Abstract
This work was conducted over three-year monitoring seasons of three almond cultivars (Guara, Marta, and Lauranne) subjected to deficit irrigation in combination with cover crops in a Mediterranean semiarid area (SW, Spain). Four water–soil treatments were evaluated based on the conjunction of two irrigation strategies: fully irrigated (FI), covering 100% of the ETC, and regulated deficit irrigation (RDI), with two soil-management systems: bare soil (BS) and cover crop based on a mixture of vetch (Vicia sativa L.) and oat (Avena sativa L.) (CC). Throughout the study period in trees, the yield, the stem water potential (Ψstem), leaf nutrient content (N, P, K, Ca, Mg, Na, Fe, Zn, Mn, and Cu) in soils, organic carbon, microbial biomass, fluoresceine diacetate, and enzymatic activities (dehydrogenase, protease, β-glucosidase, and alkaline phosphatase) were determined. In addition, the dry matter and carbon fixation by plant covers were evaluated. For Guara and Lauranne, yield reductions (22 and 26%, respectively) were found for water-stressed (RDI-CC) plots with respect to non-stressed combination (FI-CC) plots, contrasting with cv. Marta, without a significant impact on productivity in all combinations. That is, the RDI (~3.000 m3 ha−1) strategy enabled acceptable productivity, offering promising possibilities for cultivation performance under water-scarcity scenarios. Important differences in Ψstem could be observed and ascribed to irrigation strategies, especially for Guara and Lauranne, but without significant effects due to the soil-management systems applied. No differences were observed in the tree nutritional status due to the presence or absence of CC; however, its presence increased the fixation of atmospheric carbon, which was not the case under BS conditions. Additionally, CC significantly fostered the microbial processes and enzymatic activities, particularly in upper soil layers (0–10 cm) and with plenty of water supply in FI-CC plots and to a lesser extent in RDI-CC plots, which could encourage prominent aspects for soil quality and health restoration. Thus, the cover crop is congruent with RDI to facilitate soil functionality and water savings in a changing climate, contributing to resilient farming systems in the Mediterranean environment.
1. Introduction
Adaptation strategies to mitigate climate change in Mediterranean agriculture are crucial to ensure food security and reduce the vulnerability of agricultural systems [1], and this process should include integrated water and soil resources management, the promotion of sustainable agricultural practices, and crop diversification [2,3,4,5]. Resilient agriculture is an approach that involves sustainably using natural resources through crop and livestock production systems to attain long-term productivity and farm profits under climate variability [6,7].
To address these challenges, the European Union (EU) focuses on the main objective of the Common Agricultural Policy 2021–2027: promoting practices that ensure adaptation and mitigation of climate change through investments, incentives, and improvements of final returns [8]. The use of different techniques related to sustainable and regenerative agriculture and irrigation water productivity is within this set of required actions. In this sense, the implementation of regulated deficit irrigation (RDI) strategies or the implementation of cover crops (CC) is expected to promote the sustainability, profitability, and viability of Mediterranean agroecosystems [9,10]. These actions are even more necessary in regions where agricultural intensification has provoked soil degradation, as in many rural areas of Southern Europe, where natural resources are becoming even more scarce [11,12].
Recently, European initiatives such as the Green Deal or EU Mission: A soil deal for Europe have discussed agriculture as a potential sector to favor atmosphere C sequestration, improving soil organic carbon (SOC) levels and soil health. As a result of the latest Common Agricultural Policy (CAP) 2023–2027, there has been a growing interest in practices focused on more sustainable agriculture [13,14,15]. In this line, the optimal management of vegetative covers in woody crops is required to incorporate production systems into one of the eco-regimes (ER) considered in the latest CAP. Although accepting these ERs is voluntary, the budgetary amount considered in the direct aid independent of production (decoupled aid) is the second in terms of amount. It requires incorporating one of the nine ERs considered in the CAP Specific Plan (PEPAC) [16], especially practices six and seven, which are specifically devoted to woody crops.
Particularly, in the Mediterranean region, several works have been conducted to demonstrate the effect of CC, achieving environmental and agronomic benefits [10,17,18]. That is, the implementation of CC based on legume (vetch, broad beans, lentils, etc.) plants or a mixture with grasses (barley) has been demonstrated to be an adequate strategy for improving soil quality terms and reducing soil degradation [19,20]. However, the combination of CC with water-saving practices such as RDI can pose a significant challenge, as these could compete with soil water resources, inducing a higher level of stress than that already endured by the crop due to drought conditions, although the final result will be determined by the species and its adaptation capability. In this context, understanding the effects of water scarcity on the soil’s capability to increase the SOC is a key factor in supporting the policy and decision making on strategies to enhance the resilience of agriculture to climate change [21,22]. Moreover, water scarcity is a long-standing issue in Mediterranean countries, as recent years have seen more frequent and prolonged adverse conditions due to the changing climate [3]. Thus, it is essential to increase awareness of water use efficiency, adapt agroecosystem management to present and future conditions, and prevent scenarios where the resilience capacity is severely limited [23,24].
Almond (Prunus dulcis Mill.) is the second perennial crop in Spain in terms of surface (~760,000 hectares), although its cultivation has traditionally been associated with marginal and rainfed areas [25]. This crop is considered a drought-tolerant plant, and its capability of adaptation to water-scarcity scenarios offers the possibility of obtaining competitive and sustainable yields when moderate deficit-irrigation strategies are implemented [26,27].
Concretely, previous works have shown that almonds can respond positively to moderate RDI strategies, often without any reduction in yield [28,29]. This optimum capacity of adaptation to situations of moderate water stress is a great advantage, especially when the average water allocation available for this crop is low. In general terms, this supply has not exceeded 3500 m3 ha−1 in the best cases, while the average water needs for almonds in S Spain are around 7000 m3 ha−1 [30]. According to Mirás-Avalos et al. [31], a threshold of 3500 m3 ha−1 was established at which almond production values would be seriously affected. In contrast, above this threshold, losses would be drastically reduced and could even reach values very similar to those obtained by the crop under non-water-limiting conditions. However, these results depended greatly on the variety, the management system, the plantation age, the deficit-irrigation strategy, and the duration of stress over several consecutive seasons [32,33].
Given these considerations, its development under “more sustainable agricultural practices”, such as CC incorporation in the fields, must be considered and deeply studied in order to determine if this type of practice could worsen the crop’s yield response when grown under a moderate RDI strategy. It is well-known that one of the most critical periods for the application of water stress in the almond corresponds to its vegetative development and fruit growth [34,35,36], coinciding with the time of maximum growth and development of CC. Therefore, it could lead to competition for the water stored in the soil, increasing the crop stress situation and negatively affecting productivity. Thus, determining the optimal procedure for integrating the plant covers, particularly for woody crops, remains a significant challenge under water-scarcity conditions when RDI is implemented [37]. In addition, the conjunction of both strategies (RDI × CC) could enhance climate change mitigation by fixing atmospheric carbon. This approach would also help to restore soil quality and fertility while promoting agroecological biodiversity, as suggested by Ramos et al. [38] and Repullo-Ruibérriz et al. [18].
We hypothesize that an equilibrium between CC and RDI practices can be achieved, allowing us to face the double challenge of adapting to climate change (due to the scarcity of water resources) and mitigation (favoring the sequestering of atmospheric C through the biomass of plant covers) without affecting the almond yield or its nutritional status while improving soil quality and health. Thus, the objective of this study was to evaluate the effects of the application of RDI combined with CC on soil carbon storage, microbial and enzymatic activity, crop water status, and the yield of three almond cultivars (Guara, Marta, and Lauranne) throughout a three-year monitoring season in the Mediterranean environment (SW Spain).
