Micro-CT Analysis of Pore Structure in Upland Red Soil Under Different Long-Term Fertilization Regimes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Site
2.2. Sampling and Measurements
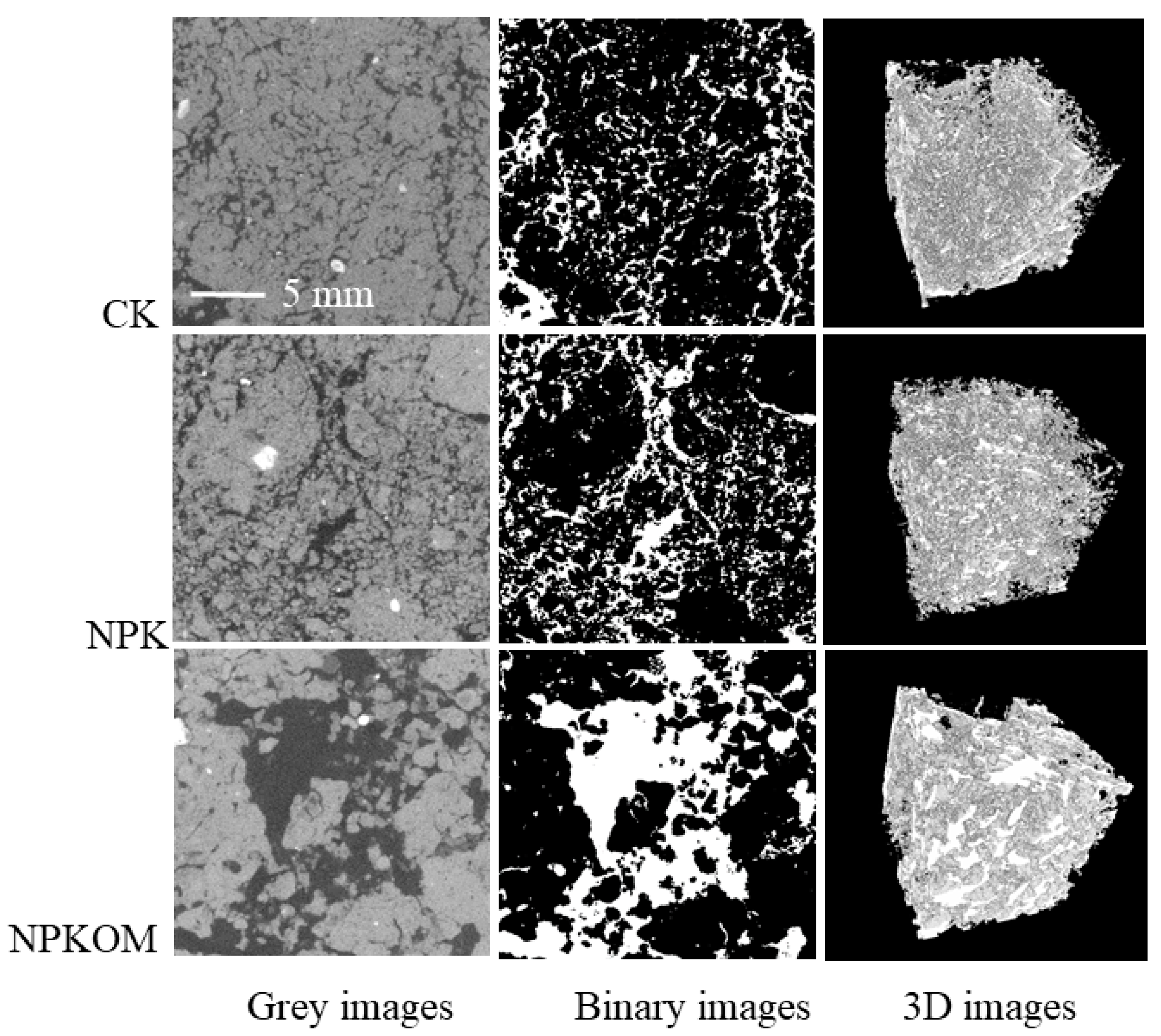
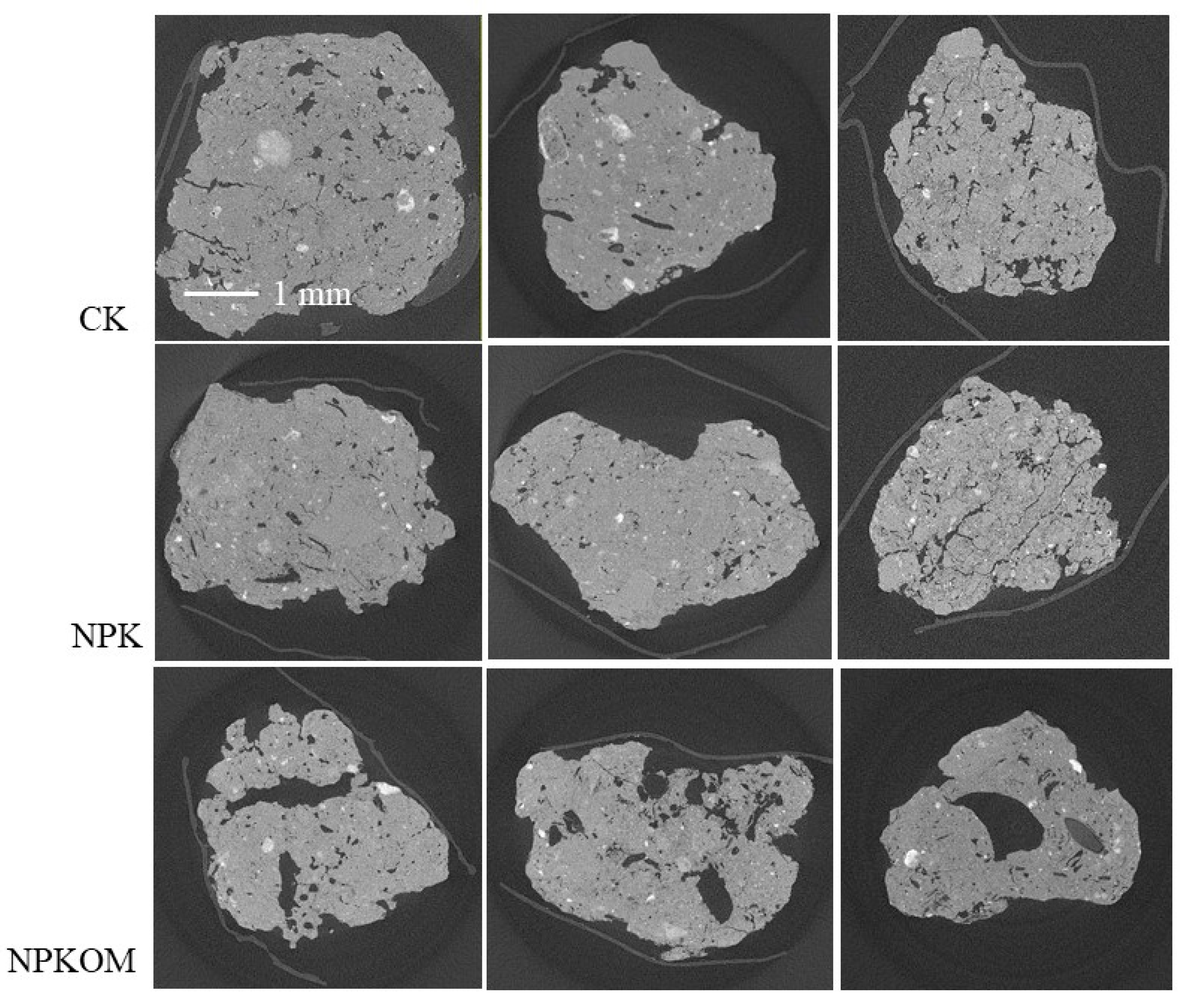
2.3. X-Ray CT Scanning and Image Processing
2.4. Statistical Analysis
3. Results
3.1. Basic Properties and Aggregate Stability of Upland Red Soil
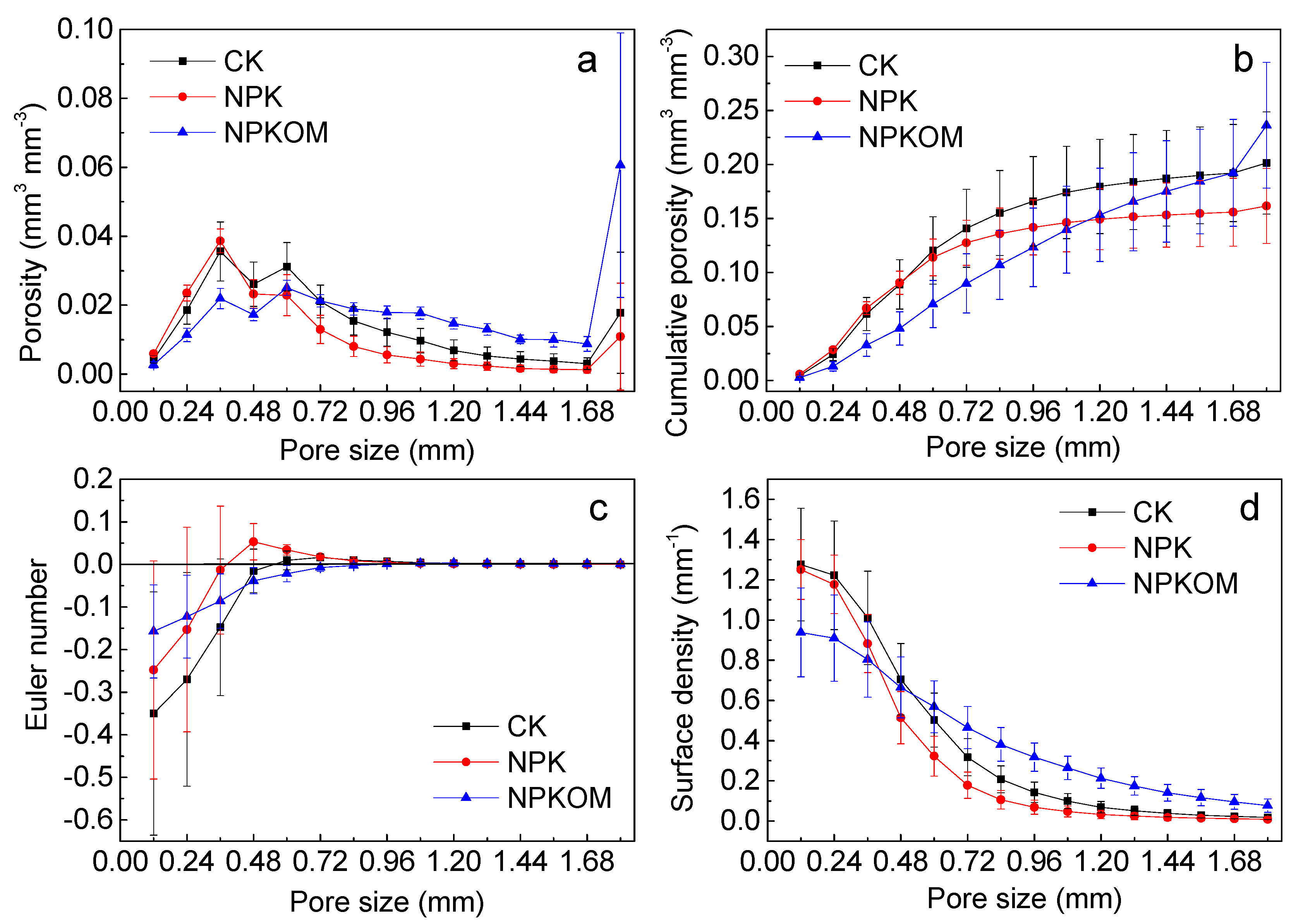
3.2. Pore Structure Characteristics of Soil Columns in Upland Red Soil
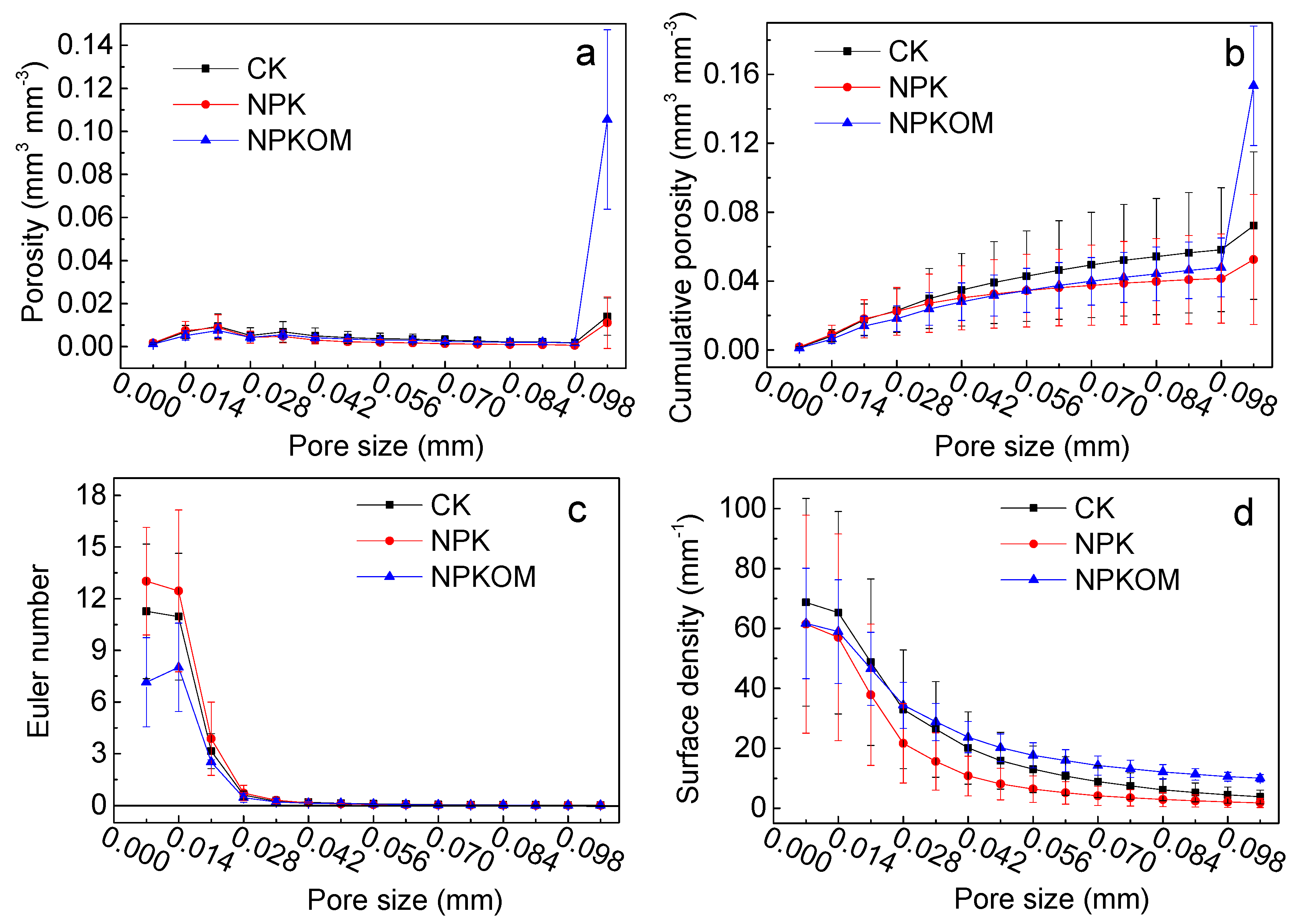
3.3. Pore Structure Characteristics of Soil Aggregates in Upland Red Soil
4. Discussion
4.1. Effects of Long-Term Fertilization on SOC and Aggregate Stability in Upland Red Soil
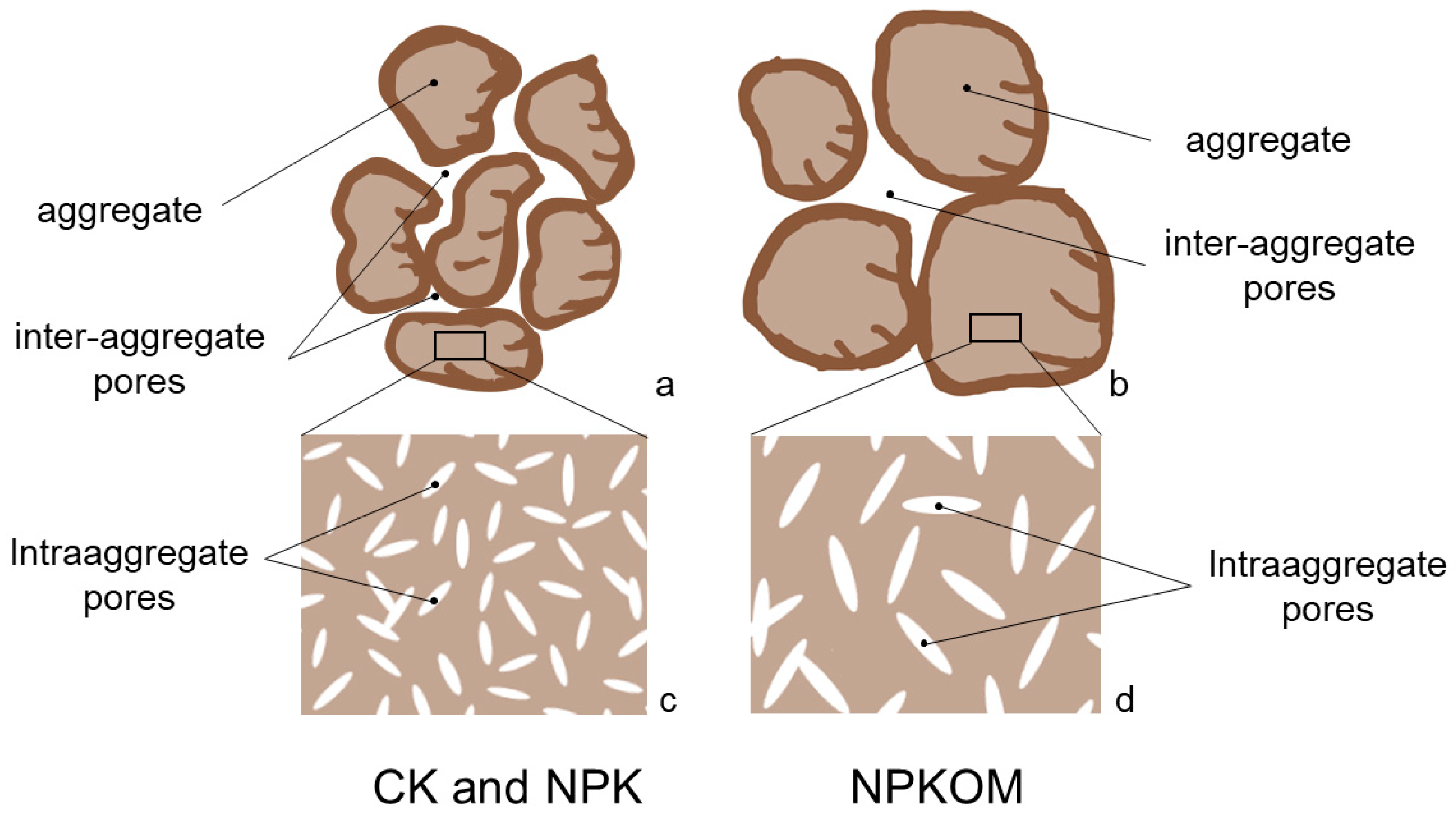
4.2. Effects of Long-Term Fertilization on the Pore Structure Characteristics of the Upland Red Soil
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Fan, R.; Zhang, X.; Zhang, Y.; Wu, D.; McLaughlin, N.; Zhang, S.; Chen, X.; Jia, S.; Liang, A. Tillage-induced effects on SOC through changes in aggregate stability and soil pore structure. Sci. Total Environ. 2020, 703, 134617. [Google Scholar] [CrossRef] [PubMed]
- Eden, M.; Moldrup, P.; Schjønning, P.; Vogel, H.J.; Scow, K.M.; de Jonge, L.W. Linking soil physical parameters along a density gradient in a loess-soil long-term experiment. Soil Sci. 2012, 177, 1–11. [Google Scholar] [CrossRef]
