Effects of Different Fertilization Measures on Bacterial Community Structure in Seed Production Corn Fields
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Experimental Design
2.3. Soil Sample Collection
2.4. Measurement of Soil Physical and Chemical Indicators
2.5. Bacterial 16S rRNA High-Throughput Sequencing
2.6. PICRUSt Gene Function Prediction
2.7. Data Processing
3. Results
3.1. Analysis of Soil Chemical Properties Under Different Fertilization Measures
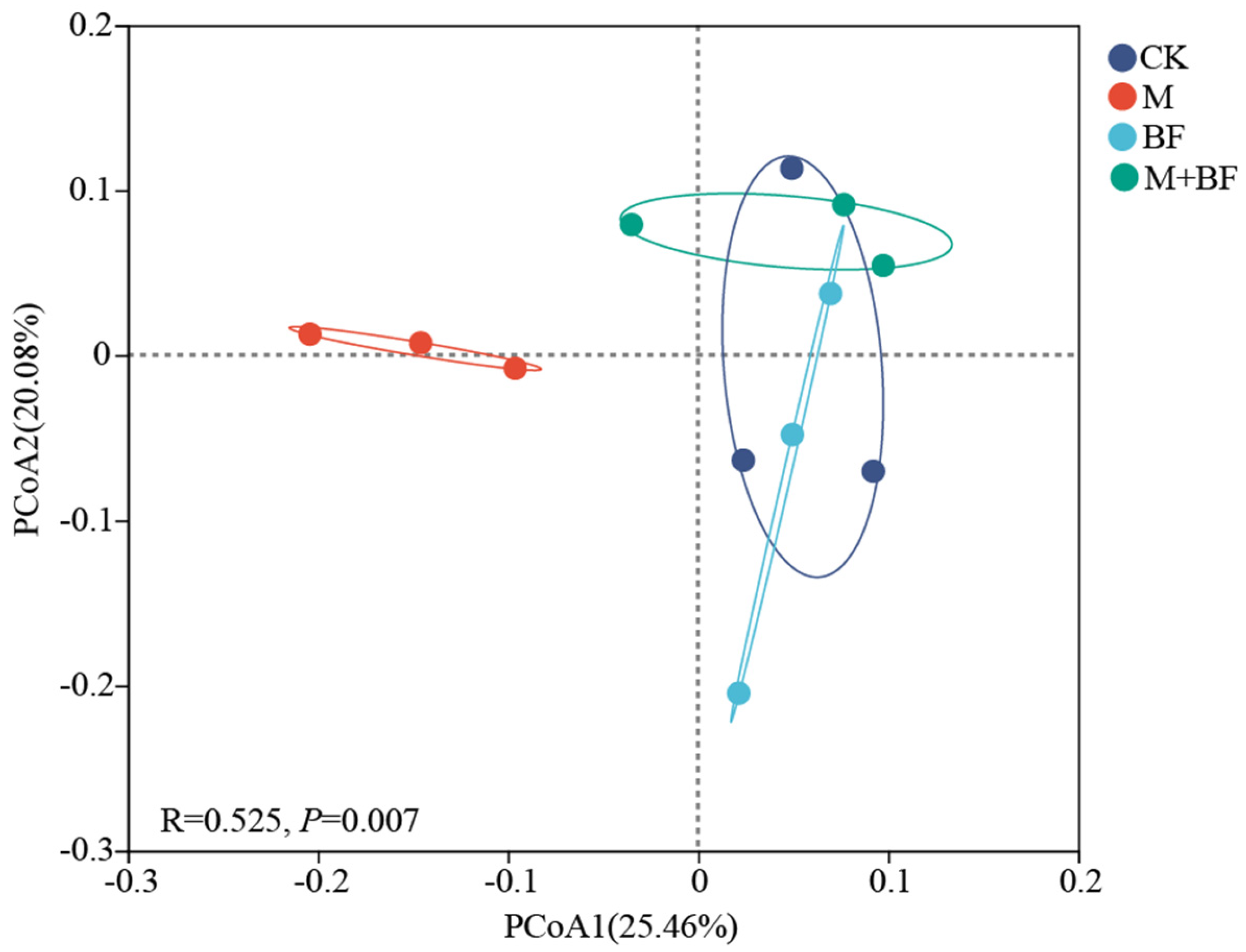
3.2. Soil Bacterial Community Diversity
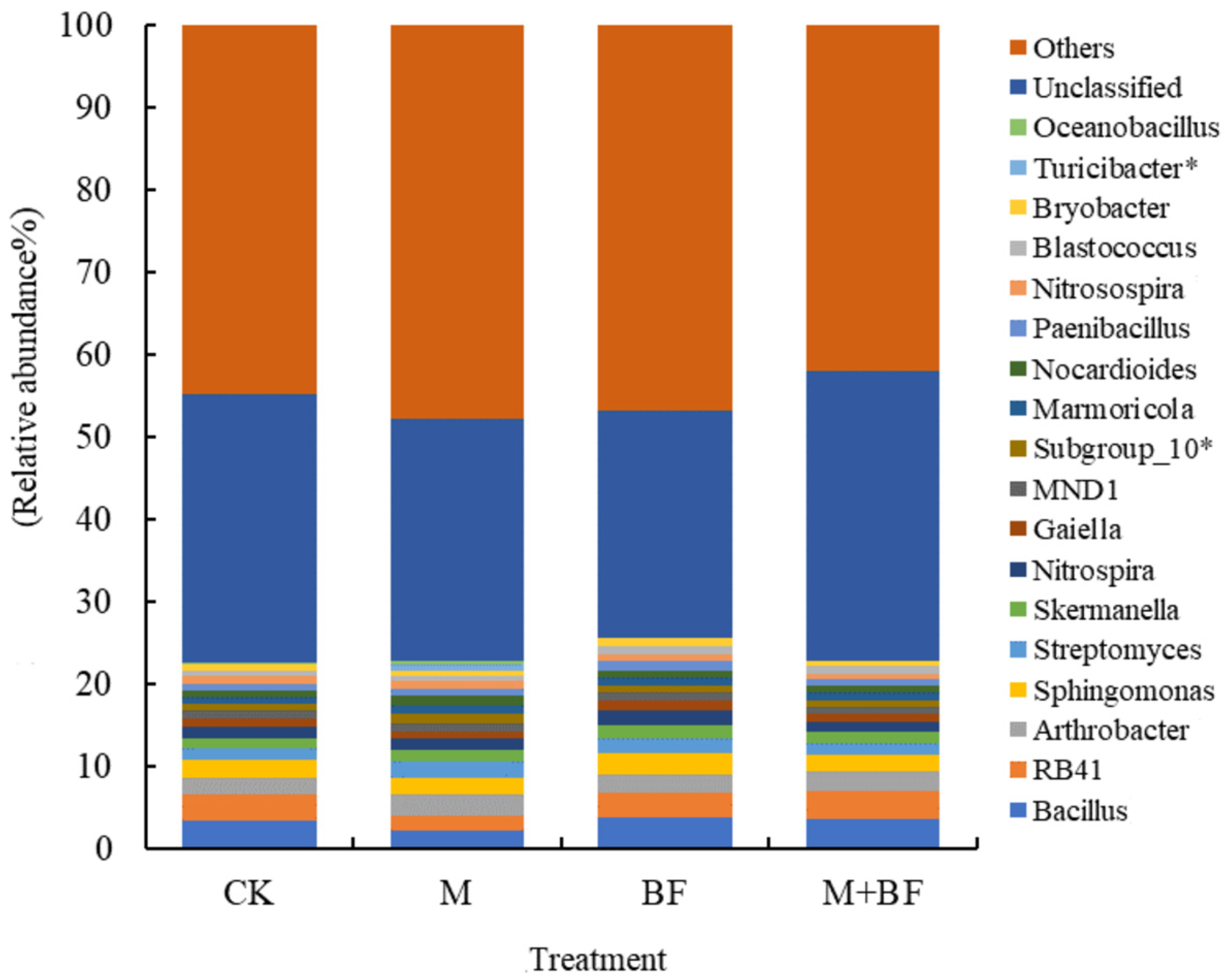
3.3. Soil Bacterial Community Composition
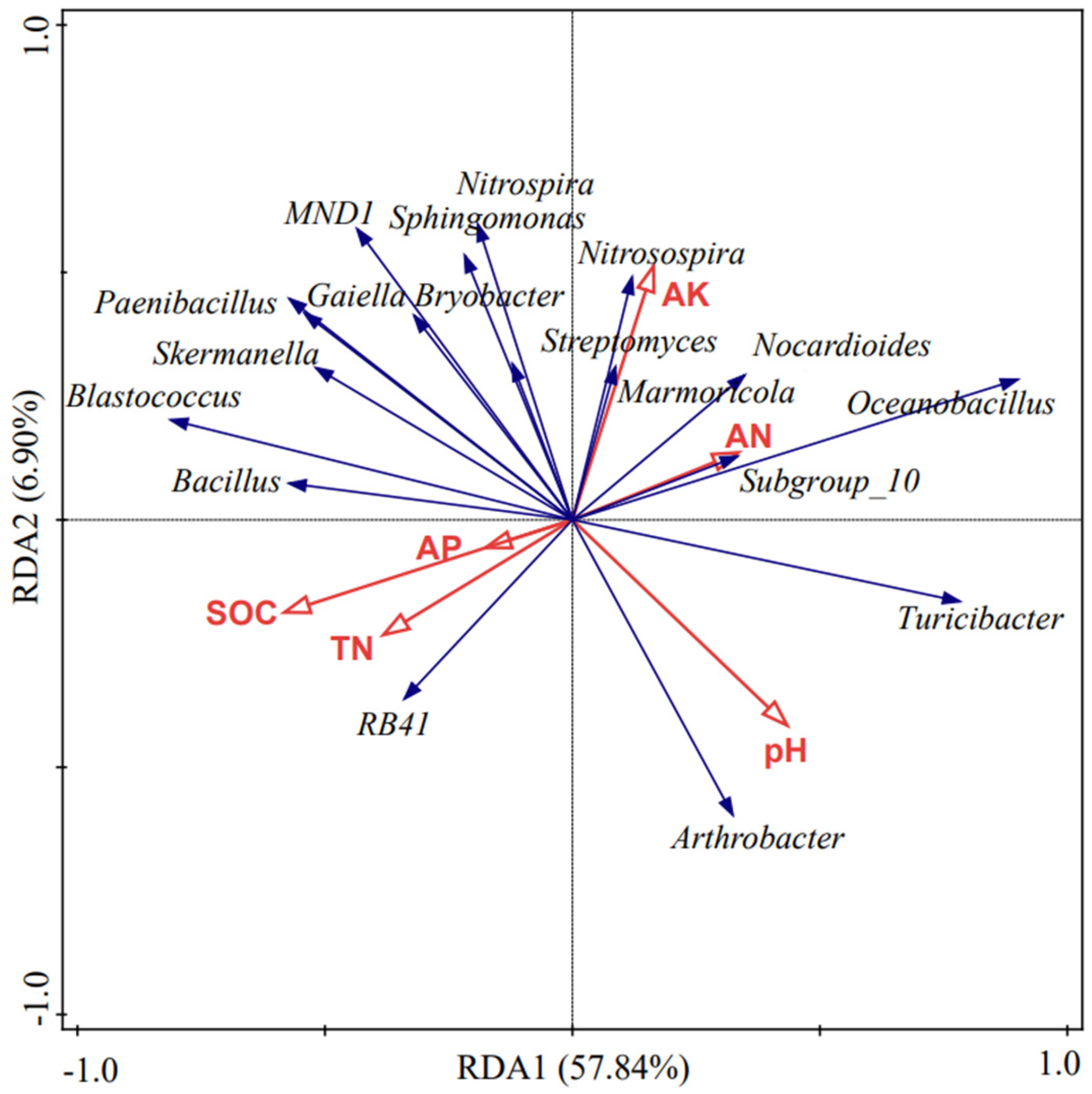
3.4. Relationship Between Soil Bacterial Communities and Environmental Factors
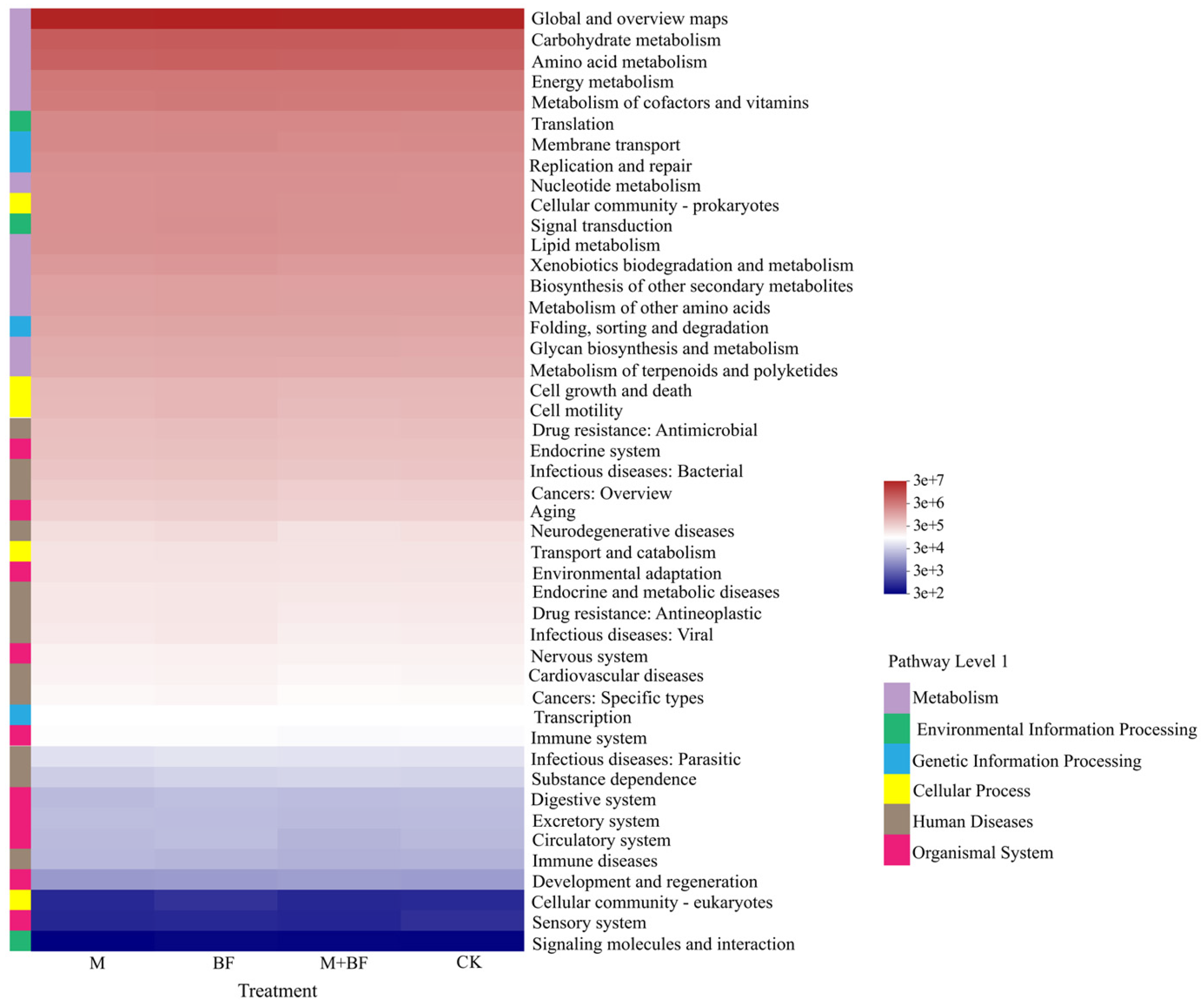
3.5. Prediction of Soil Bacterial PICRUSt Function in Different Post-Crops
4. Discussion
4.1. Effects of Different Fertilization Measures on Soil Bacterial Community Structure
4.2. Effects of Different Fertilization Measures on Soil Bacterial Ecological Functions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, H.J.; Han, Z.D.; Yang, Y.Q.; Li, Z.C.; Wang, J. Bio-organic Fertilizer: Application and Farmland Environmental Effects. China Agric. Sci. Bull. 2019, 35, 82–88. [Google Scholar]
- Du, W.Y.; Tang, B.; Wang, H. The status of organic fertilizer industry and organic fertilizer resources in China. Soil Fert. Sci. China 2020, 3, 210–219. [Google Scholar]
- Wen, Y.C.; Li, Y.Q.; Yuan, L.; Li, J.; Li, W.; Lin, Z.A.; Zhao, B.Q. Comprehensive assessment methodology of characteristics of soil fertility under different fertilization regimes in North China. Trans. China Soc. Agric. Eng. 2015, 31, 91–99. [Google Scholar]