2. Materials and Methods
2.1. Experimental Site
The experiment was conducted during three seasons (2021–2023) in an experimental mature almond orchard at the Andalusian Institute of Agricultural and Fisheries Research and Training (IFAPA), located in Alcalá del Río, Seville (SW Spain). Trees were planted in 2016, with three representative almond cultivars (Marta, Guara, and Lauranne) for the study area in the Guadalquivir River basin, and all grafted onto GN15 rootstock. The trees are 6 × 7 m spaced, with drip irrigation using two pipelines with emitters of 2.3 L h−1. The soil is a typical clayey-loam texture Fluvisol [39], with a depth of more than 2.5 m, fertile, and a field capacity and permanent wilt point of 0.42 and 0.17 m3 m−3, respectively.
Climatic classification of the area is attenuated meso-Mediterranean, with an annual potential evapotranspiration (ET0) rate of 1400 mm and an accumulated rainfall of 540 mm (average data corresponding to the last 15 years; obtained from the Andalusian Weather Information Network).
2.2. Field Experimental Design
The experimental orchard was formed by four treatments based on a combination of two water-irrigation strategies and two soil-management systems, following a randomized complete block with four replications (Table 1). Accordingly, (i) fully irrigated (FI) trees were irrigated, covering 100% of the irrigation requirements (II.RR), and (ii) the regulated deficit irrigation (RDI) strategy involved irrigation while applying 80% of II.RR during the vegetative stage (from March to June) and post-harvest (from harvesting to the end of October, coinciding with the beginning of autumn rainfall). Additionally, during the kernel-filling stage (from June to mid-August or the beginning of September, just before harvesting), trees were irrigated, applying 40% of II.RR. Each irrigation strategy was subjected to two soil-management systems, which were performed as follows: (i) bare soil (BS), trying to keep the soil without spontaneous plants from February until foliage fall-over, applying two chemical mowing at the beginning of February and June, and (ii) the implementation of cover crop (CC) with a mixture of legumes and cereal (75% Vicia sativa L. + 25% Avena sativa L.), which was planted in autumn after the first rains and mechanically controlled at the beginning of April of the following year (Figure 1). On that date, the cover crop was mowed, and mulch was spread over the soil surface. Punctual herbicide applications were performed to avoid additional competition from spontaneous plants during the maximum water-demand period.

Table 1.
The experimental treatments based on the irrigation water strategies and soil-management systems used.

Figure 1.
Experimental plots with different soil-management systems: bare soils (A) and cover crop implementation (B).
Irrigation doses were calculated according to the methodology proposed by Allen et al. [40], obtaining the values of ET0 by using a weather station close to the experimental orchards (<500 m). The local crop coefficients used during the experimental period ranged from 0.4 to 1.2, according to García-Tejero et al. [41]. Overall, RDI receives ~50% of the irrigation water supply in FI.
Finally, routine almond cultivation techniques were used according to the regulations for integrated production for the study area (Andalusia, SW Spain). In this sense, the experimental orchard annually received NPK complex fertilizer (15-15-15, 250 kg ha−1) at pre-flowering, with additional nutrients incorporated until reaching NPK units around 80-45-100; finally, pest and disease products were applied when required.
2.3. Sampling, Chemical Determinations, and Microbiological and Enzymatic Activity Measurements
Table 2 summarizes the different samplings, the timing, and the analysis developed throughout the experimental period. Soil samples were taken for each study season: (i) approximately one month after plant cover mowing (mid-May, approximately); and (ii) and at the end of the season (early October), coinciding with the beginning of leaf pulling and before the autumn sowing of plant cover for the following season. In each sampling, four replications were taken for each treatment (n = 4) and at three different soil depths (0–10, 10–25, and 25–50 cm); the samples taken for each treatment were randomly distributed throughout the experimental plot.

Table 2.
Sampling, timing, and measurements performed.
Additionally, before the plant cover was mowed, four replications (n = 4) were taken at random points of the plot using a 0.5 × 0.5 m harrow to determine the accumulated dry matter of the cover crops and its potential for C fixation via above-ground biomass. In addition, coinciding with the beginning of the kernel-filling period, four leaf samples were taken in each almond cultivar and treatment. These samples were taken around the trees, ensuring they corresponded to mature and healthy leaves.
The macro- and micronutrients in leaves were determined in accordance with the standard methods for the examination of this type of sample [42]. The SOC was analyzed following the methodology proposed by Walkley and Black [43]. Additionally, microbial activity [the hydrolysis of fluorescein diacetate activity (FDA)] was determined, as indicated by Adam and Duncan [44]. Microbial biomass carbon was analyzed using the fumigation–extraction method [45,46]. Dehydrogenase activity was determined according to Thalmann [47], and the protease was described by Ladd and Butler [48]. Finally, alkaline phosphatase activity was analyzed following the Tabatabai and Bremmer [49] protocols, and β-glucosidase was measured according to Eivazi and Tabatabai [50].
During the irrigation period, measurements of stem water potential (Ψstem) were taken at fortnightly intervals at midday (12:00 solar time). For midday Ψstem readings, the standard procedure was bagging (aluminum foil-covered plastic bags) a leaf on the tree at least 15 min before sampling from monitored trees under different treatments; this allowed it to reach equilibrium. Then, the leaves were cut off and placed in a pressure chamber with the petiole protruding out. Concretely, Ψstem was measured using a pressure chamber (Soil Moisture Equipment Corp., Sta. Barbara, CA, USA) in four trees per cultivar and treatment using one leaf per tree (n = 4) in three phenological stages: vegetative (P-I), kernel-filling (P-II), and post-harvest (P-III).
At the end of each season, almond production was measured in terms of the in-shell yield. Harvesting operations were performed using a mechanical vibrator and peeling to remove the hull. Later, almonds were air-dried and weighed once they reached a humidity content below 6%. For this, almond samples per monitored tree were weighed. Once the kernel unit weight (with and without the shell) was known, kernel yield was estimated by calculating their ratio. Ultimately, the irrigation water productivity (IWP) was estimated annually and for the whole study period as the almond yield divided by the total seasonal irrigation water applied (kg m−3).
2.4. Statistical Analysis
Considering that all of the data were obtained in three consecutive seasons, year-to-year, an exploratory and descriptive analysis of all measurements (the nutritional status of leaves, soil measurements, and dry matter of cover crops) for each treatment was performed, checking the normality and homoscedasticity using XLSTAT Premium 2016 (Addinsoft, New York, NY, USA). After, a one-way analysis of variance (ANOVA) was performed to study the single effect of irrigation treatment and soil management and a two-way ANOVA for multiple effects, with the treatment means separated using the Tukey multiple range test at a 5% significance level. For each cultivar, the kernel yield and its components were analyzed year-to-year (kernel unit weight, the ratio between kernel weight vs. almond weight, and the IWP), applying an ANOVA with Tukey’s test, considering the irrigation treatments, the soil management, and the interactions between them as factors.
As all the data were obtained in three different seasons, in order to analyze the dataset for the three seasons (n = 12, 4 replicates per treatment × 3 seasons), dataset normalization for each treatment and cultivar was performed, following the Sterk and Stein [51] procedures. In this regard, this methodology minimizes the variability associated with the particular conditions in each season to group the values. It allows applying an overall analysis with the entire set of data (n = 12) according to the following equation:
where is the normalized variable for each treatment and cultivar, is the single value obtained for each replication, is the average value for a single treatment and cultivar in a year, and is the average value for a single treatment and cultivar during all the monitored seasons.
3. Results and Discussion
3.1. Crop Water Requirements, Irrigation Doses, Crop Water Status, and Leaf Nutrient Status
Table 3 presents the crop water requirements and irrigation doses applied during the study period. The FI strategy received irrigation doses between 6060 and 6310 m3 ha−1, while the RDI amounts were between 3020 and 3620 m3 ha−1, which was approximately 50% of the amount of water provided in the fully irrigated trees.

Table 3.
The rainfall, crop water requirements and evapotranspiration, and irrigation doses during the study period in each phenological stage.
According to the treatment design, the RDI-CC and RDI-BS plots received the same irrigation doses, the only difference being the presence/absence of plant covers. The largest water withholdings in RDI were applied during the kernel-filling period, as was designed for this strategy at the beginning of the experiment. In this stage, during the first year, the RDI received 38% of the irrigation requirements (II.RR.), while during the second and third years of the study, the water allocations in that period were about 35% of the total II.RR.
