Overexpression of GmXTH1 Enhances Salt Stress Tolerance in Soybean
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Determination of Germination Percentage, Germination Potential and Germination Index
2.3. Measurement of Physiological and Biochemical Indicators
2.4. Determination of Root Phenotypes
2.5. Measurement of Relative Expression of Target Genes
2.6. Determination of Ion Content
2.7. Methods of Data Analysis
3. Results and Analysis
3.1. Effect of Different Stress Treatments on Soybean Germination Period
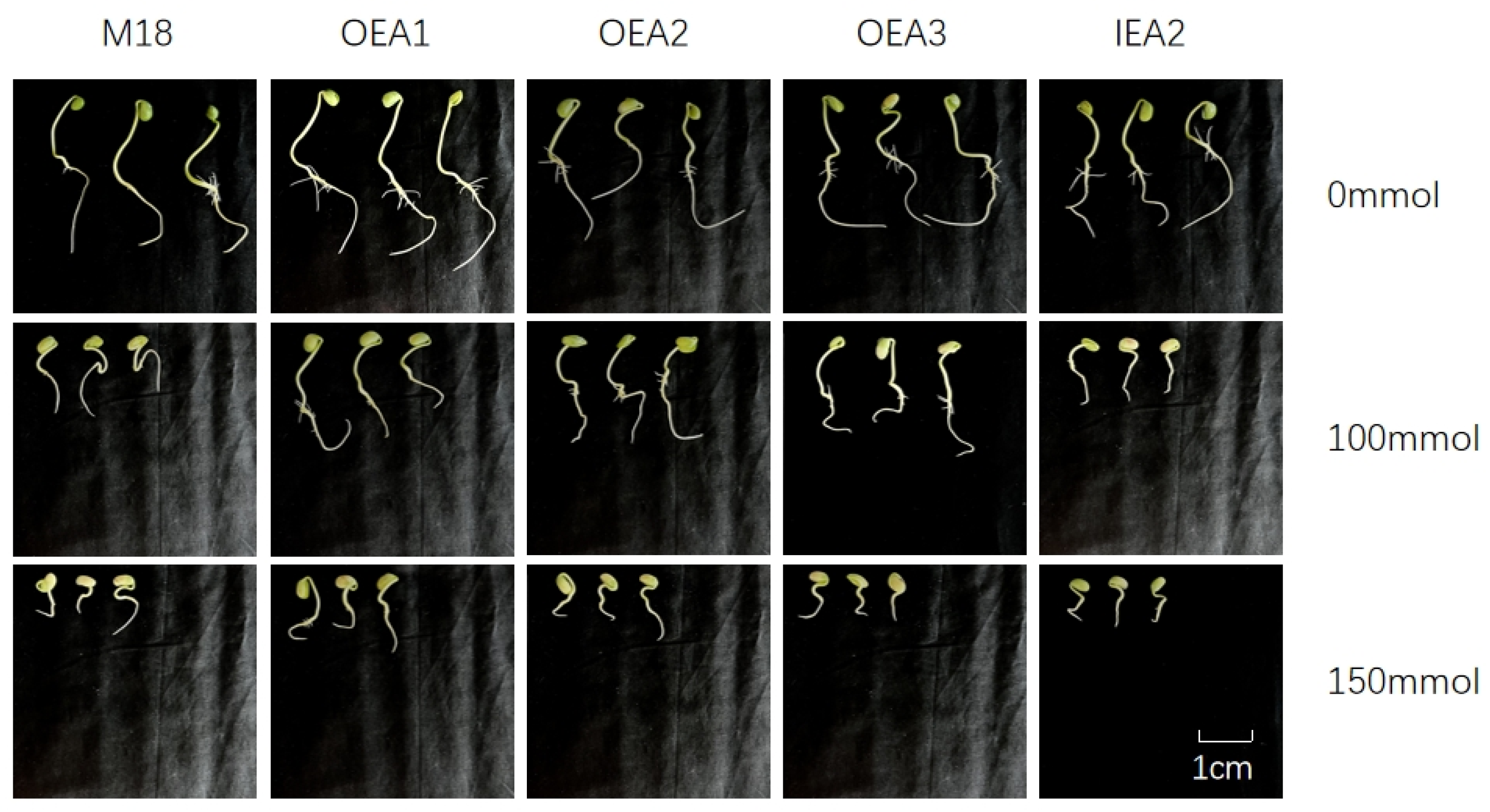
