Black Soil Quality After 19 Years of Continuous Conservation Tillage
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Experimental Design
2.3. Sample Collection and Measurement
2.3.1. Soil Capacity, Field Water-Holding Capacity, Total Porosity, and Non-Capillary Porosity
2.3.2. Water-Stable Macroaggregate Content and Mean Weight Diameter
2.3.3. Soil Organic Carbon and Soil Nutrients
2.3.4. Soil Enzymes and Soil Microbial Biomass Carbon and Nitrogen
2.3.5. High-Throughput Sequencing Analysis
2.4. Composite Index of Soil Fertility Quality
2.4.1. Affiliation Function and Affiliation Value
2.4.2. Establishment of Weights
2.4.3. Soil Quality Composite Index
2.5. Data Calculation and Analysis
3. Results
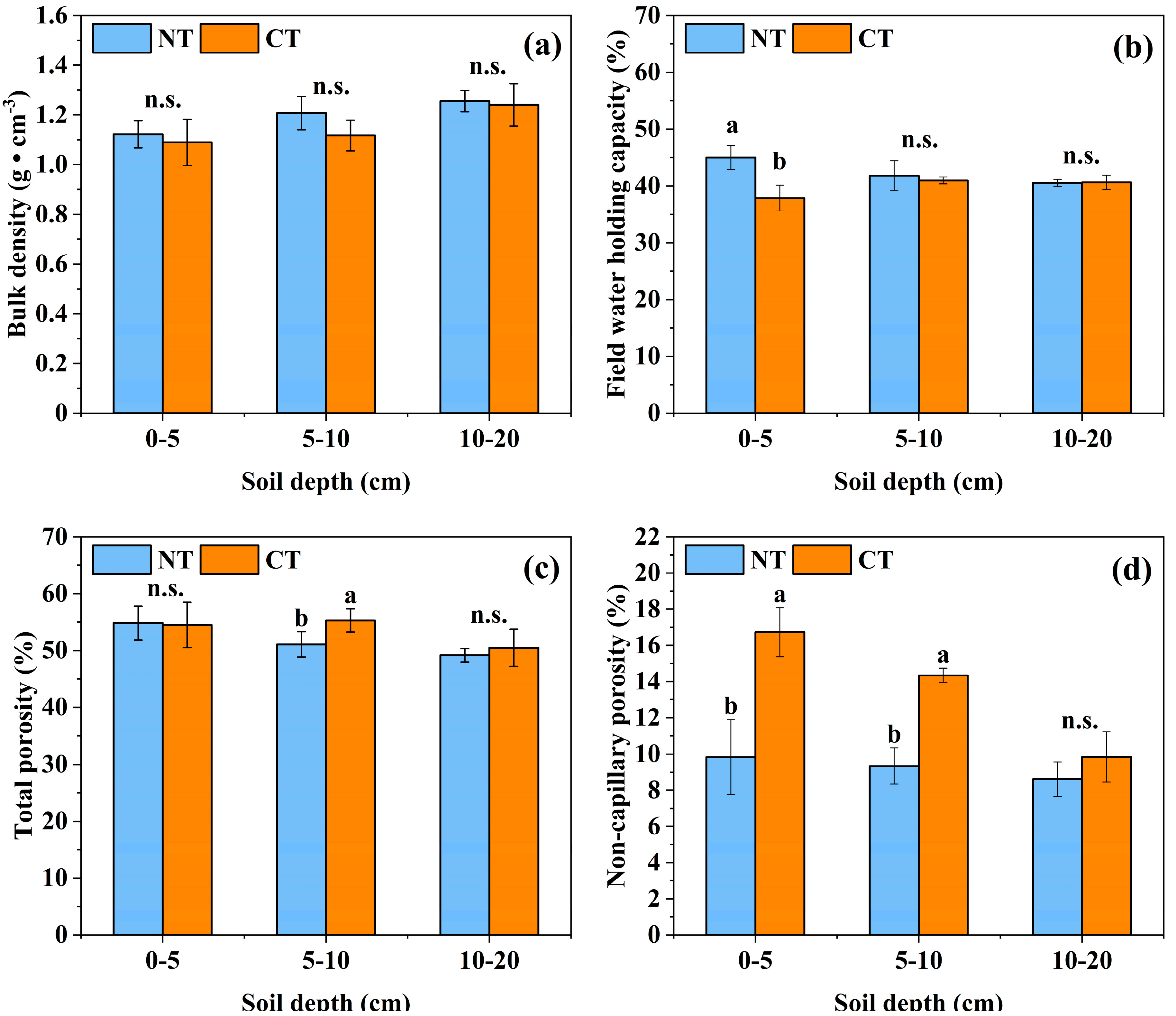
3.1. Effects of Long-Term Conservation Tillage on Soil Physical Properties
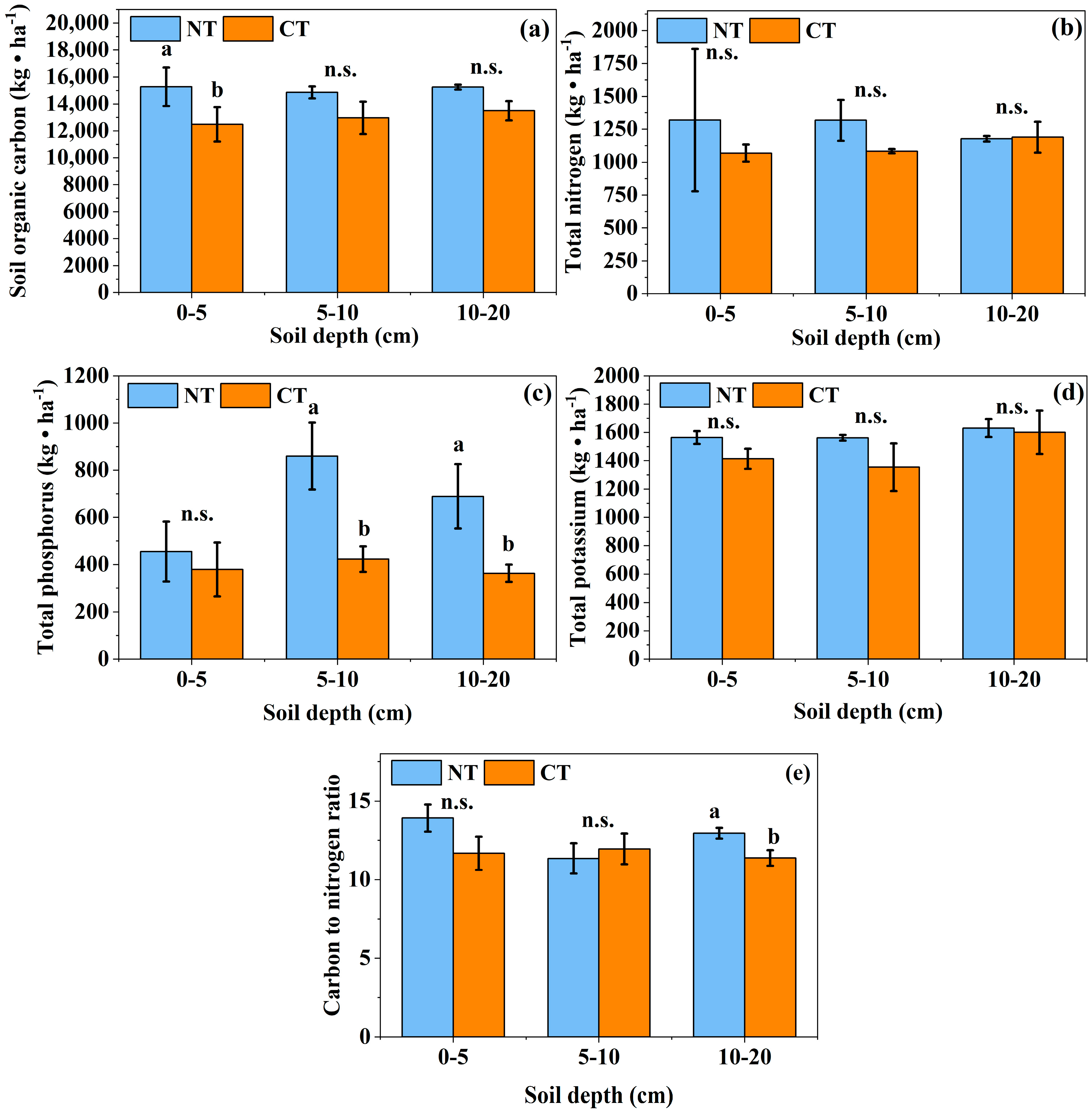
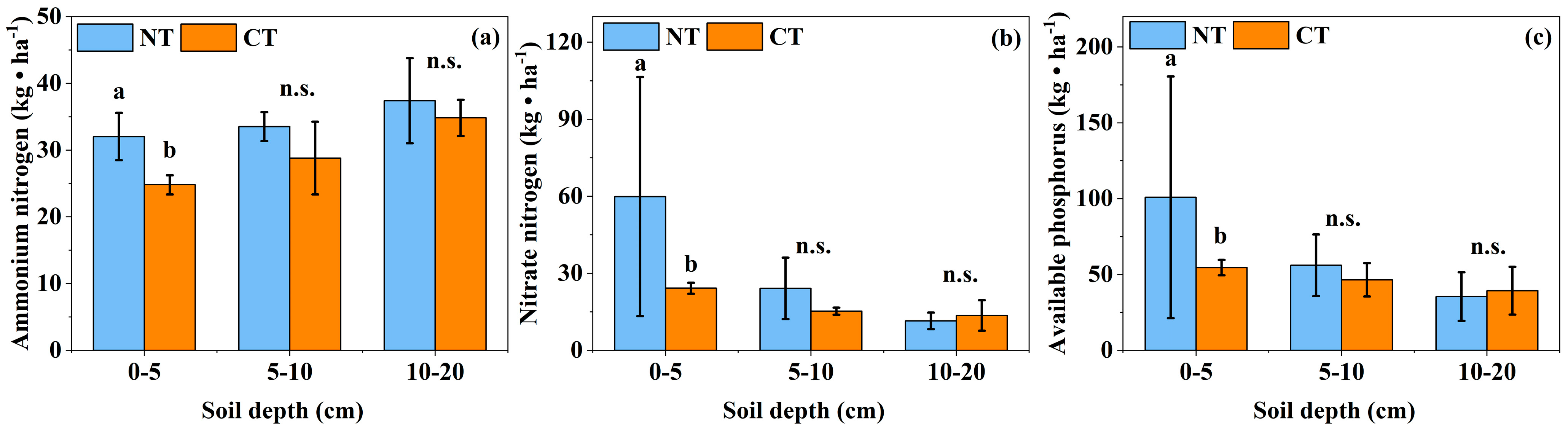
3.2. Effects of Long-Term Conservation Tillage on Soil Chemical Properties
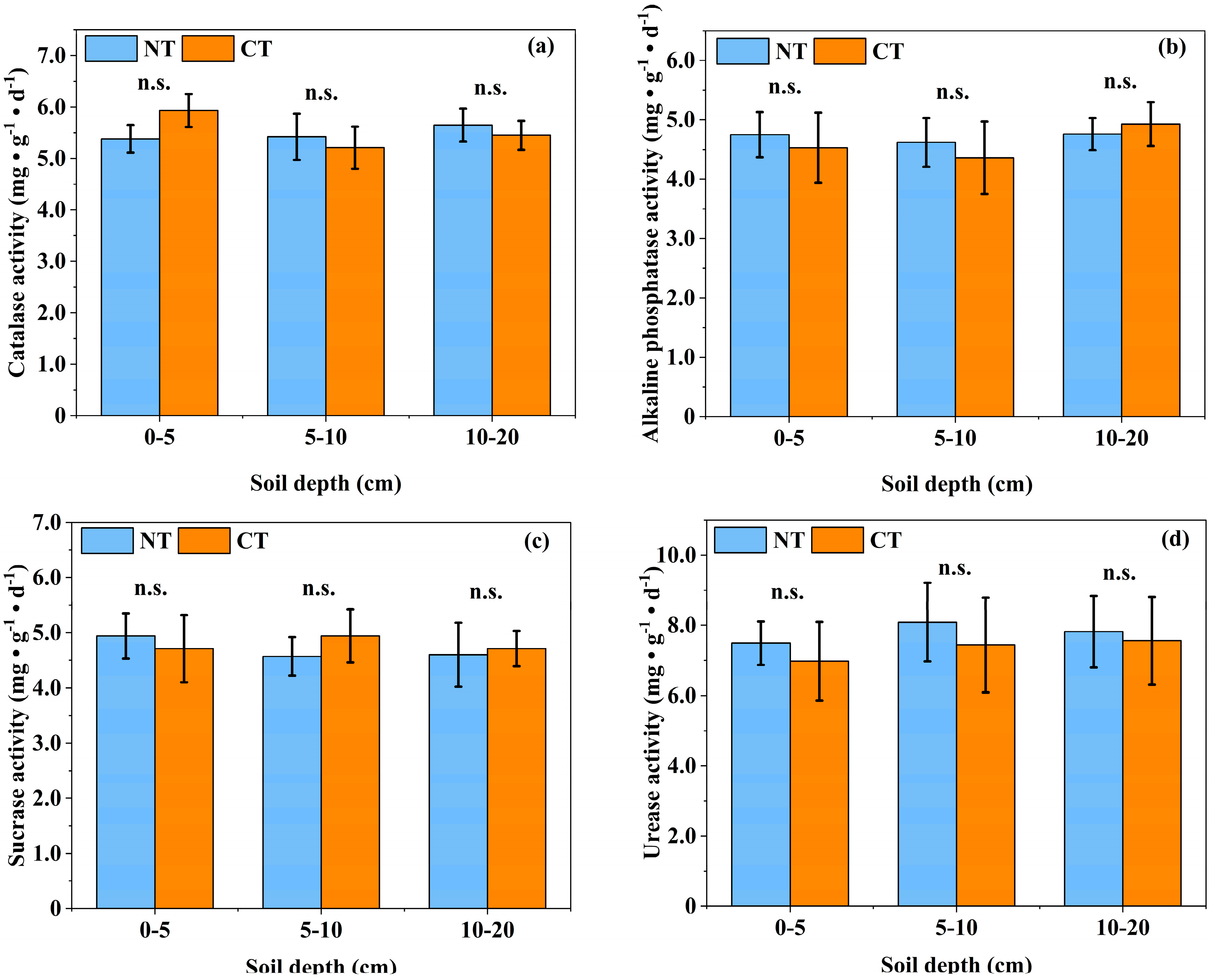
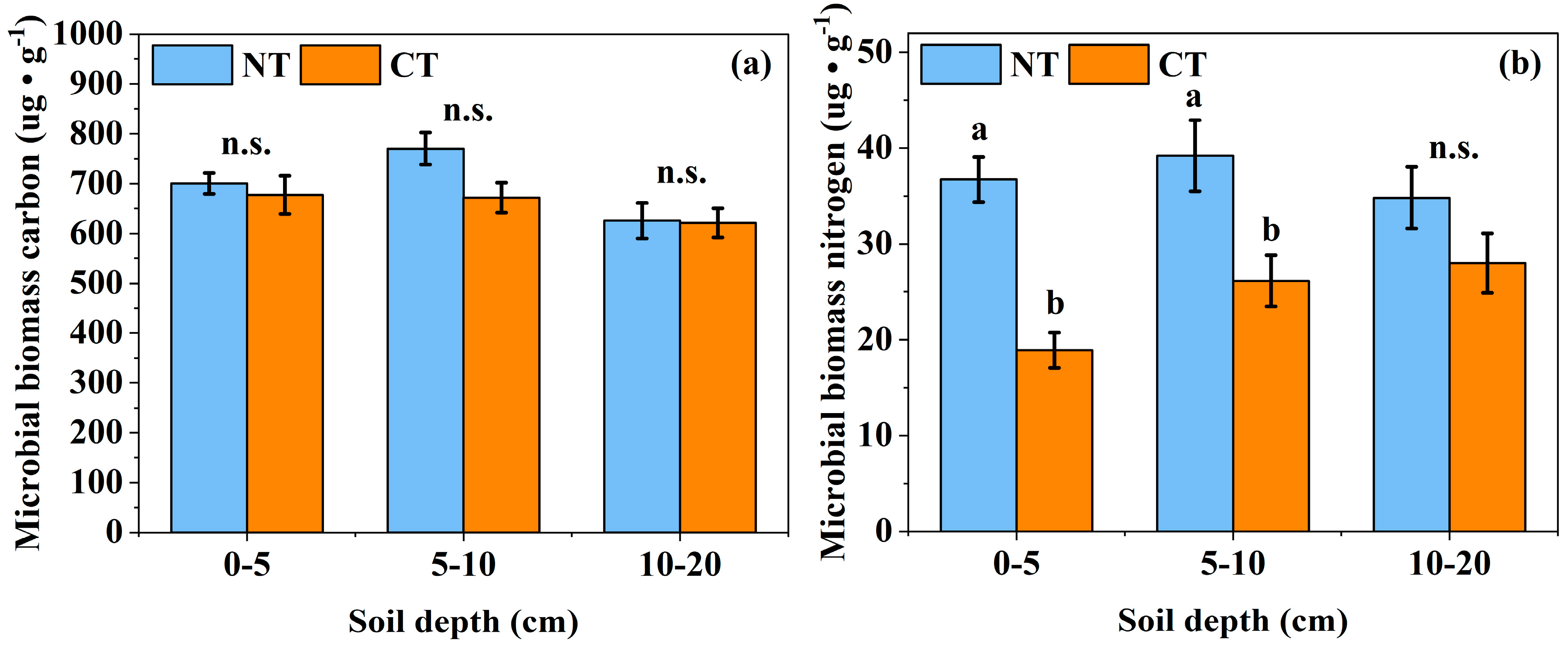
3.3. Effects of Long-Term Conservation Tillage on the Biological Properties of Soil
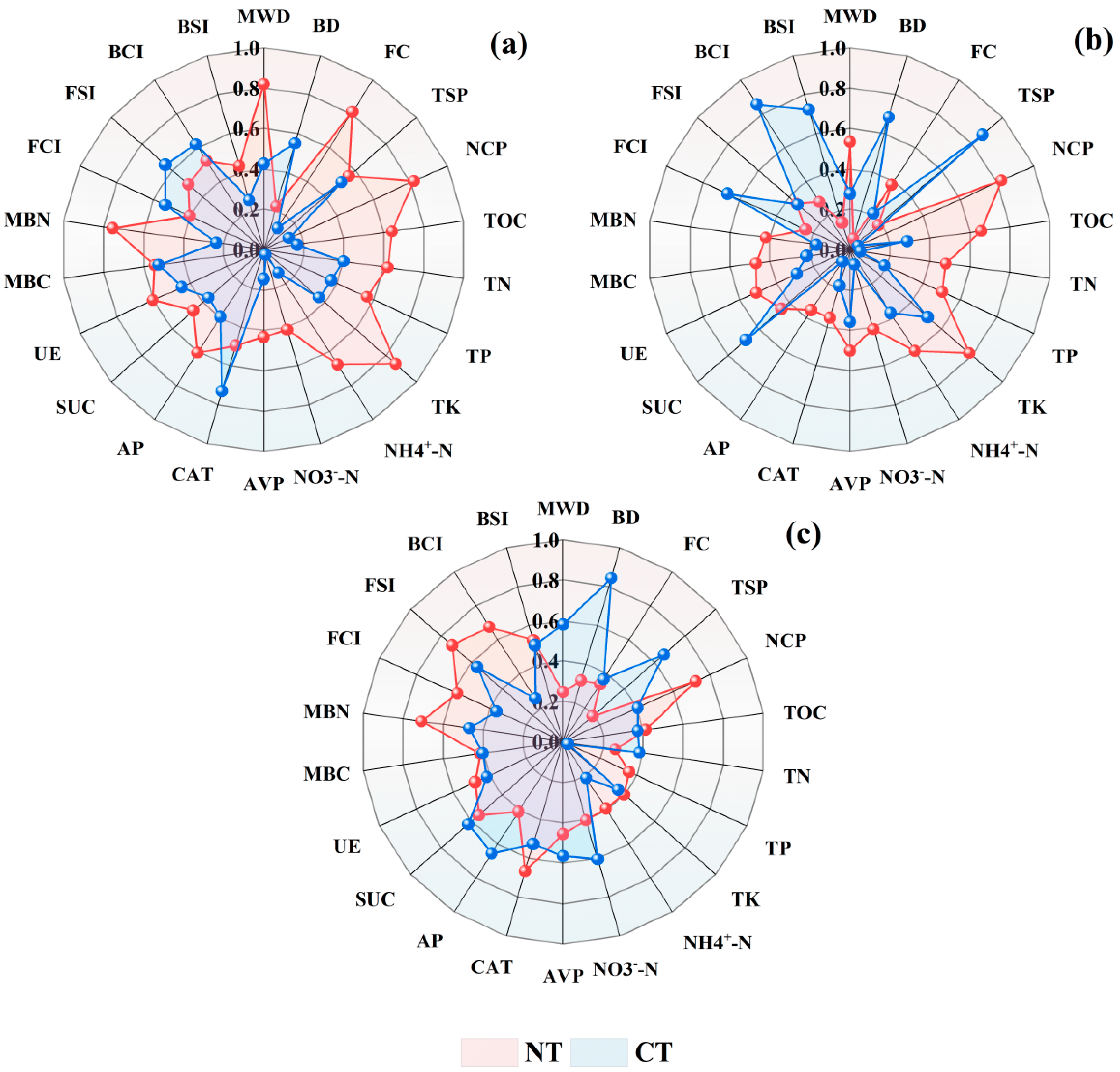
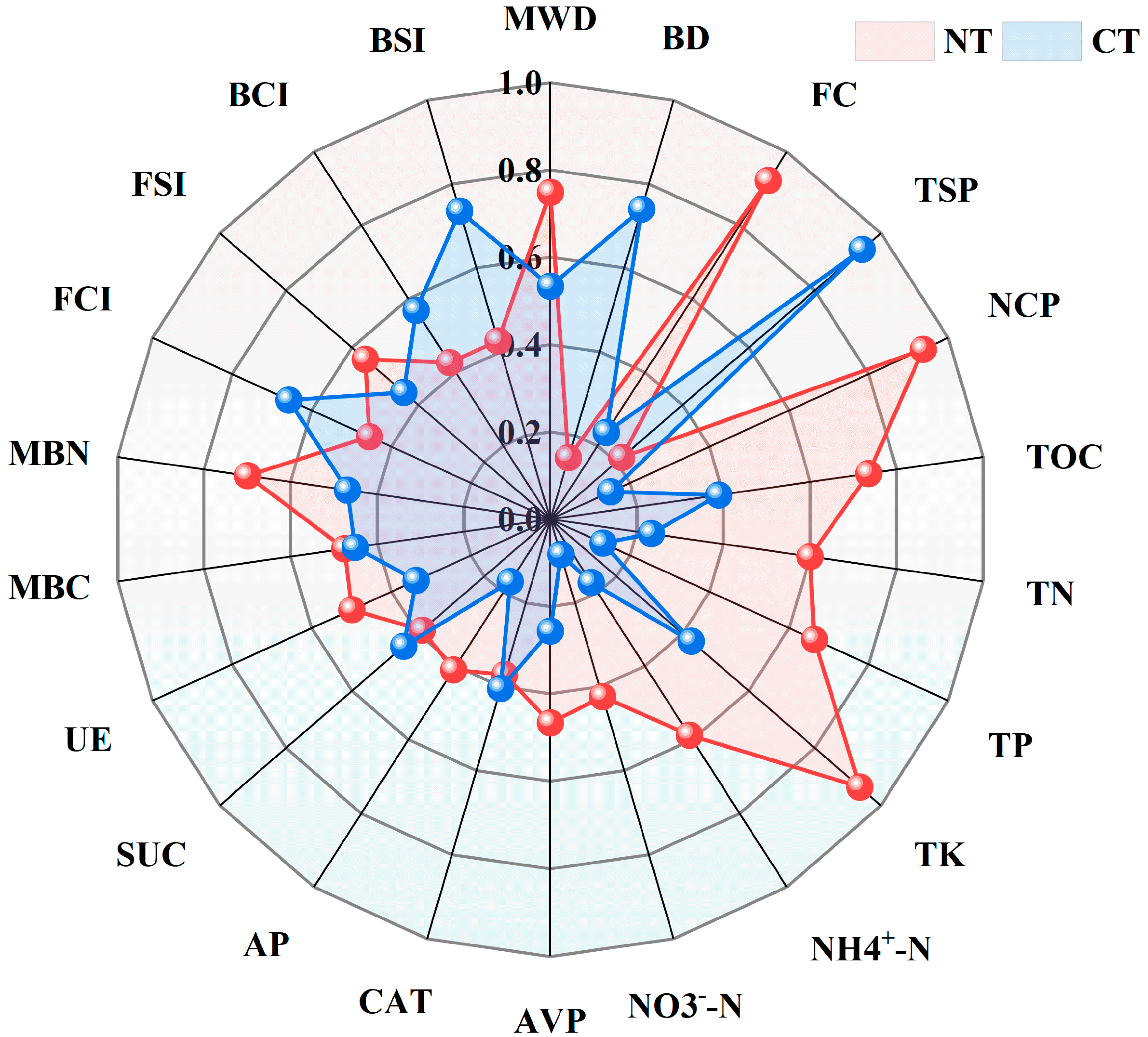
3.4. Evaluation of Soil Quality
3.5. Soil Quality Index
4. Discussion
4.1. Effect of Long-Term Conservation Tillage on the Physical Properties of Soil
4.2. Effects of Long-Term Conservation Tillage on the Chemical Properties of Soil
4.3. Effects of Long-Term Conservation Tillage on the Biological Properties of Soil
4.4. Effects of Different Tillage Practices on Soil Quality Index
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, H.; Chen, P.; Sun, Y. Analysis of the coupling and coordination between soil erosion and land use in the Northeastern black soil region of China: A case study of Lishu County. Sci. Rep. 2024, 14, 21955. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cao, S.; Sun, Z.; Wang, H.; Qu, S.; Lei, N.; He, J.; Dong, Q. Tillage effects on soil properties and crop yield after land reclamation. Sci. Rep. 2021, 11, 4611. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Nie, R.; Du, G. Responses of soil collembolans to land degradation in a black soil region in China. Int. J. Environ. Res. Public Health 2023, 20, 4820. [Google Scholar] [CrossRef]
- Toor, G.S.; Yang, Y.Y.; Das, S.; Dorsey, S.; Felton, G. Soil health in agricultural ecosystems: Current status and future perspectives. Adv. Agron. 2021, 168, 157–201. [Google Scholar]