In addition, taking advantage of the early spring rains, the RDI strategy was not subjected to stress situations during vegetative growth, except for the third year of the study, when the rainfall recorded in that period was very low. During the post-harvest period (from mid-August to the end of October) in the first year, there was hardly any stress in the RDI, while in the second and third seasons, this irrigation strategy amounted to 58 and 74% of II.RR. Thus, analyzing the whole dataset, the RDI received during the fruit-growth, kernel-filling, and post-harvest stages amounted to 78, 36, and 70% of the II.RR., respectively, very close to the irrigation strategy initially designed.
Figure 2 shows the average values of Ψstem for the three studied seasons in each cultivar and treatment. The greatest differences were found during the kernel-filling period, which were observed for all almond cultivars. Additionally, the presence of plant cover increased these differences; therefore, the lowest Ψstem were detected mainly in RDI-CC plots, with mean values between −1.5 and −1.7 MPa for all almond cultivars. In contrast, CC did not seem to affect the crop water status in the FI plot and, in the case of the FI-CC, improved these values with respect to the same treatment under FI-BS (−0.68 vs. −1.31 MPa in cv. Guara, −1.21 vs. −1.33 MPa in cv. Marta, and −1.37 vs. −1.42 MPa in cv. Lauranne). This fact was perceptible, and the decrease in soil water content induced by RDI could be triggered in extreme situations, decreasing the Ψstem in almond trees below −2 MPa and stomatal conductance at 0.15 mol m−2 s−1, as was outlined by Sperling et al. [52].
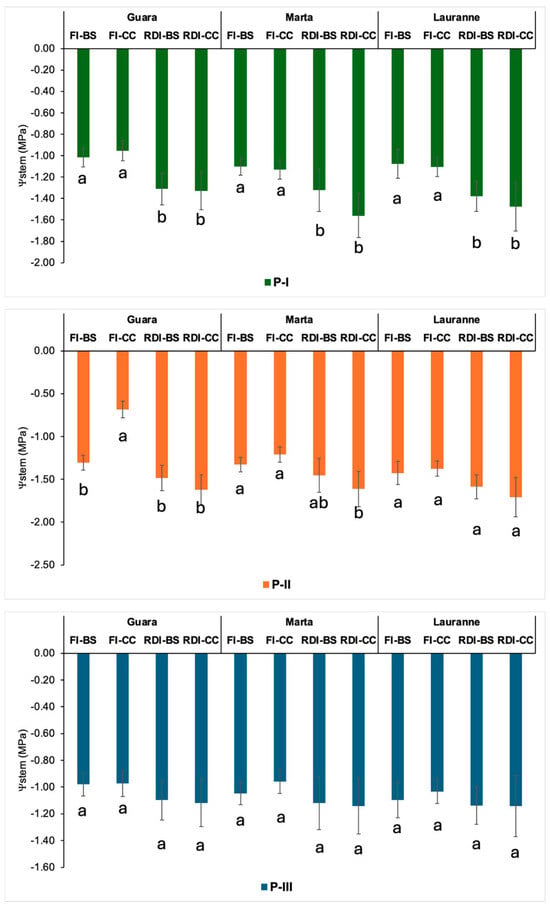
Figure 2.
The stem water potential dynamics for each treatment and phenological stage in almond cultivars. FI, full irrigation; RDI, regulated deficit irrigation; BS, bare soil; CC, cover crop. Each value corresponds to the average for the three studied seasons (n = 12). P-I, P-II, and P-III are the three main phenological stages (vegetative, kernel-filling, and post-harvest, respectively). Vertical bars are the standard error. Different letters represent significant treatment differences (p < 0.05) within each phenological period.
Throughout the vegetative growth period, even though no major irrigation restrictions were applied in RDI compared to FI, there were significant differences in the crop water status for the three cultivars. Also, throughout the post-harvest period, no significant differences were recorded among treatments in any of the monitored cultivars, showing that, at least during this period, the irrigation restrictions were not reflected, at least in terms of Ψstem, probably because of the lower crop water uptake because of the leaves’ progressive senescence.
In relation to the mineral content in leaves, no significant effects were determined for the different treatments. Concretely, the average values of macronutrients (NPK) were similar in the four treatments, comparably observed for micronutrients (Fe, Cu, Zn, and Mn) (Table 4). Minor differences were observed in the Na levels, which were significantly higher for RDI-CC in cv. Guara in comparison to the remaining treatments. With this same logic, higher levels of Ca were denoted in RDI with respect to FI, occurring in the three studied almond cultivars, whereas higher levels in RDI were found for cv. Lauranne in comparison to FI plots for the case of Mg. However, Ca, Mg, and Na are elements highly related to the crop response under drought conditions, and their increase agrees with the presence of an osmoregulatory response when stomatal conductance regulation is low, as is the case for almonds, this being considered an anhysohidric species [53].

Table 4.
Leaf mineral content for each water soil treatment.
Thus, in general, the potential competition for nutrients by plant covers that could alter the mineral nutrition of the main crop did not occur under our experimental conditions. This was presumably impossible due to the surplus of nutrient inputs associated with important amounts of the mineral fertilizers applied. However, the indisputable advantage attributable to plant cover is that they can regulate these nutrient surpluses by storing and recycling them into their tissues and, at the same time, releasing them from residues in a natural biological cycle, which is absent in bare soil.
3.2. Cover Crop Dry Mass Production and Atmospheric Carbon Fixation
The treatments based on combination of plant covers with both irrigation strategies and its effect on amount of dry mass generated was marked by the rainfall amount during the months after planting, and not so much with the irrigation supply (Figure 3). That is, during the 2021 season, with a cover crop sown in autumn 2020, the average dry mass production was of 6000 and 5000 kg ha−1 in the FI and RDI strategies, respectively. Likewise, during the subsequent seasons, dry matter production by the cover crop was about 1400 and 1800 kg ha−1 in 2022, and 2400 and 2100 kg ha−1 in 2023 for the FI and RDI plots, respectively, with no significant differences in any of monitored seasons. It should be noted that, during 2021, the rainfall recorded from planting the cover crop to harvesting amounted to 323 mm, while during the second and third seasons, this rainfall decreased to 255 and 204 mm, respectively, which would partly explain the lower production of biomass in these seasons.
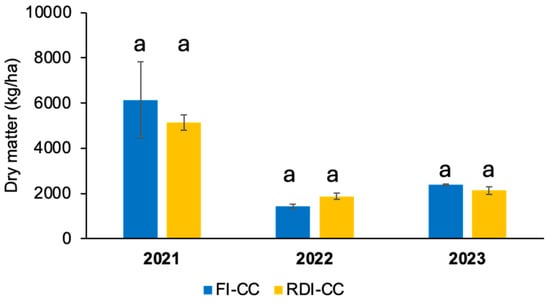
Figure 3.
Average dry matter from plant covers under each irrigation strategy and monitored season for each year. FI, full irrigation; RDI, regulated deficit irrigation; CC, cover crop. Each value corresponds to the average for each treatment and season (n = 4). Vertical bars are the standard error. Different letters represent significant differences among treatments (p < 0.05).
These results of dry matter from plant covers correspond to C fixation of 38.4 and 40% for FI-CC and RDI-CC plots, respectively, which imply a C sequestration rate of 1.2 t per hectare for both treatments, evidencing the advantage of this combination for the encouragement of agricultural activity as a climate change-mitigating activity. In this sense, for a Mediterranean environment, the cover crops contributed significantly to CO2 reduction and recycling of free-unused plant nutrients in woody fruit plantations [54].
Although the purpose of this work was not to elucidate the mechanism of plant cover-induced atmospheric C sequestration nor to appraise C balance in our production system, it was evident that above-ground plant C inputs partly explain the determined soil C gains. When considering the designed treatments, it was clear that the soil C gains and generation of biomass via C fixation were manifested in the plots with plant covers compared to those that were not grown. Therefore, implementing policies that compensate farmers for sequestering C may offset the losses in profits associated with plant cover use and provide incentives for its adoption.