- Song, J.; Liu, W.F.; Wei, X.X.; Lu, C.L.; Zhang, M.; Li, J.G.; Zhang, L. Effects of Special Jujube Microbial Agents on Soil Nutrients and Soil Enzyme Activities of Jun Jujube Orchard in Arid Area. Southwest China J. Agric. Sci. 2021, 34, 1472–1479. [Google Scholar]
- Chen, X.Y.; Wang, X.L.; Xie, X.J. Effect of Microbial Fertilizers on Corn Yield and Soil Fertility. China J. Trop. Agric. 2021, 41, 11–16. [Google Scholar]
- Li, J.J.; Liu, C.; Wang, X.X.; Zhang, R.F.; Wang, H. Effects of Increasing Microbial Agents on Growth, Quality and Soil Nutrients of Green Pepper. N. Hortic. 2021, 1–10. [Google Scholar]
- He, J.; Ma, T.H.; Bai, X.J.; Li, Q.Q.; Hao, W.L. Effects of microbial agents on growth and development, yield and quality of Lycium barbarum and soil nutrients. Jiangsu Agric. Sci. 2021, 49, 149–154. [Google Scholar]
- Li, X.G.; Guo, M.; Wang, C.G. Study on the treatment of organic fertilizer + bacterial fertilizer on peanut continuous cropping production obstacles. Agric. Henan 2018, 43–45. [Google Scholar] [CrossRef]
- Zheng, G.D.; Gong, S.; Huang, Y.X.; Huang, J.T. Effects of Different Amounts of Combination of Organic Fertilizer and Microbial Agent on Yield, Quality of Peanut and Soil Fertility. J. Pea. Sci. 2022, 51, 25–31+48. [Google Scholar]
- Wang, A.L.; Ma, R.; Ma, Y.J.; Lv, Y.X. Prediction of Soil Bacterial Community Structure and Function in Minqin Desert-oasis Ecotone Artificial Haloxylon ammodendron Forest. China J. Environ. Sci. 2024, 45, 508–519. [Google Scholar]