- Duan, J.; Liu, H.; Zhang, X.; Ren, C.; Wang, C.; Cheng, L.; Xu, J.; Gu, B. Agricultural management practices in China enhance nitrogen sustainability and benefit human health. Nat. Food 2024, 5, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zang, J.; Fu, Y.; Pan, X.; Meng, D.; Zhu, Y.; Sun, T. Influences of conservation tillage on soil macrofaunal biodiversity and trophic structure in the Mollisol region of Northeast China. Catena 2024, 236, 107750. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, W.; Zhang, Y.; Liu, X.; Zhang, Y.; Zheng, X.; Luo, J.; Zou, J. Effects of no-till on upland crop yield and soil organic carbon: A global meta-analysis. Plant Soil 2024, 499, 363–377. [Google Scholar] [CrossRef]
- Li, Y.; Song, D.; Liang, S.; Dang, P.; Qin, X.; Liao, Y.; Siddique, K.H.M. Effect of no-tillage on soil bacterial and fungal community diversity: A meta-analysis. Soil Tillage Res. 2020, 204, 104721. [Google Scholar] [CrossRef]
- Attademo, A.M.; Sanchez-Hernandez, J.C.; Lajmanovich, R.C.; Repetti, M.R.; Peltzer, P.M. Enzyme activities as indicators of soil quality: Response to intensive soybean and rice crops. Water Air Soil Pollut. 2021, 232, 295. [Google Scholar] [CrossRef]
- Si, P.; Liu, E.; He, W.; Sun, Z.; Dong, W.; Yan, C.; Zhang, Y. Effect of no-tillage with straw mulch and conventional tillage on soil organic carbon pools in Northern China. Arch. Agron. Soil Sci. 2018, 64, 398–408. [Google Scholar] [CrossRef]
- Makó, A.; Szabó, B.; Rajkai, K.; Szabó, J.; Bakacsi, Z.; Labancz, V.; Hernádi, H.; Barna, G. Evaluation of soil texture determination using soil fraction data resulting from laser diffraction method. Int. Agrophysics 2019, 33, 445–454. [Google Scholar] [CrossRef]
- Cui, J.; Guo, L.; Xiong, S.; Yang, S.; Wang, Y.; Zhang, S.; Sun, H. Soil organic carbon induces a decrease in erodibility of black soil with loess parent materials in northeast China. Quat. Res. 2024, 120, 83–92. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Li, H.; Li, X.F.; Song, C.Y.; Cruse, R.M.; Zhang, X.Y. Effects of conservation tillage on corn and soybean yield in the humid continental climate region of Northeast China. Soil Tillage Res. 2011, 115, 56–61. [Google Scholar] [CrossRef]
- Pranagal, J.; Kraska, P. 10-years studies of the soil physical condition after one-time biochar application. Agronomy 2020, 10, 1589. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, L.; Xiao, Z.; Li, C.; Wang, Z.; Zhou, P.; Sun, G.; Ye, Y.; Hu, T.; Wang, H. Rice-crayfish farming increases soil organic carbon. Agric. Ecosyst. Environ. 2022, 329, 107857. [Google Scholar] [CrossRef]
