Heat Stress Resistance in Chlorella vulgaris Enhanced by Hydrolyzed Whey Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Whey Hydrolysates
2.2. Cultured Reference Strains
2.3. Growth Assays
2.4. Chlorophyll and Carotenoid Determination
2.5. qRT-PCR of psbA and psbC
2.6. Statistical Analysis
3. Results
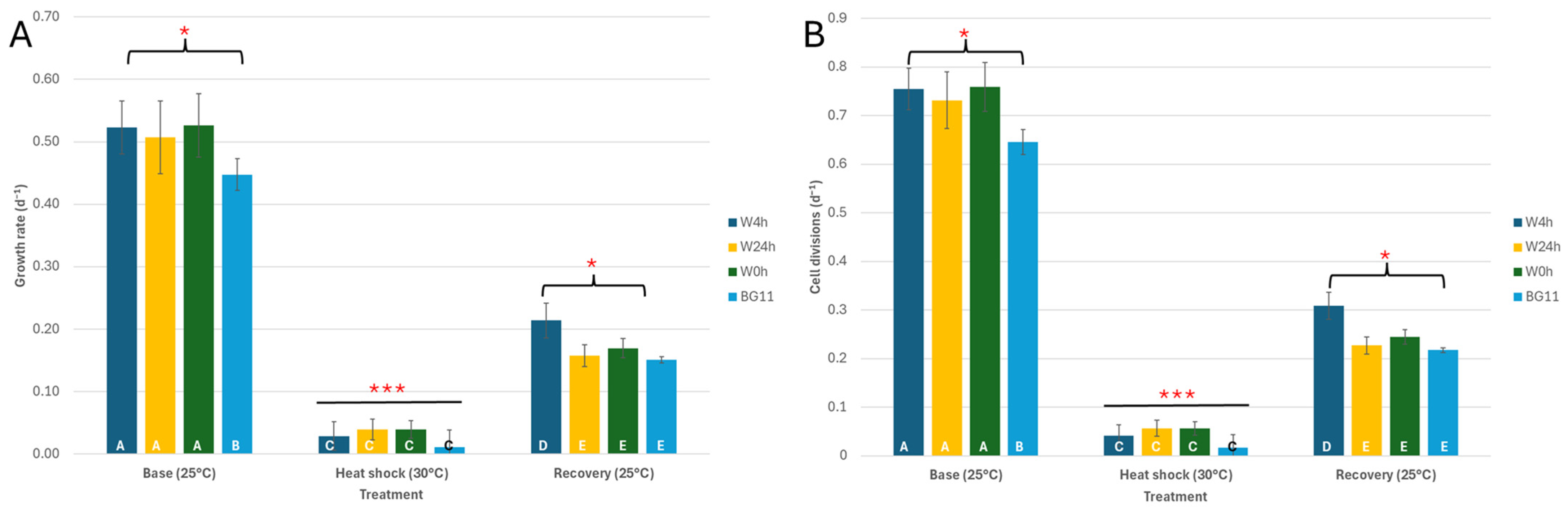
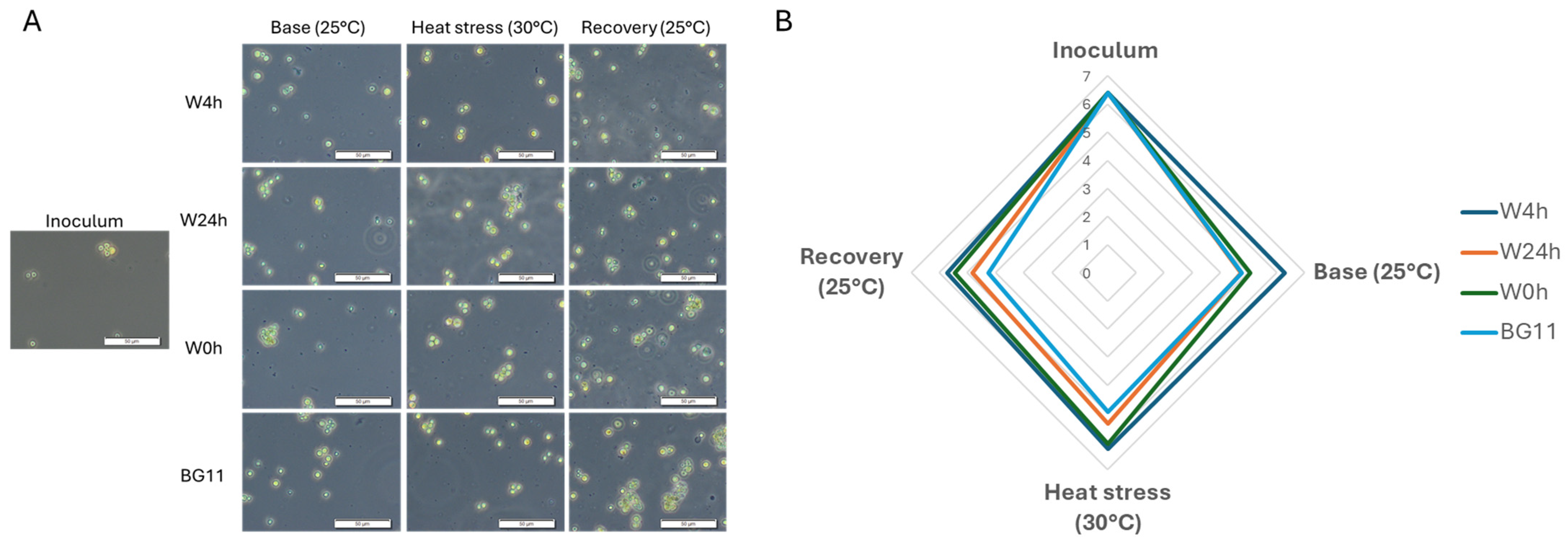
3.1. C. vulgaris Growth Under Heat Stress
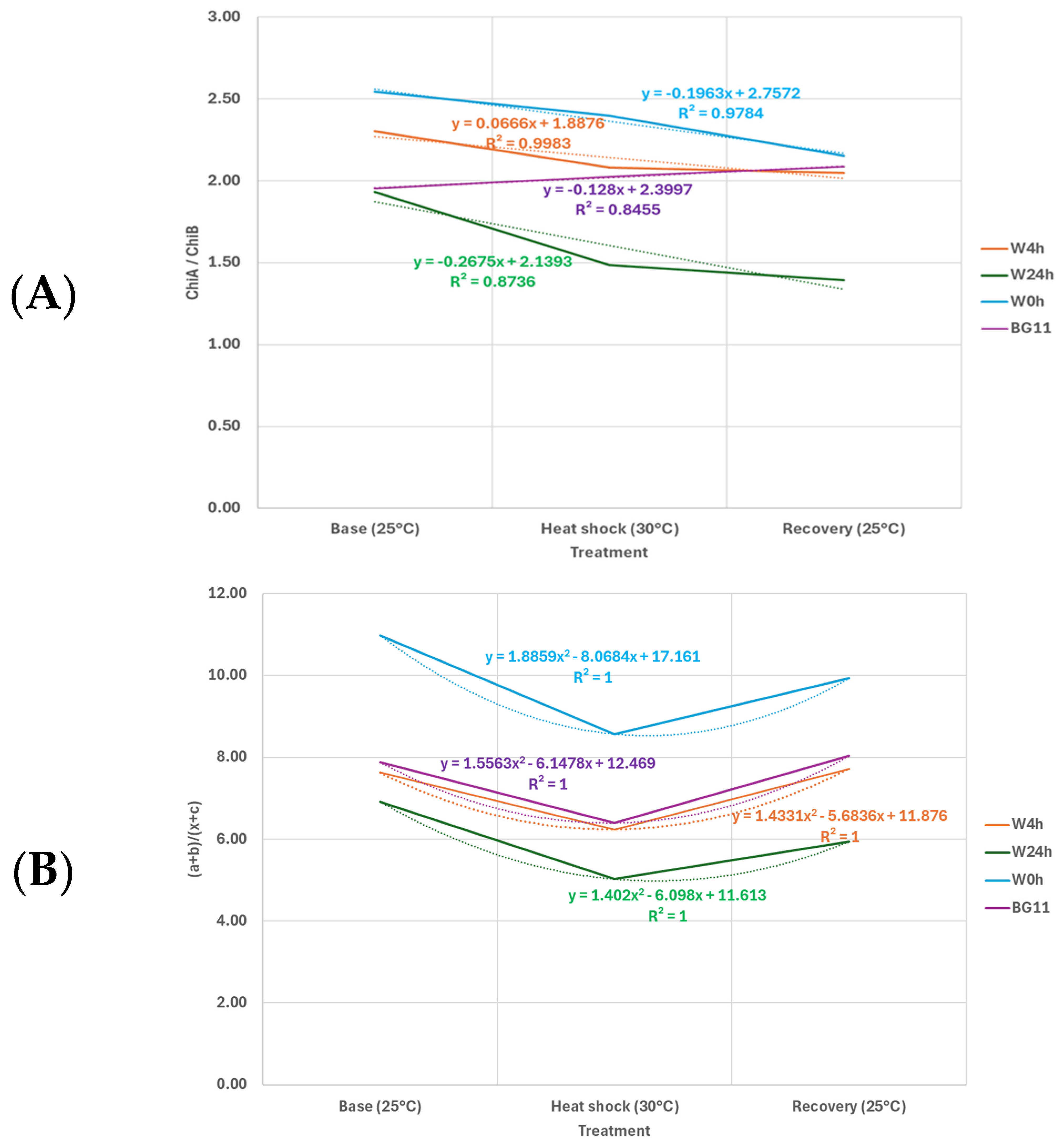
3.2. Heat Stress Induced Changes in Chlorophyll and Carotenoid Content
3.3. Heat Stress Induced Changes of psbA and psbC
4. Discussion
4.1. C. vulgaris Growth Under Heat Stress
4.2. Heat Stress Induced Changes in Chlorophyll and Carotenoid Content
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Maréchal-Drouard, L.; et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Vonshak, A. Stress physiology and cell division in Dunaliella: Implications for commercial production. J. Appl. Phycol. 2017, 29, 69–82. [Google Scholar]
- Harris, E.H.; Stern, D.B.; Witman, G.B. The Chlamydomonas Sourcebook, 2nd ed.; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Levine, R.P.; Goodenough, U.W. The genetics of photosynthesis and of the chloroplast in Chlamydomonas reinhardtii. Ann. Rev. Genet. 1970, 4, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, L.; Chu, H.; Zhou, X.; Yao, T.; Zhang, Y. Optimization for Scenedesmus obliquus Cultivation: The Effects of Temperature, Light Intensity and pH on Growth and Biochemical Composition. Microbiol. Biotechnol. Lett. 2019, 47, 614–620. [Google Scholar] [CrossRef]
- Schambach, J.Y.; Kruse, C.P.S.; Kitin, P.; Mays, W.; Hunt, C.G.; Starkenburg, S.R.; Barry, A.N. Metabolism of Scenedesmus obliquus cultivated with raw plant substrates. Front. Plant Sci. 2022, 13, 992702. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Tian, B.; Zhang, F.; Yin, J. Effects of nitrogen on growth and chlorophyll fluorescence of microalga Chlorella vulgaris in thebiomass production. Environ. Sci. Pollut. Res. 2011, 18, 563–570. [Google Scholar]
