Analysis of the Physiological Parameters of Cold Resistance in Core Winter and Spring Wheat Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Testing Content and Methods of Measurement
2.2.1. Investigation of Frost Damage During the Winter Period of Wheat Under Field Conditions
2.2.2. Measurement of Chl Content
2.2.3. Measurement of SS Content
2.2.4. Measurement of SP Content
2.2.5. Measurement of Pro Content
2.2.6. Measurement of MDA Content
2.2.7. Measurement of Wheat Yield
2.2.8. Measurement of Wheat Grain Quality Traits
2.3. Statistical Analysis
3. Results
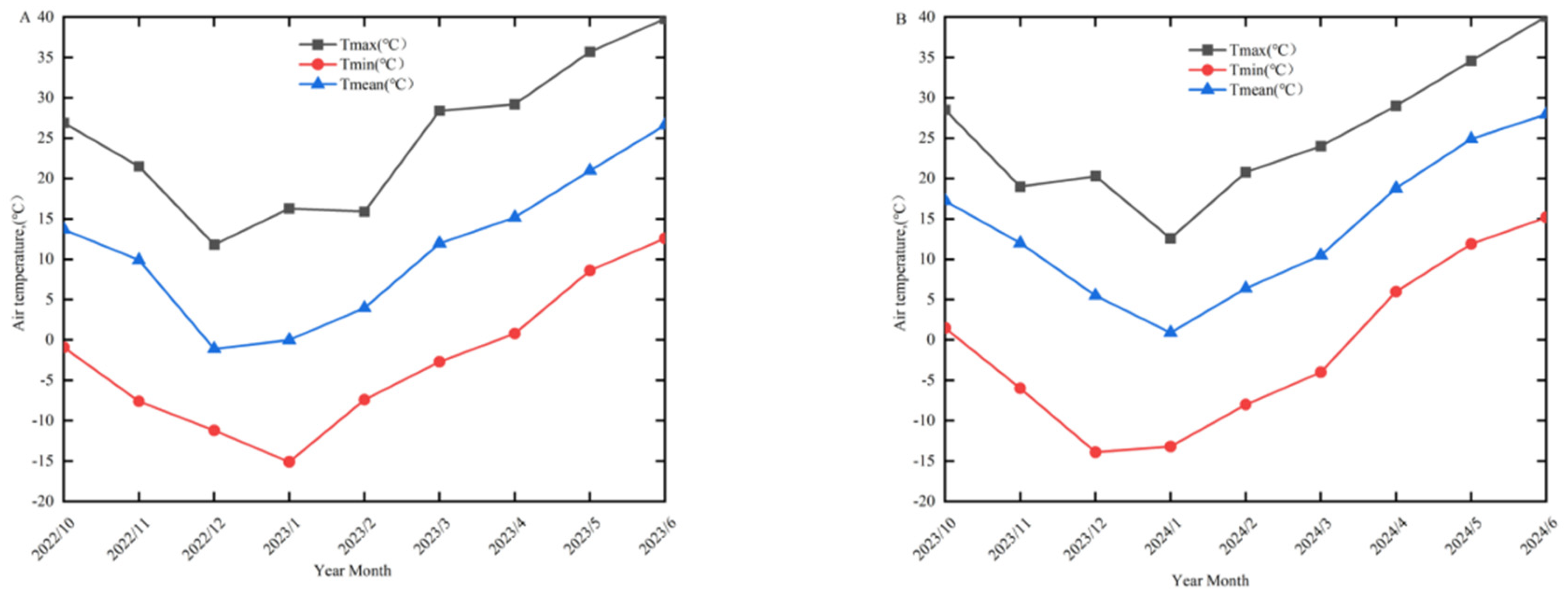
3.1. Winter–Spring Characteristics and Frost Damage Levels of Different Wheat Cultivars
3.2. Comparison of the Physiological Parameters of Different Winter–Spring Wheat Cultivars
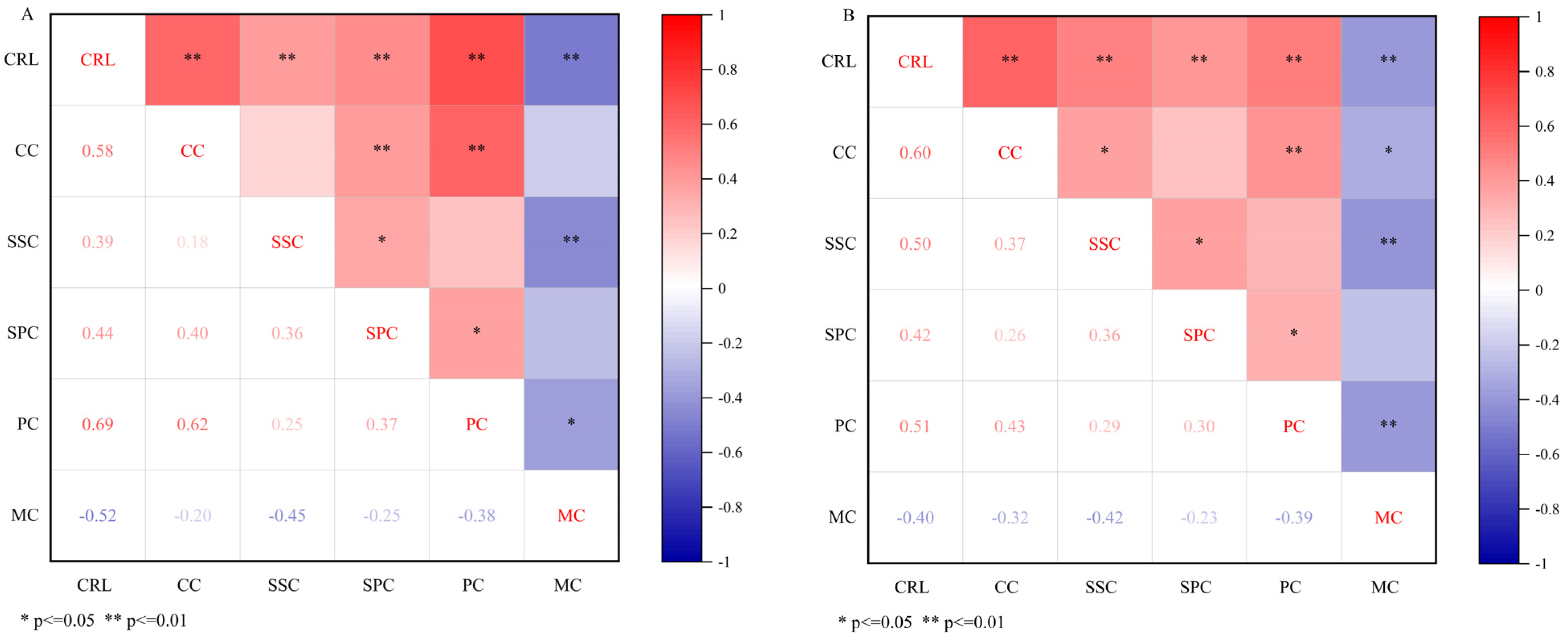
3.3. Relationship Between the Physiological Parameters of Cold Resistance of Different Winter–Spring Wheat Cultivars
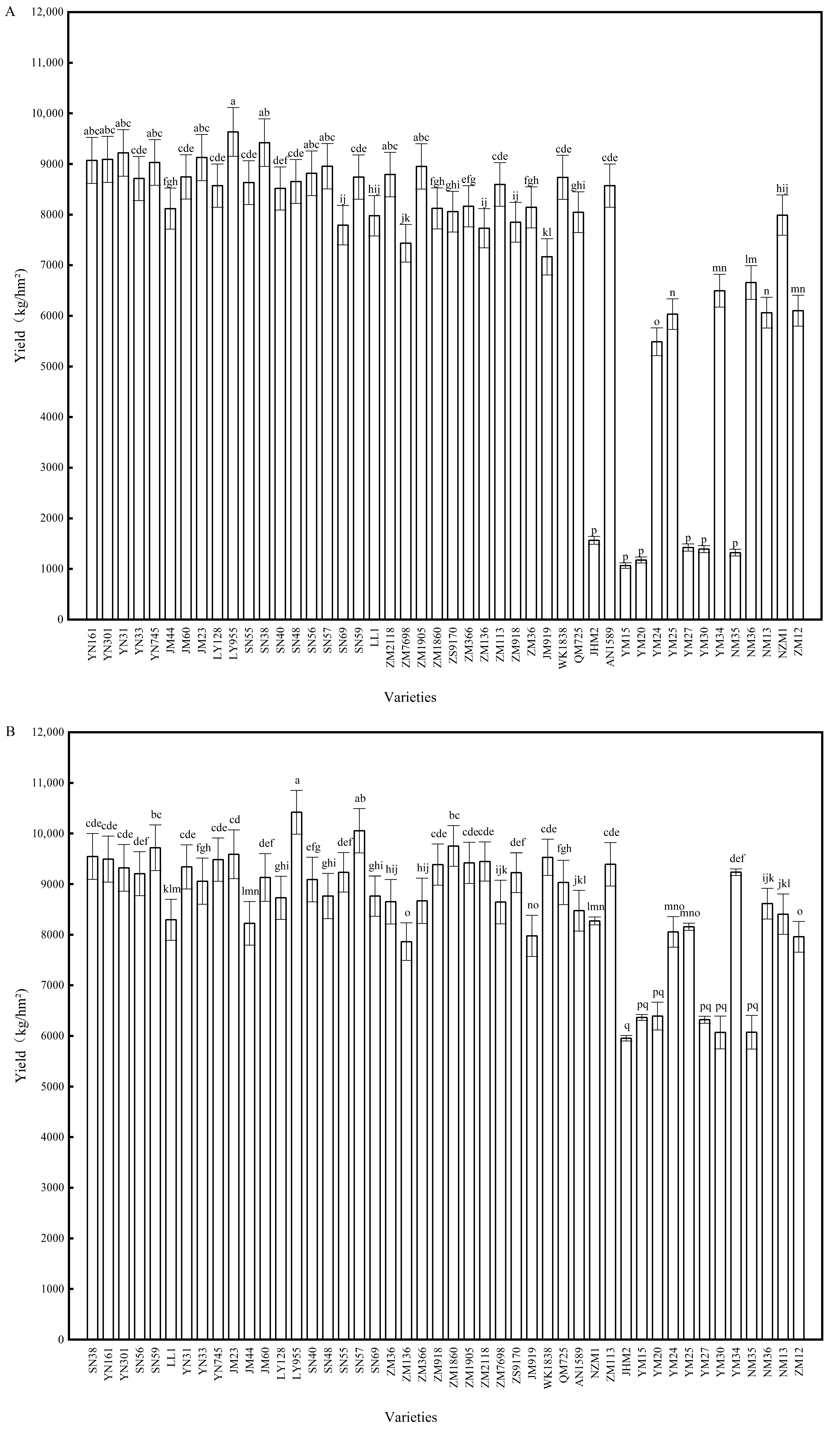
3.4. Comparison of Wheat Yields of Different Winter–Spring Cultivars
3.5. Comparison of Quality Indices of Different Winter–Spring Wheat Cultivars
3.6. Comprehensive Evaluation and Screening of Different Winter–Spring Wheat Cultivars
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gu, X.B.; Cai, H.J.; Chen, P.P.; Li, Y.P.; Fang, H.; Li, Y.N. Ridge-furrow film mulching improves water and nitrogen use efficiencies under reduced irrigation and nitrogen applications in wheat field. Field Crop Res. 2021, 270, 108214. [Google Scholar] [CrossRef]
- Wang, D.L.; Guo, M.J.; Liu, S.B.; Li, Y.; Dong, Q.G.; Gong, X.W.; Ge, J.J.; Wu, F.; Feng, H. Spatiotemporal evolution of winter wheat planting area and meteorology driven effects on yield under climate change in Henan province of China. Plants 2024, 13, 2109. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Qiu, X.L.; Kang, M.; Zhang, L.Y.; Lu, W.J.; Liu, B.; Tang, L.; Xiao, L.J.; Zhu, Y.; Cao, W.X.; et al. Evaluating the impacts of climatic factors and global climate change on the yield and resource use efficiency of winter wheat in China. Eur. J. Agron. 2024, 159, 127295. [Google Scholar] [CrossRef]
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.; Kim, K.M.; Kim, Y.; Kang, C.S.; Son, J.; Ko, J.; Kim, K.H. Low temperature effects on the growth and phytochemical properties of wheat sprouts. Agriculture 2022, 12, 745. [Google Scholar] [CrossRef]
- Yue, Y.J.; Zhou, Y.; Wang, J.A.; Ye, X.Y. Assessing wheat frost risk with the support of GIS: An approach coupling a growing season meteorological index and a hybrid fuzzy neural network model. Sustainability 2016, 8, 1308. [Google Scholar] [CrossRef]
- Skinner, D.Z. Genetics of winter wheat response to two freezing treatments. Plant Breed. 2012, 131, 380–384. [Google Scholar] [CrossRef]
- Cheong, B.E.; William, W.H.H.; Biddulph, B.; Wallace, X.; Rathjen, T.; Rupasinghe, T.W.T.; Roessner, U.; Dolferus, R. Phenotyping reproductive stage chilling and frost tolerance in wheat using targeted metabolome and lipidome profiling. Metabolomics 2019, 15, 144. [Google Scholar] [CrossRef]