- Francis, A.; Kevin, R.; Gopi, G.; Baby, R. Soil Organic Carbon Estimation Using Remote Sensing Technique. In Green Buildings and Sustainable Engineering: Proceedings of GBSE 2019; Springer: Singapore, 2020; pp. 405–412. [Google Scholar]
- Neemisha; Sharma, S. Soil enzymes and their role in nutrient cycling. In Structure and Functions of Pedosphere; Springer Nature: Singapore, 2022; pp. 173–188. [Google Scholar]
- Guo, Z.; Wan, S.; Hua, K.; Yin, Y.; Chu, H.; Wang, D.; Guo, X. Fertilization regime has a greater effect on soil microbial community structure than crop rotation and growth stage in an agroecosystem. Appl. Soil Ecol. 2020, 149, 103510. [Google Scholar] [CrossRef]
- Chaudhry, H.; Vasava, H.B.; Chen, S.; Saurette, D.; Beri, A.; Gillespie, A.; Biswas, A. Evaluating the Soil Quality Index Using Three Methods to Assess Soil Fertility. Sensors 2024, 24, 864. [Google Scholar] [CrossRef]
- Yan, X.; Luo, X. Assessment of the soil quality by fuzzy mathematics in farmland around a uranium mill tailing repository in China. Radioprotection 2016, 51, 37–41. [Google Scholar] [CrossRef]
- Ye, Y.; Sun, X.; Zhao, J.; Wang, M.; Guan, Q. Establishing a soil quality index to assess the effect of thinning on soil quality in a Chinese fir plantation. Eur. J. For. Res. 2022, 141, 999–1009. [Google Scholar] [CrossRef]
- Fu, X.; Wang, J.; Sainju, U.M.; Liu, W. Aggregate size distribution and associated carbon and nitrogen in mulched winter wheat and spring corn. Can. J. Soil Sci. 2019, 99, 367–379. [Google Scholar] [CrossRef]
- Awale, R.; Emeson, M.A.; Machado, S. Soil organic carbon pools as early indicators for soil organic matter stock changes under different tillage practices in Inland Pacific Northwest. Front. Ecol. Evol. 2017, 5, 96. [Google Scholar] [CrossRef]
- Mohammed, Y.A.; Desta, K.G. Nutrient sources and harvest frequencies impact water stable soil macro-aggregates. Commun. Soil Sci. Plant Anal. 2017, 48, 2359–2367. [Google Scholar] [CrossRef][Green Version]
- Obidike-Ugwu, E.O.; Ogunwole, J.O.; Eze, P.N. Derivation and validation of a pedotransfer function for estimating the bulk density of tropical forest soils. Model. Earth Syst. Environ. 2023, 9, 801–809. [Google Scholar] [CrossRef]
- Wang, Q.J.; Zhang, J.T.; Gai, Z.J.; Cai, L.J.; Liu, J.Q.; Zhao, G.F.; Du, J.X.; Jiao, F. Effect of long-term straw mulching and no-tillage on physical properties of meadow soil in cold region. J. Appl. Ecol. 2018, 29, 2943–2948. [Google Scholar]