3.3. Soil Organic Carbon, Microbiological Biomass, and Enzymatic Activity
Figure 4 shows the SOC and soil microbiological biomass carbon (MBC) contents for each treatment at different soil depths and during early spring and late autumn periods. The highest levels of SOC were determined, especially at the first 10 cm soil depth, following the same trend with higher contents in CC treatments than BS, with a slight increase for autumn samples with respect to the spring ones. In this line, Madejón et al. [55] stated that for a large range of soil types and environmental conditions, no-tillage operations and plant covers can effectively increase SOC rates and foster more favorable conditions, particularly in the upper soil layers. Consequently, an increase in SOC could also encourage an improvement in the water retention capacity in soils [56]. In contrast, for remaining soil depths, a declining pattern for FI-CC and FI-BS plots was found, particularly in the deeper soil layers (25–50 cm). In addition, higher SOC contents were determined in RDI-CC compared to FI-CC at 10–25 and 25–50 cm soil depths, which could be compatible with less organic matter mineralization in the most water-deficient conditions. This fact concorded with the findings of Núñez and Schipanski [57], who reported that water irrigation augments SOC contents in semi-arid agroecosystems. Finally, the amount of SOC stored in the surface soil highly depends on the cumulative effect of plant covers. In this context, Poeplau and Don [58] reported that the C inputs to the soil undergo various transformations depending on the turnover time of the plant residues, and a fraction of the added soil C remains in a stable organic form and contributes to SOC accumulation in the long term.
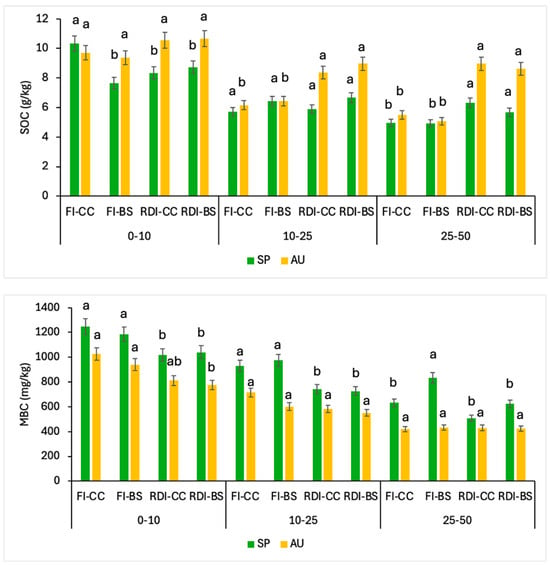
Figure 4.
The soil organic carbon (SOC) and microbial biomass carbon (MBC) contents at three soil depths (0–10, 10–25, and 25–30 cm) in early spring (SP) and late autumn (AU) for each monitored treatment. Data correspond to the average of the three years (n = 12). FI, full irrigation; RDI, regulated deficit irrigation; BS, bare soil; CC, cover crop. Vertical bars are the standard error. Different letters show significant differences (p < 0.05) among treatments within each soil depth and sampling time.
Soil MBC is widely recognized as the main force driving the decomposition of organic matter and is usually used as an early indicator of changes in soil parameters resulting from soil-management systems. Concretely, the MBC contents for the first two sampling depths (0–10 and 10–25 cm) were higher under full irrigation (FI-CC and FI-BS) plots than water deficit irrigation ones (RDI-CC and RDI-BS). These data align with the greater contents of SOC, as discussed before (Figure 5). Also, this agrees with the results found by Melero et al. [59], highlighting the strong correlation between SOC and MBC in soils. In addition, higher SOC rates in the autumn sampling corresponded to a lower MBC content, the exact opposite of the trend that took place in the spring sampling. Similarly, Lepcha and Devi [60] indicated that land use, soil depth, and season significantly influenced the microbial biomass carbon.
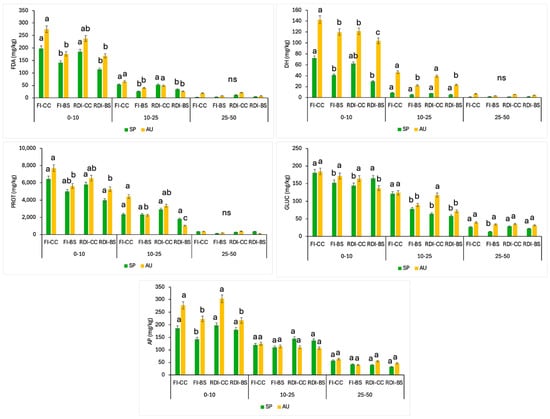
Figure 5.
The levels of fluoresceine diacetate (FDA), dehydrogenase (DH), protease (PROT), glucosidase (GLUC), and alkaline phosphatase (AP) activities at different soil depths (0–10, 10–25, and 25–30 cm) in early spring (SP) and late autumn (AU) for each monitored treatment. Data correspond to the average of the three years (n = 12). FI, full irrigation; RDI, regulated deficit irrigation; BS, bare soil; CC, cover crop. Vertical bars are the standard error. Different letters show significant differences (p < 0.05) among treatments within each soil depth and sampling time.
Overall, the presence or absence of plant covers did not seem to provoke important effects on SOC and MBC levels, and these values were impacted more by the irrigation supply; therefore, according to the findings, water-deficit scenarios promote a lower SOC mineralization capacity and a lower microbial biomass rate. In this sense, Wang and Klassen [61] determined that different irrigation doses did not cause significant changes in soil MBC, but the cover crops increased the soil microbiological biomass through the decomposition of organic C, especially with legume plants. Finally, according to Naylor and Coleman-Derr [62] or Schimel [63], water scarcity can determine the levels of SOC, affecting the retention and organic matter mineralization of agricultural ecosystems acting as potential carbon sinks. Recent works have demonstrated that higher soil moisture content is directly related to higher soil respiration rates and, hence, a higher presence of biological activity is attributable to carbon released [64,65,66]. The findings of the present experiment are in line with those reported by these authors, registering lower MBC levels and soil enzymatic activity under deficit soil water contents in RDI plots.
Figure 5 displays the levels of the main enzymatic activities registered in the spring and autumn samplings at different soil depths for each of the studied treatments. The results showed significantly higher levels of fluorescein (FDA) activity in the treatments with cover crops (FI-CC and RDI-CC), mainly at a 10 cm soil depth, for both spring and autumn samplings, with no significant effects derived from the irrigation strategies applied. With an increasing soil depth, the FDA was significantly reduced in all treatments. Similarly, Chavarría et al. [67] highlighted that soil enzyme activities was enhanced with plant cover inclusion, particularly for FDA and dehydrogenase activity up to 35.3 and 38.1%, respectively, higher than the control treatment.
In relation to dehydrogenase activity, higher levels were fixed in the autumn period, with rate improvements in treatments with plant covers. This activity was singularly relevant until the 25 cm soil depth, with negligible values for soil samples between a 25 and 50 cm depth. Similarly, Mikanová et al. [68] found that dehydrogenase activity was higher in soil layers of 0–15 cm with no tillage, decreasing with deeper soil layers. The irrigation and, therefore, higher soil water content can raise the soil dehydrogenase activity, according to Brzezinska et al. [69,70], as was determined in our experiment. However, no important alterations due to cover crops were reported by Nivelle et al. [71]. Among soil enzymes, dehydrogenase is considered a direct measure of soil microbial activity, indicating the level as a biological indicator of soil health [72], which was evident in the present study.
On the other hand, the protease activity showed significantly higher values in the treatments with plant covers. In this case, the differences were smaller than those observed in the case of dehydrogenase activity. The protease activity was imperceptible at a 25–50 cm soil depth, contrasting with the levels found until a 25 cm soil depth for all treatments. The findings of the present experiment coincide with the study by Roldán et al. [73], who reported that no-till soil and legume cover significantly enhanced the soil enzyme activities (protease, dehydrogenase, β-glucosidase, and acid phosphatase, among others), which agrees singularly with the FI-CC plots at upper soil layers. The protease activity, like any other enzyme activity, depends on the soil type and decreases with the soil depth due to a reduction in organic matter content and microbial abundance [74], which is in line with the organic carbon content found in deeper soil layers in the present experiment.
