Effects of Foliar Application of Magnesium Fertilizer on Photosynthesis and Growth in Grapes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Determination of Leaf Area, Leaf Extensibility, and Dry Weight
2.3. Determination of Chlorophyll Index and Gas Exchange Measurements
2.4. Determination of Fast Fluorescence Rise Curve OJIP and the JIP-Test
2.5. Determination of the Yield
2.6. Statistical Analyses
3. Results
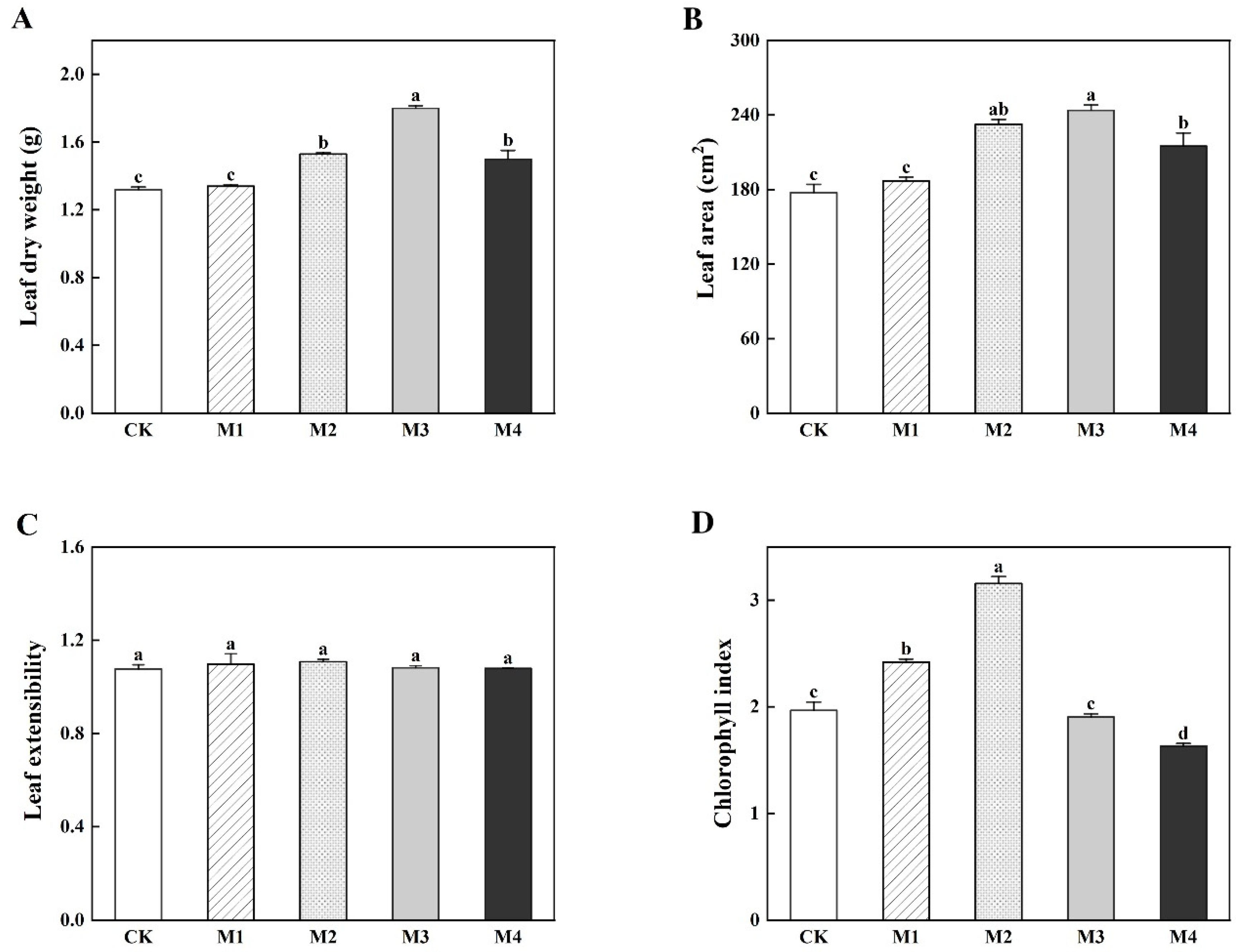
3.1. Leaf Growth
3.2. Photosynthetic Parameters
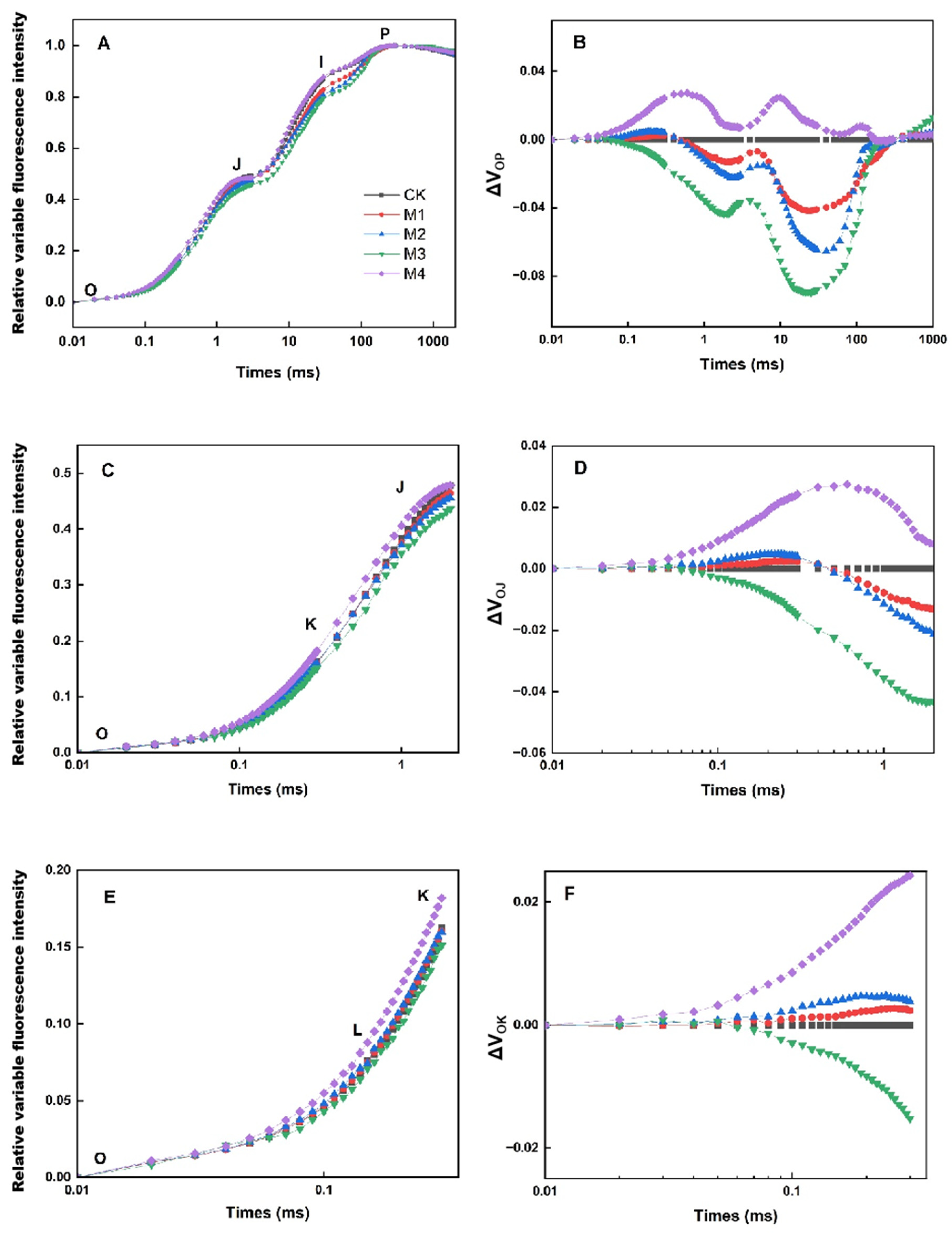
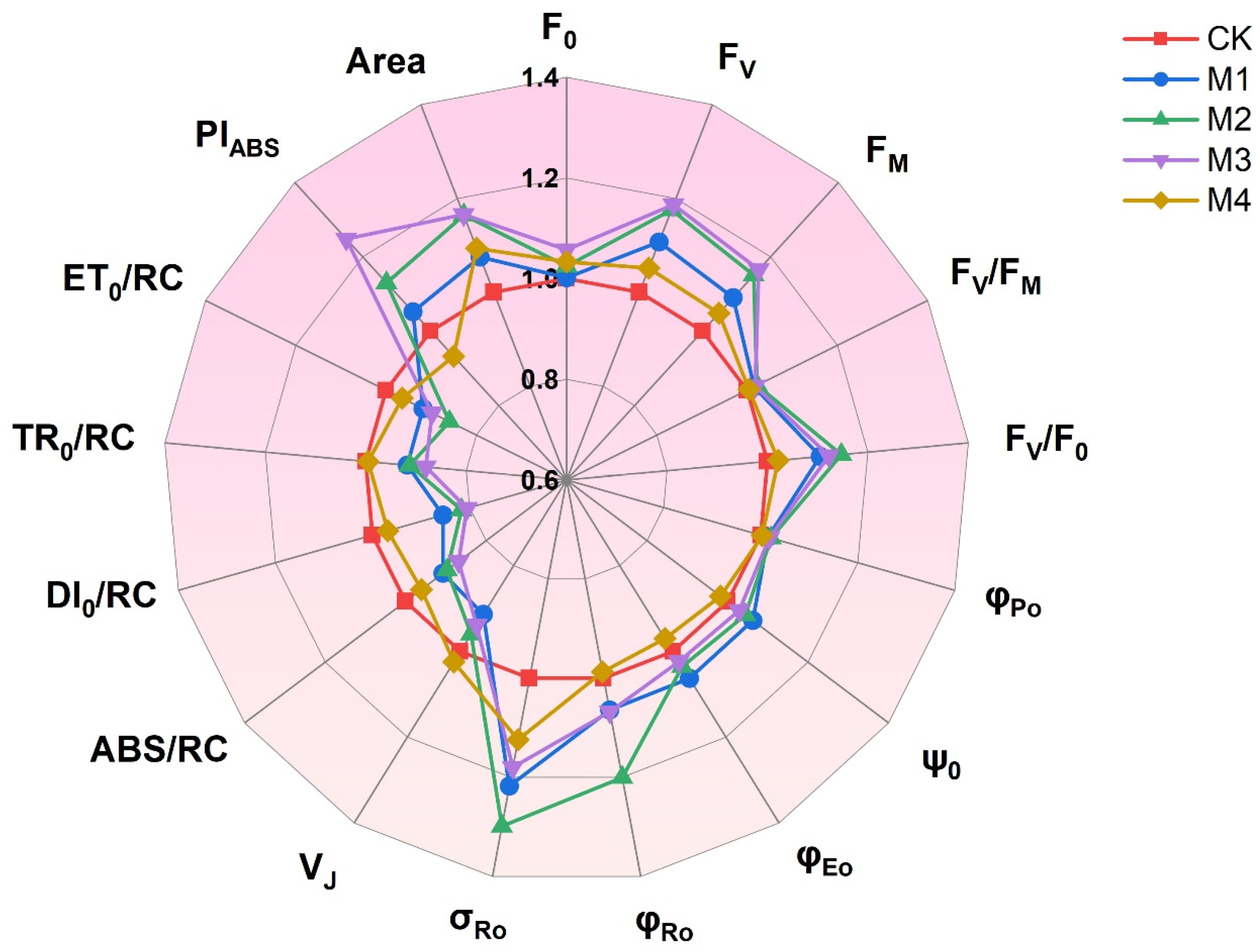
3.3. The OJIP and JIP-Tests
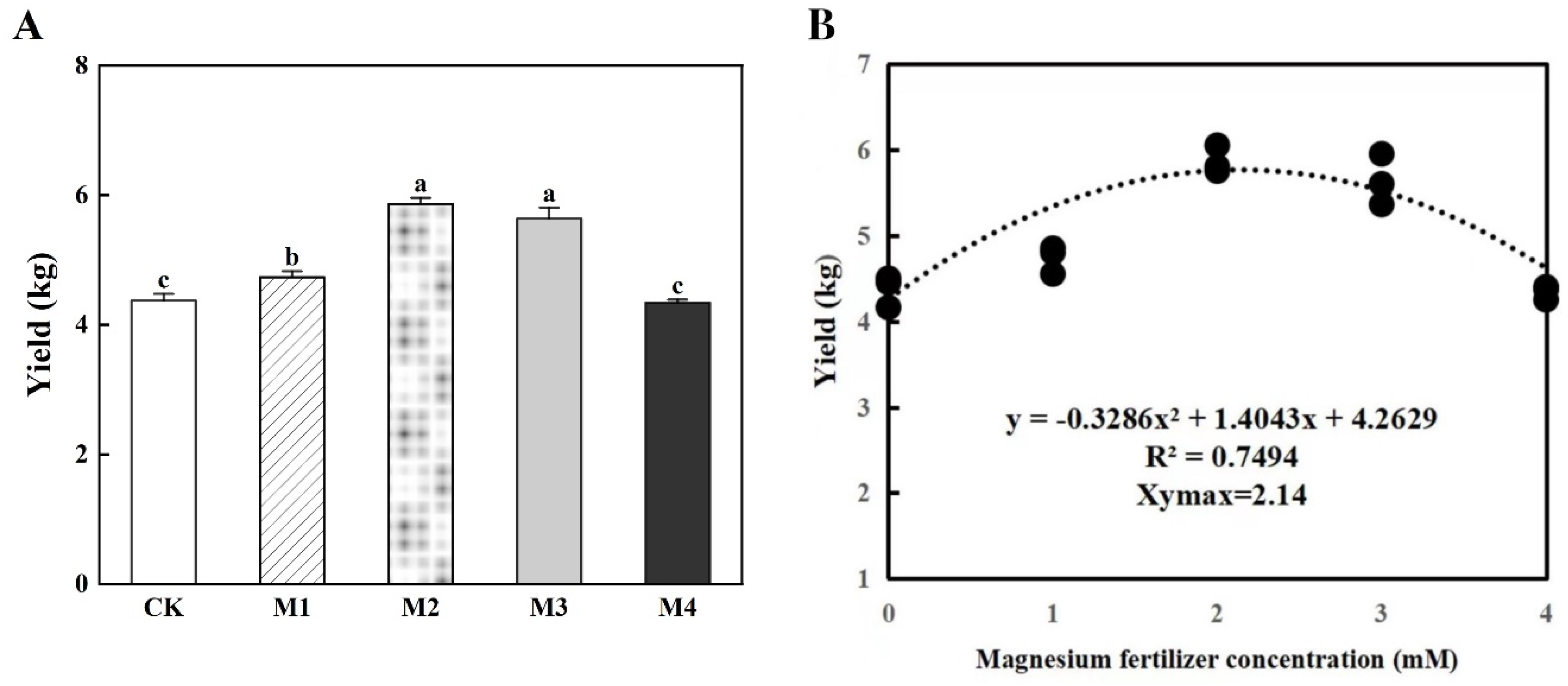
3.4. Yield
4. Discussion
4.1. Leaf Growth
4.2. Photosynthetic Parameters
4.3. OJIP
4.4. Chlorophyll Fluorescence Parameters
4.5. Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khadivi, A.; Gismondi, A.; Canini, A. Genetic characterization of Iranian grapes (Vitis vinifera L.) and their relationships with Italian ecotypes. Agrofor. Syst. 2019, 93, 435–447. [Google Scholar] [CrossRef]
- Nie, J.; Li, Y.H.; Yang, X.; Zheng, J.R.; Xie, Y.M.; Shi, L.L. Effect of fertilization treatment on growth, yield, fruit quality, and nutrition accumulation of cherry tomato. Appl. Ecol. Environ. Res. 2023, 21, 3849–3863. [Google Scholar] [CrossRef]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilisers in agriculture: Plant-soil continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, B.; Bozdar, B.; Chachar, S.; Rai, M.; Li, J.; Li, Y.; Hayat, F.; Chachar, Z.; Tu, P. The power of magnesium: Unlocking the potential for increased yield, quality, and stress tolerance of horticultural crops. Front. Plant Sci. 2023, 14, 1285512. [Google Scholar] [CrossRef]
- Meng, X.; Bai, S.; Wang, S.; Pan, Y.; Chen, K.; Xie, K.; Wang, M.; Guo, S. The sensitivity of photosynthesis to magnesium deficiency differs between rice (Oryza sativa L.) and cucumber (Cucumis sativus L.). Front. Plant Sci. 2023, 14, 1164866. [Google Scholar] [CrossRef]
- Ali, M.M.; Hu, X.; Chao, P.; Ali, S.; Akram, M.T.; Naveed, W.A.; Gull, S.; Deng, H.; Mosa, W.F.A.; Hou, Y.; et al. Magnesium’s impact on fruit quality of loquat: Insights into sugar and acid dynamics. Sci. Hortic. 2024, 328, 112972. [Google Scholar] [CrossRef]