- Xiao, L.J.; Liu, L.L.; Asseng, S.; Xia, Y.M.; Tang, L.; Liu, B.; Cao, W.X.; Zhu, Y. Estimating spring frost and its impact on yield across winter wheat in China. Agric. For. Meteorol. 2018, 260, 154–164. [Google Scholar] [CrossRef]
- Venzhik, Y.V.; Moshkov, I.E. The role of ultrastructural organization of cells in adaptation of winter wheat to low temperature. Russ. J. Plant Physiol. 2023, 70, 100. [Google Scholar] [CrossRef]
- Uemura, M.; Tominaga, Y.; Nakagawara, C.; Shigematsu, S.; Minami, A.; Kawamura, Y. Responses of the plasma membrane to low temperatures. Physiol. Plant. 2006, 126, 81–89. [Google Scholar] [CrossRef]
- Ejaz, I.; Pu, X.Y.; Naseer, M.A.; Bohoussou, Y.D.; Liu, Y.; Farooq, M.; Zhang, J.T.; Zhang, Y.H.; Wang, Z.M.; Sun, Z.C. Cold and drought stresses in wheat: A global meta-analysis of 21st century. J. Plant Growth Regul. 2023, 42, 5379–5395. [Google Scholar] [CrossRef]
- Armonienė, R.; Liatukas, Ž.; Brazauskas, G. Evaluation of freezing tolerance of winter wheat (Triticum aestivum L.) under controlled conditions and in the field. Zemdirbyste 2013, 100, 417–424. [Google Scholar] [CrossRef][Green Version]
- Ding, Y.L.; Shi, Y.T.; Yang, S.H. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Yan, C.S.; Zhang, X.Y.; Sun, G.Z.; Qian, Z.G.; Qi, X.L.; Mou, Q.H.; Xiao, S.H. Effects of low temperature in spring on wheat yield and photosynthetic characteristics. Crop J. 2018, 44, 288–296. [Google Scholar] [CrossRef]
- Watanabe, T.; Okada, R.; Tokunaga, S.; Maruyama, H.; Urayama, M. Nitrogen deficiency-induced molybdenum accumulation in wheat. J. Plant Nutr. 2022, 45, 1413–1424. [Google Scholar] [CrossRef]
- Zhang, F.F.; Gao, S.; Zhao, Y.Y.; Zhao, X.L.; Liu, X.M.; Xiao, K. Growth traits and nitrogen assimilation-associated physiological parameters of wheat (Triticum aestivum L.) under low and high N conditions. J. Int. Agric. 2015, 14, 1295–1308. [Google Scholar] [CrossRef]
- Li, X.; Pu, H.; Liu, F.; Zhou, Q.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Winter wheat photosynthesis and grain yield responses to spring freeze. Agron. J. 2015, 107, 1002–1010. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.X.; Guo, J.H.; Li, N.; Jiang, M.; Li, X.N. Low temperature tolerance is depressed in wild-type and abscisic acid-deficient mutant barley grown in Cd-contaminated soil. J. Hazard. Mater. 2022, 430, 128489. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Jiang, N.; Zhang, H.Q.; Huo, Z.G.; Yang, Z.Q. Effect of low temperature on photosynthetic characteristics, senescence characteristics, and endogenous hormones of winter wheat “Ji Mai 22” during the jointing stage. Agronomy 2023, 13, 2650. [Google Scholar] [CrossRef]
- Jiang, L.N.; Zhang, D.J.; Song, F.; Liu, P.; Fan, T.T.; Yu, H.B.; Li, C.X. Physiological responses of leaves of different wheat varieties to low temperature at jointing stage and evaluation of cold resistance. Acta Ecol. Sin. 2014, 34, 4251–4261. [Google Scholar]