The β-glucosidase activity registered the highest values for FI-CC, with declines for FI-BS and treatments with RDI strategies. A similar pattern was registered for alkaline phosphatase, with the highest values recorded at a 10 cm soil depth, with significant improvements in both FI-CC and RDI-CC plots. The β-glucosidase activity was limited due to decreased soil moisture [75], as was denoted in plots under the RDI strategy, and depended on the soil depth [76]. In this sense, Acosta et al. [77] revealed that β-glucosidase activity decreases with soil depth, greatly depending on substrate supply and the microorganisms that produce this enzyme, which are active in the topsoil [78]. In relation to the impact of plant covers, Sinsabaugh et al. [79] stated that the no-till treatment augmented β-glucosidase activity due to enhanced microbial biomass, greater substrate availability, and reduced soil disturbance combined with cover crops, as was designed in the present study. Makoi et al. [80] outlined that leguminous plants release more phosphatase enzymes than non-leguminous plants, and both protease and phosphatase activities significantly improved after two seasons of minimum soil disturbance [81,82].
Overall, the plant covers, with their root systems, can encourage the development of porosity and infiltration, thus enabling the root zone to act as a partial sink for irrigation water (FI or RDI) applied, especially at upper soil layers. This fact increases the soil water content, which strongly influences the soil’s microbial activity, community composition, and, consequently, enzymatic activities, as was pointed out by Ramos et al. [38] and Geisseler et al. [83]. This differs from non-irrigated areas, where, as soils dry, the water potential declines, and microbial activity decreases as enzyme activity slows.
3.4. Almond Yield and Its Components
Table 5 shows the almond yield in response to different treatments during the study period. According to the average data, the cv. Guara showed yield losses associated with the irrigation strategy but not with plant cover, denoting no worsening effects due to its implementation in the RDI strategy and with improvements in the case of the FI-CC plots, amounting to 1470 kg ha−1.

Table 5.
Almond production and its components and irrigation water productivity for the studied seasons under each water soil treatment.
Regarding the cv. Marta almonds, no effects were found in yield due to either the irrigation or the plant cover presence. This suggests the feasibility of combining the irrigation strategy (RDI) and soil-management system (CC) in fostering sustainable and competitive almond production. Concretely, the cv. Marta in RDI-CC plots averaged a yield of 1685 kg ha−1, which is practically similar to FI-CC plots, with 1618 kg ha−1, but with 50% irrigation water savings. In contrast, the cv. Lauranne showed significant effects in yield terms due to water stress and plant cover, with an even more significant reduction in RDI-CC with respect to RDI-BS plots (1332 vs. 1479 kg ha−1).
By analyzing the data year by year, during the first (2021), the cv. Guara displayed significant yield losses in the RDI compared to FI plots, registering the highest production values for FI-CC. In the case of the cv. Marta, the most relevant production values were registered in RDI-CC, followed by FI-CC treatments, while the cv. Lauranne in the FI-CC had the best production values. Meanwhile, the lowest values were determined in RDI-CC plots.
In the second season in 2022, the cv. Guara again yielded the highest values in the FI-CC trees, with very significant increases in relation to the previous season. The cv. Marta also showed very considerable improvements in production values during this season, with no differences observed among the treatments. In relation to the cv. Lauranne, despite fixing significant yield increases, the RDI again showed important reductions compared to FI plots without transcendent effects due to the implementation of plant covers.
During the third year, a generalized decrease in yield was found in cvs. Guara and Lauranne with respect to the previous year, probably due to the alternate and irregular bearing of almond trees that could have generated important annual production fluctuations. The lowest production values for cv. Guara were recorded in RDI-BS (1372 kg ha−1), while those for the cv. Marta were recorded in FI-CC (1709 kg ha−1) plots. Regarding the cv. Lauranne, no differences were found among treatments either due to the irrigation dose or soil-management system. Thus, according to the findings, the implementation of plant covers was compatible with RDI strategies without provoking important negative effects on yield in the medium term, even with improvements in the case of scenarios without water restriction. In general, our findings agree with those highlighted by Stewart et al. [84] in relation to the absence of important yield reductions due to the effect of RDI strategies. Many studies have reported almond yield reductions in response to deficit irrigation strategies [26,32,52,85]. Particularly, Moldero et al. [86] outlined that a shift from moderate RDI (65% ETc) to severe RDI (30% ETc) lessened yield, affecting the fruit load more than kernel weight. In this sense, Girona et al. [87] stated that a decline of 30% in irrigation water reduced the fruits per tree but not the kernel dry weight.
In relation to almond yield components, a significant effect of treatments on grain weight was determined. That is, for cv. Guara, the lowest values were recorded in RDI-CC (1.20 g), as in the case of the cv. Lauranne (1.13 g), while for cv. Marta, the decrease in grain weight was similar to that of the RDI strategy (~1.28 g). Regarding the weight ratio, only the cv. Marta showed significant effects that were associated with irrigation, reaching the lowest value of 0.24, with no significant effects with respect to the remaining studied cultivars.
On the other hand, a significant increase in IWP was determined in the RDI for all almond cultivars ranging from 0.34 to 0.53 kg m−3, which highlighted the advantages of RDI strategies to face scenarios of water scarcity. In contrast, the FI strategy recorded values between 0.21 and 0.29 kg m−3 for three almond cultivars. Thus, there is potential for improving IWP by implementing RDI, as there are many cases of the successful use of this strategy in almond plantations, showing that it not only augments water productivity but also farmers’ incomes [28,87,88]. Although there is a risk of competition for available soil water resources that could affect almond productivity, the plant covers can potentially provide improvements in rainwater infiltration and changes in root absorption zones, benefiting the tree’s root exploration of deeper soil layers due to contention with them [89,90,91].
4. Conclusions
According to the findings of the present experiment, implementing cover crops in irrigated almond plantations based on moderate water stress does not represent an important disadvantage for the crop in nut yield terms, singularly, for the cv. Marta, which showed regular productivity. The water-stressed trees of the cv. Guara and cv. Lauranne had lower yields than fully irrigated trees, although the latter was more productive among the studied cultivars. That is, the 50% reduction in irrigation water due to regulated deficit irrigation with respect to fully irrigated trees should be adopted as the most appropriate irrigation strategy for achieving sustainable water management in almond orchards.
The water status of the crop was not significantly affected by the vegetation covers in the fully irrigated plots, except for the slightly higher effect due to the combination of deficit irrigation and the presence of the covers in plots with water-stressed trees.
The deficit-irrigation strategy seems to decrease the levels of biological activity, which slows down the mineralization of organic matter, thus decreasing carbon emissions from soil microbiological respiration. The plant covers positively impacted soil organic storage, microbial processes, and enzymatic activities ascribed to changes in the quantity and quality of the plant residues that enter the soil matrix; changes in the provision of nutrients; and physical alterations. This process was more evident in the upper soil layers without irrigation restrictions, which could tend to improve prominent aspects of soil health restoration. Overall, soil enzymes catalyze and foster decomposition and plant nutrient cycling; thus, their activities are a biological sign of enhancing soil quality. Additionally, soil enzymes reacted to soil-management systems based on cover crop implementation long before other soil quality changes were detectable. Thus, the cover crops in irrigated orchards improved soil functionality and provided the possibility of protecting the natural resources of water and soil in the context of climate change.
These findings indicate the possibility of undertaking new work aimed at studying the predominant biological communities in water-stress scenarios, as well as the development of carbon balances that allow effective quantification of the effects of deficit irrigation combined with different plant covers with various soil types, cultivars, and weather scenarios over a longer period.
