Leaf Surface Micromorphology in Hybrids of Wheat and ×Trititrigia × Elymus farctus
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin of Hybrid Samples
2.2. Growing Conditions
2.3. Determination of Spike Morphology and Grain Quality Parameters
2.4. Scanning Electron Microscopy
2.5. Statistical Analysis
3. Results
3.1. Spike Morphology and Grain Quality
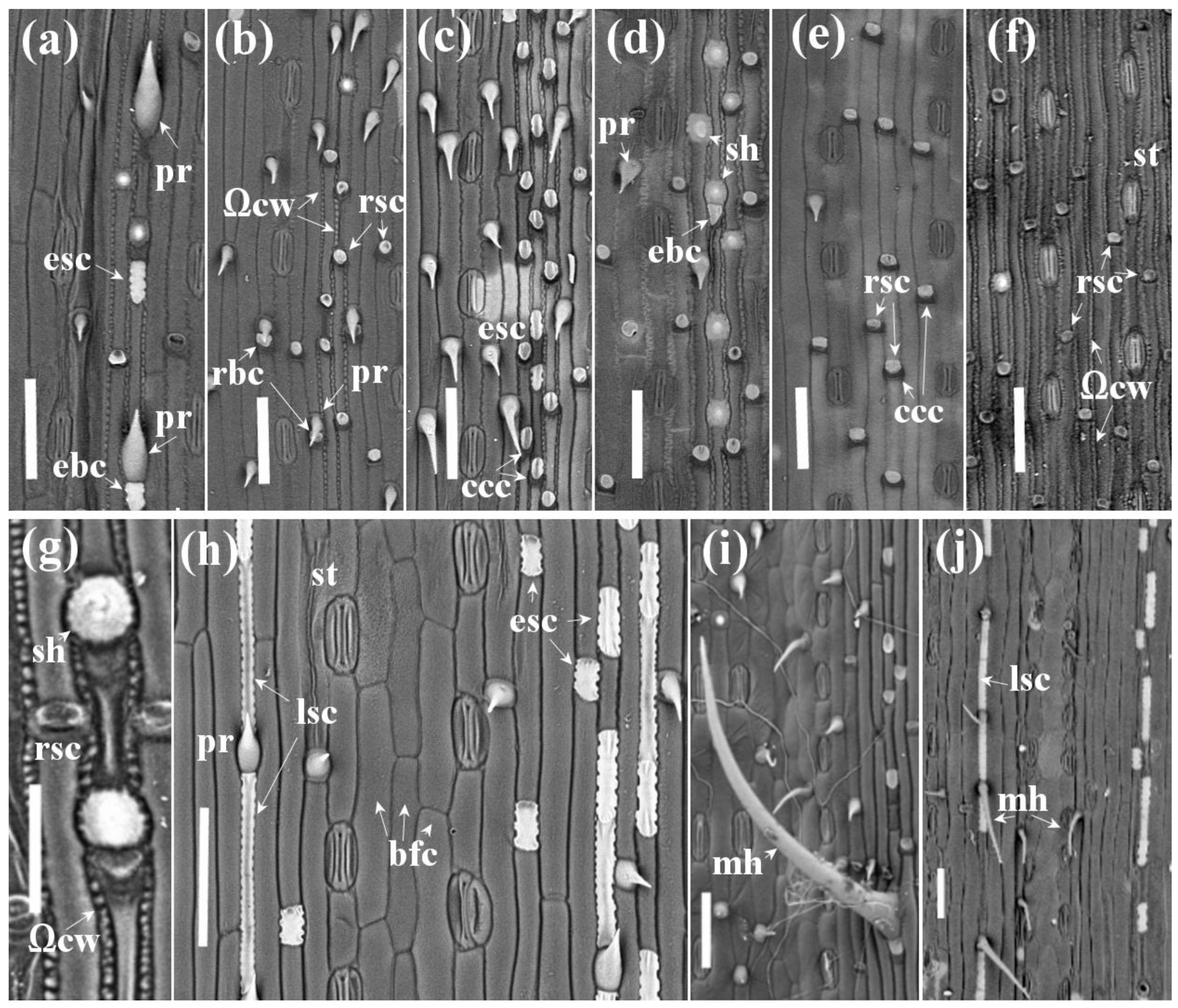
3.2. Micromorphology of Leaf Surface
3.2.1. B1. Apically Directed Prickles in the Costal Zone on the Abaxial Leaf Side (Figure 1a, −♀−♂)
3.2.2. B2. Basally Directed Prickles in the Costal and Midvein Zones on Abaxial Leaf Side (Figure 1b–d, −♀+♂)
3.2.3. B3. Basally Directed Long Thickened Macro-Hairs in the Costal and Midvein Zones (Figure 1c,j, −♀+♂)
3.2.4. B4 and B5. Shield-Shaped Prickles (Shields) in the Costal (B4) and Midvein (B5) Zones on Abaxial Leaf Side (Figure 1a,c,d,f, +♀±♂)
3.2.5. B6. Rounded Short Silica Cells with Thin Sickle-Shaped and Darker Basal Cork Cells (Figure 1a–f, +♀+♂)
3.2.6. B7. Oval or Rectangular Short Cells with Horizontally Elongated Silica Bodies with Sinuous Outline, Single or with Semicircular Basal Cell in the Costal Zone (Figure 1a,c, −♀+♂)
3.2.7. B8. Ω-Shaped Junction of Horizontal Anticlinal Walls of Long Cells in the Intercostal Zone on the Abaxial Side (Figure 1f, +♀−♂)
3.2.8. D1. Apically Directed Prickles in the Costal Zone on the Adaxial Leaf Side (+♀+♂)
3.2.9. D2. Shield-Shaped Prickles, Single or with Semicircular Basal Cell (Figure 1g and Figure 3e, +♀−♂), and Rounded Short Silica Cells (Figure 1g–i and Figure 3e) in the Costal and Midvein Zones of the Adaxial Side
3.2.10. D3. Basal Cells of the Prickles and Shield-Shaped Prickles Are Oval or Rectangular
3.2.11. D4. Long Cells with Silicified Wavy Cell Walls in the Midvein Zone on the Adaxial Leaf Side (Figure 3f, −♀+♂)
3.2.12. Maternal and Paternal Markers
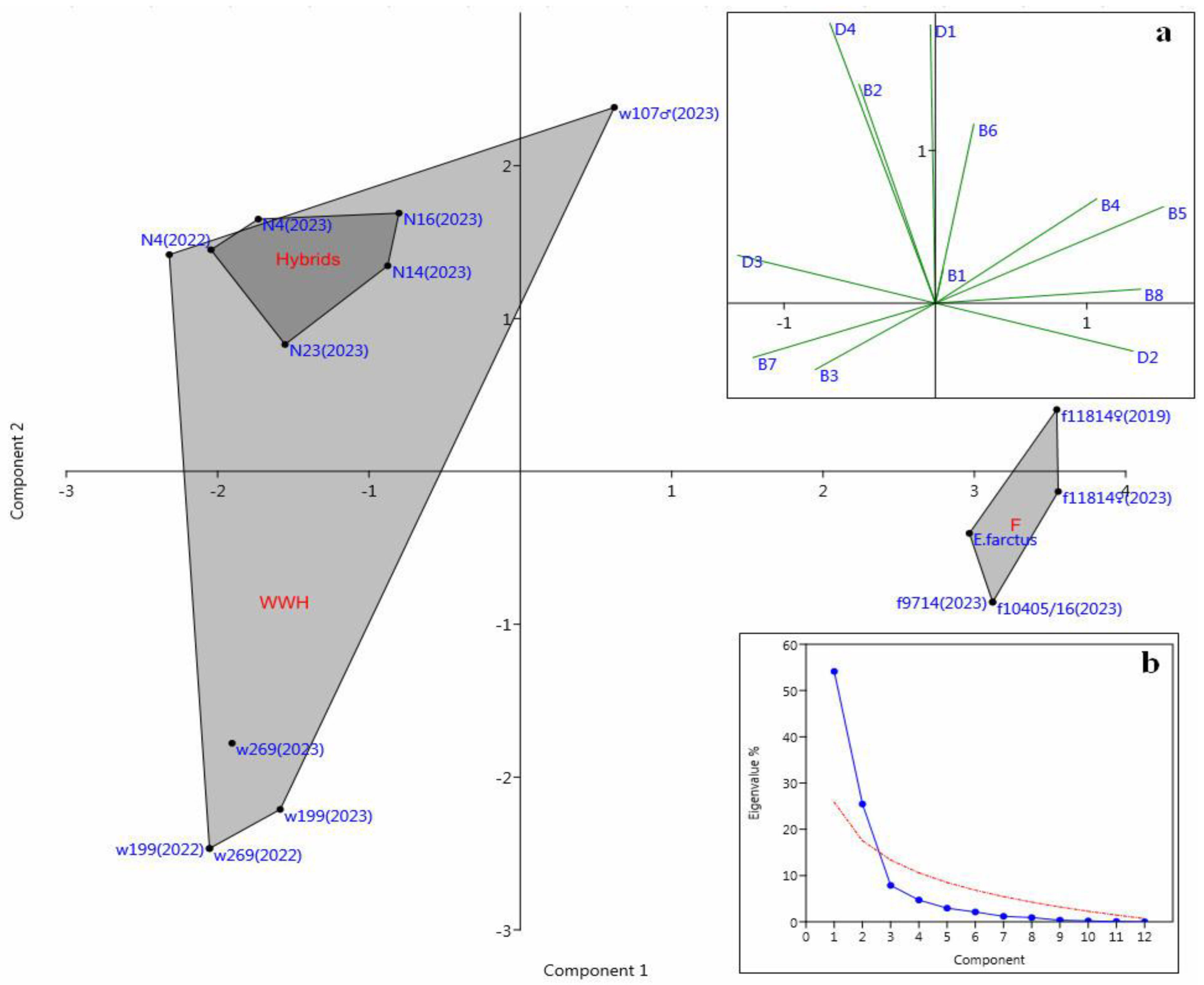
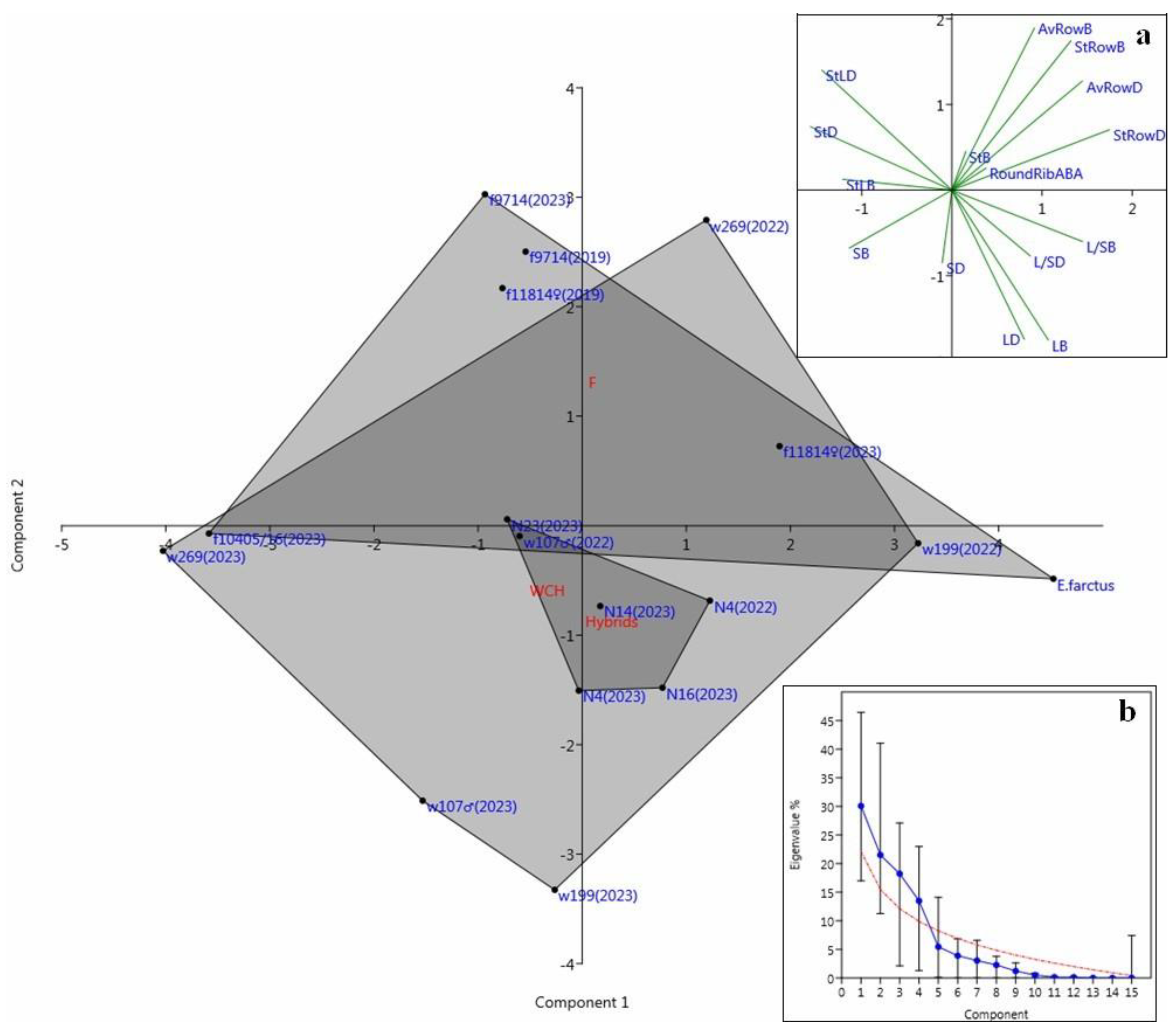
3.3. Principal Component Analysis of Micromorphologic Markers
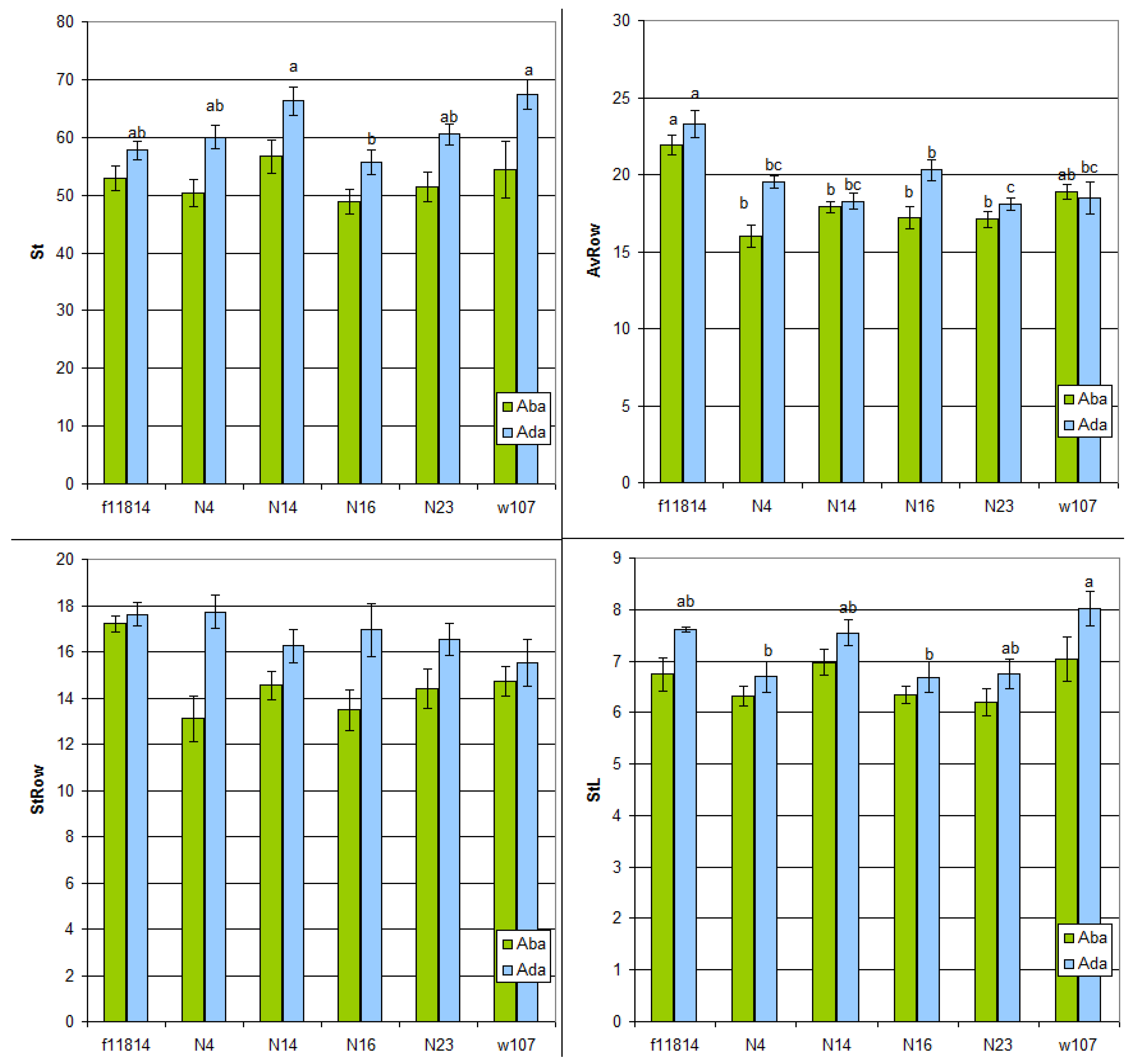
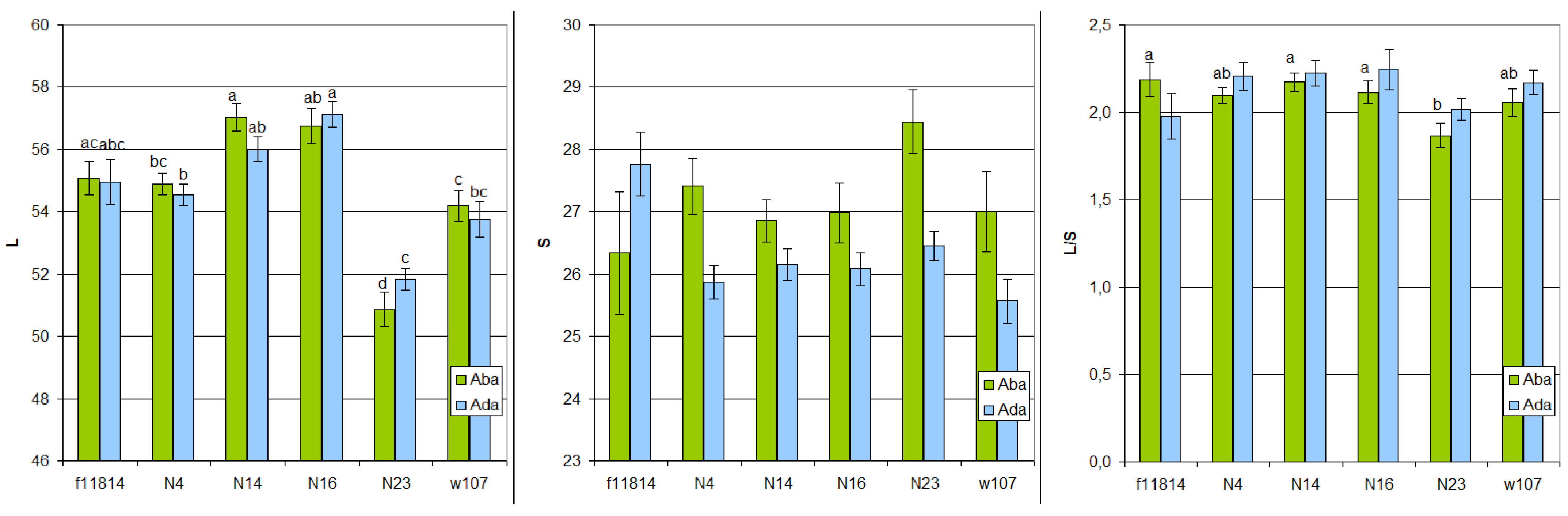
3.4. Quantitative Micromorphological Analysis of Stomatal Apparatus and Cell Row Width
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

| Sample | f11814 (♀) | w107 (♂) | N4 | N14 | N16 | N23 | Zlata | Sudarynya |