- Monteiro, A.I.; Malheiro, A.C.; Bacelar, E.A. Morphology, physiology and analysis techniques of grapevine bud fruitfulness: A Review. Agriculture 2021, 11, 127. [Google Scholar] [CrossRef]
- Rissler, H.M.; Collakova, E.; DellaPenna, D.; Whelan, J.; Pogson, B.J. Chlorophyll biosynthesis. Expression of a second chl I gene of magnesium chelatase in Arabidopsis supports only limited chlorophyll synthesis. Plant Physiol. 2002, 128, 770–779. [Google Scholar] [CrossRef]
- Karley, A.J.; White, P.J. Moving cationic minerals to edible tissues: Potassium, magnesium, calcium. Curr. Opin. Plant Biol. 2009, 12, 291–298. [Google Scholar] [CrossRef]
- Pietrzykowska, M.; Suorsa, M.; Semchonok, D.A.; Tikkanen, M.; Boekema, E.J.; Aro, E.-M.; Jansson, S. The Light-harvesting chlorophyll a/b binding proteins Lhcb1 and Lhcb2 play complementary roles during state transitions in Arabidopsis. Plant Cell 2014, 26, 3646–3660. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Y.; Bai, C.; Yong, J.W.H. The significance of chloroplast NAD(P)H dehydrogenase complex and its dependent cyclic electron transport in photosynthesis. Front. Plant Sci. 2021, 12, 661863. [Google Scholar] [CrossRef] [PubMed]
- Willows, R.D. Biosynthesis of chlorophylls from protoporphyrin IX. Nat. Prod. Rep. 2003, 20, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Johnson, G.N.; Strasser, R.J.; Verbruggen, N. Physiological characterisation of magnesium deficiency in sugar beet: Acclimation to low magnesium differentially affects photosystems I and II. Planta 2004, 220, 344–355. [Google Scholar] [CrossRef]
- He, H.; Jin, X.; Ma, H.; Deng, Y.; Huang, J.; Yin, L. Changes of plant biomass partitioning, tissue nutrients and carbohydrates status in magnesium-deficient banana seedlings and remedy potential by foliar application of magnesium. Sci. Hortic. 2020, 268, 109377. [Google Scholar] [CrossRef]
- Chaudhry, A.H.; Nayab, S.; Hussain, S.B.; Ali, M.; Pan, Z. Current understandings on magnesium deficiency and future outlooks for sustainable agriculture. Int. J. Mol. Sci. 2021, 22, 1819. [Google Scholar] [CrossRef]