- Pardo, A.; Amato, M.; Chiarandà, F.Q. Relationships between soil structure, root distribution and water uptake of chickpea (Cicer arietinum L.). Plant growth and water distribution. Eur. J. Agron. 2000, 13, 39–45. [Google Scholar] [CrossRef]
- Tong, X.; Xu, M.; Wang, X.; Bhattacharyya, R.; Zhang, W.; Cong, R. Long-term fertilization effects on organic carbon fractions in a red soil of China. Catena 2014, 113, 251–259. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B.; Xu, M.; Fan, T. Crop yield and soil responses to long-term fertilization on a red soil in southern China. Pedosphere 2009, 19, 199–207. [Google Scholar] [CrossRef]
- Baligar, V.C.; Fageria, N.K.; Eswaran, H.; Wilson, M.J.; He, Z. Nature and properties of red soils of the world. In The Red Soils of China: Their Nature, Management and Utilization; Springer: Dordrecht, The Netherlands, 2004; pp. 7–27. [Google Scholar]
- Dong, W.; Zhang, X.; Wang, H.; Dai, X.; Sun, X.; Qiu, W.; Yang, F. Effect of different fertilizer application on the soil fertility of paddy soils in red soil region of southern China. PLoS ONE 2012, 7, e44504. [Google Scholar] [CrossRef] [PubMed]
- Celik, I.; Ortas, I.; Kilic, S. Effects of compost, mycorrhiza, manure and fertilizer on some physical properties of a Chromoxerert soil. Soil Tillage Res. 2004, 78, 59–67. [Google Scholar] [CrossRef]
- Adnan, M.; Xu, M.; Syed, A.; Muhammad, M.; Sun, N.; Wang, B.; Cai, Z.; Qudsia, S.; Muhammad, N.; Khalid, M. Soil aggregation and soil aggregate stability regulate organic carbon and nitrogen storage in a red soil of southern China. J. Environ. Manag. 2020, 270, 110894. [Google Scholar]
