Unravelling Yield and Yield-Related Traits in Soybean Using GGE Biplot and Path Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Description of the Study Locations
2.3. Experimental Design
2.4. Data Collection
2.4.1. Days to Flowering (DTF) and Days to Maturity (DTM)
2.4.2. Plant Height, Pod Clearance, and Number of Branches
2.4.3. Length and Number of Internodes
2.4.4. Yield and Yield-Related Traits
2.5. Data Analysis
2.5.1. Multi-Locational Yield Data
2.5.2. Correlation Analysis
2.5.3. Path Analysis
3. Results
3.1. Grain Yield Performance of Genotypes Across Locations
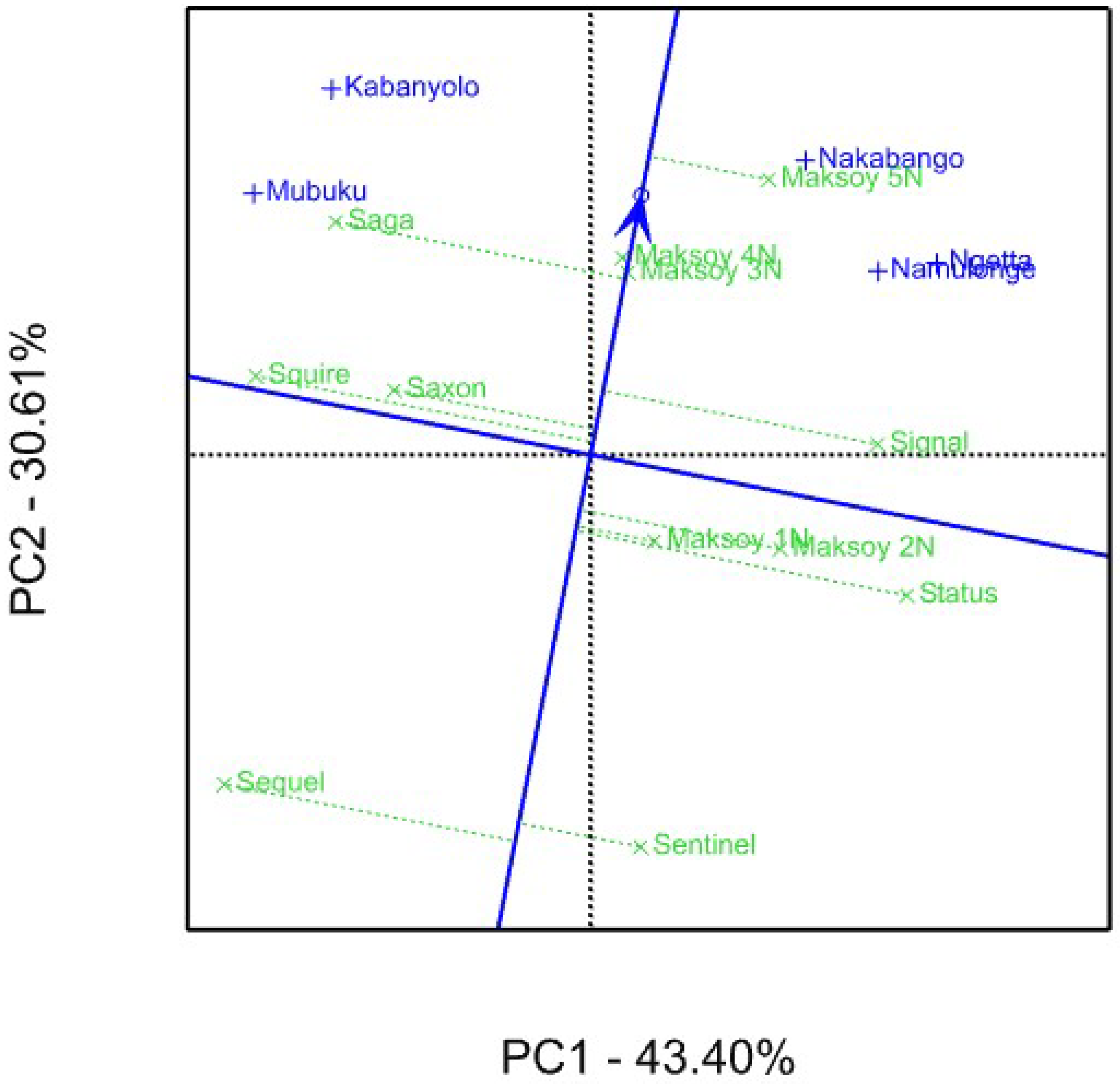
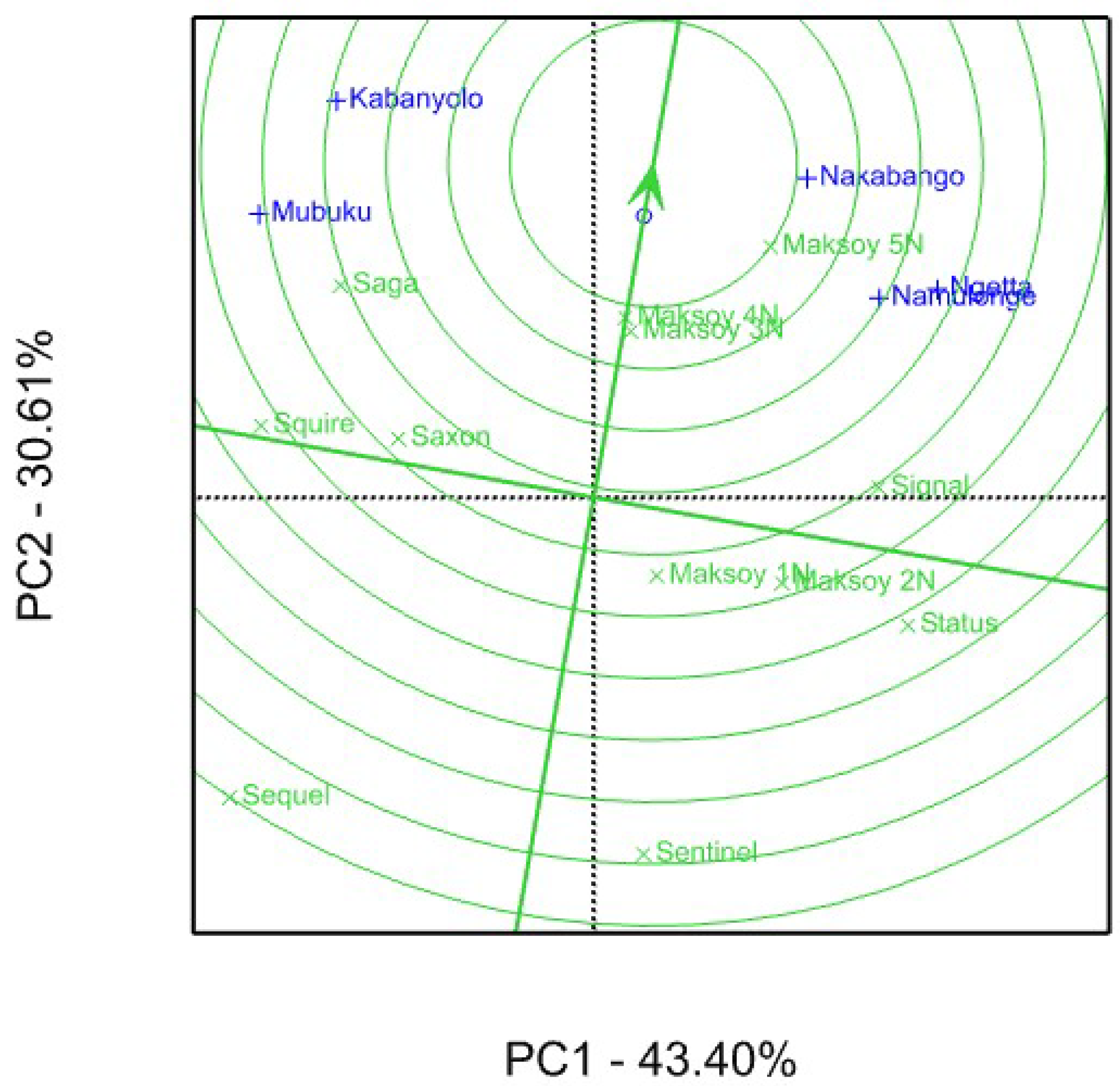
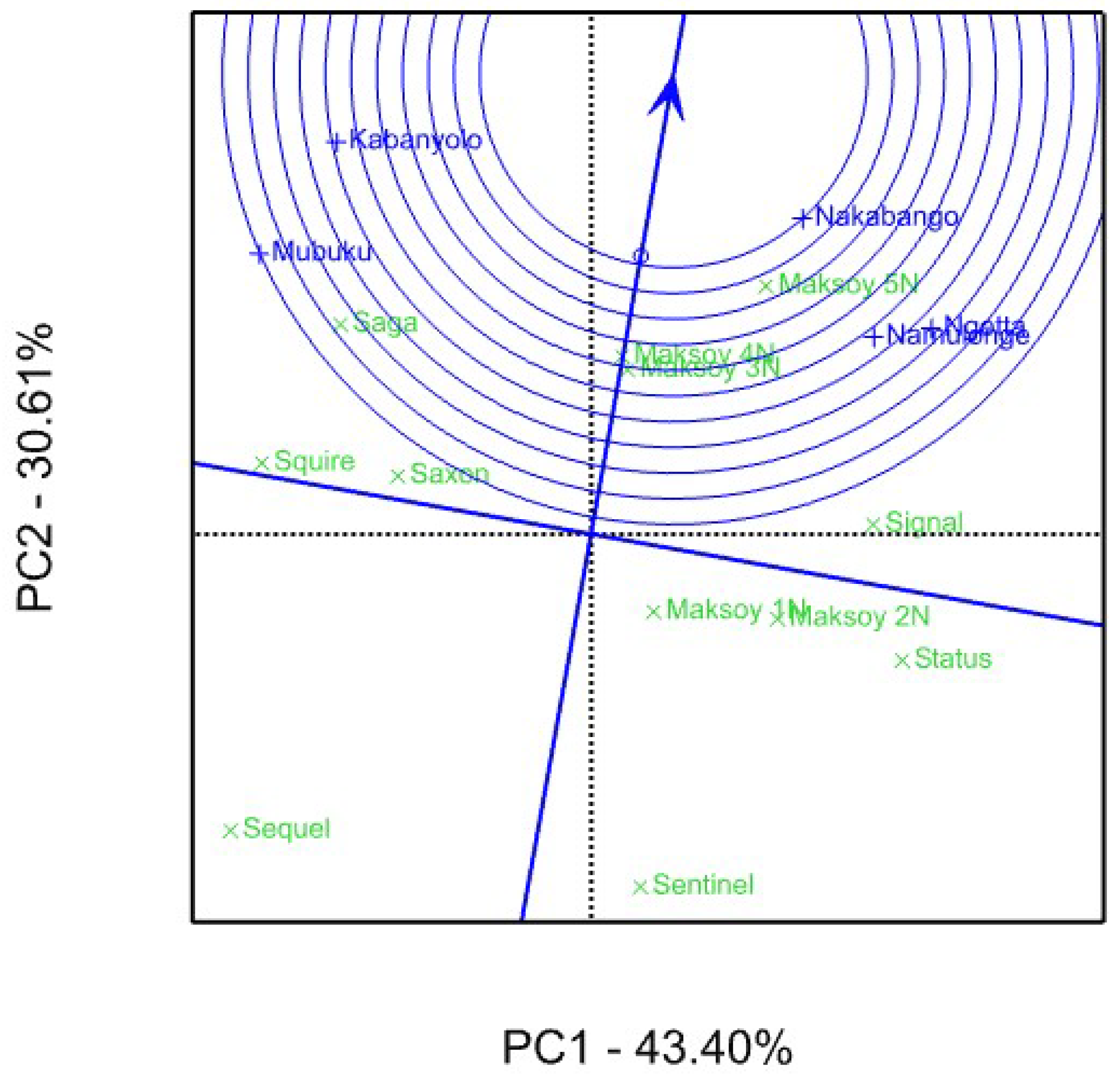
3.2. Genotype Yield Stability Using GGE Biplot Analysis
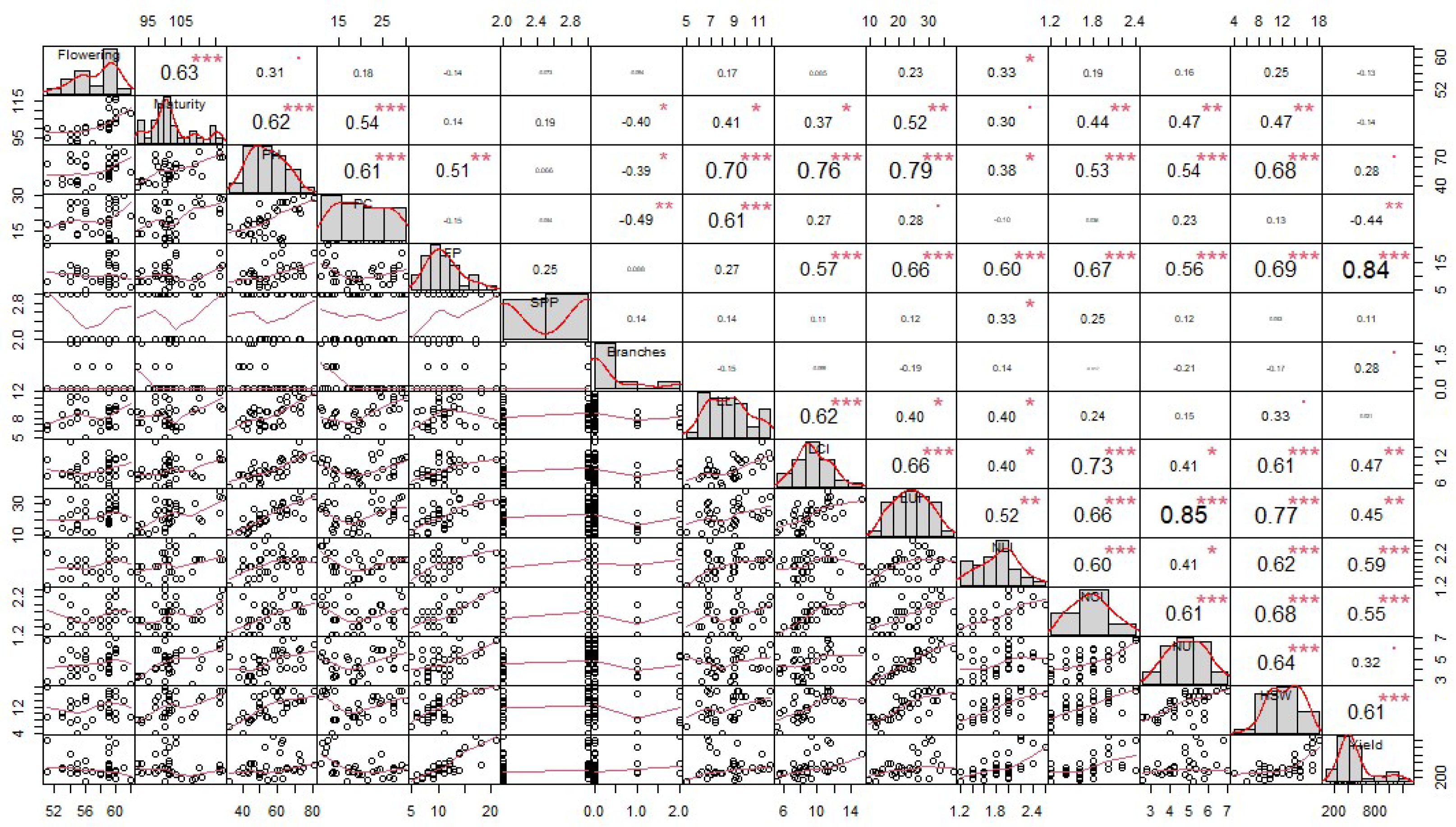
3.3. Correlations Between Grain Yield and Yield-Related Traits
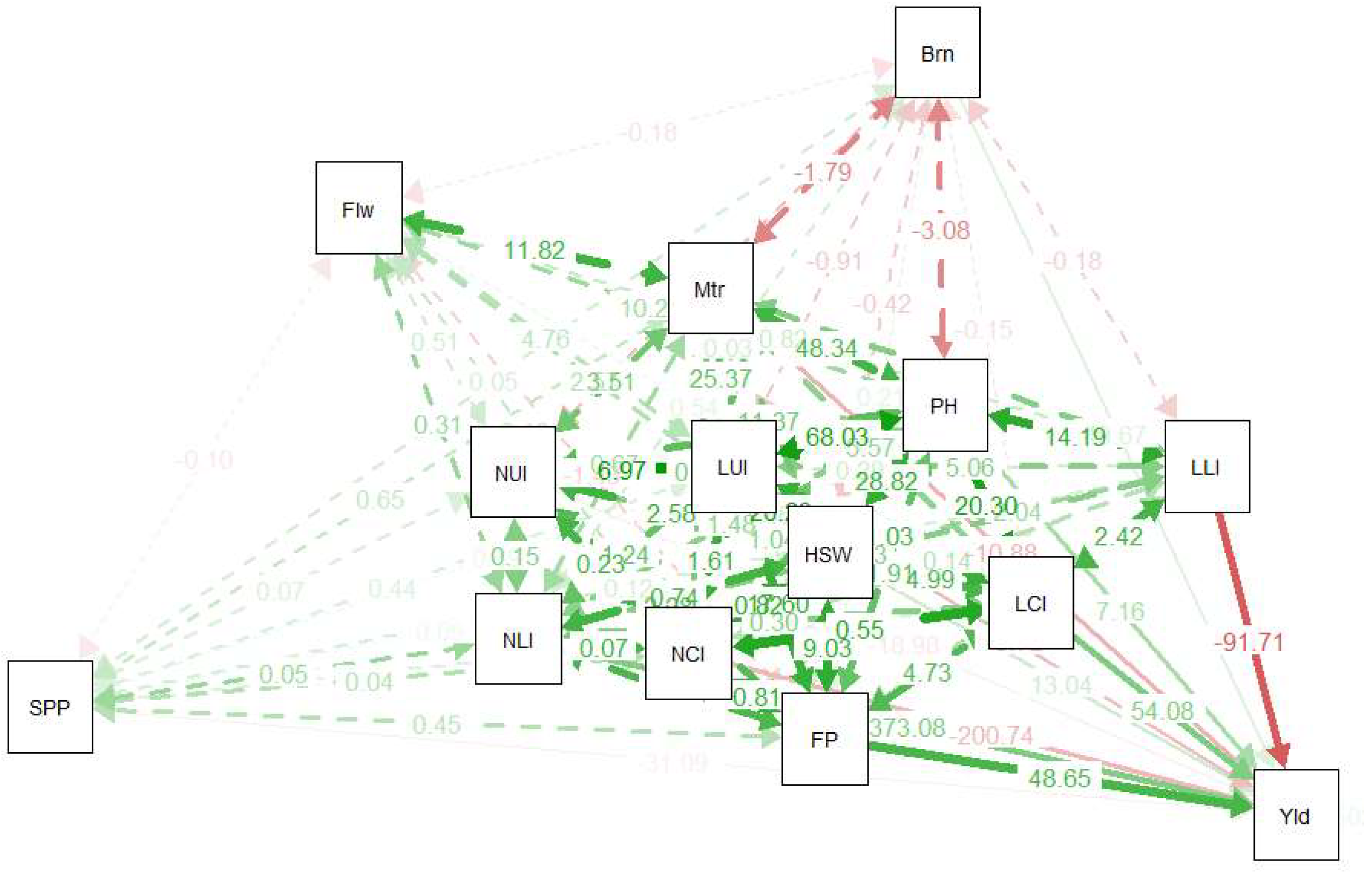
3.4. Path Analysis of Yield-Related Traits
4. Discussion
4.1. Grain Yield Performance of Genotypes Across Locations
4.2. Genotype Yield Stability Using GGE Biplot Analysis
4.3. Correlations Between Grain Yield and Yield-Related Traits
4.4. Path Analysis of Yield-Related Traits