- Vuyyuru, M.; Sandhu, H.S.; Erickson, J.E.; Ogram, A.V. Soil chemical and biological fertility, microbial community structure and dynamics in successive and fallow sugarcane planting systems. Agroecol. Sustain. Food Syst. 2020, 44, 768–794. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X. Responses of soil microbial communities to nutrient limitation in the desert-grassland ecological transition zone. Sci. Total Environ. 2018, 642, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Lejon David, P.H.; Chaussod, R.; Ranger, J.; Ranjard, L. Microbial Community Structure and Density Under Different Tree Species in an Acid Forest Soil (Morvan, France). Micro. Ecol. 2005, 50, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Xu, Z.; Tang, L.; Li, Y.H.; Song, J.Q.; Xu, J.Q. Effects of different organic fertilizers on the microbes in rhizospheric soil of flue-cured tobacco. China J. Appl. Ecol. 2013, 24, 2551–2556. [Google Scholar]
- Niu, C.C.; Geng, G.M.; Yu, L.; Xie, Q.J.; Liao, J.J.; Qi, H.Y. Reducing fertilizer input combined with the application of Trichoderma to increase yield, quality of melon, and soil microbial functional diversity. J. Plant Nutr. Fert. 2019, 25, 620–629. [Google Scholar]
- Fei, Y.C.; Wu, Q.Z.; Lu, J.; Ji, C.S.; Zheng, H.; Cao, S.J.; Lin, K.J.; Cao, G.Q. Effects of undergrowth vegetation management measures on the soil bacterial community structure of large diameter timber plantation of Cunninghamia lanceolate. China J. Appl. Ecol. 2020, 31, 407–416. [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2000; pp. 40–98. [Google Scholar]