- Furtauer, L.; Weiszmann, J.; Weckwerth, W.; Nagele, T. Dynamics of plant metabolism during cold acclimation. Int. J. Mol. Sci. 2019, 20, 5411. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Hao, Y.C.; Hao, M.; Cui, Y.J.; Kong, L.R.; Wang, H.W. Genome-wide survey of the dehydrin genes in bread wheat (Triticum aestivum L.) and its relatives: Identification, evolution and expression profiling under various abiotic stresses. BMC Genom. 2022, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- Kishor, P.B.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.R.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Zhu, Y.G.; Kneer, R.; Smith, S.E. Effect of zinc on cadmium toxicity induced oxidative stress in winter wheat seedlings. J. Plant Nutr. 2005, 28, 1947–1959. [Google Scholar] [CrossRef]
- Anna, I.; Vera, T.; Natalia, R.; Alexander, T. Exogenous salicylic acid treatment induces cold tolerance in wheat through promotion of antioxidant enzyme activity and proline accumulation. Acta Physiol. Plant. 2019, 41, 80. [Google Scholar]
- Castroverde, C.D.M.; Dina, D. Temperature regulation of plant hormone signaling during stress and development. J. Exp. Bot. 2021, 72, 7436–7458. [Google Scholar] [CrossRef]
- Li, P.F.; Ma, B.L.; Xiong, Y.C.; Zhang, W.Y. Morphological and physiological responses of different wheat genotypes to chilling stress: A cue to explain yield loss. J. Sci. Food Agric. 2017, 97, 4036–4045. [Google Scholar] [CrossRef]
- Gebbing, T.; Schnyder, H. Pre-anthesis reserve utilization for protein and carbohydrate synthesis in grains of wheat. Plant Physiol. 1999, 121, 871–878. [Google Scholar] [CrossRef]
- Liu, L.L.; Song, H.; Shi, K.J.; Liu, B.; Zhang, Y.; Tang, L.; Cao, W.X.; Zhu, Y. Response of wheat grain quality to low temperature during jointing and booting stages—On the importance of considering canopy temperature. Agric. For. Meteorol. 2019, 278, 107658. [Google Scholar] [CrossRef]
- Zheng, J.C.; Liu, T.; Zheng, Q.X.; Li, J.Q.; Qian, Y.C.; Li, J.C.; Zhan, Q.W. Identification of cold tolerance and analysis of genetic diversity for major wheat varieties in Jianghuai region of China. Pak. J. Bot. 2020, 3, 839–849. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Huang, Z.; Mi, L.; Xu, K.; Wu, J.; Fan, Y.; Ma, S.; Jiang, D. Effects of low temperature at booting stage on sucrose metabolism and endogenous hormone contents in winter wheat spikelet. Front. Plant Sci. 2019, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Uchida, S. Quality matters more than quantity: Asymmetric temperature effects on crop yield and quality grade. Am. J. Agric. Econ. 2016, 98, 1195–1209. [Google Scholar] [CrossRef]
- Liu, Y.H.; Su, L.J.; Wang, Q.J.; Zhang, J.H.; Shan, Y.Y.; Deng, M.M. Comprehensive and quantitative analysis of growth characteristics of winter wheat in China based on growing degree days. Adv. Agron. 2020, 159, 237–273. [Google Scholar]
- Zheng, D.X.; Yang, X.G.; Mínguez, I.M.; Connor, D.J.; Mu, C.Y.; Guo, E.J.; Chen, X. Tolerance of different winter wheat cultivars to prolonged freezing injury at their critical temperatures. Crop Sci. 2018, 58, 1740–1750. [Google Scholar] [CrossRef]
- Notice of the Ministry of Agriculture of the People’s Republic of China; Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2024. Available online: http://www.moa.gov.cn/ (accessed on 5 September 2024).
- NY/T 2283-2012; Rules of Field Investigation and Grading of Damage to Winter Wheat. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2012.
