Allelopathic Effects of Soil Extracts from Rhus typhina Plantations on Common Turfgrass Species in Northern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Area
2.2. Soil Sampling and Extract Preparation
2.3. Turfgrass Seed Preparation
2.4. Germination and Seedling Growth Experiments
2.5. Physiological Properties of the Seedlings
2.6. Data Processing
2.7. Statistical Analysis
3. Results
3.1. Allelopathic Effects of Extracts from the Root Zone Soil of Rt on the Germination of the Receivers
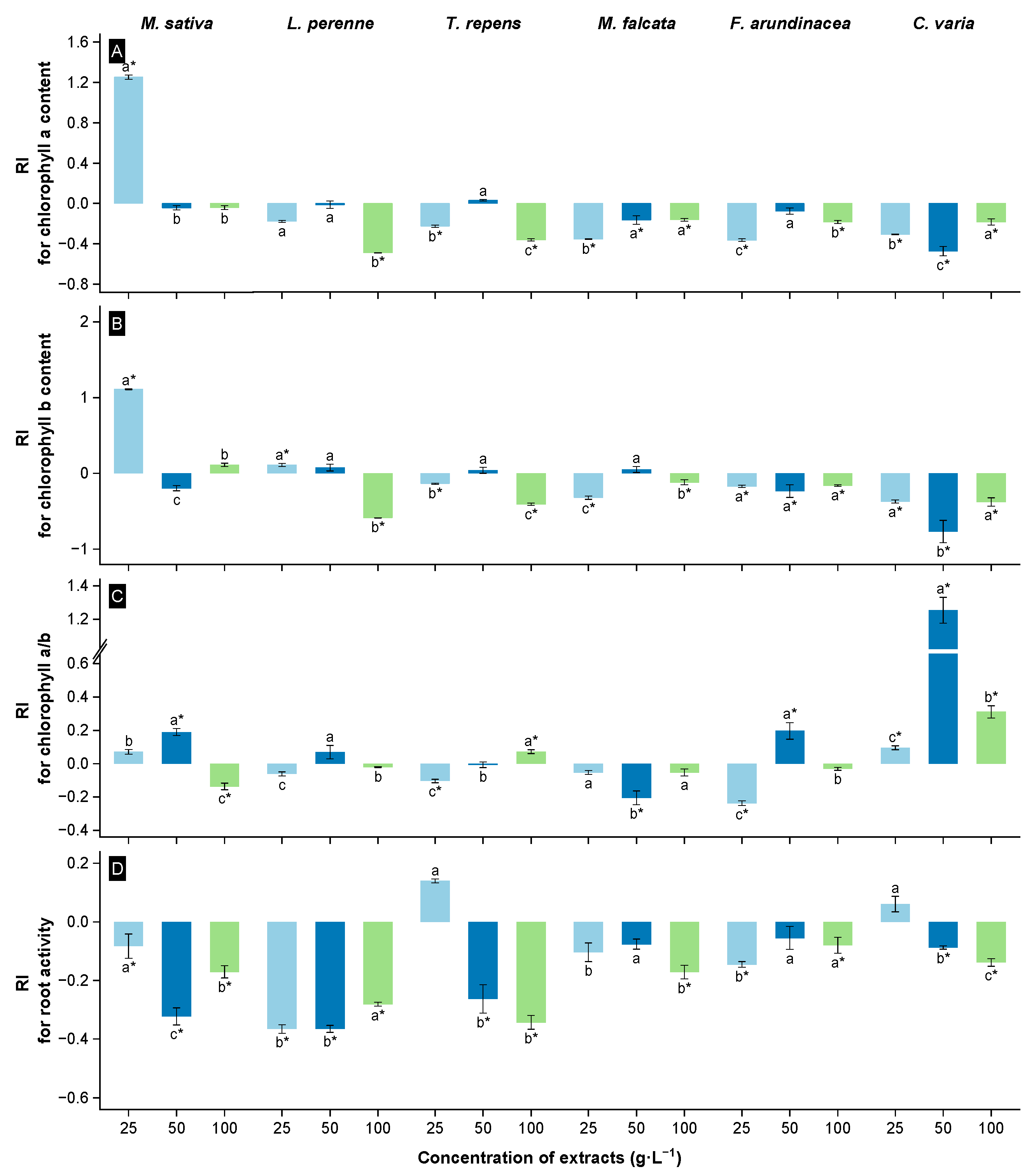
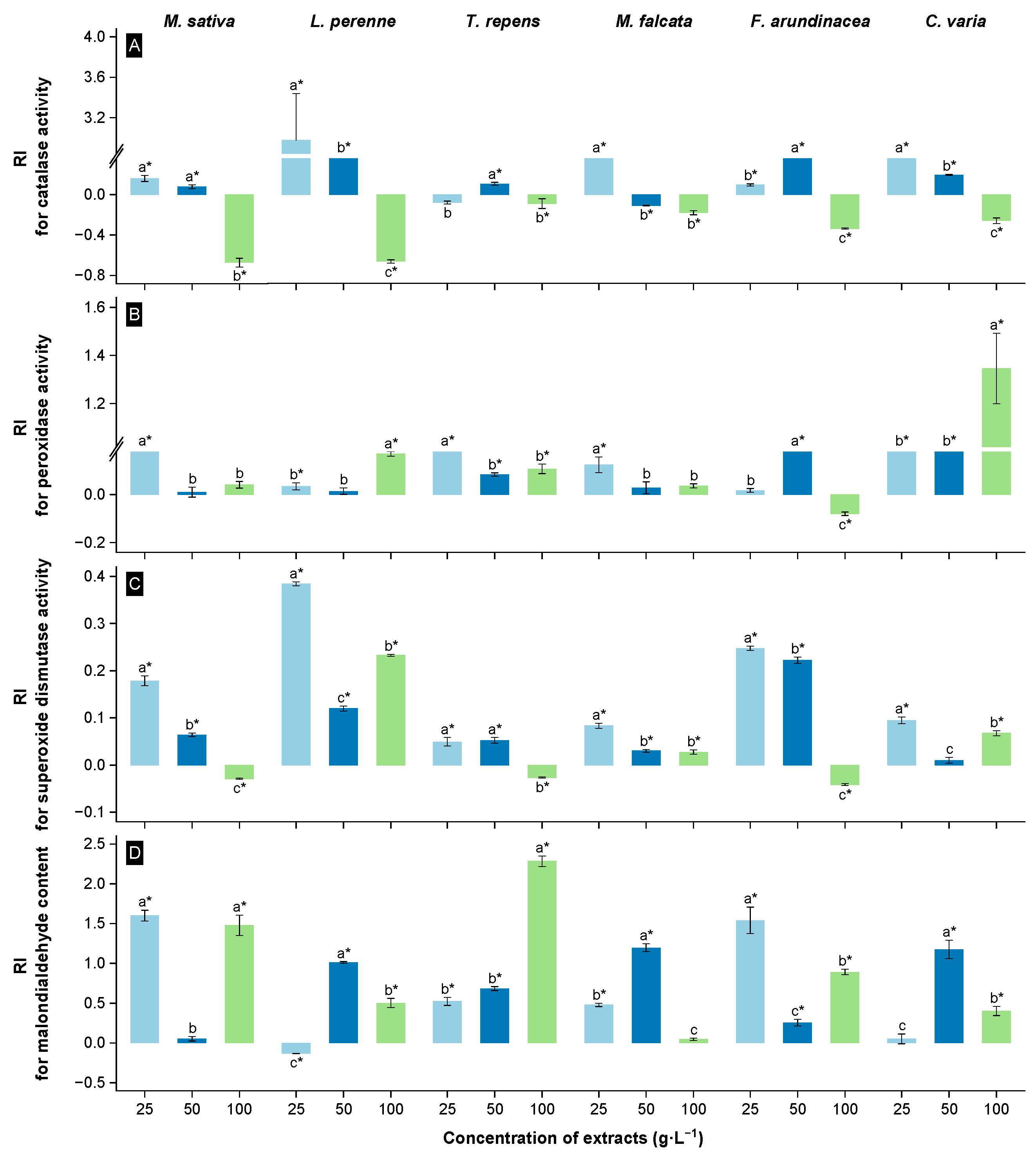
3.2. Allelopathic Effects of Extracts from the Root Zone Soil of Rt on the Physiological Properties of Receivers
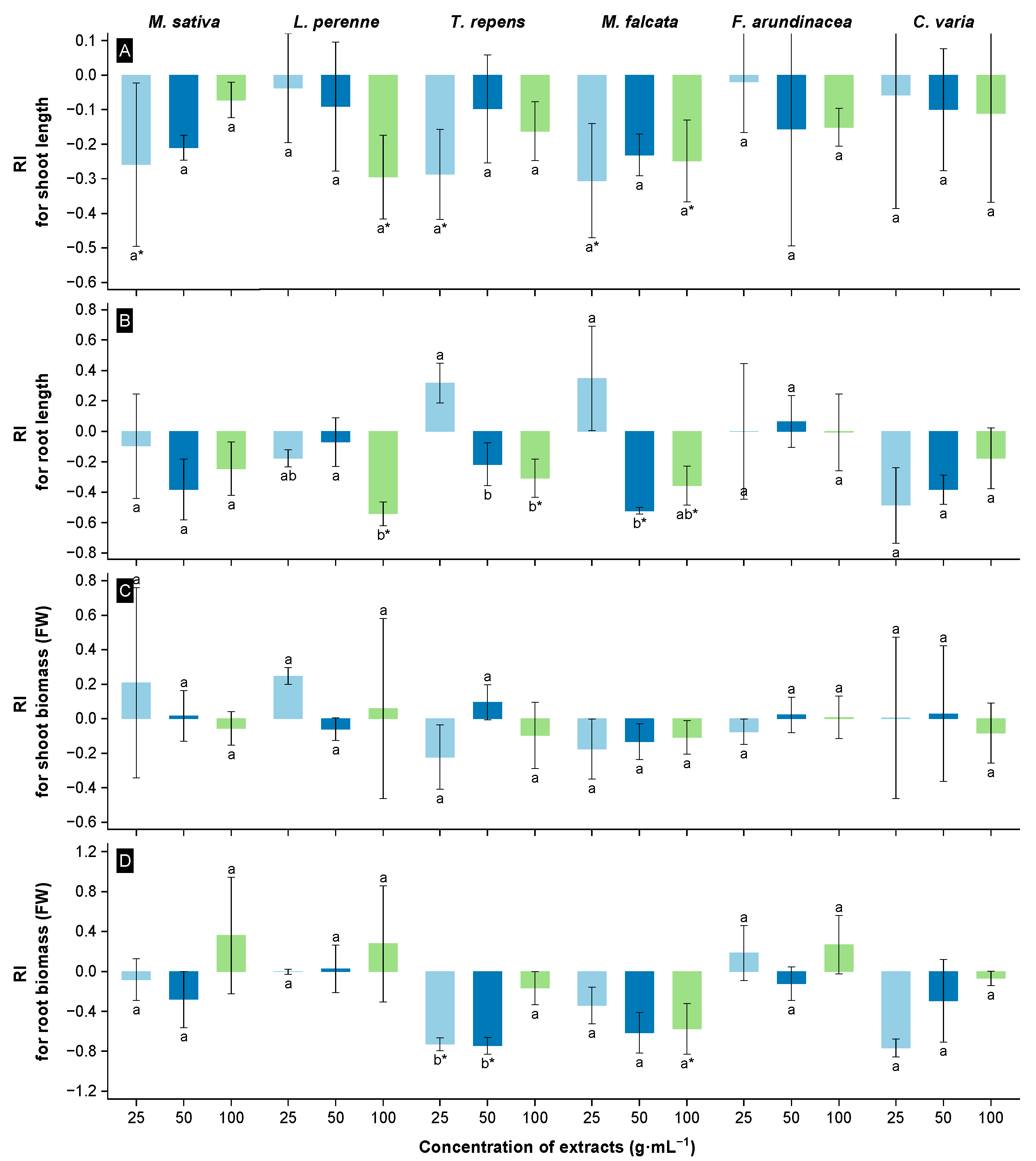
3.3. Allelopathic Effects of Extracts from the Root Zone Soil of Rt on Seedling Growth of the Receivers
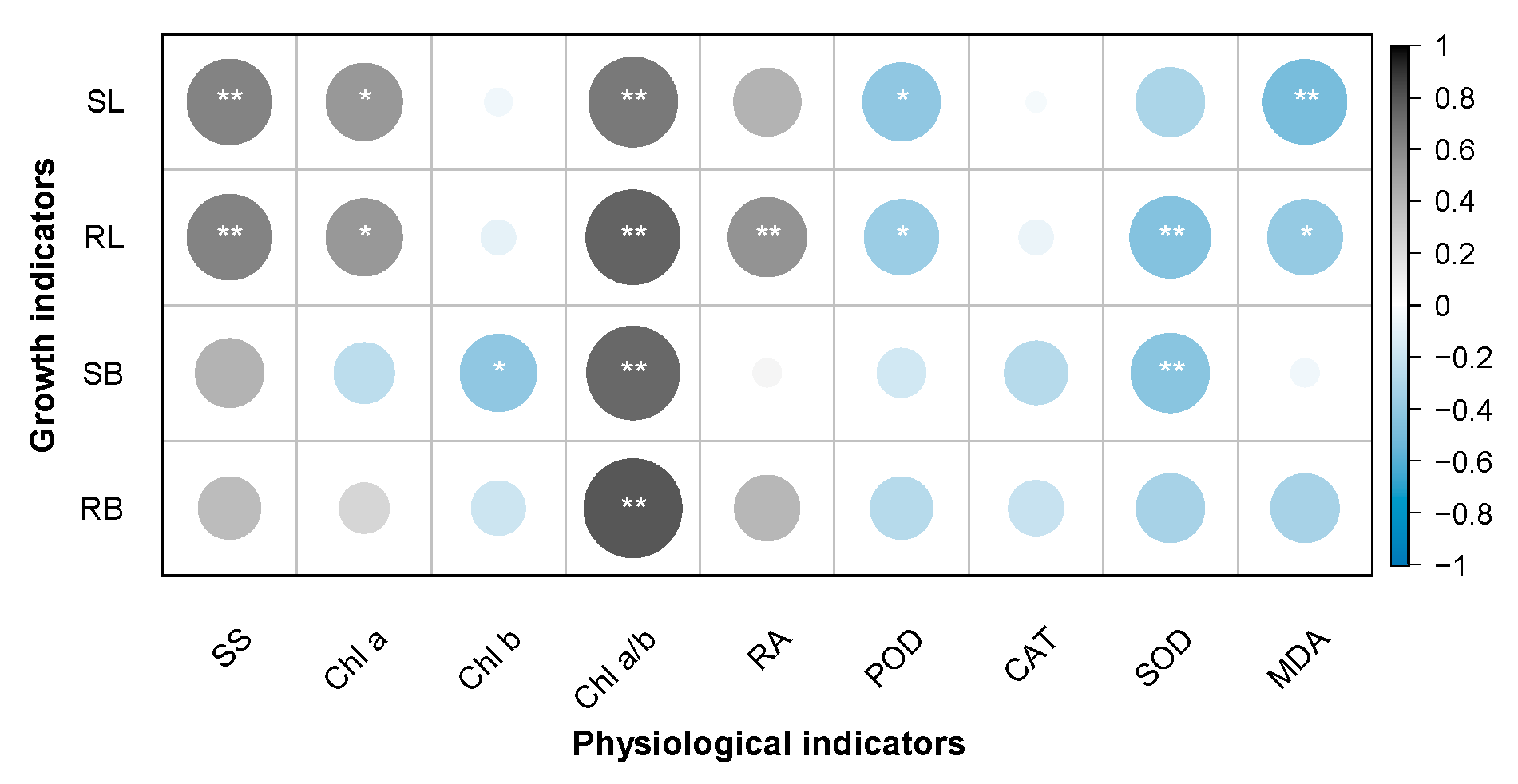
3.4. Relationships Between the Growth and Physiological Indices of the Receivers
3.5. Integrated Allelopathic Effects of Extracts from the Root Zone Soil of Rt on Receivers
4. Discussion
4.1. Allelopathic Effects of Extracts from the Root Zone Soil of Rt on the Receivers
4.2. Other Issues Should Be Addressed
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Liu, Y.; Yuan, L.; Weber, E.; van Kleunen, M. Effect of allelopathy on plant performance: A meta-analysis. Ecol. Lett. 2021, 24, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, L.; Guo, J.; Su, H.; Lan, Q.; Liu, G. The allelopathic effect of aqueous extracts of Stellera chamae jasme on seed germination and seedling growth of Allium senescens. Acta Agrestia Sin. 2022, 30, 2391–2398. [Google Scholar]