- Stošić, M.; Brozović, B.; Vinković, T.; Ravnjak, B.; Kluz, M.; Zebec, V. Soil resistance and bulk density under different tillage system. Poljoprivreda 2020, 26, 17–24. [Google Scholar] [CrossRef]
- Yin, W.; Chen, G.; Feng, F.; Guo, Y.; Hu, F.; Chen, G.; Zhao, C.; Yu, A.; Chai, Q. Straw retention combined with plastic mulching improves compensation of intercropped maize in arid environment. Field Crops Res. 2017, 204, 42–51. [Google Scholar] [CrossRef]
- Mateo-Marín, N.; Bosch-Serra, À.D.; Molina, M.G.; Poch, R.M. Impacts of tillage and nutrient management on soil porosity trends in dryland agriculture. Eur. J. Soil Sci. 2022, 73, e13139. [Google Scholar] [CrossRef]
- Cui, Y.; Shi, H.; Guo, A.; Sun, G.; Wang, G.; Wang, J.; Huang, W.; Lu, T. Effects of straw and biochar returned to the soil on soil physical properties and pH value in cold rice region. Asian Agric. Res. 2021, 13, 27–32. [Google Scholar]
- Zhao, S.; Li, K.; Zhou, W.; Qiu, S.; Huang, S.; He, P. Changes in soil microbial community, enzyme activities and organic matter fractions under long-term straw return in north-central China. Agric. Ecosyst. Environ. 2016, 216, 82–88. [Google Scholar] [CrossRef]
- Nicoloso, R.S.; Rice, C.W.; Amado, T.J.C.; Costa, C.N.; Akley, E.K. Carbon saturation and translocation in a no-till soil under organic amendments. Agric. Ecosyst. Environ. 2018, 264, 73–84. [Google Scholar] [CrossRef]
- Huang, M.; Zhou, X.; Cao, F.; Zou, Y. Long-term effect of no-tillage on soil organic carbon and nitrogen in an irrigated rice-based cropping system. Paddy Water Environ. 2016, 14, 367–371. [Google Scholar] [CrossRef]
- Liu, B.; Dai, Y.; Cheng, X.; He, X.; Bei, Q.; Wang, Y.; Zhou, Y.; Zhu, B.; Zhang, K.; Tian, X.; et al. Straw mulch improves soil carbon and nitrogen cycle by mediating microbial community structure and function in the maize field. Front. Microbiol. 2023, 14, 1217966. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Du, L.; Zhai, C.; Guan, Y.; Wu, S.; Zhang, Z.; Ogundeji, A.O.; Gu, S. Influence of tillage practices on phosphorus forms in aggregates of Mollisols from northeast China. J. Sci. Food Agric. 2021, 101, 4523–4531. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Li, C.; Cheng, K.; Shi, L.; Wen, L.; Xiao, X.; Xu, Y.; Li, W.; Wang, K. Effects of short-term soil tillage management on activity and community structure of denitrifiers under double-cropping rice field. J. Microbiol. Biotechnol. 2020, 30, 1688. [Google Scholar] [CrossRef] [PubMed]
- Kara, Z.; Yürürdurmaz, C.; Cokkızgın, A.; Keles, H.; Gonen, E. The effects of wheat straw used as mulch on some chemical properties of the soil and grain yield in durum wheat. Elixir Agric. 2021, 154, 55382–55386. [Google Scholar]