|---|---|---|---|---|---|---|---|---|
| Height, cm | 98.2 ± 1.6 a | 69.0 ± 4.0 bc | 68.2 ± 5.1 c | 98.2 ± 4.3 a | 82.0 ± 4.4 bc | 80.0 ± 2.9 bc | 84.4 ± 2.6 ab | 74.2 ± 1.2 bc |
| Parameter | Samples | |||
|---|---|---|---|---|
| N4 | N14 | N16 | N23 | |
| Spike length, cm | 10.0 ± 0.3 ab | 11.0 ± 0.4 a | 10.6 ± 0.3 ab | 9.4 ± 0.2 b |
| Number of spikelets in spike | 17.9 ± 0.2 b | 19.9 ± 0.5 a | 17.3 ± 0.4 b | 16.6 ± 0.3 b |
| Number of flowers in spikelet | 4.5 ± 0.2 | 3.8 ± 0.3 | 3.9 ± 0.1 | 4.3 ± 0.2 |
| Number of grains in spike | 51.9 ± 2.6 a | 40.3 ± 2.6 b | 41.4 ± 2.1 b | 47.2 ± 2.0 ab |
| 1000 grain weight, g | 51.3 ± 0.5 | 49.5 ± 1.8 | 48.1 ± 1.5 | 47.9 ± 2.7 |
References
- Tzvelev, N. The system of grasses (Poaceae) and their evolution. Bot. Rev. 1989, 55, 141–204. [Google Scholar] [CrossRef]
- Dobryakova, K.S. Allopolyploidy and origin of genomes in the Elymus L. species (a review). Proc. Appl. Bot. Genet. Breed. 2017, 178, 127–134. [Google Scholar] [CrossRef]
- Urfusová, R.; Mahelka, V.; Krahulec, F.; Urfus, T. Evidence of widespread hybridization among couch grasses (Elymus, Poaceae). J. Syst. Evol. 2021, 59, 113–124. [Google Scholar] [CrossRef]
- Parisod, C.; Badaeva, E.D. Chromosome restructuring among hybridizing wild wheats. New Phytol. 2020, 226, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Zemetra, R.S.; Hansen, J.; Mallory-Smith, C.A. Potential for gene transfer between wheat (Triticum aestivum) and jointed goatgrass (Aegilops cylindrica). Weed Sci. 1998, 46, 313–317. [Google Scholar] [CrossRef]
- Kocheshkova, A.A.; Kroupin, P.Y.; Bazhenov, M.S.; Karlov, G.I.; Pochtovyy, A.A.; Upelniek, V.P.; Belov, V.I.; Divashuk, M.G. Pre-harvest sprouting resistance and haplotype variation of ThVp-1 gene in the collection of wheat-wheatgrass hybrids. PLoS ONE 2017, 12, e0188049. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.; Garg, P.; Tanwar, K.; Sharma, J.; Gupta, N.C.; Ha, P.T.T.; Bhattacharya, R.C.; Mason, A.S.; Rao, M. Strategies for utilization of crop wild relatives in plant breeding programs. Theor. Appl. Genet. 2022, 135, 4151–4167. [Google Scholar] [CrossRef]
- Mathre, D.; Johnston, R.; Martin, J. Sources of resistance to Cephalosporium gramineum in Triticum and Agropyron species. Euphytica 1985, 34, 419–424. [Google Scholar] [CrossRef]
