Abstract
Wheat stripe rust is a fungal disease severely affecting wheat production. Breeding resistant cultivars is the most cost-effective and efficient way to control wheat stripe rust. YrZH84 is an all-stage resistance gene with good resistance to stripe rust. In this study, YrZH84 was introgressed from germplasm Lantian36 (LT36) into the two southwestern Chinese elite wheat cultivars Mianmai367 (MM367, carrying Yr10, Yr26), and Chuanmai104 (CM104, carrying Yr26), using marker-assisted selection. F1 seeds of the two cross-combinations were planted and self-crossed to develop the advanced generations in the field. A total of 397 introgression lines (ILs) were obtained in F6 and genotyped using molecular markers Xcfa2040, Xbarc32 (linked to YrZH84), Yr10 (linked to Yr10), We173, and Xbarc181 (linked to Yr26). The 397 ILs were also evaluated for resistance to stripe rust and agronomic traits, including plant height, number of tillers, spike length, thousand-grain weight, and spikelet number. Finally, 61 lines with appreciative agronomic traits and disease resistance were selected. Among these lines, 31 lines had stripe rust resistance gene YrZH84. These selected lines are expected to become new wheat varieties for their high resistance to stripe rust and excellent agronomic traits that will make important contributions to the control of stripe rust and wheat production.
1. Introduction
Stripe rust of wheat, caused by Puccinia striifonnis f. sp. tritici (Pst), is a serious disease that spreads through high air currents and infects wheat [1]. Pst can spread hundreds or even thousands of kilometers through the air [2]. The incidence range of stripe rust is extensive. It occurs in almost all wheat-producing regions of the world, such as China, India, the United States, Australia and so on [3,4]. Pst reduces wheat yield by infecting the leaves of wheat, leading to leaf dryness and affecting irrigation. In total, 88% of the world’s wheat production is susceptible to wheat stripe rust, resulting in global wheat losses of more than 5 million tons [1,5,6]. Wheat is the most widely grown crop in the world, second only to maize and tied for second place with rice in terms of production [7]. As one of the world’s food crops, the stable production of wheat is essential for maintaining China’s and the world’s food supply.
The most effective method to control stripe rust is to breed resistant cultivars [8]. To date, 86 resistance genes against stripe rust (Yr1-Yr86) have been officially named internationally [9,10], along with numerous unnamed resistance genes such as YrZH22 [11], Yraci [12], and YrJ22 [13], and over 300 resistance quantitative trait locus (QTL). These genes are divided into all-stage resistance (ASR) genes and adult plant resistance (APR) genes according to their functioning period [14]. ASR genes have resistance throughout the entire growth period of wheat, such as Yr10, Yr41, Yr59, and Yr82, and APR genes only show resistance during the adult plant period, such as Yr16, Yr59, and Yr80. Despite the discovery of so many disease-resistant genes, the control of wheat stripe rust remains a challenging task due to the increasing frequency of pathogenic race variation.
Traditional breeding mainly evaluates and selects crops based on their performance in the field, and the breeding process is slow and inefficient. Marker-assisted selection (MAS) is a method of detecting the presence of a target gene through the detection of molecular markers that are closely related to the target gene, and then combining it with conventional breeding to screen and breed new varieties [15,16]. MAS can significantly improve breeding efficiency. The molecular marker is independent of environmental conditions and can be detected at all stages of plant growth, determining the presence of target genes in the early generations of a breeding program [17]. At present, MAS has been widely used in wheat, rice, maize, tomato, and other crop breeding [18,19,20]. For example, Zhou et al. introduced Yr62 into four susceptible wheat cultivars and detected 22 positive wheat lines with excellent disease resistance using molecular markers Xgwm251 and Xgwm192 [21]; Zhang et al. developed an excellent cultivar containing cotton fiber strength QTLFS1 by MAS [22]; Liu developed a water-saving and drought-resistant rice (WDR) PTGMS line, huhan74S [23]. With the development of molecular biology, MAS has become an important tool for crop breeding [24].
Lantian 36 (LT36), a winter wheat cultivar developed by the Wheat Research Institute of Gansu Academy of Agricultural Sciences and Tianshui Agricultural School, possesses the stripe rust-resistant gene YrZH84, which confers good resistance to wheat stripe rust. The flanking markers of YrZH84 are Xcfa2040 and Xbarc32, with a genetic linkage distance ranging from 4.8 cM to 1.4 cM [25]. Yin et al. developed the RGAP marker Xrga-1 with a genetic linkage distance of 0.8 cM [26], which was shortened to 4.0 cM from the same-side SSR marker Xbarc32. Chuanmai104 (CM104, carrying Yr26) and Mianmai367 (MM367, carrying Yr10 and Yr26) are important high-yield varieties used in wheat production in southwest China [27,28]. In recent years, as the Pst races CRY32, CRY33, and CRY34 have evolved into epidemics, the resistance of Yr10 and Yr26 has been gradually overcome [29,30], and these wheat cultivars with excellent agronomic traits are losing their disease resistance, gradually.
The ASR gene YrZH84 has strong resistance to stripe rust. The purpose of this study was to introgress the wheat stripe rust resistance gene YrZH84 from the northwestern Chinese wheat cultivar Lantian36 into two southwestern Chinese wheat varieties, Mianmai367 and Chuanmai104, by utilizing the stripe rust resistance of YrZH84 and the excellent agronomic traits of Mianmai367 and Chuanmai104. The phenotypes of the progeny breeding population were screened, and new wheat germplasms with strong resistance to stripe rust and excellent agronomic traits were selected utilizing molecular markers closely linked to YrZH84, Yr10, and Yr26. Some selected lines contained one or more of the genes YrZH84, Yr10, and Yr26. This will enrich the genetic resources for stripe rust resistance in the wheat region of southwest China. It provides important breeding materials for the subsequent utilization of resistance genes and disease resistance breeding.