Author Contributions
Conceptualization, I.F.G.-T., J.F.H.-G. and A.C.-P.; methodology, I.F.G.-T. and J.F.H.-G.; validation, I.F.G.-T., J.F.H.-G. and V.H.D.Z.; formal analysis, I.F.G.-T., J.F.H.-G., V.H.D.Z. and A.E.R.-C.; investigation, I.F.G.-T., A.C.-P., J.F.H.-G. and A.E.R.-C.; resources, I.F.G.-T., J.F.H.-G., J.A.N. and A.C.-P.; data curation, I.F.G.-T., J.F.H.-G., A.C.-P., B.C.R. and V.H.D.Z.; writing—original draft preparation, I.F.G.-T., J.F.H.-G., B.C.R., V.H.D.Z. and A.E.R.-C.; writing—review and editing, I.F.G.-T., J.F.H.-G., B.C.R. and V.H.D.Z.; visualization, I.F.G.-T., J.F.H.-G., B.C.R. and V.H.D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was partially sponsored by the following research project: strategies to improve the adaptation of almond cultivation to different scenarios of water scarcity and management systems “NUTRESILIENCE” (AVA23.INV202301.004), co-financed by the European Regional Development Fund (ERDF) within the Operational Programme 2021/2027.
Data Availability Statement
The data are contained within this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zagaria, C.; Schulp, C.J.E.; Malek, Ž.; Verburg, P.H. Potential for land and water management adaptations in Mediterranean croplands under climate change. Agric. Syst. 2023, 205, 103586. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Durán, Z.V.H. Water Scarcity and Sustainable Agriculture. In Semiarid Environment. Tools, Strategies, and Challenges for Woody Crops; Academic Press: London, UK, 2018; p. 624. [Google Scholar]
- del Pozo, A.; Brunel-Saldias, N.; Engler, A.; Ortega-Farias, S.; Acevedo-Opazo, C.; Lobos, G.A.; Jara-Rojas, R.; Molina-Montenegro, M.A. Climate Change Impacts and Adaptation Strategies of Agriculture in Mediterranean-Climate Regions (MCRs). Sustainability 2019, 11, 2769. [Google Scholar] [CrossRef]
- Cárceles, R.B.; Durán, Z.V.H.; Soriano, R.M.; García-Tejero, I.F.; Gálvez, R.B.; Cuadros, T.S. Conservation agriculture as a sustainable system for soil health: A review. Soil Syst. 2022, 6, 87. [Google Scholar] [CrossRef]
- Hossard, L.; Blanc, L.; Lambarraa-Lehnhardt, F.; Dordas, C.; Papakaloudis, P.; Michalitsis, A.; Lampurlanes, J.; Latati, M.; Touama, R.; Kherif, O.; et al. Co-design of diversified cropping systems in the Mediterranean area. Eur. J. Agron. 2024, 153, 127050. [Google Scholar] [CrossRef]
- Reckling, M.; Watson, C.A.; Whitbread, A.; Helming, K. Diversification for sustainable and resilient agricultural landscape systems. Agron. Sustain. Dev. 2023, 4, 44. [Google Scholar] [CrossRef]
- Hellin, J.; Fisher, E.; Taylor, M.; Bhasme, S.; Loboguerrero, A.M. Transformative adaptation: From climate-smart to climate-resilient agriculture. CABI Agric. Biosci. 2023, 4, 30. [Google Scholar] [CrossRef]
- IEEP. CAP 2021-27: Proposals for Increasing Its Environmental and Climate Ambition. 2018. Available online: https://ieep.eu/wp-content/uploads/2022/12/NABU-CAP-Report-FINAL-.pdf (accessed on 8 July 2024).
- García-Tejero, I.F.; Gordillo, S.G.; Souza, L.; Cuadros-Tavira, S.; Zuazo, V.H.D. Fostering sustainable water use in almond (Prunus dulcis Mill.) orchards in a semiarid Mediterranean environment. Arch. Agron. Soil Sci. 2018, 65, 164–181. [Google Scholar] [CrossRef]
- Rodríguez, B.C.; Zuazo, V.H.D.; Rodríguez, M.S.; Ruiz, B.G.; García-Tejero, I.F. Soil Erosion and the Efficiency of the Conservation Measures in Mediterranean Hillslope Farming (SE Spain). Eurasian Soil Sci. 2021, 54, 792–806. [Google Scholar] [CrossRef]
- Ibarrola-Rivas, M.; Granados-Ramírez, R.; Nonhebel, S. Is the available cropland and water enough for food demand? A global perspective of the Land-Water-Food nexus. Adv. Water Resour. 2017, 110, 476–483. [Google Scholar] [CrossRef]
- Tójar-Hurtado, J.-C.; Mena-Rodríguez, E.; Fernández-Jiménez, M. Spanish Agriculture and Water: Educational Implications of Water Culture and Consumption from the Farmers’ Perspective. Water 2017, 9, 964. [Google Scholar] [CrossRef]
- EC. European Commission, Proposal for a Regulation of the European Parliament and the Council Establishing Rules on Support for Strategic Plans to be Drawn up by Member States under the Common Agricultural Policy (CAP Strategic Plans). COM/2018/392 Final. 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2018%3A392%3AFIN (accessed on 8 July 2024).
- EC. European Commission. The European Green Deal Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions COM (2019) 640 Final. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN (accessed on 8 July 2024).
- EC. European Commission 2023, Horizon Europe Work Programme (2023–24)—EU Missions European Commission Decision C (2023) 2178 of 31 March 2023. 2023. Available online: https://ec.europa.eu/info/funding-tenders/opportunities/docs/2021-2027/horizon/wp-call/2023-2024/wp-1-general-introduction_horizon-2023-2024_en.pdf (accessed on 9 July 2024).
- MAPA. Ministerio de Agricultura, Pesca y Alimentación, Plan Estratégico de la PAC de España 2023-2027. Resumen del Plan aprobado por la Comisión Europea. 2023. Available online: https://www.mapa.gob.es/es/pac/pac-2023-2027/divulgacion-del-plan.aspx (accessed on 5 June 2024).
- Durán, Z.V.H.; Rodríguez, P.C.R.; Francia, M.J.R.; Martínez, R.A.; Arroyo, P.L.; Cárceles, R.B.; Navarro, M.M.C. Benefits of plant strips for sustainable mountain agriculture. Agron. Sustain. Dev. 2008, 28, 497–505. [Google Scholar] [CrossRef]
- Repullo-Ruibérriz de Torres, M.A.; Moreno, G.M.; Ordóñez, F.R.; Rodríguez, L.A.; Cárceles, R.B.; García-Tejero, I.F.; Durán, Z.V.H.; Carbonell, B.R.M. Cover Crop Contributions to Improve the Soil Nitrogen and Carbon Sequestration in Almond Orchards (SW Spain). Agronomy 2021, 11, 387. [Google Scholar] [CrossRef]
- Durán, Z.V.H.; Cárceles, R.B.; García-Tejero, I.F.; Gálvez, R.B.; Cuadros, T.S. Benefits of organic olive rainfed systems to control soil erosion and runoff and improve soil health restoration. Agron. Sustain. Dev. 2020, 40, 41. [Google Scholar] [CrossRef]
- Cárceles, R.B.; Durán, Z.V.H.; Herencia, G.J.F.; Lipan, L.; Soriano, M.; Hernández, F.; Sendra, E.; Carbonell, B.Á.A.; Gálvez, R.B.; García-Tejero, I.F. Soil Management Strategies in Organic Almond Orchards: Implications for Soil Rehabilitation and Nut Quality. Agronomy 2023, 13, 749. [Google Scholar] [CrossRef]
- Bossio, D.A.; Cook-Patton, S.C.; Ellis, P.W.; Fargione, J.; Sanderman, J.; Smith, P.; Wood, S.; Zomer, R.J.; von Unger, M.; Emmer, I.M.; et al. The role of soil carbon in natural climate solutions. Nat. Sustain. 2020, 3, 391–398. [Google Scholar] [CrossRef]
- Soares, P.R.; Harrison, M.T.; Kalantari, Z.; Zhao, W.; Ferreira, C.S.S. Drought effects on soil organic carbon under different agricultural systems. Environ. Res. Commun. 2023, 5, 112001. [Google Scholar] [CrossRef]
- Iglesias, A.; Garrote, L. Local and collective actions for adaptation to use less water for agriculture in the mediterranean region. In Water Scarcity and Sustainable Agriculture in Semiarid Environment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 73–84. [Google Scholar]
- Tejero, I.F.G.; Duran-Zuazo, V.H. Optimizing Plant Water Use Efficiency for a Sustainable Environment; MDPI AG: Basel, Switzerland, 2022; p. 366. [Google Scholar] [CrossRef]
- ESYRCE. Encuesta Sobre Superficies y Rendimientos Cultivos (ESYRCE). 2023. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/esyrce/ (accessed on 17 May 2024).