- Duan, P.F.; Chen, Y.; Zhang, F.; Han, H.; Pang, F.H.; Chen, Z.J.; Tian, W. Effect of Miscanthus planting on the structure and function of soil bacterial community. China J. Appl. Ecol. 2019, 30, 2030–2203. [Google Scholar]
- Li, G.X.; Ma, K.M. PICRUSt-based predicted metagenomic analysis of treeline soil bacteria on Mount Dongling, Beijing. Acta Ecol. Sin. 2018, 38, 2180–2186. [Google Scholar]
- Shi, P.; Wang, S.P.; Jia, S.G.; Gao, Q.; Sun, X.Q. Effects of three planting patterns on soil microbial community composition. China J. Plant Ecol. 2011, 35, 965–972. [Google Scholar] [CrossRef]
- Lin, X.X. Effects of Different Years of Straw Returning on Organic Carbon and Microbial Community Structure in Black Soil. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2021. [Google Scholar]
- Ma, X.; Luo, Z.Z.; Zhang, Y.Q.; Liu, J.H.; Niu, Y.N.; Cai, L.Q. Distribution characteristics and ecological function predictions of soil bacterial communities in rainfed alfalfa fields on the Loess Plateau. Acta Prataculturae Sin. 2021, 30, 54–67. [Google Scholar]
- Jiang, X.W.; Ma, D.L.; Zang, S.Y.; Zhang, D.Y.; Sun, H.Z. Characteristics of soil bacterial and fungal community of typical forest in the Greater Khingan Mountains based on high-throughput sequencing. Microbiol. China 2021, 48, 1093–1105. [Google Scholar]
- Wang, Y.N.; Hu, Y.G.; Wamg, Z.R.; Li, Y.K.; Zhang, Z.H.; Zhou, H.K. Impacts of desertification and artificial revegetation on soil bacterial communities in alpine grassland. Acta Prataculturae Sin. 2022, 31, 26–39. [Google Scholar]