2. Materials and Methods
2.1. Plant Materials
Donor parent LT36 was developed from the cross of Aizao781/Zhou 8425B//Zhoumai9///Lantian23 by the Wheat Research Institute of Gansu Academy of Agricultural Sciences and Tianshui Agricultural School. It carries the stripe rust-resistant gene YrZH84, whose flanking markers are Xcfa2040 and Xbarc32 [25]. The recipient parents include Mianmai367 (MM367) and Chuanmai104 (CM104). CM104 was derived from the cross of Chuanmai42/Chuannong16 by the Crop Research Institute of Sichuan Academy of Agricultural Sciences and has driven the development of the wheat industry in southwest China as one of the leading varieties. MM367 is an excellent dwarf, large-spike material developed by the Mianyang Institute of Agricultural Science. CM104 contains the stripe rust resistance gene Yr26 [27,28]. MM367 contains the stripe rust resistance genes Yr10 and Yr26 [27], which are tightly linked to the molecular markers Yr10 [31], We173, and Xbarc181 [32]. The two recipient parents were used as important parental materials because of their excellent agronomy traits. Jinmai47 (JM47) was used as a susceptible control in the field.
2.2. Construction of Cross-Population and Offspring Screening
The construction of a cross-population and offspring screening were conducted from 2018 to 2024 in Mianyang (31.41° N, 104.39° E) of Sichuan province in China. Mianyang is located in the Sichuan Basin and has a flat topography. The climate is warm and humid in winter, with abundant rainfall and high soil moisture, conditions that are favorable for the growth and spread of the stripe rust fungus. Mianyang is the overwintering area of stripe rust. The experimental field naturally became infected without creating an artificial disease epiphytotic.
In March of the crop-growing season, the main stem spikes of robustly growing plants of MM367 and CM104 were selected and bagged after removing the stamens with forceps. Mature pollen from LT36 was collected the following day and imparted to the stigmas of MM367 and CM104. We baged the hybridized wheat ears again and marked the parental information on the bag. The seeds were to be harvested in May. The harvested F1 seeds were planted in the field and sealed to develop advanced generations. F2–F5 were grown in the field for consecutive years to self-cross. The F2 to F5 seeds derived from these two crosses were sown in rows of 2 m length, 30 rows with about 80 seeds per row, and 30 cm row spacing. During single-generation selection, susceptible plants were discarded and seeds from each combination were harvested in large numbers to preserve as many genotypes as possible.
In the 2022–2023 season, the F5 populations were selected based on the following criteria: infection type (IT) ≤ 6, plant height (PH) ≤ 100 cm, number of tillers (NT) ≥ 3, and spike length (SL) ≥ 10 cm. A total of 397 F5 introgression lines (ILs) were selected to produce F6 seeds. Of these 397 lines, 215 originated from MM367/LT36, and 182 from CM104/LT36. In the 2023–2024 season, F6 lines were planted with three replications, following a randomized block experimental design. Each line was planted in 2 rows, each with a length of 1.2 m and a spacing of 30 cm between rows, and approximately 30 seeds in each row. Spreader rows containing the Pst-susceptible cultivar JM47 were planted around the experimental plot to create an epidemic environment. The field was properly managed with water and fertilizer.
2.3. Genotyping Wheat Lines by Markers
The parents and progeny lines were genotyped using SSR markers linked to YrZH84, namely, Xcfa2040 and Xbarc32. In addition, the markers We173 and Xbarc181 were used for genotyping CM104/LT36 plants, and Yr10, We173 and Xbarc181 were used to genotype MM367/LT36 plants. All primers were synthesized by Shenggong Biological Engineering (Shanghai) Co., LTD. (Shanghai, China) (https://www.sangon.com) (accessed on 26 February 2024), and the sequence information is shown in Table 1. Plant genomic DNA was extracted from fresh leaves of the three parents and F6 lines of the MM367/LT36 and CM104/LT36 populations, employing the modified cetyl trimethyl ammonium bromide (CTAB) extraction method [33]. The concentration of DNA was determined by spectrophotometry and subsequently diluted to 50 ng/μL for PCR amplification.

Table 1.
Sequence and amplification information for markers linked to the YrZH84, Yr10, and Yr26.
The polymerase chain reaction (PCR) was performed in a 10 µL volume, which contained 0.5 μL DNA template, 0.5 μL primer (forward and reverse primers in a volume of 0.25 µL each), 5 μL 2 × M5 PAGE Taq PCR Mix, and 4 μL ddH2O. The PCR amplification procedure included pre-denaturing at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 25 s; annealing at 52.2 °C–60.5 °C for 25 s (annealing temperatures of different primers are shown in Table 1); extension at 72 °C for 25 s. After the cycles, a final extension was performed at 72 °C for 5 min, then the samples were kept at 10 °C for later detection. The PCR products were separated by 6% polyacrylamide gel electrophoresis (PAGE) and the expected bands were recorded.
2.4. Stripe Rust Reaction and Agronomic Traits in the Field
The stripe rust infection type (IT) of each line was recorded according to the 0–9 scale [34], where 0, 1–3, 4–6, and 7–9 were regarded as immune, resistant, moderately resistant, and susceptible, respectively. Disease severity (DS) was recorded as the percentage of infected leaf areas using a method derived from Peterson [35]. During the 2023–2024 season, IT and DS data were collected when the susceptible control JM47 exhibited about 80% severity in April. Data were collected twice, with a one-week interval between surveys. The average of the three replicate surveys was treated as the IT and DS data for the line.