- Liu, G.-Y.; Yang, Y.; Zhang, Q.; Yang, L.-L.; Liang, L.-Y.; Ma, Q.; Tong, Y.-A. Effects of three mulching modes on winter wheat productivity and soil fertility in dryland of Weibei Rainfed Area. J. Plant Nutr. Fertil. 2018, 24, 857–868. [Google Scholar]
- Xiao, J.; Lei, Z.; Deti, X.; Chaofu, W. Study on the relationship between soil microbes and soil fertility in paddy fields of long-tern no-tillage and ridge culture. Xi Nan Nong Ye Da Xue Xue Bao J. Southwest Agric. Univ. 2002, 24, 82–85. [Google Scholar]
- Singh, D.; Lenka, S.; Lenka, N.K.; Trivedi, S.K.; Bhattacharjya, S.; Sahoo, S.; Saha, J.K.; Patra, A.K. Effect of reversal of conservation tillage on soil nutrient availability and crop nutrient uptake in soybean in the vertisols of central India. Sustainability 2020, 12, 6608. [Google Scholar] [CrossRef]
- Lv, L.; Gao, Z.; Liao, K.; Zhu, Q.; Zhu, J. Impact of conservation tillage on the distribution of soil nutrients with depth. Soil Tillage Res. 2023, 225, 105527. [Google Scholar] [CrossRef]
- Pérez, W.A.; Torres-Bazurto, J. Carbon-nitrogen ratio in soils with fertilizer applications and nutrient absorption in banana (Musa spp.) cv. Williams. Agron. Colomb. 2020, 38, 253–260. [Google Scholar] [CrossRef]
- Yuan, J.; Liang, Y.; Zhuo, M.; Sadiq, M.; Liu, L.; Wu, J.; Xu, G.; Liu, S.; Li, G.; Yan, L. Soil nitrogen and carbon storages and carbon pool management index under sustainable conservation tillage strategy. Front. Ecol. Evol. 2023, 10, 1082624. [Google Scholar] [CrossRef]
- Yan, F.; Zhou, W.; Sun, Y.; Guo, C.; Xiang, K.; Li, N.; Yang, Z.; Wu, Y.; Zhang, Q.; Wang, X.; et al. No-tillage with straw mulching promotes the utilization of soil nitrogen by rice under wheat–rice and oilseed rape–rice cropping systems. Front. Plant Sci. 2023, 14, 1170739. [Google Scholar] [CrossRef]
- Ghosh, A.; Singh, A.B.; Kumar, R.V.; Manna, M.C.; Bhattacharyya, R.; Rahman, M.M.; Sharma, P.; Rajput, P.S.; Misra, S. Soil enzymes and microbial elemental stoichiometry as bio-indicators of soil quality in diverse cropping systems and nutrient management practices of Indian Vertisols. Appl. Soil Ecol. 2020, 145, 103304. [Google Scholar] [CrossRef]
- Wang, W.; Hou, Y.; Pan, W.; Vinay, N.; Mo, F.; Liao, Y.; Wen, X. Continuous application of conservation tillage affects in situ N2O emissions and nitrogen cycling gene abundances following nitrogen fertilization. Soil Biol. Biochem. 2021, 157, 108239. [Google Scholar] [CrossRef]