- Liu, K.; Han, T.; Huang, J.; Huang, Q.; Li, D.; Hu, Z.; Yu, X.; Muhammad, Q.; Ahmed, W.; Hu, H.; et al. Response of soil aggregate-associated potassium to long-term fertilization in red soil. Geoderma 2019, 352, 160–170. [Google Scholar] [CrossRef]
- Di, J.; Liu, X.; Du, Z.; Xiao, X.; Yang, G.; Ren, T. Influences of long-term organic and chemical fertilization on soil aggregation and associated organic carbon fractions in a red paddy soil. Chin. J. Eco-Agric. 2014, 22, 1129–1138. [Google Scholar]
- Huang, S.; Peng, X.; Huang, Q.; Zhang, W. Soil aggregation and organic carbon fractions affected by long-term fertilization in a red soil of subtropical China. Geoderma 2010, 154, 364–369. [Google Scholar] [CrossRef]
- Sun, M. Characteristics of Pore Size Distribution and Change in Upland Res Soil under Long-Term Manure Application. Master’s Thesis, Hunan Agriculture University, Changsha, China, 2014. (In Chinese). [Google Scholar]
- Liu, K.; Huang, J.; Zhang, H.; Li, D.; Han, T.; Cai, Z.; Wang, B.; Huang, Q. Effect of long-term fertilization on aggregation characteristics and distribution of potassium fractions in red soil. Acta Pedol. Sin. 2018, 55, 443–454. (In Chinese) [Google Scholar]
- Dal Ferro, N.; Charrier, P.; Morari, F. Dual-scale micro-CT assessment of soil structure in a long-term fertilization experiment. Geoderma 2013, 204, 84–93. [Google Scholar] [CrossRef]