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, K.S. Breeding Oilseed Crops for Sustainable Production: Opportunities and Constraints; Academic Press: Cambridge, MA, USA, 2015; ISBN 978-0-12-801309-0. [Google Scholar]
- Our World in Data. Available online: https://ourworldindata.org/drivers-of-deforestation#is-our-appetite-for-soy-driving-deforestation-in-the-amazon (accessed on 6 August 2024).
- Agralytica. Agralytica United Soybean Board Connections 2012: Soybean Market Scan; Agralytica: Washington, DC, USA, 2012; p. 97. [Google Scholar]
- FAOSTAT. 2022. Available online: http://www.fao.org/faostat (accessed on 17 November 2024).
- WWF. The Growth of Soy: Impacts and Solutions; WWF International: Gland, Switzerland, 2014; ISBN 978-2-940443-79-6. Available online: http://issuu.com/wwfsoyreport/docs/wwf_soy_report_final_jan_19/1?e=10667775/6569194 (accessed on 26 October 2024).
- Joala, R. The Rise of Soya in Zambia and the Integration of smallholder farmers. Inst. Poverty Land Agrar. Stud. 2018, 1–4. [Google Scholar]
- Masuda, T.; Goldsmith, P.D. World soybean production: Area harvested, yield, and long-term projections. Int. Food Agribus. Manag. Rev. 2009, 12, 143–162. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, R.; Sabapathy, S.N.; Bawa, A.S. Functional and edible uses of soy protein products. Compr. Rev. Food Sci. Food Saf. 2008, 7, 14–28. [Google Scholar] [CrossRef]