- Xu, Y.; Chen, X.; Ding, L.; Kong, C.-H. Allelopathy and allelochemicals in grasslands and forests. Forests 2023, 14, 562. [Google Scholar] [CrossRef]
- Khamare, Y.; Chen, J.; Marble, S.C. Allelopathy and its application as a weed management tool: A review. Front. Plant Sci. 2022, 13, 1034649. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Hu, C.; Fu, R.; Hu, G. Plant allelopathy research and development: 60 years (1960–2019). Allelopath. J. 2022, 52, 163–180. [Google Scholar] [CrossRef]
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant allelochemicals: Agronomic, nutritional and ecological relevance in the soil system. Plant Soil 2019, 442, 23–48. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Chen, Q.; Miao, Y.; Peng, Z.; Huang, B.; Guo, L.; Liu, D.; Du, H. Allelopathic effect of Artemisia argyi on the germination and growth of various weeds. Sci. Rep. 2021, 11, 4303. [Google Scholar] [CrossRef]
- Liu, C.; Chen, X.; Wu, M.; He, L.; Ye, X.; Fan, T. Allelopathic effects of Phragmites communis leaves on the growth and physiobiochemical characteristics of Solidago canadensis. Acta Pratacult. Sin. 2014, 23, 182–190. [Google Scholar]
- Jilani, G.; Mahmood, S.; Chaudhry, A.N.; Hassan, I.; Akram, M. Allelochemicals: Sources, toxicity and microbial transformation in soil—A review. Ann. Microbiol. 2008, 58, 351–357. [Google Scholar] [CrossRef]
- Strugstad, M.P.; Despotovski, S. A summary of extraction, properties, and potential uses of juglone: A literature review. J. Ecosyst. Manag. 2012, 13, 1–16. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Q.; Liu, H.; Wang, X.; Ma, Q. Research advances in microbial degradation of phenolic acids with allelopathic effects. Microbiol. China 2024, 51, 402–418. [Google Scholar]