- Adongo, J.O.; Osumba, S.; Misoi, S.; Kibet, J. Spectroscopic and Kinetic Study of Sucrose Oxidation by Cr (VI) and Its Application in the Quantitative Analysis of Soil Organic Carbon. Int. Res. J. Pure Appl. Chem. 2022, 1–15. [Google Scholar] [CrossRef]
- Zhu, Z.; Bai, Y.; Lv, M.; Tian, G.; Zhang, X.; Li, L.; Jiang, Y.; Ge, S. Soil Fertility, Microbial Biomass, and Microbial Functional Diversity Responses to Four Years Fertilization in an Apple Orchard in North China. Hortic. Plant J. 2020, 6, 223–230. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Soil microbial biomass: The eco-physiological approach. Soil Biol. Biochem. 2010, 42, 2039–2043. [Google Scholar] [CrossRef]
- Li, S.; Su, P.; Zhang, H.; Zhou, Z.; Xie, T.; Shi, R.; Gou, W. Distribution patterns of desert plant diversity and relationship to soil properties in the Heihe River Basin, China. Ecosphere 2018, 9, e02355. [Google Scholar] [CrossRef]
- Guo, L.; Shi, J.; Lin, W.; Liang, J.; Lu, Z.; Tang, X.; Liu, Y.; Wu, P.; Li, C. Soil bacteria mediate soil organic carbon sequestration under different tillage and straw management in rice-wheat cropping systems. Agriculture 2022, 12, 1552. [Google Scholar] [CrossRef]
- Dan, L.; Xia, Z.; Jun, L.; Xu-Dong, W. Effects of different tillage patterns on soil properties, maize yield and water use efficiency in Weibei Highland, China. J. Appl. Ecol. 2018, 29, 573. [Google Scholar]
- Tyler, H.L. Bacterial community composition under long-term reduced tillage and no till management. J. Appl. Microbiol. 2019, 126, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Iqbal, A.; He, L.; Zhao, Q.; Wei, S.; Ali, I.; Ullah, S.; Yan, B.; Jiang, L. Long-term no-tillage and straw retention management enhances soil bacterial community diversity and soil properties in southern China. Agronomy 2020, 10, 1233. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Luo, Y.; Awasthi, M.K.; Yang, J.; Duan, Y.; Li, H.; Zhao, Z. Mulching practices alter the bacterial-fungal community and network in favor of soil quality in a semiarid orchard system. Sci. Total Environ. 2020, 725, 138527. [Google Scholar] [CrossRef]
- Miao, S.; Zhao, H.; Qiao, Y.; Lu, X.; Li, Q. Evaluation of the Effects of Tillage Measures on the Topsoil Quality of Aeolian Sandy Soils in Northeast China Based on Tillage Index. Soil Fertil. Sci. China 2019, 9–15. [Google Scholar] [CrossRef]
- Toth, D.; Janků, J.; Marhoul, A.; Kozák, J.; Maitah, M.; Jehlička, J.; Řeháček, L.; Přikryl, R.; Herza, T.; Vopravil, J.; et al. Soil quality assessment using SAS (Soil Assessment System). Soil Water Res. 2023, 18, 1–15. [Google Scholar] [CrossRef]