- Hardarson, G.; Atkins, C. Optimising biological N2 fixation by legumes in farming systems. Plant Soil 2003, 252, 41–54. [Google Scholar] [CrossRef]
- Sanginga, N.; Woomer, P.L. Integrated Soil Fertility Management in Africa: Principles Practices and Development Process; Tropical Soil Biology and Fertility (TSBF); International Centre for Tropical Agriculture (CIAT): Nairobi, Kenya, 2009; ISBN 978-92-9059-261-7. [Google Scholar]
- Sanginga, P.C.; Adesina, A.A.; Manyong, V.M.; Otite, O.; Dashiell, K.E. Social impact of soybean in Nigeria’s southern Guinea savanna. Trop. Agric. 1999, 34. [Google Scholar]
- Sarutayophat, T. Correlation and path coefficient analysis for yield and its components in vegetable soybean. Songklanakarin J. Sci. Technol. 2012, 34, 273–277. [Google Scholar]
- Balla, M.Y.; Ibrahim, S.E. Genotypic Correlation and Path Coefficient Analysis of Soybean [Glycine max (L.) Merr.] for Yield and Its Components. Agric. Res. Technol. 2017, 7, 3–7. [Google Scholar]
- Ferrari, M.; Carvalho, I.R.; de Pelegrin, A.J.; Nardino, M.; Szareski, V.J.; Olivoto, T.; da Rosa, T.C.; Follmann, D.N.; Pegoraro, C.; da Maia, L.C.; et al. Path analysis and phenotypic correlation among yield compnents of soybean using environmental stratification methods. Aust. J. Crop Sci. 2018, 12, 193–202. [Google Scholar] [CrossRef]
- Wei, M.C.F.; Molin, J.P. Soybean yield estimation and its components: A linear regression approach. Agric. Switz. 2020, 10, 348. [Google Scholar] [CrossRef]
- Karyawati, A.S.; Puspitaningrum, E.S.V. Correlation and path analysis for agronomic traits contributing to yield in 30 genotypes of soybean. Biodiversitas 2021, 22, 1146–1151. [Google Scholar] [CrossRef]
- Lekota, M.P.; Morojele, M.E.; Motanyane, M.S. Assessment of Yield and Yield Components of Soya-Bean (Glycine max (L.) Merril) Grown Under Conventional Agronomic Practices of Lesotho. SSRN Electron. J. 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Mulugeta, A.; Kidane, S.; Abadi, S.; Fisha, Z. GGE biplots to analyze soybean multi-environment yield trial data in north Western Ethiopia. J. Plant Breed. Crop Sci. 2013, 5, 245–254. [Google Scholar] [CrossRef]
- Adie, M.M.; Krisnawati, A.; Gatut-Wahyu, A.S. Assessment of Genotype × Environment Interactions for Black Soybean Yield using Ammi and GGE Biplot. Int. J. Agric. Innov. Res. 2014, 2, 673–678. [Google Scholar]