The agronomic traits of three parents and F6 lines during the ripening period were investigated. Ten uniformly growing individual plants were randomly selected from each replicate group to measure the following traits: plant height (PH), number of tillers (NT), spike length (SL) and spikelet number (SN). The measurement method was referenced from Gai [36]. The mean value was taken as the result of the survey. After the wheat was harvested and threshed, the weight of 1000 randomly selected grains was measured three times, and the average value was recorded as the thousand-grain weight (TGW).
2.5. Statistical Analysis
The data were preliminarily sorted out and mapped using Excel 2021 (https://www.microsoft.com/zh-cn/microsoft-365/excel) (accessed on 28 May 2024), and analyzed by variance using GraphPad Prism Version 8.0.2 (https://www.graphpad.com/) (accessed on 18 June 2024). To determine the effects of YrZH84 on agronomic traits, we divided all the lines into two groups, one containing YrZH84 and the other without YrZH84. t-test analysis was performed using GraphPad Prism Version 8.0.2, with p > 0.05 indicating that the differences were not significant.
3. Results
3.1. Molecular Marker Detection
The markers Xcfa2040 and Xbarc32 were used to genotype for the three parents and the 397 ILs (Figure 1). Of the 397 lines, 235 lines contained YrZH84, of which 99 lines were from MM367/LT36 and 136 lines from CM104/LT36 (Table 2). In total, 162 lines did not contain YrZH84, of which 116 lines were from MM367/LT36 and 46 lines were from CM104/LT36. The ILs were also detected using the markers Yr10, We173, and Xbarc181. Here, 46 lines in the MM367/LT36 carried Yr10 and 84 lines carried Yr26; 81 lines in the CM104/LT36 carried Yr26.

Figure 1.
PCR amplification patterns of polymorphic molecular markers Xbarc32 (a), We173 (b), and Yr10 (c). M means marker; lanes 1–16 represent some of the ILs from the crosses of MM367/LT36 (a,c) and CM104/LT36 (b). Arrows indicate polymorphic bands.

Table 2.
The number of lines containing and not containing YrZH84 in the F6 lines from the two crosses.
3.2. Stripe Rust Resistance Evaluation
In the crop season of 2023–2024, in the field, the donor parent LT36 showed high resistance (IT 1, DS 5%), while the recipient parents MM367 and CM104 showed susceptibility (IT 7–8, DS 70–80%) (Figure 2, Table S1). Most of the 397 cross progenies had IT values ranging from 0 to 3 and DS values between 0% and 10% (Figure 3). Specifically, 86 and 21 lines from MM367/LT36 and CM104/LT36 were immune to stripe rust, respectively (Table 3); 122 and 150 lines from MM367/LT36 and CM104/LT36 were highly resistant; and 7 and 11 lines from MM367/LT36 and CM104/LT3 were moderately resistant. After the elimination of susceptibility in the previous generations, there were no susceptible lines in the 397 ILs.
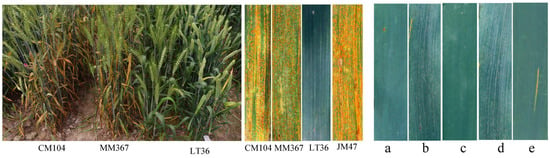
Figure 2.
Adult stage stripe rust reactions on leaves of three parents, susceptible controls (JM47), and F6 lines (a–e). (a) MM367/LT36-58; (b) MM367/LT36-95; (c) MM367/LT36-209; (d) CM104/LT36-301; (e) CM104/LT36-321.
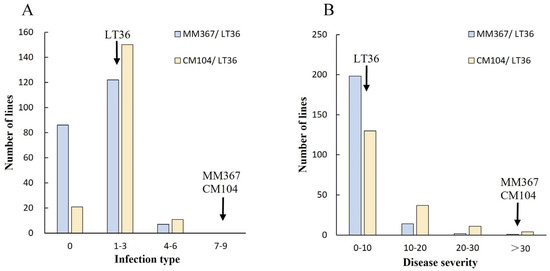
Figure 3.
Distributions of mean infection type (IT) (A) and disease severity (DS) (B) for 397 F6 lines from the crosses of MM367/LT36 and CM104/LT36.

Table 3.
Numbers of immune, resistant, intermediate, and susceptible ILs from two crosses in 2023–2024 based on the mean infection type (IT).
3.3. Agronomic Traits Performance
In the 2023–2024 crop seasons, the mean PHs of the three parents, LT36, MM367, and CM104, were 72.83, 77.67, and 88.67 cm, respectively, and the ILs of the two combinations were mainly distributed in the 70–100 cm range (Figure 4A). The mean NTs of the three parents were 4.00, 3.67, and 4.20, respectively, and the ILs of the two combinations were mainly distributed in the 4–6 range (Figure 4B). The mean SLs of the three parents were 9.73, 11.63, and 14.10 cm, respectively, and the ILs of the two combinations were mainly distributed in the range of 9.5–12.5 cm (Figure 4C). The mean TGW of the three parents were 41.50, 39.53, and 37.20 g, respectively, and the ILs of the two combinations were mainly distributed in the range of 45–60 g (Figure 4D). The mean SN of the three parents were 24.00, 17.67, and 17.33, respectively, and the ILs of the two combinations were mainly distributed in the 17–20 range (Figure 4E).