- Zhou, Q.W.; Yang, R.F.; Wang, X.H.; Quan, R.P.; Cui, G.X. Application of SPAD-502 chlorophyll meter in screening ramie germplasm with high light efficiency. Mod. Agric. Technol. 2022, 3, 40–43. [Google Scholar]
- Li, H.S. Experimental Principles and Techniques of Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2000; pp. 167–169. [Google Scholar]
- Zhang, Z.L. Laboratory Precedure of Plant Physiology; Higher Education Press: Beijing, China, 1990; p. 259. [Google Scholar]
- Hong, S.Z.; Jiao, F.L.; Kuang, N.K.; Liu, C.Y.; Ma, Y.Z.; Li, Q.Q. Simulating the effects of irrigation and tillage on soil water, evapotranspiration, and yield of winter wheat with RZWQM2. Soil Tillage Res. 2021, 214, 105170. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association of Cereal Chemists, 11th ed.; AACC: St. Paul, MN, USA, 2010. [Google Scholar]
- Guo, Z.Q.; Zhou, Q.; Lou, T.T.; Sheng, K.; Zhou, W.G.; Xu, C.; Zhu, S.Y.; Zhu, P. Comprehensive evaluation of 11 early spring fast-growing leafy vegetable varieties using membership function method. Zhejiang Agri. Sci. 2024, 1–7. [Google Scholar] [CrossRef]
- Shi, D.; Wei, X.; Chen, G. Effects of low temperature on photosynthetic characteristics in the super-high-yield hybrid rice ‘Liangyoupeijiu’ at the seedling stage. Genet. Mol. Res. 2016, 15, gmr15049021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, H.M.; Liu, L.Z.; Xu, H.; Chen, X.; Li, J.C. Screening of varieties resistant to late-spring coldness in wheat and effects of late-spring coldness on the ultrastructure of wheat cells. Agronomy 2023, 13, 3011. [Google Scholar] [CrossRef]
- Szalai, G.; Pap, M.; Janda, T. Light-induced frost tolerance differs in winter and spring wheat plants. J. Plant Physiol. 2009, 166, 1826–1831. [Google Scholar] [CrossRef]
- Mu, C.Y.; Yang, X.G.; Yang, J.; Li, K.N.; Zheng, D.X. Freezing resistance and injury indices for different cultivars of winter-spring wheat in Huang-Huai-Hai Plain. I. Comparison of freezing resistance for different cultivars of winter-spring wheat during mid-winter period. J. Appl. Ecol. 2015, 26, 3119–3125. [Google Scholar]
- Yang, H.B.; Li, F.Y.; Xu, C.Z.; Wang, F.H. Study on the strength of spring and winter characteristics of the main wheat varieties in Shandong Province. Shandong Agric. Sci. 2009, 1, 43–46. [Google Scholar]
- Venzhik, Y.; Talanova, V.; Titov, A. The effect of abscisic acid on cold tolerance and chloroplasts ultrastructure in wheat under optimal and cold stress conditions. Acta Physiol. Plant. 2016, 38, 63. [Google Scholar] [CrossRef]
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Hui, X.; Ke, Y.Y.; Bruno, A.K.; Zhang, L.L.; Li, J.C. Cold stress in wheat: Plant acclimation responses and management strategies. Front. Plant Sci. 2021, 12, 676884. [Google Scholar] [CrossRef]
- Li, C.Y.; Chen, S.S.; Xu, W.; Li, D.S.; Gu, X.; Zhu, X.K.; Guo, W.S.; Feng, C.N. Effects of low temperature stress during seedling stage on antioxidant enzymes and osmoregulation substances in leaves of Yangmai 16. Crop J. 2011, 37, 2293. [Google Scholar]
- Alexander, D.; Kseniya, Z.; Natalia, N.; Yuliya, V. Effect of low temperature on content of primary metabolites in two wheat genotypes differing in cold tolerance. Metabolites 2024, 14, 199. [Google Scholar] [CrossRef]
- Rejeb, K.B.; Abdelly, C.; Savoure, A. How reactive oxygen species and proline face stress together. Plant Physio. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Ali, A.; Dindhoria, K.; Kumar, R. Acinetobacter oleivorans IRS14 alleviates cold stress in wheat by regulating physiological and biochemical factors. J. Appl. Micrbiol. 2023, 134, lxad176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jia, D.; Gao, Z.; Dong, Q.; He, L. Physiological responses to low temperature in spring and winter wheat varieties. J. Sci. Food Agric. 2016, 96, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Lelarge-Trouverie, C.; Mhamdi, A. The metabolic of oxidative stress. Phytochemistry 2015, 112, 33–53. [Google Scholar] [CrossRef]
- Kolupaev, Y.E.; Vayner, A.A.; Yastreb, T.O.; Oboznyi, A.I.; Ryabchun, N.I. Antioxidant enzyme activity and osmolyte content in winter cereal seedlings under hardening and cryostress. Russ. J. Plant Physiol. 2015, 62, 499–506. [Google Scholar] [CrossRef]
- Liu, L.; Ji, H.; An, J.; Shi, K.; Ma, J.; Liu, B.; Tang, L.; Cao, W.; Zhu, Y. Response of biomass accumulation in wheat to low temperature stress at jointing and booting stages. Environ. Exp. Bot. 2019, 157, 46–57. [Google Scholar] [CrossRef]
- Su, H.; Tan, C.; Liu, Y.; Chen, X.; Li, X.; Jones, A.; Zhu, Y.; Song, Y. Physiology and molecular breeding in sustaining wheat grain setting and quality under spring cold stress. Int. J. Mol. Sci. 2022, 23, 14099. [Google Scholar] [CrossRef]
- Fuller, M.P.; Fuller, A.M.; Kaniouras, S.; Christophers, J.; Fredericks, T. The freezing characteristics of wheat at ear emergence. Eur. J. Agron. 2007, 26, 435–441. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, K.; Gu, D.; Zhang, S.; Wu, J. Quantifying the effect of low-temperature events on the grain quality formation of wheat. J. Cereal Sci. 2021, 100, 103257. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Al-Issawi, M.; Fuller, M.P. Advances in physiological and molecular aspects of plant cold tolerance. J. Plant Int. 2017, 12, 143–157. [Google Scholar] [CrossRef]