- Jin, X.; Ackah, M.; Wang, L.; Amoako, F.K.; Shi, Y.; Essoh, L.G.; Li, J.; Zhang, Q.; Li, H.; Zhao, W. Magnesium nutrient application induces metabolomics and physiological responses in mulberry (Morus alba) plants. Int. J. Mol. Sci. 2023, 24, 9650. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.-J.; Shin, J.-S.; Li, S.-Y. The catalytic role of Rubisco for in situ CO2 recycling in escherichia coli. Front. Bioeng. Biotechnol. 2020, 8, 543807. [Google Scholar] [CrossRef]
- Jamali Jaghdani, S.; Jahns, P.; Tränkner, M. The impact of magnesium deficiency on photosynthesis and photoprotection in Spinacia oleracea. Plant Stress 2021, 2, 100040. [Google Scholar] [CrossRef]
- Ba, Q.; Zhang, L.; Chen, S.; Li, G.; Wang, W. Effects of foliar application of magnesium sulfate on photosynthetic characteristics, dry matter accumulation and its translocation, and carbohydrate metabolism in grain during wheat grain filling. Cereal Res. Commun. 2020, 48, 157–163. [Google Scholar] [CrossRef]
- Jezek, M.; Geilfus, C.-M.; Bayer, A.; Muehling, K. Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front. Plant Sci. 2015, 5, 781. [Google Scholar] [CrossRef] [PubMed]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Esteves, E.; Maltais-Landry, G.; Zambon, F.; Ferrarezi, R.S.; Kadyampakeni, D.M. Nitrogen, calcium, and magnesium inconsistently affect tree growth, fruit yield, and juice quality of huanglongbing-affected orange trees. Hortscience 2021, 56, 1269–1277. [Google Scholar] [CrossRef]
- Hu, W.; Yang, B.; He, Z.; Li, G. Magnesium may be a key nutrient mechanism related to Fusarium wilt resistance: A new banana cultivar (Zhongjiao No. 9). PeerJ 2021, 9, e11141. [Google Scholar] [CrossRef] [PubMed]
- Grzebisz, W. Magnesium-food and human health. J. Elem. 2011, 16, 299–323. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, X.; Tian, G.; Liu, C.; Xing, Y.; Feng, Z.; Lyu, M.; Liu, J.; Xu, X.; Zhu, Z.; et al. Magnesium alleviates growth inhibition under low potassium by enhancing photosynthesis and carbon-nitrogen metabolism in apple plants. Plant Physiol. Biochem. 2024, 214, 108875. [Google Scholar] [CrossRef]
- El-Badawy, H.E. Implication of using potassium and magnesium fertilization to improve growth, yield and quality of crimson seedless grapes (Vitis vinifera L.). J. Plant Prod. 2019, 10, 133–141. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 2015, 6, 7879. [Google Scholar] [CrossRef]