- Facenda, G.; Real, M.; Galán-Pérez, J.A.; Gámiz, B.; Celis, R. Soil effects on the bioactivity of hydroxycoumarins as plant allelochemicals. Plants 2023, 12, 1278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, M. Reproduction and community dynamics of staghorn sumac (Rhus typhina) in a coal-gangue area. Acta Ecol. Sin. 2016, 36, 195–199. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, J.; Zhang, D.; Gong, C.; Wang, Q.; Zhai, C.; Dai, X. Effects of Rhus typhina invasion on soil physicochemical properties and carbon emissions in urban green spaces. Forests 2022, 13, 1827. [Google Scholar] [CrossRef]
- Diao, W. Study on Morphological Anatomy of Rhus typhina; Jilin Agriculture University: Changchun, China, 2016. [Google Scholar]
- Qu, T.; Li, Y.; Ma, W. Influence of aqueous extract of Rhus typhina on seed germination and seeding growth in Cosmos bipinnata. J. Northeast For. Univ. 2017, 45, 26–31. [Google Scholar]
- Yue, J.; Cheng, G. Allelopathy of Rhus typhina on Samolous parviflorus seedlings. J. Beihua Univ. (Nat. Sci.) 2023, 24, 16–24. [Google Scholar]
- Zuo, L.; Wang, S.; Ma, Y.; Wen, S.; Bai, G.; Yu, X. Effect of torch tree extract on seeds germination in two types of turfgrass. Acta Agrestia Sin. 2021, 29, 1927–1933. [Google Scholar]
- Xu, Z.; Zhong, S.; Yu, Y.; Wang, Y.; Cheng, H.; Du, D.; Wang, C. Rhus typhina L. triggered greater allelopathic effects than Koelreuteria paniculata Laxm under ammonium fertilization. Sci. Hortic. 2023, 309, 111703. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.; Wang, X.; Li, J.; Liu, K.; Chen, L.; Dong, Y.; Wang, Z.; Chen, J. Allelopathic effects of Rhus typhina tillering seedlings on seed germination and seedling growth of three common turf species. Acta Pratacult. Sin. 2024, 33, 47–59. [Google Scholar]