- Cheelo, P.; Lungu, D.; Mwala, M. GGE Biplot Analysis for Identification of Ideal Soybean [Glycine max L. Merrill] Test and Production Locations in Zambia. J. Exp. Agric. Int. 2017, 15, 1–15. [Google Scholar] [CrossRef]
- El-Mohsen, A. Agronomical evaluation of six soybean cultivars using correlation and regression analysis under different irrigation regime conditions. J. Plant Breed. Crop Sci. 2013, 5, 91–102. [Google Scholar] [CrossRef]
- de Carvalho, C.G.P.; Arias, C.A.A.; de Toledo, J.F.F.; de Oliveira, M.F.; Vello, N.A. Correlações e análise de trilha em linhagens de soja semeadas em diferentes épocas. Pesqui. Agropecuária Bras. 2002, 37, 311–320. [Google Scholar] [CrossRef]
- Akram, R.M.; Fares, W.M.; Fateh, H.S.A.; Rizk1, A.M.A. Genetic variability, correlation and path analysis in soybean. Legume Res. 2011, 34, 36–40. [Google Scholar]
- Iqbal, S.; Mahmood, T.; Tahira Muhammad Ali, M.A.; Sarwar, M. Path Coefficient Analysis in Different Genotypes of Soybean (Glycine max (L.) Merril). Pak. J. Biol. Sci. 2003, 6, 1085–1087. [Google Scholar] [CrossRef]