- Rabot, E.; Wiesmeier, M.; Schlüter, S.; Vogel, H.J. Soil structure as an indicator of soil functions: A review. Geoderma 2018, 314, 122–137. [Google Scholar] [CrossRef]
- Young, I.M.; Crawford, J.W. Interactions and self-organization in the soil-microbe complex. Science 2004, 304, 1634–1637. [Google Scholar] [CrossRef]
- Zhou, H.; Fang, H.; Mooney, S.J.; Peng, X. Effects of long-term inorganic and organic fertilizations on the soil micro and macro structures of rice paddies. Geoderma 2016, 266, 66–74. [Google Scholar] [CrossRef]
- Ju, X.; Gao, L.; She, D.; Jia, Y.; Pang, Z.; Wang, Y. Impacts of the soil pore structure on infiltration characteristics at the profile scale in the red soil region. Soil Tillage Res. 2024, 236, 105922. [Google Scholar] [CrossRef]
- Zhang, S.Y.; He, B.; Hao, B.; Lv, D. Quantification of red soil macropores affected by slope erosion and sediment using computed tomography. Vadose Zone J. 2023, 22, e20276. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Peng, J.; Yang, Q.; Zhang, C.; Ni, S.; Wang, J.; Cai, C. Aggregate pore structure, stability characteristics, and biochemical properties induced by different cultivation durations in the Mollisol region of Northeast China. Soil Tillage Res. 2023, 233, 105797. [Google Scholar] [CrossRef]
- Pulido-Moncada, M.; Petersen, S.O.; Clough, T.J.; Munkholm, L.J.; Squartini, A.; Longo, M.; Dal Ferro, N.; Morari, F. Soil pore network effects on the fate of nitrous oxide as influenced by soil compaction, depth and water potential. Soil Biol. Biochem. 