- Martín-Sanz, J.P.; de Santiago-Martín, A.; Valverde-Asenjo, I.; Quintana-Nieto, J.; González-Huecas, C.; L. López-Lafuente, A. Comparison of soil quality indexes calculated by network and principal component analysis for carbonated soils under different uses. Ecol. Indic. 2022, 143, 109374. [Google Scholar] [CrossRef]
- Reid, M.J. On the Accuracy of Three-dimensional Kinematic Distances. Astron. J. 2022, 164, 133. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, D.E. Radial distance estimation with tapered whisker sensors. Sensors 2017, 17, 1659. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Lü, Y.; Sun, X.; Jia, S.; Zhang, X.; Liang, W. Conservation tillage positively influences the microflora and microfauna in the black soil of Northeast China. Soil Tillage Res. 2015, 149, 46–52. [Google Scholar] [CrossRef]
- Liang, A.Z.; Yang, X.M.; Zhang, X.P.; Chen, X.W.; Mclaughlin, N.B.; Wei, S.C.; Zhang, Y.; Jia, S.X.; Zhang, S.X. Changes in soil organic carbon stocks under 10-year conservation tillage on a Black soil in Northeast China. J. Agric. Sci. 2016, 154, 1425–1436. [Google Scholar] [CrossRef]
| Soil Layer (cm) | Bulk Density (g‧cm−3) | Field Capacity (%) | Sand (%) | Silt (%) | Clay (%) |
|---|---|---|---|---|---|
| 0–20 | 1.13 | 34.9 | 31.6 | 30.8 | 37.6 |
| Soil Depth (cm) | Treatments | Aggregate Size Distribution (%) | Mean Weight Diameter (mm) | |||
|---|---|---|---|---|---|---|
| >2 mm | 0.25–2 mm | 0.053–0.25 mm | <0.053 mm | |||
| 0–5 | NT | 1.47 a | 60.63 a | 18.31 a | 19.59 a | 0.48 a |
| CT | 0.53 b | 61.24 a | 19.35 a | 18.88 a | 0.40 a | |
| 5–10 | NT | 0.88 a | 57.95 a | 19.56 a | 21.61 a | 0.42 a |
| CT | 0.55 a | 59.30 a | 16.55 a | 23.60 a | 0.40 a | |
| 10–20 | NT | 0.54 a | 57.03 a | 19.69 a | 22.74 a | 0.38 a |
| CT | 0.61 a | 59.69 a | 19.31 a | 20.40 a | 0.41 a | |
| Soil Depth (cm) | Treatments | Fungi | Bacteria | ||
|---|---|---|---|---|---|
| Chao1 Index | Shannon Index | Chao1 Index | Shannon Index | ||
| 0–5 | NT | 766.97 ± 110.41 a | 3.19 ± 0.38 a | 2439.87 ± 373.56 a | 6.08 ± 0.29 a |
| CT | 794.88 ± 52.35 a | 3.31 ± 0.08 a | 2511.66 ± 56.16 a | 5.98 ± 0.16 a | |
| 5–10 | NT | 534.00 ± 129.39 a | 3.14 ± 0.79 a | 2218.48 ± 345.23 a | 5.60 ± 0.19 b |
| CT | 791.82 ± 197.99 a | 3.13 ± 0.22 a | 2758.26 ± 123.65 a | 6.09 ± 0.23 a | |
| 10–20 | NT | 566.38 ± 114.40 a | 3.26 ± 0.44 a | 2779.74 ± 155.97 a | 6.00 ± 0.11 a |
| CT | 499.85 ± 121.47 a | 2.95 ± 0.90 a | 2556.11 ± 118.72 a | 6.00 ± 0.11 a | |