- Vu, T.T.H.; Le, T.T.C.; Vu, D.H.; Nguyen, T.T.; Ngoc, T. Correlations and Path Coefficients for Yield Related Traits in Soybean Progenies. Asian J. Crop Sci. 2019, 11, 32–39. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, G.B.; Marim, B.G.; da Silva, D.J.H.; Mattedi, A.P.; de Souza, V. Path analysis of primary and secondary yield components in tomato plants of the Salad group. Pesqui. Agropecu. Bras. 2010, 45, 155–162. [Google Scholar] [CrossRef]
- Dvorjak, D.S.; Unêda-Trevisoli, S.H.; De Sousa Leite, W.; Da Silva, A.J.; Da Silva, F.M.; Di Mauro, A.O. Correlations and path analysis in soybean progenies with resistance source to cyst nematode (race 3). Comun. Sci. 2019, 10, 168–175. [Google Scholar] [CrossRef]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-5. 2021. Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 26 October 2024).
- Yan, W.; Rajcan, I. Biplot analysis of test sites and trait relations of soybean in Ontario. Crop Sci. 2002, 42, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. R Package Version 0.8.4. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 26 October 2024).
- Taiyun, W.; Viliam, S. R package “corrplot”: Visualization of a Correlation Matrix. (Version 0.88). Available online: https://github.com/taiyun/corrplot (accessed on 26 October 2024).