2024, 197, 109536. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, M.; Wang, B.; Wang, X. Soil organic carbon, total nitrogen and grain yields under long-term fertilizations in the upland red soil of southern China. Nutr. Cycl. Agroecosystems 2009, 84, 59–69. [Google Scholar] [CrossRef]
- Le Bissonnais, Y.L. Aggregate stability and assessment of soil crustability and erodibility: I. Theory and methodology. Eur. J. Soil Sci. 1996, 47, 425–437. [Google Scholar] [CrossRef]
- Zhou, H.; Fang, H.; Hu, C.; Mooney, S.J.; Dong, W.; Peng, X. Inorganic fertilization effects on the structure of a calcareous silt loam soil. Agron. J. 2017, 109, 2871–2880. [Google Scholar] [CrossRef]
- Schlüter, S.; Weller, U.; Vogel, H.J. Soil-structure development including seasonal dynamics in a long-term fertilization experiment. J. Plant Nutr. Soil Sci. 2011, 174, 395–403. [Google Scholar] [CrossRef]
- Martínez, F.S.J.; Ortega, F.M.; Monreal, F.C.; Kravchenko, A.N.; Wang, W. Soil aggregate geometry: Measurements and morphology. Geoderma 2015, 237, 36–48. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Cai, G.; Sauheitl, L.; Xiao, M.; Shibistova, O.; Ge, T.; Guggenberger, G. Meta-analysis on the effects of types and levels of N, P, and K fertilization on organic carbon in cropland soils. Geoderma 2023, 437, 116580. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, N.; Huang, F.; Lv, Y. Long-term effects of different organic and inorganic fertilizer treatments on soil organic carbon sequestration and crop yields on the North China Plain. Soil Tillage Res. 2015, 146, 47–52. [Google Scholar] [CrossRef]