3.2. Effects of Different Stress Conditions on Soybean Seedling Growth and Development
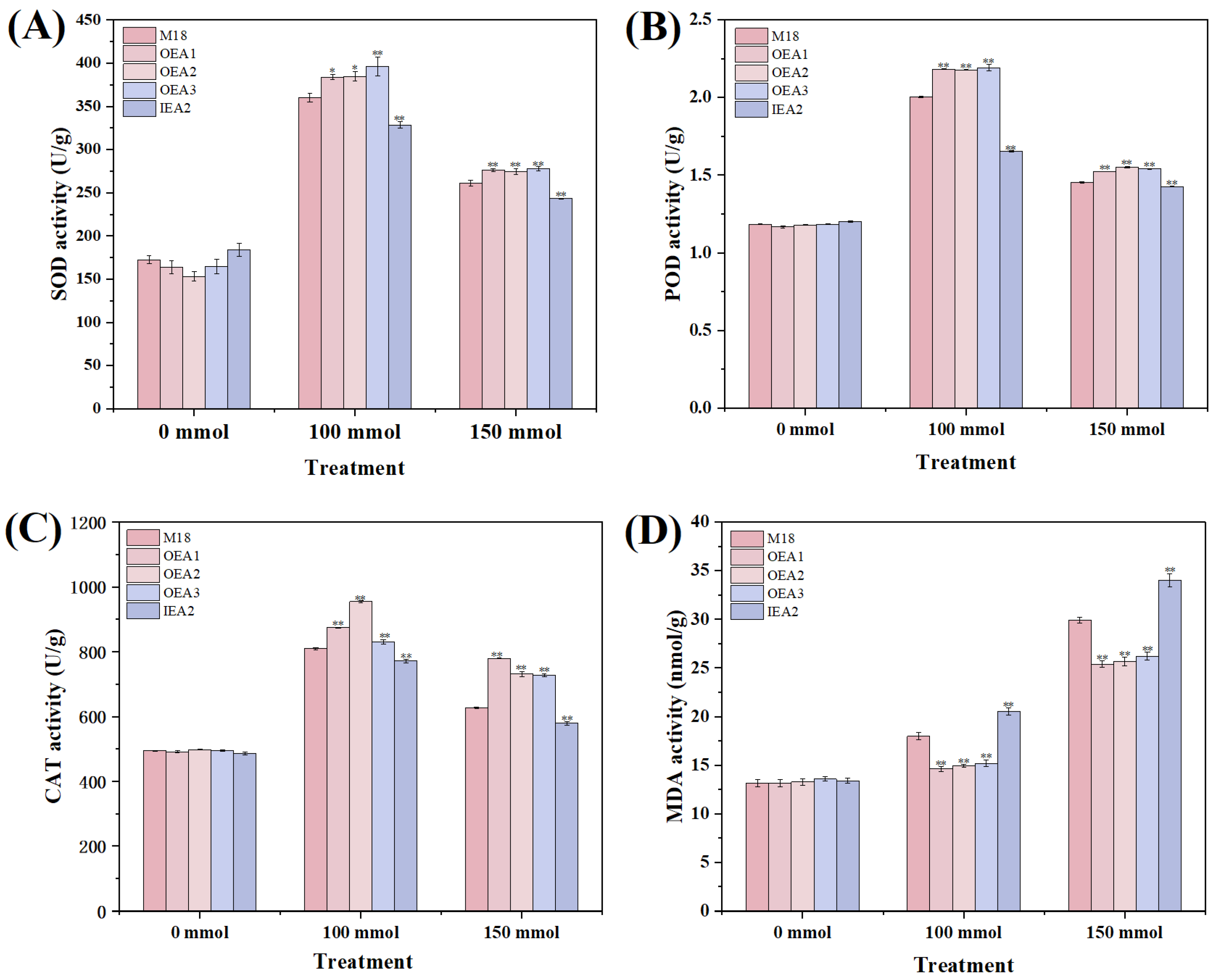
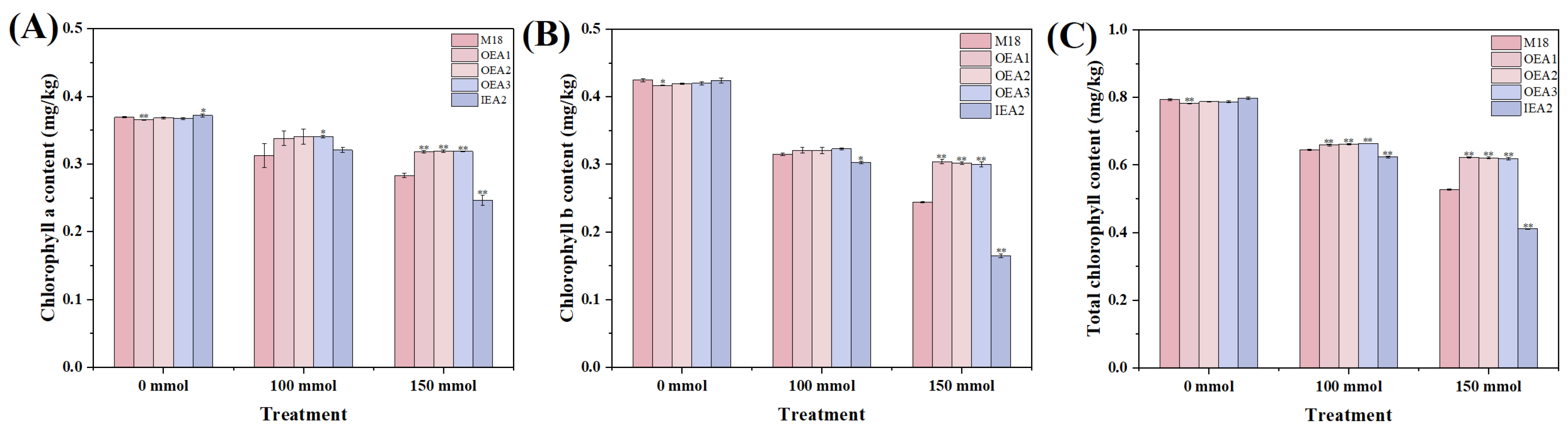
3.3. Effects of Different Stress Conditions on Physiological and Biochemical Indicators of Soybean
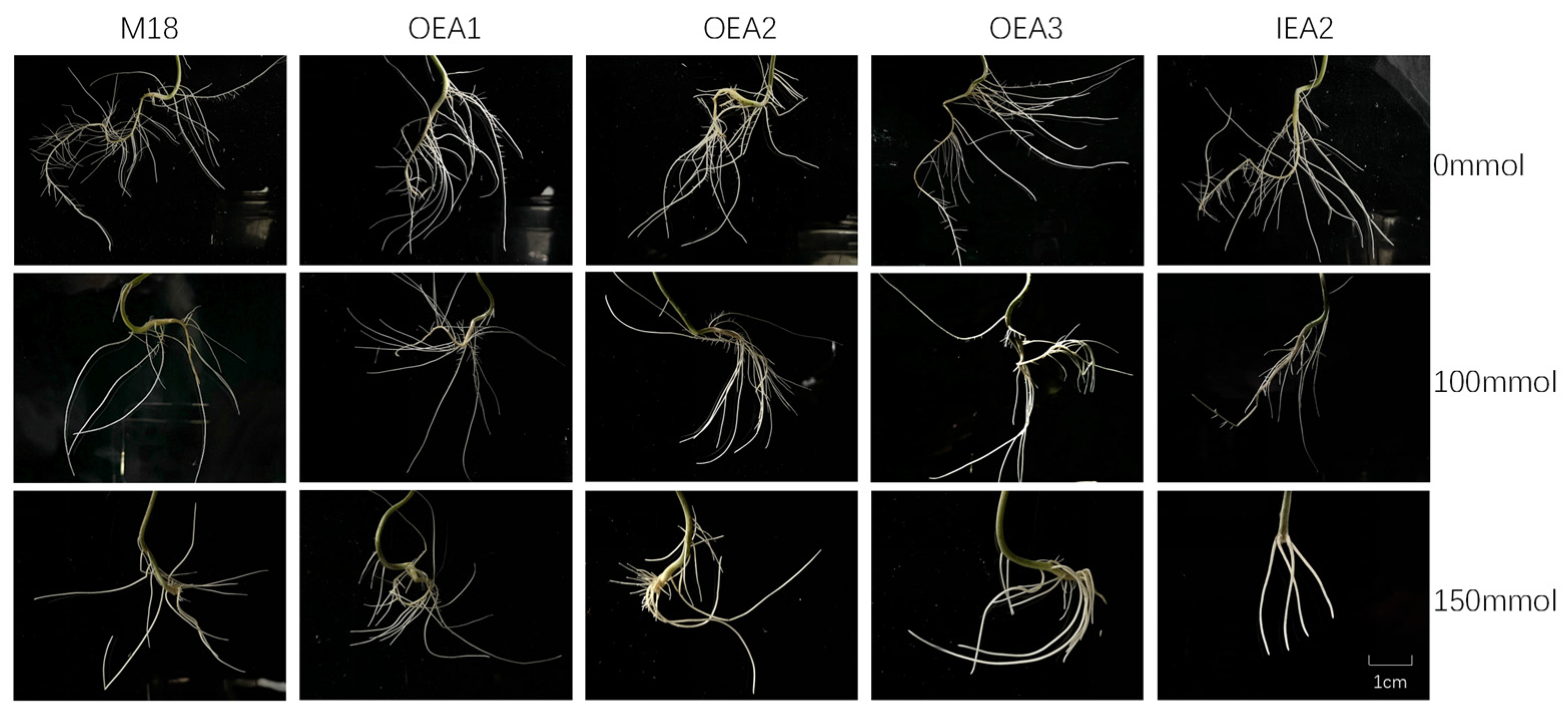
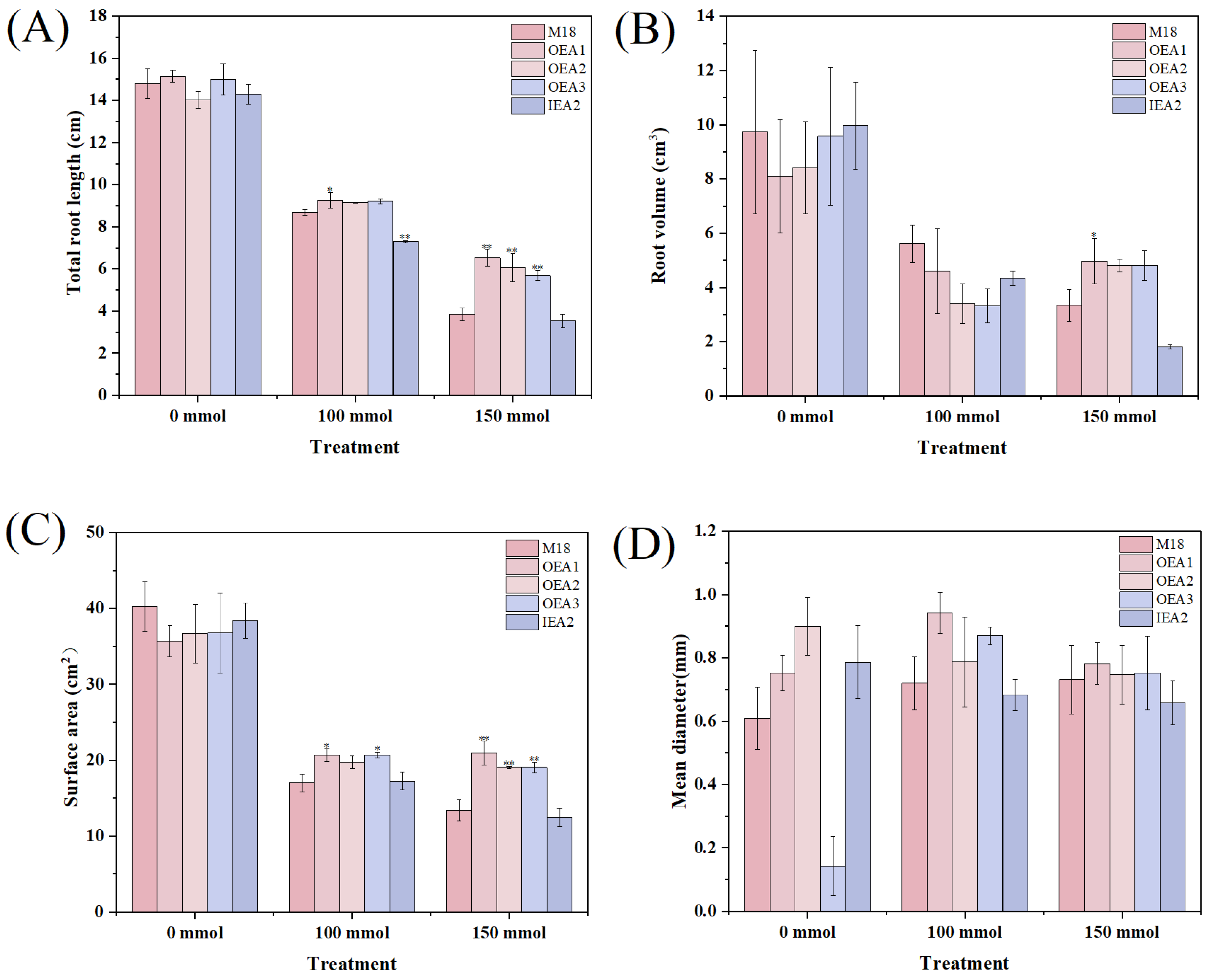
3.4. Phenotypes of the Root System of Each Strain under Different Stress Conditions
3.5. Root Indexes of Each Strain under Different Stress Conditions
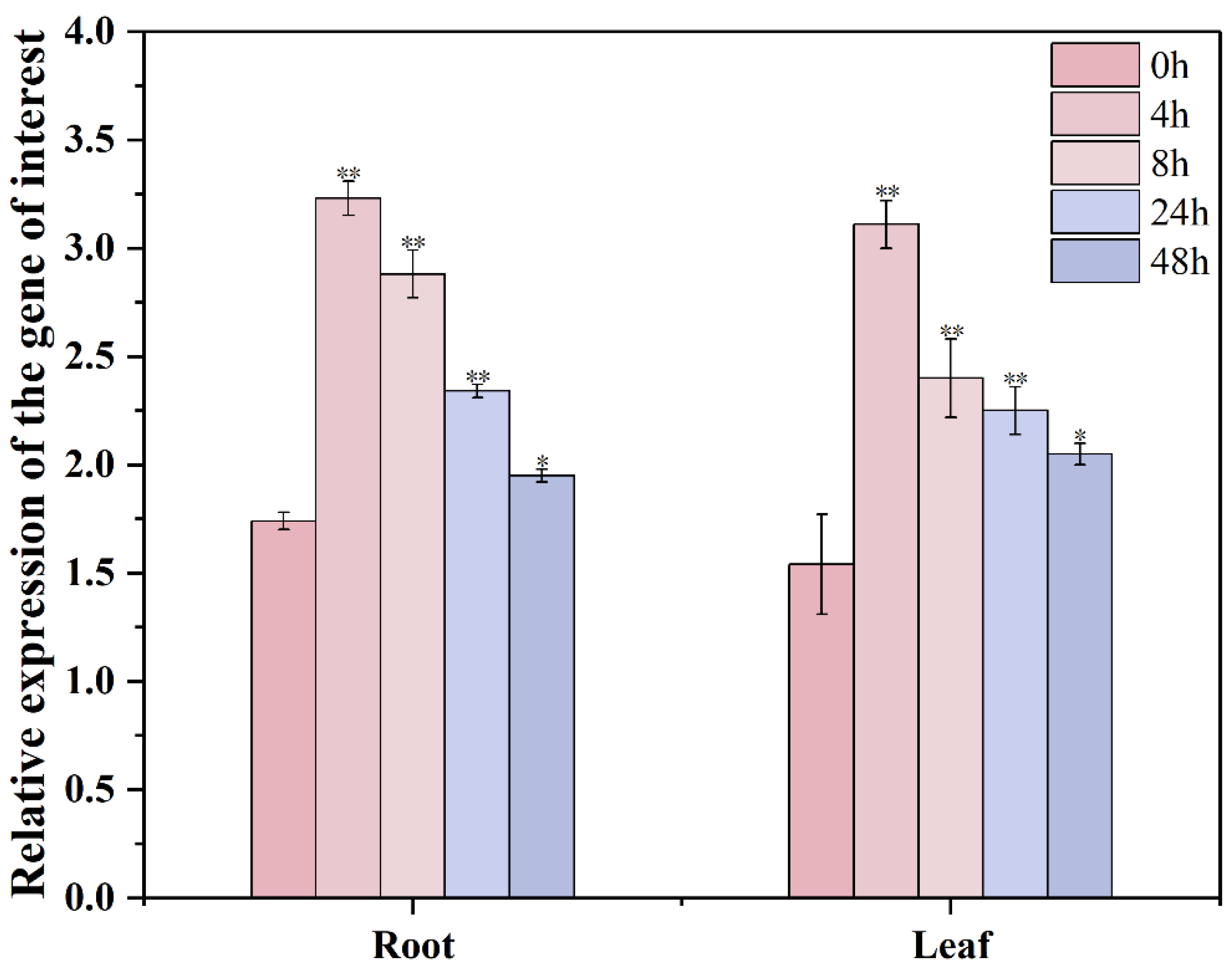