- Li, D.; Long, D.; Li, T.; Wu, Y.; Wang, Y.; Zeng, J.; Zhang, H. Cytogenetics and stripe rust resistance of wheat-Thinopyrum elongatum hybrid derivatives. Mol. Cytogenet. 2018, 11, 16. [Google Scholar] [CrossRef]
- Ullah, S.; Bramley, H.; Daetwyler, H.; He, S.; Mahmood, T.; Thistlethwaite, R.; Trethowan, R. Genetic contribution of emmer wheat (Triticum dicoccon Schrank) to heat tolerance of bread wheat. Front. Plant Sci. 2018, 9, 1529. [Google Scholar] [CrossRef]
- Suneja, Y.; Gupta, A.K.; Bains, N.S. Stress adaptive plasticity: Aegilops tauschii and Triticum dicoccoides as potential donors of drought associated morpho-physiological traits in wheat. Front. Plant Sci. 2019, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Akman, H.; Karaduman, Y. Evaluating technological quality of cultivated Triticum species, interspecific, and intergeneric hybrids for wheat-based products and breeding programs. J. Cereal Sci. 2021, 99, 103188. [Google Scholar] [CrossRef]
- Li, M.; Yuan, Y.; Ni, F.; Li, X.; Wang, H.; Bao, Y. Characterization of Two Wheat-Thinopyrum ponticum Introgression Lines With Pyramiding Resistance to Powdery Mildew. Front. Plant Sci. 2022, 13, 943669. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, C.; Feng, X.; Zhao, J.; Deng, P.; Wang, Y.; Zhang, H.; Liu, X.; Li, T.; Chen, C.; et al. Molecular cytogenetics and development of St-chromosome-specific molecular markers of novel stripe rust resistant wheat-Thinopyrum intermedium and wheat-Thinopyrum ponticum substitution lines. BMC Plant Biol. 2022, 22, 111. [Google Scholar] [CrossRef]
- Ivanova, L.P.; Kuznetsova, N.L.; Ermolenko, O.I.; Klimenkova, I.N.; Klimenkov, F.I.; Shuklina, O.A.; Upelniek, V.P. Productivity and baking properties of Trititrigia cziczinii. Agrar. Russ. 2020, 12, 14–17. [Google Scholar] [CrossRef]
- Upelniek, V.; Fisenko, A.; Ivanova, L.; Gluhova, L.; Kuzmina, N.; Loshakova, P.; Zavgorodny, S.; Gradskov, S. Biodiversity of distant hybrids of cereals in the collection of the Tsitsin MBG RAS. Acta Hortic. 2021, 1324, 233–236. [Google Scholar] [CrossRef]
- Ellis, R.P. A procedure for standardizing comparative leaf anatomy in the Poaceae. II. The epidermis as seen in surface view. Bothalia 1979, 12, 641–671. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Fu, Q.S.; Yang, R.C.; Wang, H.S.; Zhao, B.; Zhou, C.L.; Ren, S.X.; Guo, Y.-D. Leaf morphological and ultrastructural performance of eggplant (Solanum melongena L.) in response to water stress. Photosynthetica 2013, 51, 109–114. [Google Scholar] [CrossRef]