- Šimanský, V.; Juriga, M.; Jonczak, J.; Uzarowicz, Ł.; Stępień, W. How relationships between soil organic matter parameters and soil structure characteristics are affected by the long-term fertilization of a sandy soil. Geoderma 2019, 342, 75–84. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Prakash, V.; Kundu, S.; Srivastva, A.K.; Gupta, H.S.; Mitra, S. Long term effects of fertilization on carbon and nitrogen sequestration and aggregate associated carbon and nitrogen in the Indian sub-Himalayas. Nutr. Cycl. Agroecosystems 2010, 86, 1–16. [Google Scholar] [CrossRef]
- Xin, X.; Zhang, J.; Zhu, A.; Zhang, C. Effects of long-term (23 years) mineral fertilizer and compost application on physical properties of fluvo-aquic soil in the North China Plain. Soil Tillage Res. 2016, 156, 166–172. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Schlegel, A.J. Implications of inorganic fertilization of irrigated corn on soil properties: Lessons learned after 50 years. J. Environ. Qual. 2013, 42, 861–871. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Yu, X.; Hong, C.; Peng, G.; Lu, S. Response of pore structures to long-term fertilization by a combination of synchrotron radiation X-ray microcomputed tomography and a pore network model. Eur. J. Soil Sci. 2018, 69, 290–302. