, even when their embryos survive (Figure 3).
For C. liberica, there was a reduction in POX activity after the cryopreservation of the seeds that were dried to 25% and 20% moisture content and stored in either mesh fabric or aluminum foil packaging. It should be noted that seeds with 20% moisture content in the mesh packaging showed low survival rates in the germination test; however, the percentage of viable seeds was the same as the percentage observed for the seeds with 25% moisture content.
It is noteworthy that Coelho et al. [24] also found no correlation between POX and protocols related to cooling rates in the cryopreservation process of C. canephora seeds. This may indicate that for more recalcitrant seeds, such as those of C. liberica, the regulatory mechanism of POX expression does not follow the same pattern observed in seeds more tolerant to cryopreservation.
In C. racemosa, the control treatment showed virtually no EST expression. EST activation is observed along with the seed drying process and its maintenance after cryopreservation, without differences in the profiles in terms of moisture content or types of packaging (Figure 5). The exceptions were seeds cryopreserved at 18% and 20% moisture content and stored in mesh packaging (Figure 5). Esterase hydrolyzes esters and metabolizes lipids such as membrane phospholipids. Cryopreservation stress, just as seen for POX, results in lower band intensity in the treatments mentioned above.
For C. liberica, the EST pattern for control treatment was different: five isoforms were expressed, compared to the four isoforms seen in the other treatments (Figure 6). After drying, the seeds with 20% moisture content showed greater esterase activity than those with 25% moisture content. Cryopreservation in the aluminum foil packaging of seeds with 20% and 25% moisture content resulted in seeds with greater EST activity (Figure 6). Coelho et al. [25] also observed that, in C. canephora seeds, drying seeds from 22% to 17% wb favors the increased expression of this isoenzyme. The authors also found that, when the seeds were dried slowly to 22% moisture content (wb) using a NaCl solution and then cryopreserved, they also showed low levels of expression of this enzyme.
Catalase (CAT), found in the peroxisomes and mitochondria, is part of the efficient antioxidant enzyme machinery in the cells, and it is involved in protection against damage caused by oxidative stress by breaking down H2O2 into O2 and H2O [39,40,41]. CAT was already active in control treatment C. racemosa seeds and remained so after drying and cryopreservation. A decrease in catalase activity was only observed for seeds cryopreserved at 18% moisture content and stored in mesh packaging (Figure 5).
Control treatment C. liberica seeds showed low CAT activity, which increased during the drying process. Seeds with 20% moisture content showed greater intensity of CAT expression, indicating the response of this enzyme to an increased demand likely created by stress from greater water removal from the seeds (Figure 6). Although cryopreserved C. liberica seeds display low survival (Figure 4), CAT remained active in cryopreserved seeds that had 20% and 25% moisture content and were stored in mesh and aluminum foil packaging.
It is known that ROS also have a signaling effect in response to abiotic stresses, as they are necessary for cell homeostasis. The role of these reactive species is based on a delicate balance between their production and elimination [39,40]. It is noteworthy that CAT is only activated in C. liberica when water is removed from the seeds, indicating that cell signaling for protection against oxidative stress only occurs at that time (Figure 6).
In general, for C. racemosa, the GOT, POX, and EST isoenzymes showed comparatively lower activity when cryopreserved seeds with 20% or 18% moisture content were stored in mesh fabric (Figure 5). In addition to the oxidative stress damage caused by the drying process, this type of packaging led to increased stress from the direct contact of LN with the endosperm. The opposite was observed for the aluminum foil packaging, which proved to be more suitable for seed storage and in terms of physiological qualities. For C. liberica, further studies are necessary to define a cryopreservation protocol that will allow the recovery of seeds with higher germination and vigor rates.
Studies on more recalcitrant seeds are challenging as they require, at the same time, specific, appropriate methods, speed, and precision, characteristics that cannot always be ensured simultaneously. Thus, results such as those presented in this study for C. liberica are very important, despite the low survival rate of these seeds after cryopreservation. Various factors affect the quality of cryopreserved seeds, and they may interact, as was shown in this study and in the numerous results already published. The verification of factors such as the initial quality of the seeds, the drying method, the moisture content, seed preparation, the use of cryoprotectant additives, the cooling rate, and the rewarming method after removal from the cryotank is essential to mitigate oxidative stress and cellular damage, ensuring preservation.