- Kinnersley, A.M.; Lin, F. Receptor modifiers indicate that 4-aminobutyric acid (GABA) is a potential modulator of ion transport in plants. Plant Growth Regul. 2000, 32, 65–76. [Google Scholar] [CrossRef]
- Lorente-Mento, J.M.; Guillén, F.; Martínez-Romero, D.; Carrión-Antoli, A.; Valero, D.; Serrano, M. γ-Aminobutyric acid treatments of pomegranate trees increase crop yield and fruit quality at harvest. Sci. Hortic. 2023, 309, 111633. [Google Scholar] [CrossRef]
- Li, Y.; Lai, R.; Li, W.; Liu, J.; Huang, M.; Tang, Y.; Tang, X.; Pan, S.; Duan, M.; Tian, H.; et al. γ-aminobutyric acid regulates grain yield formation in different fragrant rice genotypes under different nitrogen levels. J. Plant Growth Regul. 2020, 39, 738–750. [Google Scholar] [CrossRef]
- Islam, S.N.U.; Kouser, S.; Hassan, P.; Asgher, M.; Shah, A.A.; Khan, N.A. Gamma-aminobutyric acid interactions with phytohormones and its role in modulating abiotic and biotic stress in plants. Stress Biol. 2024, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Zirek, N.S.; Uzal, O. The developmental and metabolic effects of different magnesium dozes in pepper plants under salt stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 967–977. [Google Scholar] [CrossRef]
- Ye, D.-Q. Based on mobile phone photos and imageJ software measurement of phenotypic indexes of cunninghamia lanceolata. For. Sci. Technol. 2022, 9, 32–35. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, X.; Zhang, S.; Ma, M.; Bai, R.; Liu, H.; Yi, B.; Han, X.; Liu, Y. Exogenous calcium alleviates phosphorus deficiency-induced photosynthetic inhibition in peanut (Arachis hypogaea). J. Plant Nutr. Fertil. 2022, 28, 1055–1066. [Google Scholar] [CrossRef]
- Shi, Q.; Pang, J.; Yong, J.W.H.; Bai, C.; Pereira, C.G.; Song, Q.; Wu, D.; Dong, Q.; Cheng, X.; Wang, F.; et al. Phosphorus-fertilisation has differential effects on leaf growth and photosynthetic capacity of Arachis hypogaea L. Plant Soil 2020, 447, 99–116. [Google Scholar] [CrossRef]
- Strasser, B.J. Measuring fast fluorescence transients to address environmental questions: The JIP test. In Photosynthesis: From Light to Biosphere; Kluwer Academic: Dordrecht, The Netherlands, 1995; pp. 977–980. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant haberlea rhodopensis. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Yang, X.; Wang, F.; Qi, M.; Li, T.; Liu, Y. Cyclic electron flow protects photosystem I donor side under low night temperature in tomato. Environ. Exp. Bot. 2020, 177, 104151. [Google Scholar] [CrossRef]