- Lenka, D.; Misra, B. Path Coefficient Analysis of Yield in Rice Varieties. Indian J. Agric. Sci. 1973, 43, 376–379. [Google Scholar]
- Obua, T.; Sserumaga, J.P.; Awio, B.; Nganga, F.; Odong, T.L.; Tukamuhabwa, P.; Tusiime, G.; Mukasa, S.B.; Nabasirye, M. Multi-environmental evaluation of protein content and yield stability among tropical soybean genotypes using gge biplot analysis. Agronomy 2021, 11, 1265. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S.; Ma, B.; Woods, S.; Cornelius, P.L. GGE biplot vs. AMMI analysis of genotype-by-environment data. Crop Sci. 2007, 47, 643–655. [Google Scholar] [CrossRef]
- Tukamuhabwa, P.; Oloka, H.K.; Sengooba, T.; Kabayi, P. Yield stability of rust-resistant soybean lines at four mid-altitude tropical locations. Euphytica 2012, 183, 1–10. [Google Scholar] [CrossRef]
- Valencia-Ramírez, R.A.; Ligarreto-Moreno, G.A. Phenotypic correlation and path analysis for yield in soybean (Glycine max (L.) Merril). Acta Agronómica 2012, 61, 353–363. [Google Scholar]
- Kozak, M.; Azevedo, R.A. Does using stepwise variable selection to build sequential path analysis models make sense? Physiol. Plant. 2011, 141, 197–200. [Google Scholar] [CrossRef]
- Xu, C.; Li, R.; Song, W.; Wu, T.; Sun, S.; Hu, S.; Han, T.; Wu, C. Responses of Branch Number and Yield Component of Soybean Cultivars Tested in Different Planting Densities. Agriculture 2021, 11, 69. [Google Scholar] [CrossRef]
- Allen, L.H.; Zhang, L.; Boote, K.J.; Hauser, B.A. Elevated temperature intensity, timing, and duration of exposure affect soybean internode elongation, mainstem node number, and pod number per plant. Crop J. 2018, 6, 148–161. [Google Scholar] [CrossRef]
- Pereira-Flores, M.E.; Justino, F.B. Yield Components and Biomass Partition in Soybean: Climate Change Vision; IntechOpen: London, UK, 2019. [Google Scholar]
- Van Roekel, R.J.; Purcell, L.C. Understanding and Increasing Soybean Yields. 2016, 1–6. Available online: https://dr.lib.iastate.edu/entities/publication/864f0deb-5cb3-437d-a0da-ea6b049a2e14 (accessed on 26 October 2024). [CrossRef]
- Van Roekel, R.J.; Purcell, L.C.; Salmerón, M. Physiological and management factors contributing to soybean potential yield. Field Crops Res. 2015, 182, 86–97. [Google Scholar] [CrossRef]

| Genotype | Origin |
|---|---|
| 1. Maksoy 1N | Makerere University |
| 2. Maksoy 2N | Makerere University |
| 3. Maksoy 3N | Makerere University |
| 4. Maksoy 4N | Makerere University |
| 5. Maksoy 5N | Makerere University |
| 6. Saga | Seed Co. |
| 7. Saxon | Seed Co. |
| 8. Sentinel | Seed Co. |
| 9. Sequel | Seed Co. |
| 10. Signal | Seed Co. |
| 11. Squire | Seed Co. |
| 12. Status | Seed Co. |
| Location | Region | Coordinates | Altitude (masl) | Mean Annual Temperature (°C) | Mean Annual Rainfall (mm) |
|---|---|---|---|---|---|
| Namulonge | Central | 0o32′ N/32o37′ E | 1160 | 22.6 | 1400 |
| Kabanyolo | Central | 0o28′ N/32o36′ E | 1180 | 21.4 | 1234 |
| Nakabango | Eastern | 0o29′ N/33°14′ E | 1210 | 22.8 | 1400 |
| Ngetta | Northern | 2°17′ N/32°56′ E | 1103 | 24.7 | 1200 |
| Mubuku | Western | 0o13′ N/30o08′ E | 1007 | 27.8 | 750 |
| Source of Variation | DF | Sum Sq | Mean Sq | F Value | F Pr. |
|---|---|---|---|---|---|
| Season | 1 | 5,262,177 | 5,262,177 | 84.68 | *** |
| Genotype | 11 | 3,408,556 | 309,869 | 4.99 | *** |