- Liu, H.; Wei, L.L.; Zhu, L.F.; Wei, H.; Bai, Y.X.; Liu, X.L.; Li, S.B. Research progress of Sphingomonas. Microbiol. China 2023, 50, 2738–2752. [Google Scholar]
- Liu, K.H.; Xue, Y.Q.; Zhu, L.P.; Xu, F.; Zhu, Z.H.; Zhang, T.; Zhang, F.B. Effect of Different Land Use Types on the Diversity of Soil Bacterial Community in the Coastal Zone of Jialing River. China J. Environ. Sci. 2022, 43, 1620–1629. [Google Scholar]
- Yang, P.; Zhai, Y.P.; Zhao, X.; Wang, S.M.; Liu, H.L.; Zhang, X. Effect of interaction between arbuscular mycorrhizal fungi and Rhizobium on Medicago sativa rhizosphere soil bacterial community structure and PICRUSt functional prediction. Microbiol. China 2020, 47, 3868–3879. [Google Scholar]
- Wang, X.F.; Luo, Z.Z.; Zhang, R.Z.; Niu, Y.L.; Li, L.L.; Tian, J.X.; Sun, P.Z.; Liu, J.H. Soil bacterial community characteristics and ecological function prediction of alfalfa and crop rotation systems in the Loess Plateau, Northwest China. China J. Appl. Ecol. 2022, 33, 1109–1117. [Google Scholar]
- Guo, Z.; Shi, C.D. Prediction of Bacterial Community Structure and Function in Different Compound Ratio Soils. Environ. Sci. Technol. 2021, 44, 69–76. [Google Scholar]
- Wang, T.; Cheng, K.K.; Cai, Z.H.; Zhou, J. Research advance in communication interactions among the symbionts of “bacteria-zooxanthellae-coral”. China J. Appl. Ecol. 2022, 33, 2572–2584. [Google Scholar]
- Kaiser, D.; Losick, R. How and why bacteria talk to each other. Cell 1993, 73, 873–885. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chen, C.L.; Fu, L.; Zhou, D.D. The roles of ‘cell-to-cell communication’ in phycosphere. Acta Microbiol. Sin. 2022, 62, 33–46. [Google Scholar]