- Gorepekin, I.; Fedotov, G.; Shoba, S. Allelotoxicity of soils: A review. Eurasian Soil Sci. 2022, 55, 1804–1812. [Google Scholar] [CrossRef]
- Liu, P.; Li, M.; Ding, Y. Plant Physiological Experiment; Science Press: Beijing, China, 2016. [Google Scholar]
- Ding, C.; Wei, X.; Wang, F. Effects of active allelochemicals from feral Gentiana straminea on the seed germination and seedling physiological properties of forages. Acta Pratacult. Sin. 2017, 26, 150–161. [Google Scholar]
- Zhang, H.; Yan, Y.; Zhu, Y.; Chen, Y.; Wang, J.; Cui, Z.; Yang, D.; Ren, X. Effect of stand density on understory herb diversity and soil properties in the Cornus officinalis plantation at the southern foot of Funiu Mountain. J. Southwest For. Univ. 2024, 45, 1–10. [Google Scholar]
- Możdżeń, K.; Tatoj, A.; Barabasz-Krasny, B.; Sołtys-Lelek, A.; Gruszka, W.; Zandi, P. The allelopathic potential of Rosa blanda aiton on selected wild-growing native and cultivated plants in Europe. Plants 2021, 10, 1806. [Google Scholar] [CrossRef]
- Ming, Y.; Hu, G.; Li, J.; Zhu, Z.; Fan, X.; Yuan, D. Allelopathic effects of Castanea henryi aqueous extracts on the growth and physiology of Brassica pekinensis and Zea mays. Chem. Biodivers. 2020, 17, e2000135. [Google Scholar] [CrossRef]
- Jing, R.; Peng, Z.; Li, Y.; Wang, S. Allelopathy of the litter extracts from Robinia pseudoacacia forest on its seed germination and embryo growth. J. Zhejiang AF Univ. 2023, 40, 97–106. [Google Scholar]