- García-Tejero, I.F.; Lipan, L.; Gutiérrez-Gordillo, S.; Zuazo, V.H.D.; Jančo, I.; Hernández, F.; Rodríguez, B.C.; Carbonell-Barrachina, A. Deficit Irrigation and Its Implications for HydroSOStainable Almond Production. Agronomy 2020, 10, 1632. [Google Scholar] [CrossRef]
- Gutiérrez-Gordillo, S. New Approaches to Hydrosustainable Almonds Production: Agronomical, Physiological and Quality Effects. Ph.D. Thesis, Universidad de Sevilla, Sevilla, Spain, 2022. [Google Scholar]
- Goldhamer, D.A.; Viveros, M.; Salinas, M. Regulated deficit irrigation in almonds: Effects of variations in applied water and stress timing on yield and yield components. Irrig. Sci. 2006, 24, 101–114. [Google Scholar] [CrossRef]
- Moldero, D.; López-Bernal, Á.; Testi, L.; Lorite, I.J.; Fereres, E.; Orgaz, F. Almond responses to a single season of severe irrigation water restrictions. Irrig. Sci. 2021, 40, 1–11. [Google Scholar] [CrossRef]
- López-López, M.; Espadafor, M.; Testi, L.; Lorite, I.J.; Orgaz, F.; Fereres, E. Water use of irrigated almond trees when subjected to water deficits. Agric. Water Manag. 2018, 195, 84–93. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Gonzalez-Dugo, V.; García-Tejero, I.F.; López-Urrea, R.; Intrigliolo, D.S.; Egea, G. Quantitative analysis of almond yield response to irrigation regimes in Mediterranean Spain. Agric. Water Manag. 2023, 279, 108208. [Google Scholar] [CrossRef]
- Egea, G.; Nortes, P.A.; Domingo, R.; Baille, A.; Pérez-Pastor, A.; González-Real, M.M. Almond agronomic response to long-term deficit irrigation applied since orchard establishment. Irrig. Sci. 2013, 31, 445–454. [Google Scholar] [CrossRef]
- de Oliveira, A.F.; Mameli, M.G.; De Pau, L.; Satta, D. Almond Tree Adaptation to Water Stress: Differences in Physiological Performance and Yield Responses among Four Cultivar Grown in Mediterranean Environment. Plants 2023, 12, 1131. [Google Scholar] [CrossRef]
- Egea, G.; González-Real, M.M.; Baille, A.; Nortes, P.A.; Sánchez-Bel, P.; Domingo, R. The effects of contrasted deficit irrigation strategies on the fruit growth and kernel quality of mature almond trees. Agric. Water Manag. 2009, 96, 1605–1614. [Google Scholar] [CrossRef]
- Alcon, F.; Egea, G.; Nortes, P.A. Financial feasibility of implementing regulated and sustained deficit irrigation in almond orchards. Irrig. Sci. 2013, 31, 931–941. [Google Scholar] [CrossRef]
- Mañas, F.; López-Fuster, P.; López-Urrea, R. Effects of Different Regulated and Sustained Deficit Irrigation Strategies in Almond Production. Acta Hortic. 2014, 1028, 391–394. [Google Scholar] [CrossRef]
- Rubio-Asensio, J.S.; Abbatantuono, F.; Ramírez-Cuesta, J.M.; Hortelano, D.; Ruíz, J.L.; Parra, M.; Martínez-Meroño, R.M.; Intrigliolo, D.S.; Buesa, I. Effects of Cover Crops and Drip Fertigation Regime in a Young Almond Agroecosystem. Agronomy 2022, 12, 2606. [Google Scholar] [CrossRef]
- Ramos, M.E.; Benítez, E.; García, P.A.; Robles, A.B. Cover crops under different managements vs. frequent tillage in almond orchards in semiarid conditions: Effects on soil quality. Appl. Soil Ecol. 2010, 44, 6–14. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources; World Soil Resources; Report 84; FAO: Rome, Italy, 1998. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration. FAO Irrigation and Drainage Paper; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 1998; Volume 56, p. 156. [Google Scholar]
- García-Tejero, I.F.; Hernandez, A.; Rodriguez, V.M.; Ponce, J.R.; Ramos, V.; Muriel, J.L.; Durán, Z.V.H. Estimating almond crop coefficients and physiological response to water stress in semiarid environments (SW Spain). J. Agric. Sci. Technol. 2015, 17, 1255–1266. [Google Scholar]
- MAPA. Ministerio De Agricultura Pesca y Alimentación. Métodos Oficiales De Análisis; Ministerio de Agricultura Pesca y Alimentación: Madrid, Spain, 1994. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining organic carbon in soils: Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1934, 63, 251–263. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Brookes, P.; Landman, A.; Pruden, G.; Jenkinson, D. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Thalmann, A. Methodology for determining the dehydrogenase activity in soil using triphenyltetrazolium chloride (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. (In German) [Google Scholar]
- Ladd, J.; Butler, J. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Sterk, G.; Stein, A. Mapping Wind-Blown Mass Transport by Modeling Variability in Space and Time. Soil Sci. Soc. Am. J. 1997, 61, 232–239. [Google Scholar] [CrossRef]
- Sperling, O.; Gardi, I.; Ben-Gal, A.; Kamai, T. Deficit irrigation limits almond trees’ photosynthetic productivity and compromises yields. Agric. Water Manag. 2023, 289, 108562. [Google Scholar] [CrossRef]
- Álvarez-Maldini, C.; Acevedo, M.; Estay, D.; Aros, F.; Dumroese, R.K.; Sandoval, S.; Pinto, M. Examining physiological, water relations, and hydraulic vulnerability traits to determine anisohydric and isohydric behavior in almond (Prunus dulcis) cultivars: Implications for selecting agronomic cultivars under changing climate. Front. Plant Sci. 2022, 13, 974050. [Google Scholar] [CrossRef]
- Torrús-Castillo, M.; Domouso, P.; Herrera-Rodríguez, J.M.; Calero, J.; García-Ruiz, R. Aboveground Carbon Fixation and Nutrient Retention in Temporary Spontaneous Cover Crops in Olive Groves of Andalusia. Front. Environ. Sci. 2022, 10, 868410. [Google Scholar] [CrossRef]
- Madejón, E.; Murillo, J.; Moreno, F.; López, M.; Arrue, J.; Alvaro-Fuentes, J.; Cantero, C. Effect of long-term conservation tillage on soil biochemical properties in Mediterranean Spanish areas. Soil Tillage Res. 2009, 105, 55–62. [Google Scholar] [CrossRef]
- Rawls, W.; Pachepsky, Y.; Ritchie, J.; Sobecki, T.; Bloodworth, H. Effect of soil organic carbon on soil water retention. Geoderma 2003, 116, 61–76. [Google Scholar] [CrossRef]
- Núñez, A.; Schipanski, M. Changes in soil organic matter after conversion from irrigated to dryland cropping systems. Agric. Ecosyst. Environ. 2023, 347, 108392. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops—A meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- Melero, S.; Madejón, E.; Ruiz, J.C.; Herencia, J.F. Chemical and biochemical properties of a clay soil under dryland agriculture system as affected by organic fertilization. Eur. J. Agron. 2007, 26, 327–334. [Google Scholar] [CrossRef]
- Lepcha, N.T.; Devi, N.B. Effect of land use, season, and soil depth on soil microbial biomass carbon of Eastern Himalayas. Ecol. Process. 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Wang, Q.R.; Li, Y.C.; Klassen, W. Changes of Soil Microbial Biomass Carbon and Nitrogen with Cover Crops and Irrigation in a Tomato Field. J. Plant Nutr. 2007, 30, 623–639. [Google Scholar] [CrossRef]