3.6. Changes in Relative Expression of GmXTH1 Gene in Roots and Leaves
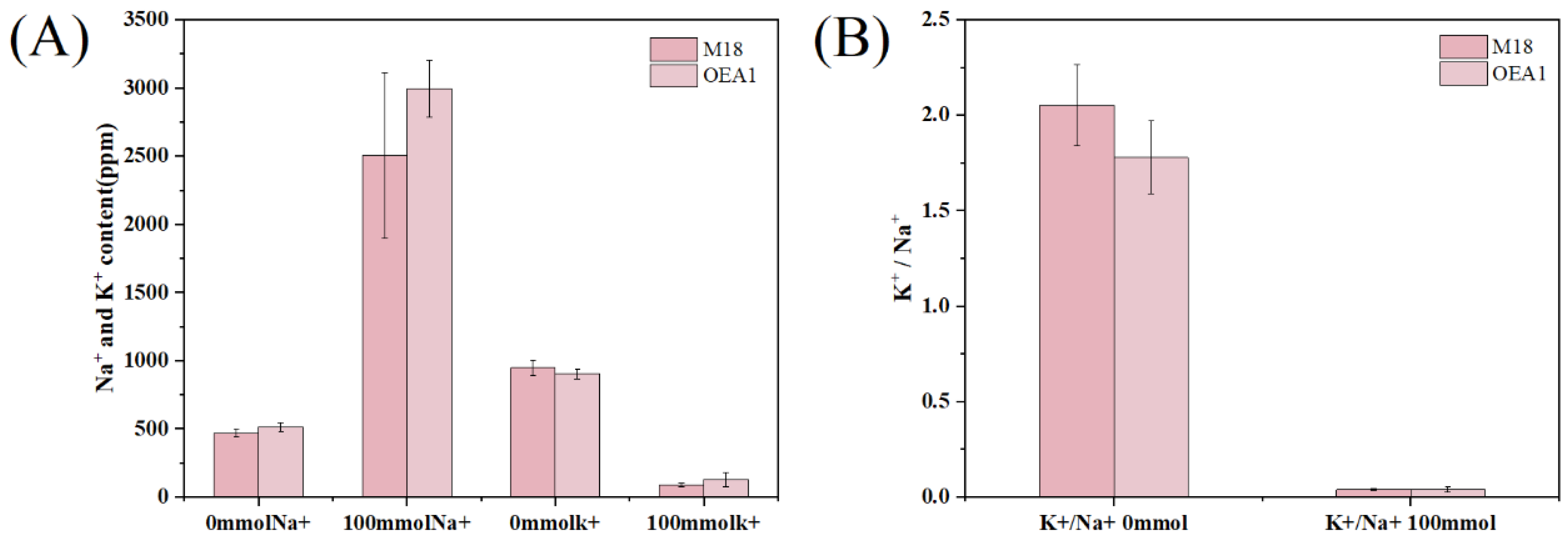
3.7. Na+ and K+ under Different Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pu, Y.; Yan, R.; Jia, D.; Che, Z.; Yang, R.; Yang, C.; Wang, H.; Cheng, H.; Yu, D. Identification of soybean mosaic virus strain SC7 resistance loci and candidate genes in soybean [Glycine max (L.) Merr.]. Mol. Genet. Genom. MGG 2024, 299, 54. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://cj.sina.com.cn/articles/view/7829375716/1d2aacae4001017boz?subch=futures (accessed on 8 September 2023).