- Chen, F.; Meng, Y.; Shuai, H.; Luo, X.; Zhou, W.; Liu, J.; Yang, W.; Shu, K. Effect of plant allelochemicals on seed germination and its ecological significance. Chin. J. Eco-Agric. 2017, 25, 36–46. [Google Scholar]
- Ma, H.; Chen, Y.; Chen, J.; Zhang, Y.; Zhang, T.; He, H. Comparison of allelopathic effects of two typical invasive plants: Mikania micrantha and Ipomoea cairica in Hainan island. Sci. Rep. 2020, 10, 11332. [Google Scholar] [CrossRef]
- Shekari, F.; Shekari, F.; Najafi, J.; Abassi, A.; Radmanesh, Z.; Bones, A.M. Phytotoxic effects of catnip (Nepeta meyeri Benth.) on early growth stages sevelopment and infection potential of field dodder (Cuscuta campestris Yunck). Plants 2022, 11, 2629. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Z.; Zou, J.; Li, Q. Allelopathic effects of sesame extracts on seed germination of moso bamboo and identification of potential allelochemicals. Sci. Rep. 2022, 12, 6661. [Google Scholar] [CrossRef]
- Xie, X.; Chen, Y.; Bo, Y.; Dai, C. A review of allelopathic researches on phenolic acids. Acta Ecol. Sin. 2014, 34, 6417–6428. [Google Scholar]
- Jin, M.; Jia, M.; Xiao, Q.; Liu, J.; Shen, C.; Shi, Y.; He, Z. Allelopathic effect of forest gap litter on the growth of Castanopsis kawakamii seedlings. Acta Ecol. Sin. 2021, 42, 8288–8299. [Google Scholar]
- Taupik, S.A.M.; Aani, S.N.A.; Wai, C.P.; Seng, C.T. Allelopathic potential of cassava (Manihot esculenta L.) extracts on germination and seedling growth of selected weeds and aerobic rice. Sains Malays. 2022, 51, 633–642. [Google Scholar] [CrossRef]
- Lang, T.; Wei, P.; Chen, X.; Fu, Y.; Tam, N.F.-y.; Hu, Z.; Chen, Z.; Li, F.; Zhou, H. Microcosm study on allelopathic effects of leaf litter leachates and purified condensed tannins from Kandelia obovata on germination and growth of Aegiceras corniculatum. Forests 2021, 12, 1000. [Google Scholar] [CrossRef]