- Ye, X.; Huang, H.; Wu, F.; Cai, L.; Lai, N.; Deng, C.; Guo, J.; Yang, L.; Chen, L. Molecular mechanisms for magnesium-deficiency-induced leaf vein lignification, enlargement and cracking in Citrus sinensis revealed by RNA-Seq. Tree Physiol 2021, 41, 280–301. [Google Scholar] [CrossRef]
- He, H.; Khan, S.; Deng, Y.; Hu, H.; Yin, L.; Huang, J. Supplemental foliar-applied magnesium reverted photosynthetic inhibition and improved biomass partitioning in magnesium-deficient banana. Horticulturae 2022, 8, 1050. [Google Scholar] [CrossRef]
- Neuhaus, C.; Geilfus, C.-M.; Zoerb, C.; Muehling, K.H. Transcript expression of Mg-chelatase and H+-ATPase isogenes in Vicia faba leaves as influenced by root and foliar magnesium supply. Plant Soil 2013, 368, 41–50. [Google Scholar] [CrossRef]
- Xie, K.; Pan, Y.; Meng, X.; Wang, M.; Guo, S. Critical leaf magnesium thresholds for growth, chlorophyll, leaf area, and photosynthesis in rice (Oryza sativa L.) and cucumber (Cucumis sativus L.). Agronomy 2024, 14, 1508. [Google Scholar] [CrossRef]
- Kosgey, J.R.; Moot, D.J.; Fletcher, A.L.; McKenzie, B.A. Dry matter accumulation and post-silking N economy of ‘stay-green’ maize (Zea mays L.) hybrids. Eur. J. Agron. 2013, 51, 43–52. [Google Scholar] [CrossRef]
- Teklić, T.; Vratarić, M.; Sudarić, A.; Kovačević, V.; Vukadinović, V.; Bertić, B. Relationships among chloroplast pigments concentration and chlorophyllmeter readings in soybean under influence of foliar magnesium application. Commun. Soil Sci. Plant Anal. 2009, 40, 706–725. [Google Scholar] [CrossRef]
- Dordas, C. Foliar application of calcium and magnesium improves growth, yield, and essential oil yield of oregano (Origanum vulgare ssp. hirtum). Ind. Crops Prod. 2009, 29, 599–608. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Y.; Bai, C.; Yang, Y.; Sun, Z.; Liu, X.; Zhang, S.; Han, X.; Yong, J.W.H. The physiological functionality of PGR5/PGRL1-dependent cyclic electron transport in sustaining photosynthesis. Front. Plant Sci. 2021, 12, 702196. [Google Scholar] [CrossRef]