- Available online: http://www.news.cn/fortune/2023-07/04/c_1129730839.htm (accessed on 4 July 2023).
- Omar, M.M.; Shitindi, M.J.; Massawe, B.J.; Fue, K.G.; Meliyo, J.L.; Pedersen, O. Salt-affected soils in Tanzanian agricultural lands: Type of soils and extent of the problem. Sustain. Environ. 2023, 9, 2205731. [Google Scholar] [CrossRef]
- Li, S.; Yang, G.; Chang, C.; Wang, H.; Zhang, H.; Zhang, N.; Peng, Z.; Song, Y. Remote Sensing Inversion of Salinization Degree Distribution and Analysis of Its Influencing Factors in an Arid Irrigated District. Land 2024, 13, 422. [Google Scholar] [CrossRef]
- Rossi, M.; Borromeo, I.; Capo, C.; Glick, B.R.; Del Gallo, M.; Pietrini, F.; Forni, C. PGPB improve photosynthetic activity and tolerance to oxidative stress in Brassica napus grown on salinized soils. Appl. Sci. 2021, 11, 11442. [Google Scholar] [CrossRef]
- Liao, X.; Shi, M.; Zhang, W.; Ye, Q.; Li, Y.; Feng, X.; Bhat, J.A.; Kan, G.; Yu, D. Association analysis of GmMAPKs and functional characterization of GmMMK1 to salt stress response in soybean. Physiol. Plant. 2021, 173, 2026–2040. [Google Scholar] [CrossRef]
- Rai, G.K.; Mishra, S.; Chouhan, R.; Mushtaq, M.; Chowdhary, A.A.; Rai, P.K.; Kumar, R.R.; Kumar, P.; Perez-Alfocea, F.; Colla, G.; et al. Plant salinity stress, sensing, and its mitigation through WRKY. Front. Plant Sci. 2023, 14, 1238507. [Google Scholar] [CrossRef]
- Yang, J.; Yao, R.; Wang, X.; Xie, W.; Zhang, X.; Zhu, W.; Zhang, L.; Sun, R. Research on saline soils in China: History, current status and prospects. Soil J. 2022, 59, 10–27. [Google Scholar]
- Available online: https://www.ecsf.ac.cn/info/1133/3547.htm (accessed on 4 July 2023).