- Jia, F.; Li, L.; Long, W.; Lan, J. Effects of fresh leaf aqueous extract from four artificial forest trees on seed germination of Pinus elliottii. J. Northwest AF Univ. (Nat. Sci. Ed.) 2023, 51, 65–72. [Google Scholar]
- Saud, S.; Jiang, Z.; Fahad, S. Significance and Exploitation of Rhizosphere Chemical Signaling Metabolites for Enhancing Soil Nutrient Transformation. J. Soil Sci. Plant Nutr. 2023, 23, 4827–4842. [Google Scholar] [CrossRef]
- Zhu, P.; Wei, W.; Bai, X.; Wu, N.; Hou, Y. Effects of invasive Rhus typhina L. on bacterial diversity and community composition in soil. Ecoscience 2020, 27, 177–184. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, L.; Wang, X.; Yan, X.; Men, H.; Li, W.; Xu, W. Allelopathic effects of aqueous extract of exotic plant Rhus typhina L. on soil microecosystem. Acta Ecol. Sin. 2013, 33, 4041–4049. [Google Scholar]
- Mendez, R.M.; Miranda, A.R. Studies on the allelopathic effect of aquatic invasive plants on Cicer arietinum L. Int. J. Eng. Sci. 2015, 4, 42–48. [Google Scholar]
- Ade, L.; Zhou, J.; Li, J.; Ma, D. Effect of allelochemical stress from Chenopodium ambrosioides L. on chloroplast ultrastructure and photosynthetic key gene expression in leaves of Vicia faba seedlings. Southwest China J. Agric. Sci. 2018, 31, 2527–2532. [Google Scholar]
- Mohammadkhani, N.; Servati, M. Nutrient concentration in wheat and soil under allelopathy treatments. J. Plant Res. 2018, 131, 143–155. [Google Scholar] [CrossRef]