- Vagujfalvi, A.; Aprile, A.; Miller, A.; Dubcovsky, J.; Delugu, G.; Galiba, G.; Cattivelli, L. The expression of several Cbf genes at the Fr-A2 locus is linked to frost resistance in wheat. Mol. Genet. Genom. 2005, 274, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Kocsy, G.; Athmer, B.; Perovic, D.; Himmelbach, A.; Szucs, A.; Vashegyi, I.; Schweizer, P.; Galiba, G.; Stein, N. Regulation of gene expression by chromosome 5A during cold hardening in wheat. Mol. Genet. Genom. 2010, 283, 351–363. [Google Scholar] [CrossRef] [PubMed]
| Cultivars | Code | Cultivar Source |
|---|---|---|
| Yannong31 | YN31 | Yantai Agricultural Science Research Institute, Shandong Province |
| Yannong33 | YN33 | |
| Yannong161 | YN161 | |
| Yannong301 | YN301 | |
| Yannong745 | YN745 | |
| Jimai23 | JM23 | Shandong Academy of Agricultural Sciences |
| Jimai44 | JM44 | |
| Jimai60 | JM60 | |
| Luyan128 | LY128 | Shandong Luyan Agricultural Variety Co., Ltd. Shandong, China |
| Luyan955 | LY955 | |
| Shannong38 | SN38 | Shandong Agricultural University |
| Shannong40 | SN40 | |
| Shannong48 | SN48 | |
| Shannong55 | SN55 | |
| Shannong56 | SN56 | |
| Shannong57 | SN57 | |
| Shannong59 | SN59 | |
| Shannong69 | SN69 | |
| Luliang1 | LL1 | |
| Zhengmai113 | ZM113 | Henan Academy of Agricultural Sciences |
| Zhengmai136 | ZM136 | |
| Zhengmai366 | ZM366 | |
| Zhengmai918 | ZM918 | |
| Zhengmai1860 | ZM1860 | |
| Zhengmai1905 | ZM1905 | |
| Zhengmai2118 | ZM2118 | |
| Zhengmai7698 | ZM7698 | |
| Zhengshi9170 | ZS9170 | |
| Zhoumai36 | ZM36 | Zhoukou Academy of Agricultural Sciences |
| Jinmai919 | JM919 | Shanxi Agricultural University |
| Wanke1838 | WK1838 | Anhui Academy of Agricultural Sciences |
| Quanmai725 | QM725 | |
| JIUhaomai2 | JHM2 | Anhui Luyan Seed Industry Co., Ltd. Anhui, China |
| Annong1589 | AN1589 | Anhui Agricultural University |
| Yangmai15 | YM15 | Jiangsu Lixiahe Agricultural Science Research Institute |
| Yangmai20 | YM20 | |
| Yangmai24 | YM24 | |
| Yangmai25 | YM25 | |
| Yangmai27 | YM27 | |
| Yangmai30 | YM30 | |
| Yangmai34 | YM34 | |
| Ningmai13 | NM13 | Jiangsu Academy of Agricultural Sciences |
| Ningmai35 | NM35 | |
| Ningmai36 | NM36 | |
| Ningzhongmai1 | NZM1 | |
| Zhenmai12 | ZM12 | Zhenjiang Agricultural Science Research Institute in Jiangsu Hilly Region |
| Cultivars | Winter-Spring Characteristics | 2022/2023 Frost Damage Level | 2023/2024 Frost Damage Level |
|---|---|---|---|
| SN38 | Strong-winter | 2 | 2 |
| YN161 | Winter | 2 | 2 |
| YN301 | 3 | 3 | |
| SN56 | 2 | 2 | |
| SN59 | 3 | 2 | |
| LL1 | 2 | 2 | |
| YN31 | Semi-winter | 2 | 2 |
| YN33 | 2 | 2 | |
| YN745 | 2 | 2 | |
| JM23 | 2 | 2 | |
| JM44 | 2 | 2 | |
| JM60 | 2 | 2 | |
| LY128 | 3 | 3 | |
| LY955 | 3 | 3 | |
| SN40 | 2 | 2 | |
| SN48 | 3 | 2 | |
| SN55 | 2 | 2 | |
| SN57 | 2 | 2 | |
| SN69 | 2 | 2 | |
| ZM36 | 3 | 3 | |
| ZM136 | 3 | 3 | |
| ZM366 | 3 | 3 | |
| ZM918 | 3 | 2 | |
| ZM1860 | 3 | 3 | |
| ZM1905 | 3 | 3 | |
| ZM2118 | 3 | 3 | |
| ZM7698 | 3 | 3 | |
| ZS9170 | 3 | 3 | |
| JM919 | 3 | 3 | |
| WK1838 | 2 | 2 | |
| QM725 | 3 | 3 | |
| AN1589 | 2 | 2 | |
| NZM1 | 3 | 3 | |
| ZM113 | Weak-spring | 2 | 2 |
| JHM2 | Spring | 5 | 4 |
| YM15 | 5 | 4 | |
| YM20 | 5 | 4 | |
| YM24 | 4 | 3 | |
| YM25 | 4 | 3 | |
| YM27 | 5 | 4 | |
| YM30 | 5 | 4 | |
| YM34 | 4 | 3 | |
| NM35 | 5 | 4 | |
| NM36 | 4 | 3 | |
| NM13 | 4 | 3 | |
| ZM12 | 4 | 3 |
| Cultivars | Year of 2022/2023 | Year of 2023/2024 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CC (SPAD) | SSC (mg·g−1 FW) | SPC (mg·g−1 FW) | PC (μg·g−1) | MC (nmol·g−1) | CC (SPAD) | SSC (mg·g−1 FW) | SPC (mg·g−1 FW) | PC (μg·g−1) | MC (nmol·g−1) | |
| SN38 | 48.47 abc | 39.59 efg | 21.63 def | 218.96 ab | 21.72 ijk | 58.70 abc | 43.53 bcd | 25.56 bcd | 252.97 a | 13.89 lmn |
| YN161 | 46.77 def | 40.42 def | 20.34 fgh | 140.52 fgh | 28.28 bcd | 56.23 abc | 48.03 abc | 26.15 bcd | 149.24 ghi | 23.62 bcd |
| YN301 | 43.47 lmn | 48.22 abc | 19.85 ghi | 82.48 klm | 24.24 efg | 52.73 ghi | 53.47 ab | 24.76 fgh | 126.31 ijk | 16.63 ghi |
| SN56 | 50.90 ab | 34.98 ijk | 21.30 def | 200.52 b | 24.60 def | 58.00 abc | 49.82 abc | 24.75 fgh | 210.68 bcd | 16.56 hij |
| SN59 | 46.90 cde | 38.24 efg | 21.98 def | 168.27 de | 22.98 ghi | 58.07 abc | 50.05 abc | 26.11 bcd | 152.22 fgh | 17.78 ghi |
| LL1 | 50.50 abc | 37.01 hij | 25.06 ab | 212.12 ab | 18.00 pq | 56.77 abc | 48.66 abc | 29.64 a | 231.53 ab | 12.78 n |
| YN31 | 42.37 nop | 51.19 a | 20.95 def | 88.09 jkl | 20.36 klm | 51.83 hij | 57.26 a | 26.02 bcd | 102.86 mno | 16.63 ghi |
| YN33 | 44.60 hij | 43.83 cde | 21.21 def | 144.04 efg | 19.88 mno | 53.60 def | 50.54 abc | 26.72 bcd | 180.15 def | 14.77 klm |
| YN745 | 41.60 opq | 33.87 klm | 19.33 jkl | 150.89 def | 20.50 jkl | 51.47 ijk | 43.56 bcd | 25.34 cde | 184.80 cde | 16.13 ijk |