- De Caroli, M.; Manno, E.; Piro, G.; Lenucci, M.S. Ride to cell wall: Arabidopsis XTH11, XTH29 and XTH33 exhibit different secretion pathways and responses to heat and drought stress. Plant J. Cell Mol. Biol. 2021, 107, 448–466. [Google Scholar] [CrossRef]
- Han, J.; Liu, Y.; Shen, Y.; Li, W. A surprising diversity of xyloglucan Endotransglucosylase/Hydrolase in wheat: New in sight to the roles in drought tolerance. Int. J. Mol. Sci. 2023, 24, 9886. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.Z.; Fu, J.Y.; Du, Y.Y.; Qu, J.; Song, Y.; Wang, P.W. The GmXTH1 gene improves drought stress resistance of soybean seedlings. Mol. Breed. New Strateg. Plant Improv. 2021, 42, 3. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, X.; Yao, W.; Gao, Y.; Zhao, K.; Guo, Q.; Zhou, B.; Jiang, T. Genome-wide identification and expression analysis of the xyloglucan endotransglucosylase/hydrolase gene family in poplar. BMC Genom. 2021, 22, 804. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Huang, Y.; He, H.; Han, T.; Di, P.; Sechet, J.; Fang, L.; Liang, Y.; Scheller, H.V.; Mortimer, J.C.; et al. Xyloglucan endotransglucosylase-hydrolase30 negatively affects salt tolerance in Arabidopsis. J. Exp. Bot. 2019, 70, 5495–5506. [Google Scholar] [CrossRef] [PubMed]
- Qiao, T.; Zhang, L.; Yu, Y.; Pang, Y.; Tang, X.; Wang, X.; Li, L.; Li, B.; Sun, Q. Identification and expression analysis of xyloglucan endotransglucosylase/hydrolase (XTH) family in grapevine (Vitis vinifera L.). Peer J. 2022, 10, e13546. [Google Scholar] [CrossRef]
- Ma, Y.; Jie, H.; Zhao, L.; He, P.; Lv, X.; Xu, Y.; Zhang, Y.; Xing, H.; Jie, Y. BnXTH1 regulates cadmium tolerance by modulating vacuolar compartmentalization and the cadmium binding capacity of cell walls in ramie (Boehmeria nivea). J. Hazard. Mater. 2024, 470, 134172. [Google Scholar] [CrossRef]
- Danso, B.; Ackah, M.; Jin, X.; Ayittey, D.M.; Amoako, F.K.; Zhao, W. Genome-Wide Analysis of the Xyloglucan Endotransglucosylase/Hydrolase (XTH) Gene Family: Expression Pattern during Magnesium Stress Treatment in the Mulberry Plant (Morus alba L.) Leaves. Plants 2024, 13, 902. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wan, H.; Zhao, H.; Dai, X.; Wu, W.; Liu, J.; Xu, J.; Yang, R.; Xu, B.; Zeng, C.; et al. Identification and expression analysis of the Xyloglucan transglycosylase/hydrolase (XTH) gene family under abiotic stress in oilseed (Brassica napus L.). BMC Plant Biol. 2024, 24, 400. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, A.; Yang, L.; Wei, J.; Bei, J.; Xu, Z.; Wang, X.; Chen, B. Identification of XTH Family Genes and Expression Analysis of Endosperm Weakening in Lettuce (Lactuca sativa L.). Agronomy 2024, 14, 324. [Google Scholar] [CrossRef]
- Takahashi, D.; Johnson, K.L.; Hao, P.; Tuong, T.; Erban, A.; Sampathkumar, A.; Bacic, A.; Livingston, D.P.; Kopka, J., 3rd; Kuroha, T.; et al. Cell wall modification by the xyloglucan endotransglucosylase/hydrolase XTH19 influences freezing tolerance after cold and sub-zero acclimation. Plant Cell Environ. 2021, 44, 915–930. [Google Scholar] [CrossRef]