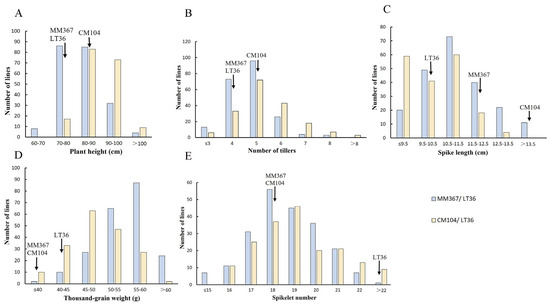
Figure 4.
Frequency distributions of agronomic traits for the 397 ILs from the two populations. (A) Plant height (PH); (B) number of tillers (NT); (C) spike length (SL); (D) thousand-grain weight (TGW); (E) spikelet number (SN).
In wheat breeding, disease resistance is a prerequisite, and high yield is the goal. In order to obtain stripe rust-resistant wheat lines, we selected lines with strong stripe rust resistance from the 397 lines, and 370 lines were selected based on IT values ≤ 3 and DS values ≤ 20. Further, among these 370 lines, the lines with excellent agronomic traits were selected according to the following criteria: 75 cm ≥ PH ≥ 95 cm, NT values ≥ 4, SL values ≥ 12 cm, TGW values ≥ 45 g, and values of SN ≥ 18. A total of 61 lines were selected (Figure 5, Table S1). These lines exhibit the potential for suitable plant height, favorable for harvesting, lodging resistance, and high yield. Of these 61 lines, 41 originated from MM367/LT36, and 20 from CM104/LT36. Furthermore, 31 of these lines introduced the stripe rust gene YrZH84, while 2, 16, and 4 introduced YrZH84 + Yr10 genes, YrZH84 + Yr26 genes and YrZH84 + Yr10 + Yr26 genes, respectively (Table S1). These 61 lines are expected to be bred into new wheat cultivars for their strong resistance and excellent agronomic traits.

Figure 5.
Grain performances of three parents and some of the 61 selected lines. 1–6 represent CM104/LT36-277, CM104/LT36-290, CM104/LT36-318, MM367/LT36-126, MM367/LT36-180 and MM367/LT36-61, respectively.
To determine whether the introduction of YrZH84 affects agronomic traits in wheat, the 397 F6 lines were divided into two groups, one containing YrZH84 and the other without YrZH84. The t-test results show that agronomic traits (PH, NT, SL, TGW, and SN) of the lines containing YrZH84 were not significantly different from those of the lines without YrZH84 (Figure 6). It was indicated that the introduction of YrZH84 into MM367 and CM104 did not affect the agronomic traits of the progeny lines. All this proves that YrZH84 is a high-quality, disease-resistant gene that can be utilized as a source material for resistance.
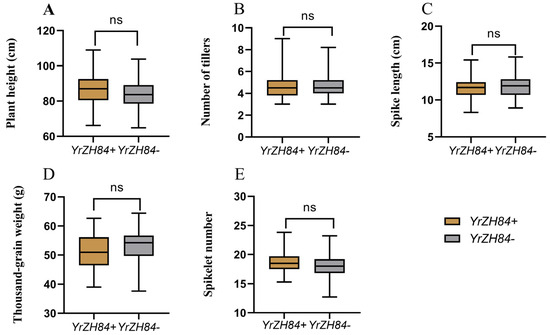
Figure 6.
Comparison of agronomic traits between lines containing YrZH84 (YrZH84+) and without YrZH84 (YrZH84−) for the 397 ILs from the two crosses. (A) Plant height (PH); (B) number of tillers (NT); (C) spike length (SL); (D) thousand-grain weight (TGW); (E) spikelet number (SN). The p-value obtained by t-test, “ns” represents p > 0.05, no significant differences.
In summary, we successfully introduced YrZH84 into two southwestern Chinese wheat varieties: MM367 and CM104. Artificial phenotypic selection was carried out on the F1–F5 generations for consecutive years to eliminate the disease-susceptible plants. After phenotypic selection in the F5, 397 plants were obtained as seeds for the F6. Three replicate plantings of F6 lines were investigated for disease resistance and agronomic traits; 370 lines were selected for stripe rust resistance using IT ≤ 3 and DS ≤ 20% as criteria; 61 lines were selected using 75 cm ≥ PH ≥ 95 cm, NT values ≥ 4, SL values ≥ 12 cm, TGW values ≥ 45 g, and SN values ≥ 18 as agronomic trait criteria. These lines had strong resistance to stripe rust and could be used for breeding wheat for disease resistance. They also have suitable plant height and thousand-grain weight, which can provide plant materials for breeding wheat with high yield, resistance to downfall, and ease of harvesting.
4. Discussion
Stripe rust resistance in wheat is a complex trait that has developed through the long-term co-evolution between wheat and the rust pathogen Pst. The extensive utilization of only a few resistance genes can lead to the emergence and dissemination of new virulent strains of Pst, which may overcome these resistance genes, resulting in a loss of resistance in the varieties. For instance, in China, the widespread application of Fan6 and its derivatives led to the breakdown of Yr3 and Yr4 resistance [37], and the overuse of the 92R lines, Guinong lines, and Chuanmai lines led to the overcoming of Yr10 and Yr24/Yr26 resistance [29]. The continuous replacement of endemic Pst races has led to the constant overcoming of disease resistance in wheat cultivars [5]. Breeding new cultivars that are resistant to diseases is an essential step in ensuring safe wheat production. In this study, the stripe rust-resistant wheat cultivar LT36 was crossed with MM367 and CM104, and after continuous breeding selection, 61 lines combining high resistance to stripe rust and excellent agronomic traits were selected in the F6 generation. These selected lines are expected to yield new wheat cultivars in future breeding programs.