- Hauser, M.-T. Molecular basis of natural variation and environmental control of trichome patterning. Front. Plant Sci. 2014, 5, 320. [Google Scholar] [CrossRef]
- Mizutani, M.; Kanaoka, M.M. Environmental sensing and morphological plasticity in plants. Semin. Cell Dev. Biol. 2018, 83, 69–77. [Google Scholar] [CrossRef]
- Galdon-Armero, J.; Fullana-Pericas, M.; Mulet, P.A.; Conesa, M.A.; Martin, C.; Galmes, J. The ratio of trichomes to stomata is associated with water use efficiency in Solanum lycopersicum (tomato). Plant J. 2018, 96, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Orlovskaya, O.A.; Koren, L.V.; Khotyleva, L.V. Morphological analysis of wheat hybrids created by remote hybridisation in the tribe Triticeae. Proc. Nat. Acad. Sci. Belarus. Ser. Biol. Sci. 2011, 3, 29–33. (In Russian) [Google Scholar]
- Tyryshkin, L.G.; Sjukov, V.V.; Zaharov, V.G.; Zuev, E.V.; Gashimov, M.J.; Kolesova, M.A.; Chikida, N.N.; Ershova, M.A.; Belousova, M.H. Sources of effective resistance of soft wheat and its relatives to fungal diseases-search, creation and use in breeding. Tr. Po Prikl. Bot. Genet. I Sel. 2012, 170, 186–199. (In Russian) [Google Scholar]
- Davojan, R.O.; Bebjakina, I.V.; Davojan, O.R.; Zinchenko, A.N.; Davojan, J.R.; Kravchenko, A.M.; Zubanova, J.S. Synthetic forms as the basis for the conservation and use of the gene pool of wild relatives of common wheat. Vavilovskij Zhurnal Genet. I Sel. 2014, 16, 44–51. (In Russian) [Google Scholar]
- Klimko, M.; Wysakowska, I. Epidermal features of glumes and florets in Aegilops geniculata Roth and Aegilops peregrina (Hack.) Maire et Weiller x Secale cereale L. hybrids, amphiploids and parental forms. Steciana 2015, 19, 13–24. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, Y.; Röder, M.S.; Reif, J.C.; Ganal, M.W.; Chen, D.; Schnurbusch, T. Manipulation and prediction of spike morphology traits for the improvement of grain yield in wheat. Sci. Rep. 2018, 8, 14435. [Google Scholar] [CrossRef]
- Klimko, M.; Pudelska, H.; Wojciechowska, B.; Klimko, W. Variation of micromorphological characters of lemma and palea in Aegilops kotschyi and Aegilops biuncialis × Secale cereale hybrids, amphiploids and parental forms. Rocz. AR Pozn. Bot.-Stec. 2009, 13, 167–176. [Google Scholar]