| Treatment | pH | Organic Carbon (g·kg−1) | Effective Phosphorus (mg·kg−1) | Total Nitrogen (g·kg−1) | Quick-Acting Potassium (mg·kg−1) |
|---|---|---|---|---|---|
| CK | 8.49 | 10.8 | 18.9 | 0.63 | 201 |
| M | 8.43 | 10.9 | 19.2 | 0.57 | 235 |
| BF | 8.50 | 10.5 | 16.4 | 0.60 | 236 |
| M + BF | 8.50 | 10.7 | 16.4 | 0.61 | 231 |
| Year | Treatment | Organic Carbon (g·kg−1) | Total Nitrogen (g·kg−1) | Available Nitrogen (mg·kg−1) | Available Phosphorus (mg·kg−1) | Available Potassium (mg·kg−1) | pH |
|---|---|---|---|---|---|---|---|
| 2021 | CK | 9.50 ± 0.03 d | 0.62 ± 0.01 ab | 54.04 ± 0.25 c | 17.61 ± 0.05 b | 238.33 ± 2.03 b | 8.49 ± 0.02 a |
| M | 11.03 ± 0.05 c | 0.60 ± 0.02 b | 59.54 ± 0.44 b | 20.51 ± 0.36 a | 243.67 ± 2.73 b | 8.44 ± 0.02 b | |
| BF | 11.21 ± 0.13 b | 0.62 ± 0.02 ab | 59.57 ± 1.13 b | 20.64 ± 0.29 a | 254.33 ± 2.96 a | 8.39 ± 0.01 c | |
| M + BF | 13.3 ± 0.07 a | 0.67 ± 0.02 a | 62.54 ± 0.63 a | 21.43 ± 0.33 a | 227.00 ± 1.15 c | 8.48 ± 0.03 a | |
| 2022 | CK | 8.90 ± 0.20 c | 0.61 ± 0.01 b | 59.38 ± 0.73 a | 17.17 ± 0.62 b | 250.67 ± 6.17 b | 8.62 ± 0.02 a |
| M | 11.05 ± 0.49 bc | 0.60 ± 0.01 b | 61.93 ± 3.92 a | 21.16 ± 1.30 a | 247.33 ± 1.86 b | 8.49 ± 0.02 a | |
| BF | 11.56 ± 0.67 b | 0.59 ± 0.04 b | 59.62 ± 1.87 a | 21.72 ± 0.94 a | 271.00 ± 3.51 a | 8.39 ± 0.09 a | |
| M + BF | 16.72 ± 0.11 a | 0.75 ± 0.01 a | 64.05 ± 0.49 a | 22.56 ± 0.90 a | 202.67 ± 2.73 c | 8.49 ± 0.01 a |
| Level 1 Function | Relative Abundance (%) | |||
|---|---|---|---|---|
| CK | M | BF | M + BF | |