- Kumar, K.; Mishra, S.K.; Shrivastav, A.; Park, M.S.; Yang, J.W. Recent trends in the mass cultivation of algae in raceway ponds. Renew. Sustain. Energy Rev. 2011, 15, 3462–3470. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta—Bioenerg. 1997, 975, 384–394. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Prot. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Xu, H.; Liu, G.; Liu, G.; Yan, B.; Duan, W.; Wang, L.; Li, S. Comparison of investigation methods of heat injury in grapevine (Vitis) and assessment to heat tolerance in different cultivars and species. BMC Plant Biol. 2014, 14, 156. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998, 3, 147–151. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoprotection and other responses of plants to highlight stress. Ann. Rev. Plant. Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Aro, E.M.; McCaffery, S.; Anderson, J.M. Photoinhibition and D1 protein degradation in vivo: Kinetics and dose dependence. Proc. Natl. Acad. Sci. USA 1993, 90, 7356–7360. [Google Scholar]
- Baniulis, D.; Janda, T.; Žukauskaitė, G. Oxidative stress in plants under environmental conditions. Environ. Exp. Bot. 2008, 63, 52–63. [Google Scholar]
- Haldimann, P.; Feller, U. Inhibition of photosynthesis by heat stress: Effects on chloroplast proteins and electron transport. J. Exp. Bot. 2005, 56, 3037–3047. [Google Scholar]
- Zhou, J.; Jiang, X.; Wei, L. Impact of heat stress on Chlorella vulgaris: Photosynthesis and growth responses. Biotech. Biofuels 2017, 10, 45–53. [Google Scholar]
- Cheng, D.; He, Q.; Qiu, C. Responses of Chlorella vulgaris to heat stress and role of carotenoids in protecting PSII. J. Appl. Phycol. 2016, 28, 2081–2090. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; DePascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Meggio, F.; Trevisan, S.; Manoli, A.; Ruperti, B.; Quaggiotti, S. Systematic Investigation of the Effects of a Novel Protein Hydrolysate on the Growth, Physiological Parameters, Fruit Development and Yield of Grapevine (Vitis vinifera L., cv Sauvignon Blanc) under Water Stress Conditions. Agronomy 2020, 10, 1785. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed]