- Tatagiba, S.D.; DaMatta, F.M.; Rodrigues, F.A. Magnesium decreases leaf scald symptoms on rice leaves and preserves their photosynthetic performance. Plant Physiol. Biochem. 2016, 108, 49–56. [Google Scholar] [CrossRef]
- Zhou, H.; Peng, J.; Zhao, W.; Zeng, Y.; Xie, K.; Huang, G. Leaf diffusional capacity largely contributes to the reduced photosynthesis in rice plants under magnesium deficiency. Plant Physiol. Biochem. 2024, 209, 108565. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yang, L.; Jiang, H.; Li, Y.; Wang, P.; Chen, L. Physiological impacts of magnesium-deficiency in Citrus seedlings: Photosynthesis, antioxidant system and carbohydrates. Trees 2012, 26, 1237–1250. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Hua, X.; Zhang, Z.; Fan, T.; Yao, W.; Zhang, M.; Zhang, J. Transcriptome dynamics underlying magnesium deficiency stress in three founding Saccharum Species. Int. J. Mol. Sci. 2022, 23, 9681. [Google Scholar] [CrossRef]
- Kan, B.; Yang, Y.; Du, P.; Li, X.; Lai, W.; Hu, H. Chlorophyll decomposition is accelerated in banana leaves after the long-term magnesium deficiency according to transcriptome analysis. PLoS ONE 2022, 17, e0270610. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, C.; Zhang, H.; Chen, S.; Wang, X.; Gan, L. Endogenous γ-Aminobutyric acid accumulation enhances salinity tolerance in rice. Plants 2024, 13, 2750. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Huang, P.; Huang, S.; Younis, U.; Hussain, G.S.; Fahad, S.; Danish, S.; Elshikh, M.S.; Rizwana, H. γ-Aminobutyric acid (GABA) and ectoine (ECT) impacts with and without AMF on antioxidants, gas exchange attributes and nutrients of cotton cultivated in salt affected soil. BMC Plant Biol. 2023, 23, 476. [Google Scholar] [CrossRef]
- Faseela, P.; Sinisha, A.; Brestič, M.; Puthur, J. Chlorophyll a fluorescence parameters as indicators of a particular abiotic stress in rice. Photosynthetica 2019, 57, 108–115. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Pereira, W.E.; de Siqueira, D.L.; Martínez, C.A.; Puiatti, M. Gas exchange and chlorophyll fluorescence in four citrus rootstocks under aluminium stress. J. Plant Physiol. 2000, 157, 513–520. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Xie, R.; Gao, J.; Yang, Z.; Wang, Y.; Tong, L.; Ke, Y.; Li, C.; Zheng, C.; Li, W. Improving high light tolerance of tobacco plants: Adequate magnesium supply enhances photosynthetic performance. Agronomy 2024, 14, 1396. [Google Scholar] [CrossRef]