The recipient parent CM104 is a high-yielding, disease-resistant, and stress-resistant wheat cultivar [38]. Because of its excellent traits, it has been used as a parental material to produce a number of new wheat varieties such as Chuanmai 69, Chuanmai 93, Chuanmai 96, and Chuanmai 1532 [39]. MM367 is a high-yielding wheat cultivar with large spikes. MM367 and CM104 were once popularized as high-yielding wheat varieties in the southwestern wheat region of China. The emergence of Pst race CRY34 led to the loss of resistance to stripe rust in MM367 and CM104. The donor parent LT36 is a compact, short-stalked wheat cultivar resistant to stripe rust and powdery mildew in the wheat region of northwest China. In this study, LT36 was crossed with MM367 and CM104. Agronomic traits of progeny incorporated the following parental traits: PH (70–90 cm); NT (4–5); SL (9.5–12.5 cm); and SN (18–20) (Figure 4). The TGWs of LT36, MM367, and CM104 were 41.50, 39.53, and 37.20 g, respectively, while the F6 lines were mostly distributed 45–60 g. Selected lines were both highly resistant to stripe rust and also possessed agronomic traits superior to those of the parents (ST 1).
YrZH84, derived from the backbone wheat Zhou8425B, has gained widespread application in China in recent years due to its robust disease resistance and excellent agronomic traits [40]. The detection of stripe rust resistance genes in important winter wheat in the source region of stripe rust in northwest China by Bai et al. [41] revealed that YrZH84 showed resistance to the disease at both seedling and adult plant stages. Good resistance to YrZH84 was also demonstrated by Li [42]. In this study, YrZH84 was introduced into MM367 and CM104, and the progeny with stripe rust resistance (IT 0–3) were significantly enhanced compared to the two parents (IT 7–8) (Figure 2 and Figure 3). The results also indicate that YrZH84 is a strong resistance gene to stripe rust. Crops have complex response mechanisms to adversity stresses [43]. Quality genes are valuable for improving crop agronomic traits [44]. Yan et al. [45] introduced the oat (Avena sativa L.) dwarfing gene Dw6 into tall oats, and Dw6 significantly reduced plant height and spike length, but adversely affected hundred-kernel weight and kernel length. In the present study, as shown in Figure 6, the differences in agronomic traits between lines containing YrZH84 (YrZH84+) and those not containing YrZH84 (YrZH84−) were not significant. YrZH84 had no adverse effect on agronomic traits in progeny lines. This indicates that it is a promising gene for stripe rust resistance and can be used as a high-quality resistance source for breeding wheat for stripe rust resistance.
An ideal molecular marker should not only exhibit high polymorphism, but also necessitate the shortest linkage distance to the target gene. If linkage markers maintain a certain physical distance from the target gene, recombination events may occur between them, leading to potential false positives. This phenomenon may be why some lines in this study, such as MM367/LT 36–61, MM367/LT36-182, CM104/LT36-370, CM104/LT36-397, and so on, showed resistance when no Yr gene was detected. Also, it is possible that these parental materials and lines contained other unknown genes, thus also contributing. The genetic distances of the molecular markers Xcfa2040 and Xbarc32 linked to YrZH84 used in this study were 4.8 cM and 1.4 cM, respectively, and genetic recombination might have occurred at long distances. In future molecular assessments of YrZH84, consideration should be given to the RGAP marker Xrga-1 [26], which has a closer genetic distance and higher marker detection accuracy. In addition, the development of more accurate and effective molecular markers is needed for MAS.
The prevalence of the Pst race CRY34 has resulted in the loss of resistance to Yr10 and Yr26 [29]. However, in this study, some lines such as MM367/LT36-36, MM367/LT36-125, CM104/LT36-366, and so on, which carry Yr10 or Yr26, exhibited excellent resistance to stripe rust. It was presumed that this might be due to the presence of other undetected or unknown resistance genes in these lines.
In gene aggregation breeding strategies, an increased richness of Yr genes correlates with reduced selection pressure from stripe rust [46]. Alma et al. [47] detected wheat cultivars carrying the Yr gene in Kazakhstan, and found that cultivars containing three genes, Yr5 + Yr17 + Yr18 and Yr5 + Yr10 + Yr18, had medium and high levels of resistance. Yr9 + Yr18 and Yr30 + Yr36 were all pyramided genes that produced additive effects and strong resistance to the Pst races [48]. Zhu et al. [49] pyramided several plant defense response genes and anti-apoptosis genes in maize to obtain the transgenic maize line 910 with significantly enhanced resistance to both maize blight and southern maize leaf blight. Mbinda et al. [50] also concluded that the use of gene pyramiding would be useful in controlling finger millet blast disease. In this study, the donor of YrZH84 was crossed with donors of Yr10 and Yr26. Among the offspring, we obtained 18 wheat lines with excellent agronomic characteristics that carry stripe rust-resistant genes YrZH84 + Yr10 or YrZH84 + Yr26, and 4 wheat lines with excellent agronomic characteristics that carry stripe rust-resistant genes YrZH84 + Yr10 + Yr26 (Table S1). Although the resistance of Yr10 and Yr26 is currently overcome by the Pst race CRY34, the pyramiding of multiple genes increases the abundance of resistance in wheat to the Pst races of Stripe Rust. This is favorable for maintaining durable resistance to stripe rust in wheat.