- Tsitsin, N.V. Perennial Wheat; Nauka: Moscow, Russia, 1978; p. 287. (In Russian) [Google Scholar]
- Tzvelev, N. Grasses of the Soviet Union; Nauka: Leningrad, Russia, 1976. (In Russian) [Google Scholar]
- Loshakova, P.O.; Badayeva, E.D.; Gevorkyan, M.M.; Kalmykova, L.P.; Babosha, A.V.; Upelniek, V.P. New promising hybrids for breeding soft wheat. Bull. Main Bot. Gard. 2020, 2, 69–79. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Hu, Z.; Zhang, J.; Larson, S.; Zhang, X.; Grieve, C.; Shannon, M. Development of salinity tolerant wheat recombinant lines from a wheat disomic addition line carrying a Thinopyrum junceum chromosome. Int. J. Plant Sci. 2003, 164, 25–33. [Google Scholar] [CrossRef]
- McArthur, R.; Zhu, X.; Oliver, R.; Klindworth, D.; Xu, S.; Stack, R.; Wang, R.; Cai, X. Homoeology of Thinopyrum junceum and Elymus rectisetus chromosomes to wheat and disease resistance conferred by the Thinopyrum and Elymus chromosomes in wheat. Chromosome Res. 2012, 20, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Loshakova, P.O.; Fisenko, A.V.; Kalmykova, L.P.; Kuznetsova, N.L.; Upelniek, V.P. Intergeneric hybrids xTrititrigia cziczinii x Elymus farctus and prospects of their se in breeding. Dostizheniya Nauk. I Tekhniki APK 2018, 32, 28–31. (In Russian) [Google Scholar] [CrossRef]
- Kalmykova, L.P.; Loshakova, P.O.; Fisenko, A.V.; Shchuklina, O.A.; Vineshanker, T.S.; Kuzmina, N.P.; Upelniek, V.P. Hybrids of younger generations (xTrititrigia x Elymus farctus) x Triticum aestivum. Bull. Main Bot. Gard. 2019, 205, 48–56. (In Russian) [Google Scholar]
- Loshakova, P.O.; Kalmykova, L.P.; Upelniek, V.P. Grain quality of hybrid F5, obtained by crossing incomplete wheat-wheatgrass amphidiploid (IWWAD) with Elymus farctus Runemark ex Melderis. Bull. Main Bot. Gard. 2016, 202, 52–56. (In Russian) [Google Scholar]
- FR.1.31.2009.06615. M 04-43-2006 (as Amended in 2009) Wheat flour. Determination of Protein, Moisture, Ash, Whiteness, Quantity and Quality of Raw Gluten Using Spectroscopy in the Near Infrared Region Using the InfraLUM FT-10 Analyzer. Available online: https://www.russiangost.com/p-273475-fr131200906615.aspx (accessed on 20 October 2024).