4. Conclusions
The cryopreservation protocol for Coffea racemosa seeds established in this study was found to effectively ensure their long-term viability. However, for the species Coffea liberica var dewevrei, whose seeds have more recalcitrant characteristics, future studies are needed to obtain a protocol that ensures higher survival rates for this species. In the present study, the protocol that provided the best results for both species tested involved drying the seeds to 20% moisture content on a dry basis and packaging them in envelopes made of three folded sheets of aluminum foil, with direct immersion in liquid nitrogen. This study demonstrates that seeds of different species depend on specific moisture contents and types of packaging for greater survival after cryopreservation, effectively contributing to the conservation of the genetic diversity of the genus Coffea.
Author Contributions
Conceptualization, S.V.B.C., S.D.V.F.d.R., L.P., O.G.F. and M.T.B.; methodology, S.V.B.C., S.D.V.F.d.R., A.L.d.O.V. and J.G.R.d.A.; formal analysis, writing—original draft preparation, S.V.B.C. and S.D.V.F.d.R.; writing—review and editing, L.P., A.L.d.O.V. and A.M.O.F.; supervision and project administration S.D.V.F.d.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work study was supported by the Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA); Consórcio Brasileiro de Pesquisa e Desenvolvimento do Café (Consórcio Pesquisa Café—234/2019-889547/2019); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES—code 001); Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Instituto Nacional de Ciência e Tecnologia do Café (INCT Café—384775/2024-1).
Data Availability Statement
The data used in this study were self-collected. The dataset is undergoing further improvement; thus, it is unavailable at present.
Acknowledgments
We thank the Federal University of Lavras (UFLA) for the use of the facilities during this investigation and the Agronomic Institute of Campinas (IAC) for donating the seeds in our experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- International Coffee Organization. Sustainability e Resilience of the Coffee Global Value Chain: Towards a Coffee Investment Vehicle. 2024. Available online: https://www.icocoffee.org/documents/cy2023-24/report-global-coffee-funding-mechanisms-june-2024-e.pdf (accessed on 16 August 2024).
- Companhia Nacional de Abastecimento (CONAB). Acompanhamento da Safra Brasileira de Café, terceiro levantamento, setembro 2024. v. 11, 3rd ed.; CONAB: Brasília, Brazil, 2024. Available online: http://www.conab.gov.br (accessed on 20 September 2024).
- Davis, A.P.; Rakotonasolo, F. Six new species of coffee (Coffea) from northern Madagascar. Kew Bull. 2021, 76, 497–511. [Google Scholar] [CrossRef]
- Fazuoli, L.C.; Braghini, M.T.; Silvarolla, M.B.; Goncalves, W.; Mistro, J.C.; Gallo, P.B.; Guerreiro Filho, O. IAC Catuaí SH3: A dwarf Arabica coffee cultivar with leaf rust resistance and drought tolerance. Crop Breed. Appl. Biotechnol. 2019, 19, 356–359. [Google Scholar] [CrossRef]
- Medina Filho, H.P.; Carvalho, A.; Sondahl, M.R.; Fazuoli, L.C.; Costa, W.M. Coffee breeding and related evolutionary aspects. Pl. Breed. Rev. 1984, 2, 157–193. [Google Scholar] [CrossRef]
- Fazuoli, L.C.; Braghini, M.T.; Silvarolla, M.B.; Gonçalves, W.; Mistro, J.C.; Gallo, P.B.; Guerreiro Filho, O. IAC 125 RN: A dwarf coffee cultivar resistant to leaf rust and root-knot nematode. Crop Breed. Appl. Biotechnol. 2018, 18, 237–240. [Google Scholar] [CrossRef]
- Guerreiro Filho, O. Coffee leaf miner resistance. Braz. J. Plant Physiol. 2006, 18, 109–117. [Google Scholar] [CrossRef]
- Eira, M.T.S.; Walters, C.; Caldas, L.S.; Fazuoli, L.C.; Sampaio, J.B.; Dias, M.C.L.L. Tolerance of Coffea spp. seeds to desiccation and low temperature. Rev. Bras. Fis. Veg. 1999, 11, 97–105. [Google Scholar]
- Kaviani, B. Conservation of plant genetic resources by cryopreservation. Aust. J. Crop Sci. 2011, 5, 778–800. Available online: https://www.cropj.com/kaviani_5_6_2011_778_800.pdf (accessed on 10 March 2024).