- Ceppi, M.G.; Oukarroum, A.; Çiçek, N.; Strasser, R.J.; Schansker, G. The IP amplitude of the fluorescence rise OJIP is sensitive to changes in the photosystem I content of leaves: A study on plants exposed to magnesium and sulfate deficiencies, drought stress and salt stress. Physiol. Plant. 2012, 144, 277–288. [Google Scholar] [CrossRef]
- Zivcak, M.; Kalaji, H.M.; Shao, H.-B.; Olsovska, K.; Brestic, M. Photosynthetic proton and electron transport in wheat leaves under prolonged moderate drought stress. J. Photochem. Photobiol. B Biol. 2014, 137, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrov, V.; Krasteva, V.; Paunov, M.; Chepisheva, M.; Kousmanova, M.; Kalaji, H.; Goltsev, V. Deficiency of some nutrient elements in bean and maize plants analyzed by luminescent method. Bulg. J. Agric. Sci. 2014, 20, 24–30. [Google Scholar]
- Kanjana, D. Foliar application of magnesium oxide nanoparticles on nutrient element concentrations, growth, physiological, and yield parameters of cotton. J. Plant Nutr. 2020, 43, 3035–3049. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Muneer, M.A.; Jin, D.; Zhang, H.; Cai, Y.; Ma, C.; Wang, C.; Chen, X.; Huang, C. Characterization of different magnesium fertilizers and their effect on yield and quality of soybean and pomelo. Agronomy 2022, 12, 2693. [Google Scholar] [CrossRef]
- Liu, X.; Hu, C.; Liu, X.; Riaz, M.; Liu, Y.; Dong, Z.; Tan, Q.; Sun, X.; Wu, S.; Tan, Z. Effect of magnesium application on the fruit coloration and sugar accumulation of navel orange (Citrus sinensis Osb.). Sci. Hortic. 2022, 304, 111282. [Google Scholar] [CrossRef]

| Depth (cm) | 0–20 | 20–40 |
|---|---|---|
| pH | 6.14 ± 0.01 | 6.46 ± 0.01 |
| Organic matter (%) | 0.54% ± 0.01 | 0.38% ± 0.01 |
| Alkaline hydrolysis nitrogen (mg/kg) | 75.73 ± 1.75 | 62.00 ± 1.00 |
| Effective phosphorus (mg/kg) | 137.40 ± 2.28 | 130.35 ± 0.57 |
| Rapidly available potassium (mg/kg) | 1215.51 ± 28.98 | 2104.97 ± 35.18 |
| Exchangeable Mg (g/kg) | 0.46 ± 0.01 | 0.30 ± 0.01 |