- Koz’mina, N.P. (Ed.) Methods of Evaluation of Technological Properties of Wheat Grains, Cereals and Legumes; Gosplan RSFSR: Moscow, Russia, 1961; 147p. (In Russian) [Google Scholar]
- GOST 54478-2011 Grain; Methods for Determining the Quantity and Quality of Gluten in Wheat. Introduced 2011-10-21. Gosstandart Rossii: Moscow, Russia, 2012; p. 23. (In Russian)
- Ryabchenko, A.S.; Babosha, A.V. Application of Thermal Paste as an Adhesive and Heat Conducting Composition in the Study of Biological Samples on a Scanning Electron Microscope Using a Freezing Attachment. Patent RU. No. 2445660, 11 March 2010. Available online: https://patents.google.com/patent/RU2445660C2/en (accessed on 20 October 2024).
- Rapid Publication-Ready MS Word Tables Using One-Way ANOVA 2.0. Available online: https://houssein-assaad.shinyapps.io/TableReport/ (accessed on 25 September 2024).
- Semenov, V.I.; Semenova, E.V. Use of telocentric analysis to detect structural reorganisation of wheat chromosomes in incomplete wheat-pyrene amphidiploids (2n = 8x = 56, AABBDDXX). In Distant Hybridisation Results of Research; Moscow, Russia, 2001; pp. 17–39. (In Russian) [Google Scholar]
- Kozhakhmetov, K.K. Some cytological features of intergeneric hybrids of Triticum × Aegilops. Soil Sci. Agrochem. 2009, 4, 45–48. (In Russian) [Google Scholar]
- Gevorkyan, M.M.; Babosha, A.V.; Loshakova, P.O.; Pogost, A.A.; Komarova, G.I.; Wineshenker, T.S.; Upelniek, V.P. The leaf surface micromorphology of plants obtained from crosses between Elymus farctus and the stable form × Trititrigia cziczinii × wheat cultivar ‘Botanicheskaya 3’. Genet. Resour. Crop Evol. 2024, 1–17. [Google Scholar] [CrossRef]
- Kasha, K.J.; Kao, K.N. High frequency haploid production in barley (Hordeum vulgare L.). Nature 1970, 225, 874–876. [Google Scholar] [CrossRef]
- Rodionov, A.V.; Amosova, A.V.; Belyakov, E.A.; Zhurbenko, P.M.; Mikhailova, Y.V.; Punina, E.O.; Shneyera, V.S.; Loskutov, I.G.; Muravenko, O.V. Genetic consequences of interspecific hybridization, its role in speciation and phenotypic diversity of plants. Rus. J. Genet. 2019, 55, 278–294. [Google Scholar] [CrossRef]
- Vimala, Y.; Lavania, U.C. Genomic territories in inter-genomic hybrids: The winners and losers with hybrid fixation. Nucleus 2021, 64, 1–6. [Google Scholar] [CrossRef]
- Tikhenko, N.; Haupt, M.; Fuchs, J.; Perovic, D.; Himmelbach, A.; Mascher, M.; Houben, A.; Rutten, T.; Nagel, M.; Tsvetkova, N.V.; et al. Major chromosome rearrangements in intergeneric wheat × rye hybrids in compatible and incompatible crosses detected by GBS read coverage analysis. Sci. Rep. 2024, 14, 11010. [Google Scholar] [CrossRef]
- Gernand, D.; Rutten, T.; Varshney, A.; Rubtsova, M.; Prodanovic, S.; Brüß, C.; Kumlehn, J.; Matzk, F.; Houben, A. Uniparental chromosome elimination at mitosis and interphase in wheat and pearl millet crosses involves micronucleus formation, progressive heterochromatinization, and DNA fragmentation. Plant Cell 2005, 17, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Polgari, D.; Mihók, E.; Sági, L. Composition and random elimination of paternal chromosomes in a large population of wheat × barley (Triticum aestivum L. × Hordeum vulgare L.) hybrids. Plant Cell Rep. 2019, 38, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Ren, Y.; Murray, T.D.; Yan, W.; Guo, Q.; Niu, Y.; Li, H. Development of perennial wheat through hybridization between wheat and wheatgrasses: A review. Engineering 2018, 4, 507–513. [Google Scholar] [CrossRef]
- Babosha, A.V.; Gevorkyan, M.M.; Ivanova, L.P.; Upelniek, V.P. Anatomic structure and surface micromorphology of leaf surface of wheat, developed by interspecific crossings. Dostizheniya Nauk. I Teh. APK 2018, 32, 32–36. (In Russian) [Google Scholar] [CrossRef]
- de Boer, H.J.; Price, C.A.; Wagner-Cremer, F.; Dekker, S.C.; Franks, P.J.; Veneklaas, E.J. Optimal allocation of leaf epidermal area for gas exchange. New Phytol. 2016, 210, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Beerling, D.J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Yang, X.; Cui, X.; Niu, H. An intrinsic geometric constraint on morphological stomatal traits. Front. Plant Sci. 2021, 12, 628. [Google Scholar] [CrossRef]
- Franks, P.J.; Farquhar, G.D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2007, 143, 78–87. [Google Scholar] [CrossRef]
- Shahinnia, F.; Le Roy, J.; Laborde, B.; Sznajder, B.; Kalambettu, P.; Mahjourimajd, S.; Tilbrook, J.; Fleury, D. Genetic association of stomatal traits and yield in wheat grown in low rainfall environments. BMC Plant Biol. 2016, 16, 150. [Google Scholar] [CrossRef]