- Jenderek, M.M.; Reed, B.M. Cryopreserved storage of clonal germplasm in the USDA national plant germplasm system. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 299–308. [Google Scholar] [CrossRef]
- Vettorazzi, R.G.; Carvalho, V.S.; Teixeira, M.C.; Campostrini, E.; Cunha, M.; Matos, E.M.; Viccini, L.F. Cryopreservation of immature and mature seeds of Brazilian orchids of the genus Cattleya. Sci. Hortic. 2019, 256, 108603. [Google Scholar] [CrossRef]
- Silva, S.S.S.; Souza, E.H.; Souza, F.V.D.; Max, D.A.S.; Rossi, M.L.; Costa, M.A.P.C. Post-seminal development and cryopreservation of endemic or endangered bromeliads. An. Acad. Bras. Ciênc. 2021, 93, e20191133. [Google Scholar] [CrossRef]
- Pammenter, N.W.; Berjak, P. Physiology of desiccation-sensitive (recalcitrant) seeds and the implications for cryopreservation. Int. J. Plant Sci. 2014, 175, 21–28. [Google Scholar] [CrossRef]
- Jaiswal, A.N.; Vagga, A. Cryopreservation: A Review Article. Cureus 2022, 14, e31564. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P. Freezing of living cells: Mechanisms and implications. Am. J. Physiol. 1984, 247, C125–C142. [Google Scholar] [CrossRef] [PubMed]
- Hor, Y.L.; Stanwood, P.C.; Chin, H.F. Cryopreservation of Coffea liberica seeds and embryos following desiccation and freezing treatments. Pertanika J. Trop. Agric. Sci. 1993, 16, 75–80. [Google Scholar]
- Dussert, S.; Chabrillange, N.; Engelmann, F.; Anthony, F.; Louarn, J.; Hamon, S. Cryopreservation of seeds of four coffee species (Coffea arabica, C. costatifructa, C. racemosa and C. sessiliflora): Importance of water content and cooling rate. Seed Sci. Res. 1998, 8, 9–15. [Google Scholar] [CrossRef]
- Dussert, S.; Chabrillange, N.; Rocquelin, G.; Engelmann, F.; Lopez, M.; Hamon, S. Tolerance of coffee (Coffea spp.) seeds to ultra-low temperature exposure in relation to calorimetric properties of tissue water, lipid composition, and cooling procedure. Physiol. Plant. 2001, 112, 495–504. [Google Scholar] [CrossRef]
- Brasil. Ministério da Agricultura. Pecuária e Abastecimento. Regras Para Análise de Sementes; MAPA: Brasília, Brazil, 2009; 395p. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf (accessed on 9 April 2024).