- Roche, D.; Rickson, J.R.; Pawlett, M. Moving towards a mechanistic understanding of biostimulant impacts on soil properties and processes: A semi-systematic review. Front. Agron. 2024, 6, 1271672. [Google Scholar] [CrossRef]
- ISO 8968-3:2007/IDF 20-3:2007; Milk. Determination of Nitrogen Content. International Organization for Standardization: Geneva, Switzerland, 2007.
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of Light Intensity and Quality on Growth Rate and Composition of Chlorella vulgaris. Plants 2019, 9, 31. [Google Scholar] [CrossRef]
- Fogg, G.E.; Thake, B. Algal Cultures and Phytoplankton Ecology; University of Wisconsin Press: Madison, WI, USA, 1987. [Google Scholar]
- Levasseur, M.; Thompson, P.A.; Harrison, P.J. Physiological acclimation of marine phytoplankton to different nitrogen sources. J. Phycol. 1993, 29, 587–595. [Google Scholar] [CrossRef]
- Chai, S.; Shi, J.; Huang, T.; Guo, Y.; Wei, J.; Guo, M.; Li, L.; Dou, S.; Liu, L.; Liu, G. Characterization of Chlorella sorokiniana growth properties in monosaccharide-supplemented batch culture. PLoS ONE 2018, 13, e0199873. [Google Scholar] [CrossRef]
- Barati, B.; Lim, P.E.; Gan, S.Y.; Poong, S.W.; Phang, S.M. Gene expression profile of marine Chlorella strains from different latitudes: Stress and recovery under elevated temperatures. J. Appl. Phycol. 2018, 30, 3121–3130. [Google Scholar] [CrossRef]
- Chatelain, P.; Blanchard, C.; Astier, J.; Klinguer, A.; Wendehenne, D.; Jeandroz, S.; Rosnoblet, C. Reliable reference genes and abiotic stress marker genes in Klebsormidium nitens. Sci. Rep. 2022, 12, 18988. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Tukey’s Honestly Significant Difference (HSD) Test. In Encyclopedia of Research Design; Salkind, N.J., Ed.; Sage: Thousand Oaks, CA, USA, 2010. [Google Scholar]
- Van der Wiel, J.B.; Mikulicz, J.D.; Boysen, M.R.; Hashemi, N.; Kalgren, P.; Nauman, L.M.; Baetzold, S.J.; Powell, G.G.; He, Q.; Hashemi, N.N. Characterization of Chlorella vulgaris and Chlorella protothecoides using multi-pixel photon counters in a 3D focusing optofluidic system. RSC Adv. 2017, 7, 4402–4408. [Google Scholar] [CrossRef]
- Cassidy, K.O. Evaluating Algal Growth at Different Temperatures. Master’s Thesis, Biosystems and Agricultural Engineering, University of Kentucky, Lexington, KY, USA, 2011. [Google Scholar]