| JM23 | 47.70 bcd | 38.66 efg | 21.34 def | 174.77 cd | 22.18 hij | 57.63 abc | 47.21 abc | 26.09 bcd | 89.27 op | 15.63 ijk |
| JM44 | 47.57 bcd | 42.60 cde | 21.67 def | 202.65 b | 17.35 q | 57.40 abc | 51.51 abc | 26.75 bcd | 131.34 hij | 13.45 mn |
| JM60 | 48.23 abc | 38.03 fgh | 21.61 def | 199.96 b | 22.52 hij | 56.20 abc | 48.44 abc | 26.94 bcd | 138.98 op | 19.28 efg |
| LY128 | 50.13 abc | 35.55 ijk | 24.35 bc | 80.89 klm | 25.23 def | 55.97 abc | 50.67 abc | 24.16 ijk | 87.78 op | 19.14 efg |
| LY955 | 41.10 pqr | 50.83 ab | 26.13 a | 114.77 hij | 19.28 opq | 59.13 ab | 31.74 jkl | 23.95 jkl | 109.90 klm | 18.42 fgh |
| SN40 | 49.90 abc | 43.27 cde | 20.85 def | 170.94 cd | 19.52 nop | 60.33 a | 43.97 bcd | 25.14 efg | 219.80 ab | 15.38 jkl |
| SN48 | 47.13 cde | 38.07 fgh | 20.73 efg | 208.76 b | 27.56 bcd | 56.37 abc | 44.81 bcd | 27.11 bcd | 205.28 bcd | 18.67 fgh |
| SN55 | 51.53 a | 32.68 klm | 21.45 def | 235.38 a | 24.66 def | 58.00 abc | 41.35 cde | 24.80 fgh | 249.73 a | 20.65 cde |
| SN57 | 48.47 abc | 33.24 klm | 21.58 def | 215.61 ab | 23.45 fgh | 58.93 abc | 40.29 def | 27.71 b | 226.31 ab | 18.78 fgh |
| SN69 | 45.93 fgh | 42.18 def | 21.49 def | 194.98 bc | 27.37 bcd | 55.93 abc | 45.31 bcd | 27.52 bcd | 201.55 bcd | 16.25 ijk |
| ZM36 | 50.33 abc | 45.72 bcd | 21.28 def | 209.51 b | 30.02 bcd | 59.87 ab | 52.90 ab | 25.44 bcd | 215.97 bc | 22.08 bcd |
| ZM136 | 45.07 ghi | 33.31 klm | 22.43 de | 121.49 ghi | 28.89 bcd | 53.77 def | 45.82 bcd | 26.74 bcd | 143.97 hij | 18.28 fgh |
| ZM366 | 47.30 bcd | 36.86 hij | 22.47 de | 130.22 fgh | 29.60 bcd | 57.20 abc | 46.86 bcd | 27.6 bc | 108.07 lmn | 19.21 efg |
| ZM918 | 42.90 mon | 38.46 efg | 21.28 def | 121.55 ghi | 25.90 def | 51.07 jkl | 45.7 bcd | 25.35 cde | 144.74 hij | 22.08 bcd |
| ZM1860 | 44.27 ijk | 44.30 cde | 20.24 fgh | 141.22 fgh | 19.99 lmn | 53.60 def | 51.18 abc | 25.61 bcd | 106.95 mno | 16.13 ijk |
| ZM1905 | 45.07 ghi | 37.06 hij | 19.66 ab | 156.96 def | 22.59 hij | 49.10 lmn | 46.70 bcd | 24.14 ijk | 112.71 klm | 18.25 fgh |
| ZM2118 | 44.23 jkl | 37.64 ghi | 20.67 efg | 143.53 efg | 24.08 efg | 53.93 def | 47.44 abc | 25.52 bcd | 170.44 efg | 16.48 hij |
| ZM7698 | 41.33 pqr | 36.01 ijk | 20.37 fgh | 144.42 efg | 25.54 def | 49.77 klm | 46.68 bcd | 25.77 bcd | 205.05 bcd | 16.44 hij |
| ZS9170 | 49.93 abc | 39.88 def | 21.45 def | 102.63 ijk | 24.83 def | 58.50 abc | 49.47 abc | 26.56 bcd | 121.66 jkl | 16.91 ghi |
| JM919 | 44.65 hij | 42.28 def | 20.81 def | 141.56 fgh | 28.78 bcd | 53.40 efg | 44.78 bcd | 24.34 hij | 180.70 def | 24.93 bcd |
| WK1838 | 46.20 fgh | 28.40 pq | 20.83 def | 114.91 ghi | 29.65 bcd | 55.67 bcd | 44.24 bcd | 26.34 bcd | 102.67 mno | 25.16 bcd |
| QM725 | 47.77 bcd | 39.83 def | 19.23 lmn | 82.15 klm | 21.03 jkl | 56.27 abc | 49.17 abc | 23.77 lmn | 158.75 fgh | 15.64 ijk |
| AN1589 | 41.77 opq | 28.57 pq | 20.74 efg | 97.29 ijk | 25.18 def | 51.37 ijk | 35.10 ijk | 26.24 bcd | 87.41 op | 21.36 bcd |
| NZM1 | 47.93 bcd | 43.38 cde | 20.63 efg | 139.35 fgh | 31.79 abc | 56.60 abc | 47.65 abc | 26.18 bcd | 83.10 p | 24.66 bcd |
| ZM113 | 48.80 abc | 31.35 mno | 22.81 cd | 139.51 fgh | 32.85 ab | 58.83 abc | 45.47 bcd | 26.06 bcd | 154.97 fgh | 26.74 ab |
| JHM2 | 40.44 rs | 30.07 nop | 18.98 mno | 92.97 jkl | 28.72 bcd | 42.23 o | 39.82 efg | 25.33 cde | 99.66 nop | 25.05 bcd |
| YM15 | 42.73 mno | 37.58 ghi | 21.91 def | 77.14 klm | 32.49 abc | 50.73 jkl | 36.63 hij | 26.89 bcd | 126.66 ijk | 23.51 bcd |
| YM20 | 40.73 qrs | 29.46 opq | 20.89 def | 82.62 klm | 28.31 bcd | 50.47 jkl | 39.20 fgh | 25.76 bcd | 101.34 nop | 26.02 abc |
| YM24 | 43.47 lmn | 29.44 opq | 18.94 mno | 85.22 klm | 35.36 a | 53.07 fgh | 34.75 ijk | 23.93 jkl | 98.46 nop | 30.97 a |
| YM25 | 45.17 fgh | 38.78 efg | 19.41 ijk | 94.59 jkl | 25.16 def | 54.43 cde | 47.05 abc | 25.25 def | 86.01 op | 22.33 bcd |
| YM27 | 39.70 s | 27.43 q | 18.9 nop | 80.46 klm | 30.2 bcd | 46.33 n | 27.72 m | 23.63 mn | 75.92 p | 23.55 bcd |
| YM30 | 40.77 qrs | 28.70 pq | 18.92 mno | 66.11 m | 29.00 bcd | 50.27 jkl | 29.49 klm | 24.61 ghi | 126.10 ijk | 22.08 bcd |
| YM34 | 44.40 ijk | 30.70 nop | 17.97 p | 82.68 klm | 25.23 def | 53.13 dfg | 39.71 efg | 23.12 n | 130.20 ijk | 17.67 ghi |
| NM35 | 40.47 rs | 31.94 lmn | 18.31 op | 83.16 klm | 28.62 bcd | 47.37 mn | 28.56 lm | 24.25 ijk | 76.49 p | 20.36 def |
| NM36 | 45.97 fgh | 31.84 lmn | 20.04 fgh | 88.06 jkl | 25.99 def | 54.40 cde | 37.92 ghu | 25.68 bcd | 85.71 op | 18.08 fgh |
| NM13 | 46.63 edf | 30.74 nop | 19.28 klm | 68.05 lm | 27.08 cde | 57.23 abc | 33.41 jll | 23.89 klm | 86.45 op | 21.36 bcd |
| ZM12 | 43.77 klm | 38.68 efg | 21.1 def | 89.47 jkl | 32.48 abc | 50.93 jkl | 43.63 bcd | 26.15 bcd | 125.36 ijk | 26.38 ab |
| Cultivars | Year of 2022/2023 | Year of 2023/2024 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GPC (%) | WGC (%) | DFT (min) | DST (min) | GHI | SV (mL) | GPC (%) | WGC (%) | DFT (min) | DST (min) | GHI | SV (mL) | |
| SN38 | 12.87 pqr | 27.80 ijk | 4.03 klm | 5.33 hi | 74.92 def | 32.24 ghi | 14.00 jkl | 25.02 nop | 4.10 klm | 6.97 ghi | 73.08 jkl | 28.40 ijk |