- Li, J.; Zhao, T.; Chen, L.; Chen, H.; Luo, D.; Chen, C.; Miao, Y.; Liu, D. Artemisia argyi allelopathy: A generalist compromises hormone balance, element absorption, and photosynthesis of receptor plants. BMC Plant Biol. 2022, 22, 368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Rutherford, S.; Qi, S.; Huang, P.; Dai, Z.; Du, D. Transcriptome profiling of Arabidopsis thaliana roots in response to allelopathic effects of Conyza canadensis. Ecotoxicology 2022, 31, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ruan, K.; Wang, T.; Bi, N.; Shi, S.; Li, S.; Liu, Z. Allelopathic effects of aqueous extracts of fallen leaves of Acer truncatum on three medicinal plants. Acta Pratacult. Sin. 2024, 33, 151–159. [Google Scholar]
- Chen, Y.; Wang, J.; Huang, H.; Yue, Y.; Luo, X.; Zheng, Z. Effects of 2-fluorobiphenyl and gallic acid on the physiology and biochemistry in Microcystis Aeruginosa. J. Fudan Univ. (Nat. Sci.) 2024. [Google Scholar] [CrossRef]
- Chen, B.; Wang, Y.; Ma, D.; Hu, Z.; He, Y.; Zhou, J. The antioxidant enzyme activities and their gene expression in maize radicle under the allelochemical stress from Chenopodium ambrosioides L. Ecol. Environ. Sci. 2015, 24, 1640–1646. [Google Scholar]
- Shi, X.; Chen, Y.; Yan, Z.; Luo, Y.; Li, Y.; Ding, J.; Xie, H. Research progress on plant allelopathy. Biotechnol. Bull. 2020, 36, 215–222. [Google Scholar]
- Ge, J.; Ye, Y.; Lou, X.; Zhang, R.; Yang, Z.; Wang, Y.; Huang, X.; Jin, H.; Yang, Y.; Ai, J.; et al. Effects of phenolic scidification on physiological characteristics and rhizosphere microecology of Trichosanthes kirilowii Maxim. J. Soil Water Conserv. 2023, 37, 258–266. [Google Scholar]