- Sánchez-Luna, L.D.; Bezerra, R.P.; Matsudo, M.C.; Sato, S.; Converti, A.; Carvalho, J.C.M. Influence of pH, temperature, and urea molar flowrate on Arthrospira platensis fed-batch cultivation: A kinetic and thermodynamic approach. Biotechnol. Bioeng. 2007, 96, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Serra-Maia, R.; Bernard, O.; Gonçalves, A.; Bensalem, S.; Lopes, F. Influence of temperature on Chlorella vulgaris growth and mortality rates in a photobioreactor. Algal. Res. 2016, 18, 352–359. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Zlotnik, I.; Dubinsky, Z. The effect of light and temperature on DOC excretion by phytoplankton. Limnol. Oceanogr. 1989, 34, 831–839. [Google Scholar] [CrossRef]
- Song, K.; Zhou, Z.; Huang, Y.; Chen, L.; Cong, W. Multi-omics insights into the mechanism of the high-temperature tolerance in a thermotolerant Chlorella sorokiniana. Bioresour. Technol. 2023, 390, 129859. [Google Scholar] [CrossRef]
- Ahmad, S.; Kothari, R.; Shankarayan, R.; Tyagi, V.V. Temperature dependent morphological changes on algal growth and cell surface with dairy industry wastewater: An experimental investigation. 3 Biotech 2020, 10, 24. [Google Scholar] [CrossRef]
- Ras, M.; Steyer, J.P.; Bernard, O. Temperature effect on microalgae: A crucial factor for outdoor production. Rev. Environ. Sci. Biotech. 2013, 12, 153–164. [Google Scholar] [CrossRef]
- Peter, K.H.; Sommer, U. Interactive effect of warming, nitrogen and phosphorus limitation on phytoplankton cell size. Ecol. Evol. 2015, 5, 1011–1024. [Google Scholar] [CrossRef]
- Piontek, J.; Handel, N.; Langer, G.; Wohlers, J.; Riebesell, U.; Engel, A. Effects of rising temperature on the formation and microbial degradation of marine diatom aggregates. Aquat. Microb. Ecol. 2009, 54, 305–318. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Gahlaut, V.; Mangal, V.; Kumar, A.; Singh, M.P.; Paul, V.; Kumar, S.; Singh, B.; Zinta, G. Physiological and molecular insights on wheat responses to heat stress. Plant Cell Rep. 2022, 41, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Zuppini, A.; Andreoli, C.; Baldan, B. Heat stress: An inducer of programmed cell death in Chlorella saccharophila. Plant Cell Physiol. 2007, 48, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar]