| YN161 | 13.79 jkl | 34.54 b | 4.37 ghi | 7.40 f | 70.67 lm | 30.70 ghi | 14.07 ghi | 34.86 a | 4.40 ghi | 7.60 f | 70.99 mn | 29.58 hij |
| YN301 | 14.16 hij | 24.65 pq | 3.90 nop | 4.00 opq | 70.81 lm | 27.42 klm | 14.45 def | 25.95 lm | 3.87 no | 4.63 opq | 71.24 mn | 27.04 kl |
| SN56 | 13.65 klm | 29.52 ef | 4.90 def | 4.40 klm | 71.95 ijk | 39.05 bc | 14.27 efg | 25.92 lm | 4.20 jkl | 6.90 ghi | 73.92 ij | 29.74 hij |
| SN59 | 14.36 ghi | 31.72 d | 5.03 cde | 4.77 jkl | 67.20 n | 37.25 cde | 14.82 cd | 33.00 b | 3.50 qr | 6.40 jkl | 69.81 n | 37.59 a |
| LL1 | 13.56 lmn | 33.14 c | 3.97 mno | 14.60 c | 70.08 m | 43.97 a | 14.03 hij | 33.50 b | 3.87 no | 7.13 fgh | 70.03 n | 38.23 a |
| YN31 | 14.24 ghi | 25.80 no | 4.00 lmn | 4.00 opq | 73.80 fgh | 25.55 nop | 14.34 efg | 25.82 lm | 3.97 mno | 4.10 rst | 74.67 ghi | 26.64 klm |
| YN33 | 13.28 nop | 24.39 pqr | 3.60 rst | 4.17 nop | 73.43 ghi | 26.29 mno | 13.87 lmn | 24.29 pqr | 3.77 op | 4.10 rst | 74.16 hij | 23.96 opq |
| YN745 | 14.08 hij | 25.29 op | 3.53 st | 4.27 lmn | 73.05 hij | 24.11 qr | 14.21 fgh | 25.29 mno | 3.60 pq | 4.07 rst | 73.71 ijk | 24.59 nop |
| JM23 | 14.93 cde | 35.47 b | 4.77 def | 7.37 f | 77.27 bc | 30.41 hij | 14.63 def | 35.45 a | 5.00 b | 9.03 d | 77.00 bcd | 30.73 fgh |
| JM44 | 15.64 b | 30.40 e | 4.77 def | 39.20 a | 76.32 cde | 30.10 ijk | 15.43 b | 29.99 cd | 5.03 b | 42.20 a | 76.13 def | 31.80 efg |
| JM60 | 13.47 mno | 36.97 a | 4.30 hij | 3.53 t | 72.53 ijk | 29.60 ijk | 14.29 efg | 35.48 a | 4.10 klm | 3.57 tu | 72.07 klm | 25.33 lmn |
| LY128 | 13.69 klm | 33.01 c | 4.30 hij | 5.40 hi | 73.70 fgh | 32.40 ghi | 13.29 pq | 33.71 b | 4.40 ghi | 6.63 hij | 73.94 ij | 34.93 bc |
| LY955 | 14.26 ghi | 33.10 c | 4.47 ghi | 4.20 mno | 75.71 cde | 30.43 hij | 14.48 def | 35.36 a | 4.50 fgh | 8.23 e | 75.78 efg | 32.52 def |
| SN40 | 12.60 qr | 27.10 klm | 4.60 fgh | 4.63 klm | 71.10 lm | 34.26 def | 12.99 qr | 27.51 ij | 4.67 def | 7.03 fgh | 70.51 mn | 32.16 def |
| SN48 | 14.11 hij | 30.54 e | 4.43 ghi | 4.37 klm | 78.97 a | 33.70 efg | 14.34 efg | 28.08 hi | 4.00 lmn | 4.17 qrs | 78.40 abc | 27.62 k |
| SN55 | 13.45 mno | 29.12 fg | 4.47 ghi | 4.88 ijk | 79.60 a | 31.76 ghi | 14.79 cd | 27.52 ij | 4.40 ghi | 8.63 de | 78.60 ab | 28.50 1ijk |
| SN57 | 15.22 bcd | 33.13 c | 5.47 ab | 4.65 klm | 71.72 jkl | 42.43 ab | 14.31 efg | 24.87 opq | 4.33 hij | 5.97 klm | 71.82 lm | 25.23 lmn |
| SN69 | 13.46 mno | 28.78 fgh | 3.93 mno | 4.98 ijk | 71.46 klm | 28.68 jkl | 13.97 klm | 27.54 ij | 3.97 mno | 5.97 klm | 71.82 lm | 25.23 lmn |
| ZM36 | 14.44 fgh | 27.38 jkl | 5.33 abc | 18.52 b | 75.63 cde | 37.46 cde | 14.25 efg | 27.58 ij | 5.70 a | 19.56 b | 76.06 def | 37.29 a |
| ZM136 | 15.04 cde | 30.47 e | 5.60 a | 7.43 f | 75.81 cde | 38.23 cd | 15.36 b | 29.94 cd | 5.63 a | 8.70 de | 76.74 cde | 37.71 a |
| ZM366 | 13.88 ijk | 29.33 fg | 4.30 hij | 9.63 d | 73.64 fgh | 26.04 nop | 13.87 lmn | 29.22 def | 4.40 ghi | 9.03 d | 73.23 jkl | 26.78 klm |
| ZM918 | 14.32 ghi | 27.47 jkl | 3.77 opq | 3.73 rst | 61.04 p | 26.51 lmn | 14.15 ghi | 26.29 kl | 3.83 no | 5.20 mno | 60.54 pq | 21.34 rst |
| ZM1860 | 13.68 klm | 28.4 ghi | 4.10 jkl | 7.90 ef | 76.49 cde | 24.37 pqr | 14.46 def | 28.35 ghi | 3.80 nop | 7.57 f | 75.06 fgh | 25.06 lmn |
| ZM1905 | 13.53 mno | 24.53 pq | 4.13 ijk | 5.77 h | 71.04 lm | 25.18 opq | 13.45 op | 24.65 opq | 3.80 nop | 6.50 ijk | 71.36 mn | 27.01 kl |
| ZM2118 | 14.17 hij | 29.46 efg | 3.97 mno | 7.73 ef | 76.47 cde | 37.63 cde | 13.95 klm | 29.63 cde | 4.20 jkl | 7.57 f | 77.54 abc | 31.46 efg |
| ZM7698 | 14.73 efg | 30.48 e | 5.17 bcd | 9.23 d | 74.70 efg | 37.40 cde | 14.85 cd | 30.20 c | 4.97 bc | 8.43 e | 75.35 efg | 33.23 cde |
| ZS9170 | 14.29 ghi | 28.91 fgh | 4.43 ghi | 18.53 b | 78.29 ab | 33.48 fgh | 14.44 def | 28.61 fgh | 4.30 ijk | 19.18 b | 78.05 abc | 32.10 def |
| JM919 | 15.26 bcd | 37.83 a | 5.57 a | 4.83 ijk | 76.20 cde | 34.60 def | 15.12 bc | 35.58 a | 5.70 a | 7.20 fgh | 76.06 def | 35.29 b |
| WK1838 | 14.48 fgh | 27.01 klm | 3.87 nop | 4.60 klm | 64.52 o | 25.55 nop | 14.79 cd | 26.94 jk | 3.97 mno | 4.83 op | 65.23 o | 25.14 lmn |
| QM725 | 15.34 cde | 33.40 c | 4.67 efg | 4.40 klm | 60.29 p | 28.54 jkl | 15.39 b | 28.33 ghi | 4.73 de | 4.73 opq | 61.22 p | 27.94 jk |
| AN1589 | 14.50 fgh | 27.43 jkl | 4.50 fgh | 6.43 g | 76.75 bcd | 33.45 fgh | 14.50 def | 28.94 efg | 4.80 cd | 7.37 fg | 78.91 a | 33.87 bcd |
| NZM1 | 13.38 mno | 26.45 mn | 3.67 pqr | 3.90 pqr | 60.92 p | 24.54 opq | 13.20 pq | 26.74 k | 3.90 mno | 5.47l m | 60.72 pq | 24.01 opq |
| ZM113 | 13.40 mno | 26.71 lmn | 4.07 jkl | 8.20 e | 76.00 cde | 22.45 r | 14.06 ghi | 26.41 kl | 4.20 jkl | 8.33 e | 75.18 fgh | 23.74 opq |
| JHM2 | 12.60 qr | 25.32 op | 3.67 pqr | 4.73 jkl | 57.98 q | 23.39 qr | 12.74 r | 25.71 lmn | 3.87 no | 4.70 opq | 58.56 rs | 25.37 lmn |
| YM15 | 13.25 nop | 24.49 pq | 3.67 pqr | 6.33 g | 57.53 qr | 23.44 qr | 13.85 mn | 24.58 opq | 3.30 r | 4.50 pqr | 57.02 st | 22.27 qrs |
| YM20 | 12.37 r | 23.54 qr | 4.10 jkl | 4.23 mno | 56.90 qrs | 24.66 opq | 12.74 r | 25.71 lmn | 3.87 no | 4.70 opq | 58.56 rs | 25.37 lmn |