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, S.; Udawatta, R.P.; Anderson, S.H.; de Jonge, L.W.; Katuwal, S. X-ray micro-computed tomography characterized soil pore network as influenced by long-term application of manure and fertilizer. Geoderma 2021, 385, 114872. [Google Scholar] [CrossRef]
- Pagenkemper, S.K.; Athmann, M.; Uteau, D.; Kautz, T.; Peth, S.; Horn, R. The effect of earthworm activity on soil bioporosity–Investigated with X-ray computed tomography and endoscopy. Soil Tillage Res. 2015, 146, 79–88. [Google Scholar] [CrossRef]
- Colombi, T.; Braun, S.; Keller, T.; Walter, A. Artificial macropores attract crop roots and enhance plant productivity on compacted soils. Sci. Total Environ. 2017, 574, 1283–1293. [Google Scholar] [CrossRef]
- Wendel, A.S.; Bauke, S.L.; Amelung, W.; Knief, C. Root-rhizosphere-soil interactions in biopores. Plant Soil 2022, 475, 253–277. [Google Scholar] [CrossRef]
- Siedt, M.; Schäffer, A.; Smith, K.E.; Nabel, M.; Roß-Nickoll, M.; Van Dongen, J.T. Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef]
- Ozlu, E.; Kumar, S. Response of soil organic carbon, pH, electrical conductivity, and water stable aggregates to long-term annual manure and inorganic fertilizer. Soil Sci. Soc. Am. J. 2018, 82, 1243–1251. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil structure and soil organic matter II. A normalized stability index and the effect of mineralogy. Soil Sci. Soc. Am. J. 2000, 64, 1042–1049. [Google Scholar] [CrossRef]

| Samples | Sample Size/ (mm) | Voltage/ (kV) | Current/ (μA) | Resolution/ (mm) |