- Gitelson, A.A. Wide dynamic range vegetation index for remote quantification of biophysical characteristics of vegetation. J. Plant. Physiol. 1999, 161, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef]
- Napaumpaiporn, P.; Sirikhachornkit, A. Effects of high temperature on Carotenoid Accumulation and Gene Expression in the Model Green Alga Chlamydomonas reinhardtii. Chiang Mai J. Sci. 2016, 43, 453–461. [Google Scholar]
- Chettri, M.K.; Cook, C.M.; Vardaka, E.; Sawidis, T.; Lanaras, T. The effect of Cu, Zn, and Pb on the chlorophyll content of the lichens Cladonia convoluta and Cladonia rangiformis. Environ. Exp. Bot. 1988, 39, 1–10. [Google Scholar] [CrossRef]
- Yamane, Y.; Kashino, Y.; Koike, H.; Satoh, K. Increases in the fluorescence Fo level and reversible inhibition of photosystem II reaction center by high-temperature treatments in higher plants. Photosynth. Res. 1997, 52, 57–64. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, B.; Liu, Y. Differential accumulation of chlorophyll and alteration of chlorophyll a/b ratio in response to drought and heat stress in creeping bentgrass. J. Am. Soc. Hortic. Sci. 2017, 142, 295–302. [Google Scholar]
- Nakamura, K.; Ogawa, T.; Shibata, K. Chlorophyll and peptide compositions in the two photosystems of marine green algae. Biochim. Biophys. Acta 1976, 423, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Kizilkaya, I.T.; Akcaalan, S.; Unal, D. Determination of Photosynthesis-Related and Ascorbate Peroxidase Gene Expression in the Green Algae (Chlorella vulgaris) Under High-Temperature Conditions. Int. J. Second Metab. 2021, 8, 59–69. [Google Scholar] [CrossRef]
- Elwazuru, E.; Ismail, H.; Abou El-Khairi, E.-S.; Al_Qahtani, S.M.; Al-Harbi, N.-A.; Abd El-Gawad, H.G.; Omar, W.A.; Abdelaal, K.; Osman, A. Biostimulant application of whey protein hydrolysates and potassium fertilization enhances the productivity and tuber quality of sweet potato. Not. Bot. Horti. Agrobo. 2023, 51, 13122. [Google Scholar] [CrossRef]