- Jäger, K.; Fábián, A.; Eitel, G.; Szabó, L.; Deák, C.; Barnabás, B.; Papp, I. A morpho-physiological approach differentiates bread wheat cultivars of contrasting tolerance under cyclic water stress. J. Plant Physiol. 2014, 171, 1256–1266. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Umar, M.; Fulton, T.; Biswal, A.K.; Dionora, J.; et al. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Hunt, L.; Afsharinafar, M.; Meselmani, M.A.; Mitchell, A.; Howells, R.; Wallington, E.; Fleming, A.J.; Gray, J.E. Reduced stomatal density in bread wheat leads to increased water-use efficiency. J. Exp. Bot. 2019, 70, 4737–4748. [Google Scholar] [CrossRef] [PubMed]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef]
- Khazaei, H.; Mohammady, S.; Monneveux, P.; Stoddard, F. The determination of direct and indirect effects of carbon isotope discrimination (Delta), stomatal characteristics and water use efficiency on grain yield in wheat using sequential path analysis. Aust. J. Crop Sci. 2011, 5, 466–472. [Google Scholar]
- Limochi, K.; Eskandari, H. Effect of planting date on the performance of flag leaf stomata and grain yield of rice cultivars. Int. J. Agron. Plant Prod. 2013, 4, 769–773. [Google Scholar]
- Ohsumi, A.; Kanemura, T.; Homma, K.; Horie, T.; Shiraiwa, T. Genotypic variation of stomatal conductance in relation to stomatal density and length in rice (Oryza sativa L.). Plant Prod. Sci. 2007, 10, 322–328. [Google Scholar] [CrossRef]
- Aminian, R.; Mohammadi, S.; Hoshmand, S.A.; Khodambashi, M. The genetic analysis of stomatal frequency and size, stomatal conductance, photosynthetic rate and yield in wheat (Triticum aestivum L.) using substitution lines series. Wheat Inf. Serv. 2010, 110, 25–34. [Google Scholar]
- Rajendra, B.R.; Mujeeb, K.A.; Bates, L.S. Relationships between 2X hordeum sp., 2X Secale sp. and 2X, 4X, 6X triticum spp. for stomatal frequency, size and distribution. Environ. Exp. Bot. 1978, 18, 33–37. [Google Scholar] [CrossRef]
- Aryavand, A.; Ehdaie, B.; Tran, B.; Waines, J.G. Stomatal frequency and size differentiate ploidy levels in Aegilops neglecta. Genet. Resour. Crop Evol. 2003, 50, 175–182. [Google Scholar] [CrossRef]
- Maosong, L.; Chunyan, W.; Jiqing, S.; Yonggang, C.; Xiufen, W.; Yongfeng, W. Evolutional trends of leaf stomatal and photosynthetic characteristics in wheat evolutions. Acta Ecol. Sin. 2008, 28, 5385–5391. [Google Scholar] [CrossRef]


| Parameter | Samples | ||||||
|---|---|---|---|---|---|---|---|
| f11814 (♀) | w107 (♂) | N4 | N14 | N16 | N23 | Sudarynya | |
| Spike length, cm | 14.7 ± 0.4 a | 8.4 ± 0.5 c | 9.6 ± 0.4 bc | 10.8 ± 0.4 b | 10.3 ± 0.3 b | 9.9 ± 0.4 bc | 10.0 ± 0.4 bc |
| Number of spikelets in spike | 18.4 ± 0.7 ab | 14.6 ± 0.7 c | 17.0 ± 0.5 ac | 19.4 ± 0.6 a | 17.0 ± 0.6 ac | 16.6 ± 0.6 bc | 17.3 ± 0.6 ac |
| Number of flowers in spikelet | 5.4 ± 0.2 a | 4.2 ± 0.2 bc | 4.4 ± 0.2 bc | 3.6 ± 0.2 c | 3.9 ± 0.2 c | 4.0 ± 0.2 bc | 4.8 ± 0.2 ab |
| Number of grains in spike | 47.4 ± 3.1 a | 30.4 ± 3.5 b | 36.4 ± 2.5 ab | 32.2 ± 2.5 b | 36.5 ± 2.5 ab | 35.2 ± 2.5 ab | 46.5 ± 2.9 a |
| 1000 grain weight, g | 34.8 ± 1.8 b | 49.8 ± 1.8 a | 45.3 ± 1.5 a | 46.6 ± 1.5 a | 44.5 ± 1.5 a | 43.9 ± 1.5 a | 28.8 ± 1.7 b |
| Parameter | Sample | |||
|---|---|---|---|---|
| N4 | N14 | N16 | N23 | |
| Vegetation, days | 90 | 105 | 90 | 100 |
| Sedimentation number (Zeleny test) | 55 | 41 | 41 | 55 |
| Gluten, % | 30.7 | 40 | 32.9 | 39 |
| Grain vitreousness, % | 56.3 | 61.4 | 53.8 | 55.3 |
| Protein, % | 17.2 | 19.6 | 17.4 | 18.9 |
| Leaf Side | N | Marker | F11814 (♀) | W107 (♂) | N4 | N14 | N16 | N23 |
|---|---|---|---|---|---|---|---|---|
| abaxial | B1 | Apically directed prickles in the costal zone, solitary or adjacent to an elongated basal cell | 0 | 0 | 0 | 1 | 1 | 0 |
| B2 | Basally directed prickles in the costal and midvein zones | 0 | 2 | 2 | 1 | 1 | 0 | |
| B3 | Basally directed long macro-hairs on midvein | 0 | 1-2 | 1 | 0 | 0 | 0 | |
| B4 | Shield-shaped prickles, single or with semicircular basal cell in the costal zone | 2 | 1-2 | 0-1 | 1 | 1 | 1 | |
| B5 | Shield-shaped prickles, single or with semicircular basal cell in the midvein zone | 3 | 0-2 | 0 | 2 | 2 | 0 | |
| B6 | Rounded short silica cells with thin basal cork cell in the costal and midvein zones | 2 | 2 | 2 | 1 | 2 | 2 | |
| B7 | Oval or rectangular short cells with horizontally elongated silica bodies with sinuous outline, single or with semicircular basal cell in the costal zone | 0 | 0-2 | 1 | 2 | 2 | 2 | |
| B8 | Ω-shaped junction of horizontal anticlinal walls of long cells in the intercostal zone | 2 | 0-2 | 0 | 0 | 0 | 0 | |
| adaxial | D1 | Apically directed prickles in the costal zone | 1-2 | 2 | 2 | 2 | 2 | 2 |
| D2 | Shield-shaped prickles, single or with semicircular basal cell, and also rounded short silica cells in the costal and midvein zones | 2 | 0 | 0 | 0 | 0 | 0 | |