Obviously, against the background of global climate change and the rapid evolution of Pst, it is not enough to control stripe rust only by gene pyramiding and breeding new cultivars. We should mine new disease-resistant genetic resources, utilize molecular breeding technology and the rational layout of disease-resistant genes, and thus strengthen pathogen detection and other measures. The control of stripe rust is challenging, but it is of great significance to human food security and requires the joint efforts of all mankind.
5. Conclusions
In this study, we employed MAS to cross the wheat cultivar LT36 (containing the stripe rust resistance gene YrZH84) with two southwestern Chinese maincrop cultivars—MM367 and CM104. After extensive selection, we successfully obtained 61 F6 lines with strong stripe rust resistance and excellent agronomic traits. Among these lines, 31 lines contained the stripe rust resistance gene YrZH84. These wheat lines not only enrich the resources for developing disease-resistant wheat cultivars, but also present promising applications. This will probably be of great value to food production in China and even to mankind.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14112672/s1, Table S1: Gene, mean IT, and agronomic traits of the final 61 lines selected.
Author Contributions
M.H. detected the markers, analyzed data and wrote the first draft of the manuscript. X.F., X.L., Q.C., X.Y., B.Y. and C.C. collected samples and phenotype data. S.Y. and K.H. contributed to the crosses, selected of target lines and evaluated the populations. S.Y. revised the draft. M.H. and S.Y. conceived the project and generated the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Major Program of National Agricultural Science and Technology of China (NK20220607) and the Breakthrough in Wheat Breeding Material and Method Innovation and New Variety Breeding (Breeding Research Project, 2021YFYZ0002).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank the anonymous reviewers for their valuable review and comments on the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wellings, C.R. Global status of stripe rust: A review of historical and current threats. Euphytica 2011, 179, 129–141. [Google Scholar] [CrossRef]
- Bouvet, L.; Holdgate, S.; James, L.; Thomas, J.; Mackay, I.J.; Cockram, J. The evolving battle between yellow rust and wheat: Implications for global food security. Theor. Appl. Genet. 2022, 135, 741–753. [Google Scholar] [CrossRef]
- Wan, A.M.; Zhao, Z.H.; Chen, X.M.; He, Z.H.; Jin, S.L.; Jia, Q.Z.; Yao, G.; X, Y.J.; Wang, B.T.; Li, G.B.; et al. Wheat stripe rust epidemic and virulence of Puccinia striiformis f. sp. tritici in China in 2002. Plant Dis. 2004, 88, 896–904. [Google Scholar] [CrossRef]
- Ali, S.; Rodriguez-Algaba, J.; Thach, T.; Sørensen, C.K.; Hansen, J.G.; Lassen, P.; Nazari, K.; Hodson, D.P.; Justesen, A.F.; Hovmøller, M.S. Yellow rust epidemics worldwide were caused by pathogen paces from divergent genetic lineages. Front. Plant Sci. 2017, 8, 1057. [Google Scholar] [CrossRef]
- Schwessinger, B. Fundamental wheat stripe rust research in the 21st century. New Phytol 2017, 213, 1625–1631. [Google Scholar] [CrossRef]
- Gebremariam, T.G.; Wang, F.T.; Lin, R.M.; Li, H.J. Comparative analysis of virulence and molecular diversity of Puccinia striiformis f. sp. tritici isolates collected in 2016 and 2023 in the western region of China. Genes 2024, 15, 542. [Google Scholar] [CrossRef]
- Erenstein, O.; Chamberlin, J.; Sonder, K. Estimating the global number and distribution of maize and wheat farms. Glob. Food Secur. 2021, 30, 100558. [Google Scholar] [CrossRef]
- Singh, J.; Chhabra, B.; Raza, A.; Yang, S.H.; Sandhu, K.S. Important wheat diseases in the US and their management in the 21st century. Front. Plant Sci. 2023, 13, 1010191. [Google Scholar] [CrossRef]
- Mcintosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Xia, X.C.; Raupp, W.J. Catalogue of gene symbols for wheat: 2021 supplement. Annu. Wheat Newsl. 2021, 67, 104–113. Available online: https://wheat.pw.usda.gov/GG3/wgc (accessed on 10 June 2024).