- Naylor, D.; Coleman-Derr, D. Drought Stress and Root-Associated Bacterial Communities. Front. Plant Sci. 2018, 8, 2223. [Google Scholar] [CrossRef]
- Schimel, J.P. Life in Dry Soils: Effects of Drought on Soil Microbial Communities and Processes. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 409–432. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, X.; Ling, Q.; Li, H.; Gao, X. Revegetation modifies patterns of temporal soil respiration responses to extreme-drying-and-rewetting in a semiarid ecosystem. Plant Soil 2018, 433, 227–241. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, S.; Wang, R. Diverse soil respiration responses to extreme precipitation patterns in arid and semiarid ecosystems. Appl. Soil Ecol. 2021, 163, 103928. [Google Scholar] [CrossRef]
- Bhandari, K.B.; Acosta-Martínez, V.; Pérez-Guzmán, L.; West, C.P. Soil health within transitions from irrigation to limited irrigation and dryland management. Agric. Environ. Lett. 2022, 7, e20077. [Google Scholar] [CrossRef]
- Chavarría, D.N.; Verdenelli, R.A.; Muñoz, E.J.; Conforto, C.; Restovich, S.B.; Andriulo, A.E.; Meriles, J.M.; Vargas-Gil, S. Soil microbial functionality in response to the inclusion of cover crop mixtures in agricultural systems. Span. J. Agric. Res. 2016, 14, e0304. [Google Scholar] [CrossRef]
- Mikanová, O.; Javůrek, M.; Šimon, T.; Friedlová, M.; Vach, M. The effect of tillage systems on some microbial characteristics. Soil Tillage Res. 2009, 105, 72–76. [Google Scholar] [CrossRef]
- Brzezińska, M.; Stępniewska, Z.; Stępniewski, W. Soil oxygen status and dehydrogenase activity. Soil Biol. Biochem. 1998, 30, 1783–1790. [Google Scholar] [CrossRef]
- Brzezinska, M.; Stepniewska, Z.; Stepniewski, W. Dehydrogenase and catalase activity of soil irrigated with municipal wastewater. Pol. J. Environ. Stud. 2001, 10, 307–311. [Google Scholar]
- Nivelle, E.; Verzeaux, J.; Habbib, H.; Kuzyakov, Y.; Decocq, G.; Roger, D.; Lacoux, J.; Duclercq, J.; Spicher, F.; Nava-Saucedo, J.-E.; et al. Functional response of soil microbial communities to tillage, cover crops and nitrogen fertilization. Appl. Soil Ecol. 2016, 108, 147–155. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, G. Dehydrogenase activity as a biological indicator of soil health. Chem. Sci. Rev. Lett. 2021, 10, 326–329. [Google Scholar] [CrossRef]
- Roldán, A.; Caravaca, F.; Hernández, M.; Garcıa, C.; Sánchez-Brito, C.; Velásquez, M.; Tiscareño, M. No-tillage, crop residue additions, and legume cover cropping effects on soil quality characteristics under maize in Patzcuaro watershed (Mexico). Soil Tillage Res. 2003, 72, 65–73. [Google Scholar] [CrossRef]
- Bausenwein, U.; Gattinger, A.; Langer, U.; Embacher, A.; Hartmann, H.-P.; Sommer, M.; Munch, J.; Schloter, M. Exploring soil microbial communities and soil organic matter: Variability and interactions in arable soils under minimum tillage practice. Appl. Soil Ecol. 2008, 40, 67–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Wu, Z.; Sun, C. Kinetic parameters of soil β-glucosidase response to environmental temperature and moisture regimes. Rev. Bras. Cienc. Solo 2011, 35, 1285–1291. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. Forest. Soil Biol. Biochem. 2005, 37, 455–461. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Klose, S.; Zobeck, T.M. Enzyme activities in semiarid soils under conservation reserve program, native rangeland, and cropland. J. Plant Nutr. Soil Sci. 2003, 166, 699–707. [Google Scholar] [CrossRef]
- Wang, X.-C.; Lu, Q. Beta-Glucosidase Activity in Paddy Soils of the Taihu Lake Region, China. Pedosphere 2006, 16, 118–124. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Makoi, J.H.J.R.; Chimphango, S.B.M.; Dakora, F.D. Elevated levels of acid and alkaline phosphatase activity in roots and rhizosphere of cowpea (Vigna unguiculata L. Walp.) genotypes grown in mixed culture and at different densities with sorghum (Sorghum bicolor L.). Crop. Pasture Sci. 2010, 61, 279–286. [Google Scholar] [CrossRef]
- Kandeler, E.; Palli, S.; Stemmer, M.; Gerzabek, M.H. Tillage changes microbial biomass and enzyme activities in particle-size fractions of a Haplic Chernozem. Soil Biol. Biochem. 1999, 31, 1253–1264. [Google Scholar] [CrossRef]
- Green, V.; Stott, D.; Cruz, J.; Curi, N. Tillage impacts on soil biological activity and aggregation in a Brazilian Cerrado Oxisol. Soil Tillage Res. 2007, 92, 114–121. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R.; Scow, K.M. Soil moisture and plant residue addition interact in their effect on extracellular enzyme activity. Pedobiologia 2011, 54, 71–78. [Google Scholar] [CrossRef]
- Stewart, W.L.; Fulton, A.E.; Krueger, W.H.; Lampinen, B.D.; Shackel, K.A. Regulated deficit irrigation reduces water use of almonds without affecting yield. Calif. Agric. 2011, 65, 90–95. [Google Scholar] [CrossRef]
- Barreales, D.; Capitão, S.; Bento, A.A.; Casquero, P.A.; Ribeiro, A.C. Adapting Almond Production to Climate Change through Deficit Irrigation and Foliar Kaolin Application in a Mediterranean Climate. Atmosphere 2023, 14, 1593. [Google Scholar] [CrossRef]
- Moldero, D.; López-Bernal, Á.; Testi, L.; Lorite, I.J.; Fereres, E.; Orgaz, F. Long-term almond yield response to deficit irrigation. Irrig. Sci. 2021, 39, 409–420. [Google Scholar] [CrossRef]
- Girona, J.; Mata, M.; Marsal, J. Regulated deficit irrigation during the kernel-filling period and optimal irrigation rates in almond. Agric. Water Manag. 2005, 75, 152–167. [Google Scholar] [CrossRef]
- García, J.; Romero, P.; Botía, P.; García, F. Cost-benefit analysis of almond orchard under regulated deficit irrigation (RDI) in SE Spain. Span. J. Agric. Res. 2004, 2, 157–165. [Google Scholar] [CrossRef]
- Koumanov, K.S.; Hopmans, J.W.; Schwankl, L.W. Spatial and temporal distribution of root water uptake of an almond tree under microsprinkler irrigation. Irrig. Sci. 2006, 24, 267–278. [Google Scholar] [CrossRef]
- Durán-Zuazo, V.H.; Pleguezuelo, C.R.; Panadero, L.A.; Raya, A.M.; Martínez, J.F.; Rodríguez, B.C. Soil Conservation Measures in Rainfed Olive Orchards in South-Eastern Spain: Impacts of Plant Strips on Soil Water Dynamics. Pedosphere 2009, 19, 453–464. [Google Scholar] [CrossRef]
- Palese, A.; Vignozzi, N.; Celano, G.; Agnelli, A.; Pagliai, M.; Xiloyannis, C. Influence of soil management on soil physical characteristics and water storage in a mature rainfed olive orchard. Soil Tillage Res. 2014, 144, 96–109. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).