| D3 | Basal cells of the prickles and shield-shaped prickles are oval or rectangular. Oval or rectangular short cells with horizontally elongated silica bodies with sinuous outline in the costal and midvein zones. Long cells with silicified wavy cell walls in the costal zone (but may be absent on the midvein) | 0 | 2 | 2 | 2 | 2 | 2 | |
| D4 | Long cells with silicified wavy cell walls in the midvein zone | 0 | 2 | 2 | 2 | 2 | 2 |
| Marker | f11814 (♀) | w107 (♂) | N4 | N14 | N16 | N23 |
|---|---|---|---|---|---|---|
| Diameter of the base of shield-shaped prickles in the costal and midvein zones on abaxial leaf side (B4 and B5) | 24.6 ± 1.0 b | 30.1 ± 1.3 a | 24.4 ± 0.9 b | 22.5 ± 0.3 b | 24.2 ± 0.7 b | 24.8 ± 1.8 b |
| Diameter of rounded short silica cells (abaxial intercostal zone) (B6) | 14.9 ± 0.4 b | 16.9 ± 0.2 ab | 16.0 ± 0.4 ab | 16.3 ± 0.4 ab | 17.7 ± 0.6 a | 17.2 ± 0.9 ab |
| Diameter of rounded short silica cells (abaxial costal and midvein zones) (B6, RoundRibABA) | 15.8 ± 0.7 | 16.2 ± 0.7 | 15.3 ± 0.4 | 16 ± 0.6 | 16.9 ± 0.7 | 16.4 ± 0.7 |
| Length of elongated silica bodies in the abaxial costal zone (B7) | - | 33.5 ± 2.6 ab | 29.4 ± 1.2 b | 37.1 ± 1.7 a | 30 ± 1.7 ab | 36.7 ± 3.1 ab |
| Length of elongated silica bodies in the abaxial midvein zone (B7) | - | 24.6 ± 1.3 | 25.6 ± 1.1 | 24.4 ± 0.8 | 23.2 ± 0.8 | 26.1 ± 2.4 |
| Length of apically directed prickles in the adaxial costal zone (D1) | 49.9 ± 3.6 ab | 44.4 ± 1.8 bc | 39.4 ± 0.8 c | 43.9 ± 1.3 bc | 54.7 ± 2.1 a | 47.2 ± 2.2 ab |
| Parameters | Component Loadings | ||||
|---|---|---|---|---|---|
| RC1 | RC2 | RC3 | RC4 | Uniqueness | |
| StB | 0.850 | 0.2348 | |||
| StRowB | 0.913 | 0.0988 | |||
| AvRowB | 0.899 | 0.1698 | |||
| LB | 0.789 | 0.3366 | |||
| SB | −0.320 | 0.569 | 0.4032 | ||
| StLB | −0.365 | 0.312 | 0.772 | 0.1081 | |
| L/SB | 0.689 | −0.379 | 0.3169 | ||
| StD | −0.751 | 0.3298 | |||
| StRowD | 0.777 | 0.323 | 0.1947 | ||
| AvRowD | 0.947 | 0.0639 | |||
| LD | 0.703 | 0.535 | 0.0716 | ||
| SD | 0.922 | 0.1125 | |||
| StLD | −0.891 | 0.1910 | |||
| L/SD | 0.450 | −0.751 | 0.1513 | ||
| RoundRibABA | 0.426 | 0.495 | |||
| PCA 1 * | 0.539 | 0.5591 | |||
| PCA 2 | −0.305 | 0.7531 | |||
| StB | StRowB | AvRowB | LB | SB | StLB | L/SB | StD | StRowD | AvRowD | LD | SD | StLD | L/SD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| StB | ||||||||||||||
| StRowB | 0.247 | |||||||||||||
| AvRowB | −0.041 | 0.902 *** | ||||||||||||
| LB | −0.123 | −0.121 | −0.165 | |||||||||||
| SB | −0.372 | −0.430 | −0.207 | −0.030 | ||||||||||
| StLB | 0.570 * | −0.299 | −0.231 | −0.212 | 0.278 | |||||||||
| L/SB | 0.298 | 0.208 | −0.028 | 0.544 * | −0.784 *** | −0.278 | ||||||||
| StD | −0.150 | −0.284 | −0.133 | −0.566 * | 0.168 | 0.211 | −0.546 * | |||||||
| StRowD | −0.049 | 0.682 ** | 0.564 * | 0.155 | −0.343 | −0.552 * | 0.298 | −0.456 | ||||||
| AvRowD | −0.090 | 0.767 *** | 0.788 *** | 0.013 | −0.260 | −0.347 | 0.172 | −0.419 | 0.874 *** | |||||
| LD | −0.337 | −0.190 | −0.185 | 0.765 *** | 0.242 | −0.284 | 0.222 | −0.462 | 0.228 | 0.109 | — | |||
| SD | −0.229 | −0.220 | −0.107 | 0.259 | 0.492 * | 0.143 | −0.155 | −0.178 | 0.042 | 0.166 | 0.664 ** | — | ||
| StLD | −0.174 | −0.034 | 0.249 | −0.613 ** | 0.179 | 0.363 | −0.539 * | 0.734 *** | −0.476 | −0.119 | −0.546 * | −0.051 | — | |
| L/SD | 0.086 | 0.037 | −0.182 | 0.387 | −0.366 | −0.421 | 0.444 | −0.243 | 0.150 | −0.190 | 0.047 | −0.671 ** | −0.576 * | — |
| RoundRib ABA | 0.317 | 0.141 | 0.141 | 0.062 | −0.004 | 0.387 | −0.004 | −0.327 | 0.239 | 0.336 | 0.129 | 0.295 | −0.130 | −0.352 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babosha, A.V.; Loshakova, P.O.; Pogost, A.A.; Gevorkyan, M.M.; Alenicheva, A.D.; Komarova, G.I.; Wineshenker, T.S.; Klimenkova, I.N.; Upelniek, V.P. Leaf Surface Micromorphology in Hybrids of Wheat and ×Trititrigia × Elymus farctus. Agronomy 2024, 14, 2490. https://doi.org/10.3390/agronomy14112490
Babosha AV, Loshakova PO, Pogost AA, Gevorkyan MM, Alenicheva AD, Komarova GI, Wineshenker TS, Klimenkova IN, Upelniek VP. Leaf Surface Micromorphology in Hybrids of Wheat and ×Trititrigia × Elymus farctus. Agronomy. 2024; 14(11):2490. https://doi.org/10.3390/agronomy14112490
Chicago/Turabian StyleBabosha, Alexander V., Pavla O. Loshakova, Alina A. Pogost, Margarita M. Gevorkyan, Anastasia D. Alenicheva, Galina I. Komarova, Tatyana S. Wineshenker, Irina N. Klimenkova, and Vladimir P. Upelniek. 2024. "Leaf Surface Micromorphology in Hybrids of Wheat and ×Trititrigia × Elymus farctus" Agronomy 14, no. 11: 2490. https://doi.org/10.3390/agronomy14112490
APA StyleBabosha, A. V., Loshakova, P. O., Pogost, A. A., Gevorkyan, M. M., Alenicheva, A. D., Komarova, G. I., Wineshenker, T. S., Klimenkova, I. N., & Upelniek, V. P. (2024). Leaf Surface Micromorphology in Hybrids of Wheat and ×Trititrigia × Elymus farctus. Agronomy, 14(11), 2490. https://doi.org/10.3390/agronomy14112490