|---|---|---|---|---|
| Soil columns | Diameter 50, height 50 | 110 | 110 | 0.06 |
| Soil aggregates | Diameter 3–5 | 110 | 120 | 0.0035 |
| Treatments | SOC/ (g kg−1) | Bulk Density/ (g cm−3) | Total Porosity/ (mm3 mm−3) | MWD/ (mm) |
|---|---|---|---|---|
| CK | 7.53 ± 0.33 c | 1.30 ± 0.05 b | 0.51 ± 0.02 b | 0.64 ± 0.10 b |
| NPK | 10.9 ± 0.23 b | 1.11 ± 0.03 c | 0.58 ± 0.01 a | 0.67 ± 0.19 b |
| NPKOM | 16.0 ± 1.20 a | 1.41 ± 0.04 a | 0.47 ± 0.01 c | 0.97 ± 0.14 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, H.; Zhang, N.; Yu, Z.; Li, D.; Peng, X.; Zhou, H. Micro-CT Analysis of Pore Structure in Upland Red Soil Under Different Long-Term Fertilization Regimes. Agronomy 2024, 14, 2668. https://doi.org/10.3390/agronomy14112668
Fang H, Zhang N, Yu Z, Li D, Peng X, Zhou H. Micro-CT Analysis of Pore Structure in Upland Red Soil Under Different Long-Term Fertilization Regimes. Agronomy. 2024; 14(11):2668. https://doi.org/10.3390/agronomy14112668
Chicago/Turabian StyleFang, Huan, Na Zhang, Zhenghong Yu, Dongchu Li, Xinhua Peng, and Hu Zhou. 2024. "Micro-CT Analysis of Pore Structure in Upland Red Soil Under Different Long-Term Fertilization Regimes" Agronomy 14, no. 11: 2668. https://doi.org/10.3390/agronomy14112668
APA StyleFang, H., Zhang, N., Yu, Z., Li, D., Peng, X., & Zhou, H. (2024). Micro-CT Analysis of Pore Structure in Upland Red Soil Under Different Long-Term Fertilization Regimes. Agronomy, 14(11), 2668. https://doi.org/10.3390/agronomy14112668