- Clemente, A.C.S.; Carvalho, M.L.M.; Guimarães, R.M.; Zeviani, W.M. Preparo das sementes de café para a avaliação da viabilidade pelo teste de tetrazólio. Rev. Bras. Sementes 2011, 33, 38–44. [Google Scholar] [CrossRef]
- Coelho, S.V.B.; Rosa, S.D.V.F.; Fernandes, J.S. Cryopreservation of coffee seeds: A simplified method. Seed Sci. Technol. 2017, 45, 638–649. [Google Scholar] [CrossRef]
- Figueiredo, M.A.; Coelho, S.V.B.; Rosa, S.D.V.F.; Vilela, A.L.; Silva, L.C. Exploratory studies for cryopreservation of Coffea arabica L. seeds. J. Seed Sci. 2017, 39, 150–158. [Google Scholar] [CrossRef]
- Figueiredo, M.A.; Rosa, S.D.V.F.; Ricaldoni, M.A.; Pereira, C.C.; Coelho, S.V.B.; Silva, L.C. Physiological, biochemical, and ultrastructural aspects of Coffea arabica L. seeds under different cryopreservation protocols. Ciênc. Agrotec. 2021, 45, e027020. [Google Scholar] [CrossRef]
- Coelho, S.V.B.; Rosa, S.D.V.F.; Fantazzini, T.B.; Baute, J.L.; Silva, L.C. Cryopreservation in Coffea canephora Pierre seeds: Slow and fast cooling. Ciênc. Agrotec. 2018, 42, 588–597. [Google Scholar] [CrossRef]
- Coelho, S.V.B.; da Rosa, S.D.V.F.; Fantazzini, T.B.; Silva, L.C. Cryopreservation of Coffea canephora Pierre ex A. Froehner seeds: Importance of drying rate and moisture content. Aust. J. Crop Sci. 2019, 13, 1335–1342. [Google Scholar] [CrossRef]
- Alfenas, A.C. (Ed.) Eletroforese e Marcadores Bioquímicos em Plantas e Microorganismos; Editora UFV: Viçosa, Brazil, 2006; 627p. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.3.1; R Foundation for Statistical Computing: Viena, Austria, 2021. [Google Scholar]
- Wesley-Smith, J.; Berjak, P.; Pammenter, N.W.; Walters, C. Intracellular ice and cell survival in cryo-exposed embryonic axes of recalcitrant seeds of Acer saccharinum: An ultrastructural study of factors affecting cell and ice structures. Ann. Bot. 2014, 113, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Pammenter, N.W.; Vertucci, C.W.; Berjak, P. Responses to dehydration in relation to non-freezable water in desiccation-sensitive and tolerant seeds. In Proceedings of the Fourth International Workshop on Seeds: Basic and Applied Aspects of Seed Biology; Côme, D., Corbineau, F., Eds.; AFSIS: Paris, France, 1993; pp. 867–872. [Google Scholar]
- Eira, M.T.S.; Reis, R.B.; Ribeiro, F.N.S. Banco de sementes de café em criopreservação: Experiência inédita do Brasil. Circ. Técnica 2005, 42. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/187030/1/ct042.pdf (accessed on 20 December 2023).
- Takahashi, D.; Willick, I.R.; Kasuga, J.; Livingston III, D.P. Responses of the plant cell wall to sub-zero temperatures: A brief update. Plant Cell Physiol. 2021, 62, 1858–1866. [Google Scholar] [CrossRef]
- Chevalier, A. Systematique des caféiers et faux-caféiers. In Les Caféiers du Globe, 3rd ed.; Encyclopédie Biologique XXVIII; Paul Lechevalier Publishers: Paris, France, 1947; 257p. [Google Scholar]
- Carvalho, A.; Medina Filho, H.P.; Fazuoli, L.C.; Guerreiro Filho, O.; Lima, M.M.A. Aspectos genéticos do cafeeiro. Rev. Bras. Genética 1991, 14, 135–183. [Google Scholar]
- Davis, A.P.; Govaerts, R.; Bridson, D.M.; Stoffelen, P. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 2006, 152, 465–512. [Google Scholar] [CrossRef]
- Normah, M.N.; Vengadasalam, M. Effects of moisture content on cryopreservation of Coffea and Vigna seeds and embryos. Cryo-Letters 1992, 13, 199–208. [Google Scholar] [CrossRef]
- Vilela, A.L.O.; Franco da Rosa, S.D.V.; Coelho, S.V.B.; Pereira, C.C.; de Souza, A.C.; Ribeiro, F.A.S. Acclimatization of coffee seedlings obtained from zygotic embryos of aged seeds. Aust. J. Crop Sci. 2022, 16, 1127–1134. [Google Scholar] [CrossRef]
- Goodman, M.M.; Stuber, C.W. Maize. In Isozymes in Plant Genetics and Breeding; Tanksley, S.D., Orton, T.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1983; pp. 9–10. [Google Scholar]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Sahu, B.; Sahu, A.K.; Thomas, V.; Naithani, S.C. Reactive oxygen species, lipid peroxidation, protein oxidation, and antioxidative enzymes in dehydrating Karanj (Pongamia pinnata) seeds during storage. S. Afr. J. Bot. 2017, 112, 383–390. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Gill, S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).