- Klymiuk, V.; Chawla, H.S.; Wiebe, K.; Ens, J.; Fatiukha, A.; Govta, L.; Fahima, T.; Pozniak, C.J. Discovery of stripe rust resistance with incomplete dominance in wild emmer wheat using bulked segregant analysis sequencing. Commun. Biol. 2022, 5, 826. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, J.Z.; Zhang, H.Z.; Guo, B.; Ning, S.Z.; Chen, Y.X.; Lu, P.; Wu, Q.H.; Li, M.M.; Zhang, D.Y.; et al. Mapping stripe rust resistance gene YrZH22 in Chinese wheat cultivar Zhoumai22 by bulked segregant RNA-Seq (BSR-Seq) and comparative genomics analyses. Theor. Appl. Genet. 2017, 130, 2191–2201. [Google Scholar] [CrossRef]
- Grover, G.; Sharma, A.; Mackay, I.; Srivastava, P.; Kaur, S.; Kaur, J.; Burridge, A.; Allen, S.P.; Bentley, A.R.; Chhuneja, P.; et al. Identification of a novel stripe rust resistance gene from the European winter wheat cultivar ‘Acienda’: A step towards rust proofing wheat cultivation. PLoS ONE 2022, 17, e0264027. [Google Scholar] [CrossRef]
- Chen, C.; He, Z.H.; Lu, J.L.; Li, J.; Ren, Y.; Ma, C.X.; Xia, X.C. Molecular mapping of stripe rust resistance gene YrJ22 in Chinese wheat cultivar Jimai22. Mol. Breed. 2016, 36, 118. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zeng, S.M. Wheat Rust in China; China Agriculture Press: Beijing, China, 2002; pp. 164–167. [Google Scholar]
- Tian, J.C. Genetic Analyses of Wheat Major Traits and Molecular Marker-Assisted Breeding; Science Press: Beijing, China, 2015; pp. 474–476. [Google Scholar]
- Hasan, N.; Choudhary, S.; Naaz, N.; Sharma, N.; Laskar, R.A. Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes. J. Genet. Eng. Biotechnol. 2021, 19, 128. [Google Scholar] [CrossRef]
- Nana, V.; Peter Skov, K.; Jeppe Reitan, A.; Ahmed, J.; Jihad, O. Marker-assisted breeding in wheat. In Next Generation Plant Breeding; Yelda Özden, Ç., Ed.; IntechOpen: Rijeka, Croatia, 2018; pp. 4–10. [Google Scholar]
- Varshney, R.K.; Langridge, P.; Graner, A. Application of genomics to molecular breeding of wheat and barley. Adv. Genet. 2007, 58, 121–155. [Google Scholar] [CrossRef]
- Utomo, H.S.; Linscombe, S.D. Current patents and future development underlying marker-assisted breeding in major grain crops. Recent Pat. DNA Gene Seq. 2009, 3, 53–62. [Google Scholar] [CrossRef]
- Qutub, M.; Chandran, S.; Rathinavel, K.; Sampathrajan, V.; Rajasekaran, R.; Manickam, S.; Adhimoolam, K.; Muniyandi, S.J.; Natesan, S. Improvement of a Yairipok Chujak Maize Landrace from north eastern Himalayan region for β-Carotene content through molecular marker-assisted backcross breeding. Genes 2021, 12, 762. [Google Scholar] [CrossRef]
- Zhou, J.N.; Zheng, X.C.; Zhong, X.; Tan, W.J.; Ma, C.H.; Wang, Y.Q.; Tian, R.; Yang, S.Z.; Li, X.; Xia, C.J.; et al. Transfer of the high-temperature adult-plant stripe rust resistance gene Yr62 in four Chinese wheat cultivars. Mol. Breed. 2023, 43, 44. [Google Scholar] [CrossRef]
- Zhang, T.Z.; Yuan, Y.L.; Yu, J.; Guo, W.Z.; Kohel, R.J. Molecular tagging of a major QTL for fiber strength in upland cotton and its marker-assisted selection. Theor. Appl. Genet. 2003, 106, 262–268. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, F.Y.; Luo, X.X.; Kong, D.Y.; Zhang, A.N.; Wang, F.M.; Pan, Z.Q.; Wang, J.H.; Bi, J.G.; Luo, L.J.; et al. Molecular breeding of a novel PTGMS line of WDR for broad-spectrum resistance to Blast using Pi9, Pi5, and Pi54 genes. Rice 2021, 14, 96. [Google Scholar] [CrossRef]
- De Mori, G.; Cipriani, G. Marker-assisted selection in breeding for fruit trait improvement: A review. Int. J. Mol. Sci. 2023, 24, 8984. [Google Scholar] [CrossRef]
- Li, Z.F.; Zheng, T.C.; He, Z.H.; Li, G.Q.; Xu, S.C.; Li, X.P.; Yang, G.Y.; Singh, R.P.; Xia, X.C. Molecular tagging of stripe rust resistance gene YrZH84 in Chinese wheat line Zhou8425B. Theor. Appl. Genet. 2006, 112, 1098–1103. [Google Scholar] [CrossRef]
- Yin, G.H.; Wang, J.W.; Wen, W.E.; He, Z.H.; Li, Z.F.; Wang, H.; Xia, X.C. Mapping of wheat stripe rust resistance gene YrZH84 with RGAP markers and its application. Acta Agron. Sin. 2009, 35, 1274–1281. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, Y.; He, Y.J.; Zheng, S.H.; Wu, G.; Zhou, F.L.; Lei, J.R.; Du, X.Y.; Tao, J.; Ou, J.M. Molecular detection of disease resistance genes in 153 Sichuan wheat varieties and lines. J. Triticeae Crops 2022, 42, 26–35. [Google Scholar] [CrossRef]
- Zhang, H.P.; Ye, X.L.; Guan, F.N.; Huang, L.; Li, W.; Deng, M.; Wei, Y.M.; Jiang, Y.F.; Chen, G.Y. Identification and evaluation of stripe rust resistance in 220 Sichuan wheat germplasms. J. Sichuan Agric. Univ. 2023, 41, 1020–1031. [Google Scholar] [CrossRef]
- McIntosh, R.; Mu, J.M.; Han, D.J.; Kang, Z.S. Wheat stripe rust resistance gene Yr24/Yr26: A retrospective review. Crop J. 2018, 6, 321–329. [Google Scholar] [CrossRef]