| Sports Event | Principal Component | Weights | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Characteristic root | 7.407 | 6.917 | 3.557 | 2.578 | 1.54 | |
| Variance explained rate | 33.67% | 31.44% | 16.17% | 11.72% | 7.00% | |
| Cumulative contribution rate | 33.67% | 65.11% | 81.28% | 93.00% | 100.00% | |
| Factor loading | ||||||
| MWD | 0.1479 | 0.0718 | 0.0679 | 0.5452 | 0.1129 | 3.93% |
| BD | 0.0906 | 0.2829 | 0.2425 | 0.2547 | 0.0777 | 4.91% |
| FC | 0.2275 | 0.2763 | 0.0852 | 0.0358 | 0.1969 | 4.94% |
| TSP | 0.0056 | 0.3511 | 0.1362 | 0.0427 | 0.2227 | 3.92% |
| NCP | 0.1215 | 0.3208 | 0.1034 | 0.095 | 0.2759 | 4.78% |
| TOC | 0.1908 | 0.1721 | 0.3306 | 0.2171 | 0.099 | 5.17% |
| TN | 0.2376 | 0.0713 | 0.3624 | 0.1074 | 0.1803 | 4.71% |
| TP | 0.0749 | 0.3568 | 0.1226 | 0.0958 | 0.0221 | 4.30% |
| TK | 0.116 | 0.307 | 0.0298 | 0.1296 | 0.3621 | 4.58% |
| NH4+-N | 0.0565 | 0.3314 | 0.0089 | 0.0919 | 0.3553 | 4.06% |
| NO3−-N | 0.3053 | 0.0431 | 0.2002 | 0.2443 | 0.0137 | 4.51% |
| AVP | 0.3088 | 0.005 | 0.2627 | 0.0555 | 0.1615 | 4.20% |
| CAT | 0.2876 | 0.1522 | 0.2225 | 0.1158 | 0.1025 | 5.10% |
| AP | 0.3514 | 0.0035 | 0.1485 | 0.0254 | 0.0586 | 3.81% |
| SUC | 0.2955 | 0.2021 | 0.0764 | 0.1392 | 0.0056 | 4.86% |
| UE | 0.3421 | 0.0239 | 0.0833 | 0.0912 | 0.2318 | 4.13% |
| MBC | 0.2469 | 0.0515 | 0.3305 | 0.1267 | 0.2549 | 4.69% |
| MBN | 0.2213 | 0.0275 | 0.1207 | 0.46 | 0.1503 | 4.23% |
| FCI | 0.2357 | 0.2243 | 0.1927 | 0.0501 | 0.2575 | 5.19% |
| FSI | 0.0765 | 0.2445 | 0.173 | 0.2793 | 0.3912 | 4.83% |
| BCI | 0.075 | 0.1336 | 0.4665 | 0.1507 | 0.037 | 4.12% |
| BSI | 0.1107 | 0.2198 | 0.2038 | 0.3121 | 0.3382 | 5.05% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, J.; Sosa, F.A.; Chen, Q.; Zhang, X. Black Soil Quality After 19 Years of Continuous Conservation Tillage. Agronomy 2024, 14, 2859. https://doi.org/10.3390/agronomy14122859
Zhang C, Li J, Sosa FA, Chen Q, Zhang X. Black Soil Quality After 19 Years of Continuous Conservation Tillage. Agronomy. 2024; 14(12):2859. https://doi.org/10.3390/agronomy14122859
Chicago/Turabian StyleZhang, Chengyuan, Jianye Li, Francisco Alberto Sosa, Qiang Chen, and Xingyi Zhang. 2024. "Black Soil Quality After 19 Years of Continuous Conservation Tillage" Agronomy 14, no. 12: 2859. https://doi.org/10.3390/agronomy14122859
APA StyleZhang, C., Li, J., Sosa, F. A., Chen, Q., & Zhang, X. (2024). Black Soil Quality After 19 Years of Continuous Conservation Tillage. Agronomy, 14(12), 2859. https://doi.org/10.3390/agronomy14122859