| Metabolism | 78.67 a | 78.51 a | 78.45 a | 78.94 a |
| Genetic Information Processing | 6.86 a | 6.84 a | 6.72 a | 6.90 a |
| Environmental Information Processing | 5.12 a | 5.17 a | 5.24 a | 5.01 a |
| Cellular Processes | 4.26 a | 4.31 a | 4.36 a | 4.17 a |
| Human Diseases | 3.30 a | 3.36 a | 3.42 a | 3.21 a |
| Organismal Systems | 1.78 a | 1.81 a | 1.81 a | 1.76 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhao, Z.; Dong, B.; Zhang, R.; Jiang, J.; Ma, F.; Zhang, Y.; Zhao, J.; Du, D.; Qiu, J.; et al. Effects of Different Fertilization Measures on Bacterial Community Structure in Seed Production Corn Fields. Agronomy 2024, 14, 2459. https://doi.org/10.3390/agronomy14112459
Yang Y, Zhao Z, Dong B, Zhang R, Jiang J, Ma F, Zhang Y, Zhao J, Du D, Qiu J, et al. Effects of Different Fertilization Measures on Bacterial Community Structure in Seed Production Corn Fields. Agronomy. 2024; 14(11):2459. https://doi.org/10.3390/agronomy14112459
Chicago/Turabian StyleYang, Yirong, Zhenhua Zhao, Bo Dong, Rui Zhang, Jing Jiang, Fengjie Ma, Yingying Zhang, Jianhua Zhao, Dandan Du, Jindong Qiu, and et al. 2024. "Effects of Different Fertilization Measures on Bacterial Community Structure in Seed Production Corn Fields" Agronomy 14, no. 11: 2459. https://doi.org/10.3390/agronomy14112459
APA StyleYang, Y., Zhao, Z., Dong, B., Zhang, R., Jiang, J., Ma, F., Zhang, Y., Zhao, J., Du, D., Qiu, J., & Li, C. (2024). Effects of Different Fertilization Measures on Bacterial Community Structure in Seed Production Corn Fields. Agronomy, 14(11), 2459. https://doi.org/10.3390/agronomy14112459