- Cheng, B.; Gao, X.; Cao, N.; Ding, Y.; Gao, Y.; Chen, T.; Xin, Z.; Zhang, L. Genome-wide association analysis of stripe rust resistance loci in wheat accessions from southwestern China. J. Appl. Genet. 2020, 61, 37–50. [Google Scholar] [CrossRef]
- Liu, W.; Frick, M.; Huel, R.; Nykiforuk, C.L.; Wang, X.; Gaudet, D.A.; Eudes, F.; Conner, R.L.; Kuzyk, A.; Chen, Q.; et al. The stripe rust resistance gene Yr10 encodes an evolutionary-conserved and unique CC-NBS-LRR sequence in wheat. Mol. Plant. 2014, 7, 1740–1755. [Google Scholar] [CrossRef]
- Wang, C.M.; Zhang, Y.P.; Han, D.J.; Kang, Z.S.; Li, G.P.; Cao, A.Z.; Chen, P.D. SSR and STS markers for wheat stripe rust resistance gene Yr26. Euphytica 2008, 159, 359–366. [Google Scholar] [CrossRef]
- Mace, E.S.; Buhariwalla, K.K.; Buhariwalla, H.K.; Crouch, J.H. A high-throughput DNA extraction protocol for tropical molecular breeding programs. Plant Mol. Biol. Report. 2003, 21, 459–460. [Google Scholar] [CrossRef]
- Qayoum, A.; Line, R.F. High-temperature, adult-plant resistance to stripe rust of wheat. Phytopathology 1985, 75, 1121–1125. [Google Scholar] [CrossRef]
- Peterson, R.F.; Campbell, A.B.; Hannah, A.E. A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can. J. Res. 2011, 26, 496–500. [Google Scholar] [CrossRef]
- Gai, J.Y. Methods and Standards for Recording Major Agronomic Traits of Wheat; China Agriculture Press: Beijing, China, 2009. [Google Scholar]
- Wang, Z.M. Regularity of wheat stripe rust and integrated control strategy. Neijiang Ke Ji 2003, 2, 19–20. [Google Scholar]
- Wu, X.L.; Tang, Y.L.; Li, C.S.; McHugh, A.D.; Li, Z.; Wu, C.X. Individual and combined effects of soil waterlogging and compaction on physiological characteristics of wheat in southwestern China. Field Crops Res. 2018, 215, 163–172. [Google Scholar] [CrossRef]
- Li, S.Z.; Wang, Q.; Zheng, J.M.; Zhu, H.Z.; Li, J.; Wan, H.S.; Luo, J.T.; Liu, Z.H.; Wu, L. Analysis of genetic components in the major wheat cultivar Chuanmai104 in southwest wheat region based on three wheat SNP arrays. J. Triticeae Crops 2021, 41, 665–672. [Google Scholar] [CrossRef]
- Li, Y.J.; Xu, H.; Yu, S.N.; Tang, J.W.; Li, Q.Y.; Gao, Y.; Zheng, J.Z.; Dong, C.H.; Yuan, Y.H.; Zheng, T.C.; et al. Genetic analysis of elite stripe rust resistance genes of founder parent Zhou8425B in its derived varieties. Acta Agron. Sin. 2024, 50, 16–31. [Google Scholar] [CrossRef]
- Bai, B.; Zhang, H.Z.; Du, J.Y.; Zhang, X.Y.; He, R.; Wu, L.; Zhang, Z.; Zhang, Y.H.; Cao, S.Q.; Liu, Z.Y. Current situation and strategy of stripe rust resistance genes untilization in winter wheat cultivars of northwestern oversummering region for Puccinia striiformis f. sp. tritici in China. Sci. Agric. Sin. 2024, 57, 4–17. [Google Scholar] [CrossRef]
- LI, Z.; Huang, Y.C.; Zhang, C.L.; Yu, R.; Liu, S.J.; Wu, J.H.; Li, C.L.; Zheng, W.J.; Zeng, Q.D.; Kang, Z.S.; et al. Evaluation of stripe rust resistance and analysis of disease resistance genes of the new wheat lines in the middle and lower reaches of the Yangtze River. J. Triticeae Crops 2024, 44, 835–845. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef]
- Rakszegi, M.; Molnár, I.; Darkó, É.; Tiwari, V.K.; Shewry, P. Editorial: Aegilops: Promising genesources to improve agronomical and quality traits of wheat. Front. Plant Sci. 2020, 11, 1060. [Google Scholar] [CrossRef]
- Yan, H.H.; Yu, K.Q.; Xu, Y.h.; Zhou, P.P.; Zhao, J.; Li, Y.; Liu, X.M.; Ren, C.Z.; Peng, Y.Y. Position validation of the dwarfing gene Dw6 in oat (Avena sativa L.) and its correlated effects on agronomic traits. Front. Plant Sci. 2021, 12, 668847. [Google Scholar] [CrossRef]
- Burdon, J.J.; Barrett, L.G.; G, R.; Thrall, P.H. Guiding deployment of resistance in cereals using evolutionary principles. Evol. Appl. 2014, 7, 609–624. [Google Scholar] [CrossRef]
- Alma, K.; Aralbek, R.; Angelina, M.; Makpal, A.; Madina, K.; Zhenis, K. Identification of stripe rust resistance genes in common wheat cultivars and breeding lines from Kazakhstan. Plants 2021, 10, 2303. [Google Scholar] [CrossRef]
- Zheng, S.G.; Li, Y.F.; Lu, L.; Liu, Z.H.; Zhang, C.H.; Ao, D.H.; Li, L.R.; Zhang, C.Y.; Liu, R.; Luo, C.P.; et al. Evaluating the contribution of Yr genes to stripe rust resistance breeding through marker-assisted detection in wheat. Euphytica 2017, 213, 50. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, J.; Abbas, H.M.K.; Liu, Y.; Cheng, M.; Huang, J.; Cheng, W.; Wang, B.; Bai, C.; Wang, G.; et al. Pyramiding of nine transgenes in maize generates high-level resistance against necrotrophic maize pathogens. Theor. Appl. Genet. 2018, 131, 2145–2156. [Google Scholar] [CrossRef]
- Mbinda, W.; Masaki, H. Breeding strategies and challenges in the improvement of blast disease resistance in finger millet. A current review. Front. Plant Sci. 2021, 11, 602882. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).