| Location | 4 | 32,565,992 | 8,141,498 | 131.01 | *** |
| Genotype × Season | 11 | 1,275,954 | 115,996 | 1.87 | ns |
| Genotype × Location | 44 | 9,570,005 | 217,500 | 3.5 | *** |
| Location × Season | 4 | 15,471,863 | 3,867,966 | 62.24 | *** |
| Genotype × Location × Season | 44 | 2,585,386 | 58,759 | 0.95 | ns |
| Rep | 2 | 51,681 | 25,840 | 0.42 | |
| Residual | 232 | 14,417,735 | 62,145 | ||
| Total | 353 | 83,847,837 |
| Genotype | Kabanyolo | Mubuku | Nakabango | Namulonge | Ngetta | Mean |
|---|---|---|---|---|---|---|
| Maksoy 1N | 666 | 1067 | 859 | 286 | 1421 | 860 abc |
| Maksoy 2N | 686 | 947 | 689 | 570 | 1234 | 825 abc |
| Maksoy 3N | 729 | 1242 | 808 | 523 | 1349 | 930 bc |
| Maksoy 4N | 774 | 1187 | 699 | 459 | 1771 | 978 c |
| Maksoy 5N | 739 | 1094 | 1088 | 406 | 1569 | 979 c |
| Saga | 809 | 1342 | 765 | 379 | 1142 | 887 abc |
| Saxon | 763 | 1237 | 726 | 378 | 1031 | 827 abc |
| Sentinel | 646 | 844 | 648 | 362 | 906 | 681 a |
| Sequel | 674 | 1262 | 507 | 212 | 804 | 692 a |
| Signal | 675 | 989 | 787 | 526 | 1672 | 930 bc |
| Squire | 816 | 1195 | 770 | 343 | 599 | 745 ab |
| Status | 636 | 873 | 844 | 428 | 1571 | 870 abc |
| Mean | 718 | 1107 | 766 | 406 | 1256 | 850 |
| Response Trait | Estimate | Std.Err | Z-Value | p (>|z|) | Std.Lv | Std.All |
|---|---|---|---|---|---|---|
| Flowering | 1.206 | 8.280 | 0.146 | 0.884 | 1.206 | 0.012 |
| Maturity | −10.884 | 3.736 | −2.913 | 0.004 | −10.884 | −0.250 |
| PH | 7.159 | 3.400 | 2.105 | 0.035 | 7.159 | 0.291 |
| Branches | 49.671 | 25.396 | 1.956 | 0.050 | 49.671 | 0.114 |
| FP | 48.654 | 6.944 | 7.007 | 0.000 | 48.654 | 0.608 |
| SPP | −31.087 | 29.998 | −1.036 | 0.300 | −31.087 | −0.054 |
| LLI | −91.707 | 15.286 | −5.999 | 0.000 | −91.707 | −0.548 |
| LCI | 54.076 | 14.225 | 3.801 | 0.000 | 54.076 | 0.425 |
| LUI | −8.724 | 5.676 | −1.537 | 0.124 | −8.724 | −0.220 |
| NLI | 373.075 | 80.081 | 4.659 | 0.000 | 373.075 | 0.423 |
| NCI | −200.742 | 90.325 | −2.222 | 0.026 | −200.742 | −0.232 |
| NUI | −18.975 | 26.594 | −0.714 | 0.476 | −18.975 | −0.074 |
| HSW | 13.042 | 6.744 | 1.934 | 0.053 | 13.042 | 0.163 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obua, T.; Sserumaga, J.P.; Tukamuhabwa, P.; Namara, M.; Awio, B.; Mugarra, J.; Tusiime, G.; Chigeza, G. Unravelling Yield and Yield-Related Traits in Soybean Using GGE Biplot and Path Analysis. Agronomy 2024, 14, 2826. https://doi.org/10.3390/agronomy14122826
Obua T, Sserumaga JP, Tukamuhabwa P, Namara M, Awio B, Mugarra J, Tusiime G, Chigeza G. Unravelling Yield and Yield-Related Traits in Soybean Using GGE Biplot and Path Analysis. Agronomy. 2024; 14(12):2826. https://doi.org/10.3390/agronomy14122826
Chicago/Turabian StyleObua, Tonny, Julius Pyton Sserumaga, Phinehas Tukamuhabwa, Mercy Namara, Bruno Awio, Johnson Mugarra, Geoffrey Tusiime, and Godfree Chigeza. 2024. "Unravelling Yield and Yield-Related Traits in Soybean Using GGE Biplot and Path Analysis" Agronomy 14, no. 12: 2826. https://doi.org/10.3390/agronomy14122826
APA StyleObua, T., Sserumaga, J. P., Tukamuhabwa, P., Namara, M., Awio, B., Mugarra, J., Tusiime, G., & Chigeza, G. (2024). Unravelling Yield and Yield-Related Traits in Soybean Using GGE Biplot and Path Analysis. Agronomy, 14(12), 2826. https://doi.org/10.3390/agronomy14122826