| YM24 | 13.63 klm | 23.7 qr | 3.67 pqr | 3.83 qrs | 55.33 s | 18.73 s | 13.78 no | 23.62 st | 3.47 qr | 3.73 stu | 56.58 t | 20.17 t |
| YM25 | 13.53 mno | 27.9 hij | 3.53 st | 5.33 hi | 61.37 p | 27.14 klm | 14.11 ghi | 24.13 qrs | 3.60 pq | 5.53 lm | 62.11 p | 20.78 st |
| YM27 | 16.39 a | 34.85 b | 4.57 fgh | 4.53 klm | 58.22 q | 25.54 nop | 16.22 a | 33.46 b | 4.50 fgh | 5.40 lmn | 60.66 pq | 29.97 ghi |
| YM30 | 14.78 def | 25.36 op | 3.63 qrs | 4.33 lmn | 56.98 qrs | 17.64 s | 14.70 de | 25.23 mno | 3.33 r | 4.33 pqr | 58.05 rst | 17.62 u |
| YM34 | 13.79 jkl | 23.72 qr | 3.47 t | 3.63 st | 56.14 rs | 17.45 s | 14.01 ijk | 23.46 t | 3.47 qr | 3.47 u | 57.15 st | 17.45 u |
| NM35 | 12.99 opq | 28.46 fgh | 4.57 fgh | 7.73 ef | 64.96 o | 25.59 nop | 13.90 klm | 28.98 efg | 4.53 fgh | 7.20 fgh | 65.93 o | 25.65 lmn |
| NM36 | 12.52 qr | 23.38 r | 3.97 mno | 3.53 t | 61.21 p | 24.41 pqr | 12.59 r | 23.85 rst | 3.97 mno | 4.90 nop | 59.53 qr | 23.11 pqr |
| NM13 | 13.37 mno | 26.91 klm | 4.37 ghi | 6.37 g | 71.19 lm | 32.23 ghi | 13.29 pq | 25.84 lm | 4.60 efg | 7.13 fgh | 72.14 klm | 30.70 fgh |
| ZM12 | 15.16 bcd | 31.72 d | 4.07 jkl | 14.38 c | 75.35 def | 25.52 nop | 15.31 b | 29.69 cde | 4.33 hij | 14.86 c | 77.81 abc | 24.90 mno |
| Cultivars | Year of 2022/2023 | Year of 2023/2024 | ||
|---|---|---|---|---|
| Average Membership Value | Comprehensive Score | Average Membership Value | Comprehensive Value | |
| SN38 | 0.51 | 1.92 | 0.5 | 1.42 |
| YN161 | 0.59 | 2.03 | 0.62 | 1.83 |
| YN301 | 0.42 | 1.53 | 0.42 | 1.21 |
| SN56 | 0.61 | 2.11 | 0.53 | 1.52 |
| SN59 | 0.58 | 1.9 | 0.62 | 1.82 |
| LL1 | 0.62 | 2.09 | 0.59 | 1.8 |
| YN31 | 0.49 | 1.8 | 0.5 | 1.41 |
| YN33 | 0.42 | 1.64 | 0.43 | 1.19 |
| YN745 | 0.44 | 1.67 | 0.45 | 1.25 |
| JM23 | 0.69 | 2.3 | 0.72 | 2.17 |
| JM44 | 0.76 | 2.3 | 0.77 | 2.16 |
| JM60 | 0.58 | 2.04 | 0.57 | 1.68 |
| LY128 | 0.54 | 1.85 | 0.54 | 1.75 |
| LY955 | 0.58 | 1.98 | 0.65 | 1.99 |
| SN40 | 0.5 | 1.86 | 0.52 | 1.56 |
| SN48 | 0.57 | 1.97 | 0.53 | 1.57 |
| SN55 | 0.57 | 2.08 | 0.6 | 1.74 |
| SN57 | 0.74 | 2.4 | 0.51 | 1.4 |
| SN69 | 0.47 | 1.73 | 0.47 | 1.33 |
| ZM36 | 0.65 | 2.07 | 0.64 | 2 |
| ZM136 | 0.67 | 2.13 | 0.65 | 2.07 |
| ZM366 | 0.49 | 1.68 | 0.47 | 1.41 |
| ZM918 | 0.37 | 1.28 | 0.38 | 0.97 |
| ZM1860 | 0.46 | 1.65 | 0.47 | 1.36 |
| ZM1905 | 0.41 | 1.5 | 0.38 | 1.1 |
| ZM2118 | 0.55 | 1.92 | 0.53 | 1.64 |
| ZM7698 | 0.62 | 2.01 | 0.59 | 1.82 |
| ZS9170 | 0.6 | 1.96 | 0.58 | 1.74 |
| JM919 | 0.7 | 2.2 | 0.68 | 2.19 |
| WK1838 | 0.45 | 1.59 | 0.47 | 1.26 |
| QM725 | 0.52 | 1.61 | 0.46 | 1.31 |
| AN1589 | 0.59 | 2.07 | 0.63 | 1.93 |
| NZM1 | 0.32 | 1.17 | 0.28 | 0.8 |
| ZM113 | 0.48 | 1.77 | 0.51 | 1.42 |
| JHM2 | 0.09 | 0.26 | 0.12 | 0.44 |
| YM15 | 0.1 | 0.25 | 0.1 | 0.29 |
| YM20 | 0.08 | 0.24 | 0.13 | 0.47 |
| YM24 | 0.17 | 0.58 | 0.19 | 0.44 |
| YM25 | 0.28 | 0.94 | 0.26 | 0.66 |
| YM27 | 0.35 | 0.8 | 0.41 | 1.27 |
| YM30 | 0.12 | 0.24 | 0.11 | 0.26 |
| YM34 | 0.17 | 0.62 | 0.22 | 0.46 |
| NM35 | 0.23 | 0.66 | 0.28 | 0.95 |
| NM36 | 0.22 | 0.84 | 0.23 | 0.64 |
| NM13 | 0.39 | 1.32 | 0.43 | 1.33 |
| ZM12 | 0.49 | 1.5 | 0.53 | 1.56 |
| Yeas | Class-Number | Group Classification | Final Cluster Center Average Membership Value | Final Cluster Center Comprehensive Value |
|---|---|---|---|---|
| 2022/2023 | 1 | JM23, JM44, SN57, JM919 | 0.72 | 2.30 |
| 2 | YN161, YN31, JM60, LY128, LY955, SN55, SN38, SN40, SN48, SN56, SN59, LL1, ZM2118, ZM7698, ZS9170, ZM136, ZM36, AN1589 | 0.58 | 1.99 | |
| 3 | YN301, YN33, YN745, SN69, ZM1905, ZM1860, ZM366, ZM113, ZM918, WK1838, QM725, NM13, ZM12 | 0.45 | 1.57 | |
| 4 | YM25, YM27, YM34, NM35, NM36, NZM1 | 0.26 | 0.84 | |
| 5 | JHM2, YM15, YM20, YM24, YM30 | 0.11 | 0.31 | |
| 2023/2024 | 1 | JM23, JM44, LY955, ZM136, ZM36, JM919, AN1589 | 0.68 | 2.07 |
| 2 | YN161, JM60, LY128, SN55, SN40, SN48, SN56, SN59, LL1, ZM2118, ZM7698, ZS9170, ZM12 | 0.57 | 1.69 | |
| 3 | YN301, YN31, YN33, YN745, SN38, SN57, SN69, ZM1905, ZM1860, ZM366, ZM113, WK1838, QM725, YM27, NM13 | 0.46 | 1.31 | |
| 4 | ZM918, YM25, NM35, NM36, NZM1 | 0.29 | 0.80 | |
| 5 | JHM2, YM15, YM20, YM24, YM30, YM34 | 0.14 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Bo, C.; Wang, X.; Yang, X.; Wang, H. Analysis of the Physiological Parameters of Cold Resistance in Core Winter and Spring Wheat Cultivars. Agronomy 2024, 14, 2438. https://doi.org/10.3390/agronomy14102438
Wang Y, Bo C, Wang X, Yang X, Wang H. Analysis of the Physiological Parameters of Cold Resistance in Core Winter and Spring Wheat Cultivars. Agronomy. 2024; 14(10):2438. https://doi.org/10.3390/agronomy14102438
Chicago/Turabian StyleWang, Yunhe, Cunyao Bo, Xiaohua Wang, Xincheng Yang, and Hongwei Wang. 2024. "Analysis of the Physiological Parameters of Cold Resistance in Core Winter and Spring Wheat Cultivars" Agronomy 14, no. 10: 2438. https://doi.org/10.3390/agronomy14102438
APA StyleWang, Y., Bo, C., Wang, X., Yang, X., & Wang, H. (2024). Analysis of the Physiological Parameters of Cold Resistance in Core Winter and Spring Wheat Cultivars. Agronomy, 14(10), 2438. https://doi.org/10.3390/agronomy14102438