| Exchangeable Ca (g/kg) | 5.25 ± 0.01 | 3.61 ± 0.01 |
| Fluorescence Parameters | Description |
|---|---|
| F0 | Minimum fluorescence at 20 ms (all RCs were supposed to be opened) |
| FV | Variable fluorescence (Fm − F0) |
| Fm | Maximum fluorescence intensity at the P phase of OJIP (maximum RCs are supposed to be closed) |
| FV/Fm | Maximum quantum yield of PSII |
| FV/F0 | Efficiency of electron donation to PSII |
| VJ | Relative variable fluorescence at the J-step |
| Area | The area between the fluorescence curve and Fm |
| ΦPo | Maximum quantum yield of primary photochemistry (t = 0) |
| ψ0 | The probability that the trapped electron was transferred to ETC beyond QA |
| ΦEo | Quantum yield of electron transported to ETC beyond QA |
| ΦRo | Quantum yield for the reduction in end electron acceptors at the PSI acceptor side (RE) |
| σRo | Efficiency/probability with which an electron from the intersystem electron carriers moved to reduce end electron acceptors at the PSI acceptor side (RE) |
| ABS/RC | Absorption per reaction center at PSII/ratio of active reaction centers in PSII |
| DI0/RC | Dissipation energy flux per reaction center (t = 0) |
| TR0/RC | Trapped energy flux per reaction center (t = 0) |
| ET0/RC | Electron transport flux per reaction center (t = 0) |
| PIABS | Performance index on absorption basis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, R.; Liu, H.; Liu, Y.; Yong, J.W.H. Effects of Foliar Application of Magnesium Fertilizer on Photosynthesis and Growth in Grapes. Agronomy 2024, 14, 2659. https://doi.org/10.3390/agronomy14112659
Bai R, Liu H, Liu Y, Yong JWH. Effects of Foliar Application of Magnesium Fertilizer on Photosynthesis and Growth in Grapes. Agronomy. 2024; 14(11):2659. https://doi.org/10.3390/agronomy14112659
Chicago/Turabian StyleBai, Rui, Huan Liu, Yifei Liu, and Jean Wan Hong Yong. 2024. "Effects of Foliar Application of Magnesium Fertilizer on Photosynthesis and Growth in Grapes" Agronomy 14, no. 11: 2659. https://doi.org/10.3390/agronomy14112659
APA StyleBai, R., Liu, H., Liu, Y., & Yong, J. W. H. (2024). Effects of Foliar Application of Magnesium Fertilizer on Photosynthesis and Growth in Grapes. Agronomy, 14(11), 2659. https://doi.org/10.3390/agronomy14112659