- Riseh, R.A.; Vazvani, M.G.; Hassanisaadi, M.; Thakur, V.K.; Kennedy, J.F. Use of whey protein as a natural polymer for the encapsulation of plant biocontrol bacteria: A review. Int. J. Biol. Macromol. 2023, 234, 123708. [Google Scholar] [CrossRef]


| Primer Sequence (5′–3′) | |||||
|---|---|---|---|---|---|
| Function | Gene Symbol | Forward | Reverse | Amplicon Size (bp) | Reference |
| D1 synthesis | psbA | GGTGGTCCTTACCAACTTATCGTTTG | GGTCCTTACCAACTTATCGTTTG | 98 | [29] |
| PSII subunit | psbC | GAACATCACCACCACCAGGA | CGGTGCTTGGCTTTTAGTTTG | 79 | [29] |
| Endogenous control | H3 | GAGATCCGCAAGTACCAGAAG | GGTCTTGAAGTCCTGGGC | 93 | [29] |
| A | Base (25 ± 1 °C) | Heat Stress (30 ± 1 °C) | Recovery (25 ± 1 °C) | |
| Generation time (d) ± SD | W4 h | 1.32 ± 0.39 | 23.99 ± 0.74 | 3.24 ± 0.33 |
| W24 h | 1.37 ± 0.46 | 17.58 ± 0.87 | 4.40 ± 0.62 | |
| W0 h | 1.32 ± 0.54 | 17.63 ± 0.99 | 4.09 ± 0.52 | |
| BG11 | 1.55 ± 0.43 | 61.12 ± 0.47 | 4.59 ± 0.35 | |
| B | ||||
| Productivity (g/L/d) ± SD | W4 h | 0.081 ± 0.023 | 0.012 ± 0.026 | 0.320 ± 0.055 |
| W24 h | 0.053 ± 0.019 | 0.011 ± 0.032 | 0.147 ± 0.042 | |
| W0 h | 0.082 ± 0.022 | 0.020 ± 0.042 | 0.274 ± 0.066 | |
| BG11 | 0.056 ± 0.003 | 0.001 ± 0.034 | 0.152 ± 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brück, W.M.; Alfonso, E.; Rienth, M.; Andlauer, W. Heat Stress Resistance in Chlorella vulgaris Enhanced by Hydrolyzed Whey Proteins. Agronomy 2024, 14, 2854. https://doi.org/10.3390/agronomy14122854
Brück WM, Alfonso E, Rienth M, Andlauer W. Heat Stress Resistance in Chlorella vulgaris Enhanced by Hydrolyzed Whey Proteins. Agronomy. 2024; 14(12):2854. https://doi.org/10.3390/agronomy14122854
Chicago/Turabian StyleBrück, Wolfram Manuel, Esteban Alfonso, Markus Rienth, and Wilfried Andlauer. 2024. "Heat Stress Resistance in Chlorella vulgaris Enhanced by Hydrolyzed Whey Proteins" Agronomy 14, no. 12: 2854. https://doi.org/10.3390/agronomy14122854
APA StyleBrück, W. M., Alfonso, E., Rienth, M., & Andlauer, W. (2024). Heat Stress Resistance in Chlorella vulgaris Enhanced by Hydrolyzed Whey Proteins. Agronomy, 14(12), 2854. https://doi.org/10.3390/agronomy14122854