- Bundit, A.; Ostlie, M.; Prom-U-Thai, C. Sunn hemp (Crotalaria juncea) weed suppression and allelopathy at different timings. Biocontrol Sci. Technol. 2021, 31, 694–704. [Google Scholar] [CrossRef]
- Huang, W.; Reddy, G.V.; Shi, P.; Huang, J.; Hu, H.; Hu, T. Allelopathic effects of Cinnamomum septentrionale leaf litter on Eucalyptus grandis saplings. Glob. Ecol. Conserv. 2020, 21, e00872. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Liu, Y.; Liu, Q.; Xu, W.; Long, Y.; Xu, X. Allelopathic effects on seed germination and seedling growth of Brassica pekinensi, caused by water extracts of branches and leaves from Davidia involucrata and Bothrocaryum controversum. Bull. Bot. Res. 2022, 42, 866–875. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Fang, L.; Li, L.; Dong, Y.; Chen, L.; Zhang, X. Allelopathic Effects of Soil Extracts from Rhus typhina Plantations on Common Turfgrass Species in Northern China. Agronomy 2024, 14, 2561. https://doi.org/10.3390/agronomy14112561
Li J, Fang L, Li L, Dong Y, Chen L, Zhang X. Allelopathic Effects of Soil Extracts from Rhus typhina Plantations on Common Turfgrass Species in Northern China. Agronomy. 2024; 14(11):2561. https://doi.org/10.3390/agronomy14112561
Chicago/Turabian StyleLi, Jiahao, Liang Fang, Liping Li, Yuxin Dong, Lingsu Chen, and Xiaoxi Zhang. 2024. "Allelopathic Effects of Soil Extracts from Rhus typhina Plantations on Common Turfgrass Species in Northern China" Agronomy 14, no. 11: 2561. https://doi.org/10.3390/agronomy14112561
APA StyleLi, J., Fang, L., Li, L., Dong, Y., Chen, L., & Zhang, X. (2024). Allelopathic Effects of Soil Extracts from Rhus typhina Plantations on Common Turfgrass Species in Northern China. Agronomy, 14(11), 2561. https://doi.org/10.3390/agronomy14112561