- Fu, C.; Han, C.; Wei, Y.; Liu, D.; Han, Y. Two NAC transcription factors regulated fruit softening through activating xyloglucan endotransglucosylase/hydrolase genes during kiwifruit ripening. Int. J. Biol. Macromol. 2024, 263 Pt 1, 130678. [Google Scholar] [CrossRef]
- Zhai, Z.; Feng, C.; Wang, Y.; Sun, Y.; Peng, X.; Xiao, Y.; Zhang, X.; Zhou, X.; Jiao, J.; Wang, W.; et al. Genome-Wide Identification of the Xyloglucan endotransglucosylase/Hydrolase (XTH) and Polygalacturonase (PG) Genes and Characterization of Their Role in Fruit Softening of Sweet Cherry. Int. J. Mol. Sci. 2021, 22, 12331. [Google Scholar] [CrossRef]
- Li, X.; Su, Q.; Feng, Y.; Gao, X.; Wang, B.; Tahir, M.M.; Yang, H.; Zhao, Z. Identification and analysis of the xyloglucan endotransferase/hydrolase (XTH) family genes in apple. Sci. Hortic. 2023, 315, 111990. [Google Scholar] [CrossRef]
- Atkinson, R.G.; Johnston, S.L.; Yauk, Y.K.; Sharma, N.N.; Schröder, R. Analysis of xyloglucan endotransglucosylase/hydrolase (XTH) gene families in kiwifruit and apple. Postharvest Biol. Technol. 2009, 51, 149–157. [Google Scholar] [CrossRef]
- Pitaksaringkarn, W.; Matsuoka, K.; Asahina, M.; Miura, K.; Sage-Ono, K.; Ono, M.; Yokoyama, R.; Nishitani, K.; Ishii, T.; Iwai, H.; et al. XTH20 and XTH19 regulated by ANAC071 under auxin flow are involved in cell proliferation in incised Arabidopsis inflorescence stems. Plant J. Cell Mol. Biol. 2014, 80, 604–614. [Google Scholar] [CrossRef]
- Malinowski, R.; Fry, S.C.; Zuzga, S.; Wiśniewska, A.; Godlewski, M.; Noyszewski, A.; Barczak-Brzyżek, A.; Malepszy, S.; Filipecki, M. Developmental expression of the cucumber Cs-XTH1 and Cs-XTH3 genes, encoding xyloglucan endotransglucosylase/hydrolases, can be influenced by mechanical stimuli. Acta Physiol. Plant 2018, 40, 130. [Google Scholar] [CrossRef]
- Kushwah, S.; Banasiak, A.; Nishikubo, N.; Derba-Maceluch, M.; Majda, M.; Endo, S.; Kumar, V.; Gomez, L.; Gorzsas, A.; McQueen-Mason, S.; et al. Arabidopsis XTH4 and XTH9 Contribute to Wood Cell Expansion and Secondary Wall Formation. Plant Physiol. 2020, 182, 1946–1965. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Lv, Y.; Li, X. Interspaced Repeat Sequences Confer the Regulatory Functions of AtXTH10, Important for Root Growth in Arabidopsis. Plants 2019, 8, 130. [Google Scholar] [CrossRef]
- Xu, P.; Fang, S.; Chen, H.; Cai, W. The brassinosteroid-responsive xyloglucan endotransglucosylase/hydrolase 19 (XTH19) and XTH23 genes are involved in lateral root development under salt stress in Arabidopsis. Plant J. Cell Mol. Biol. 2020, 104, 59–75. [Google Scholar] [CrossRef]
- Zhang, J.Z.; He, P.W.; Xu, X.M.; Lü, Z.F.; Cui, P.; George, M.S.; Lu, G.Q. Genome-Wide Identification and Expression Analysis of the Xyloglucan Endotransglucosylase/Hydrolase Gene Family in Sweet Potato [Ipomoea batatas (L.) Lam]. Int. J. Mol. Sci. 2023, 24, 775. [Google Scholar] [CrossRef]
- Niraula, P.M.; Lawrence, K.S.; Klink, V.P. The heterologous expression of a soybean (Glycine max) xyloglucan endotransglycosylase/hydrolase (XTH) in cotton (Gossypium hirsutum) suppresses parasitism by the root knot nematode Meloidogyne incognita. PLoS ONE 2020, 15, e0235344. [Google Scholar] [CrossRef]
- Bouslama, M.; Schapaugh, W.T., Jr. Stress tolerance in soybeans. I. Evaluation of three screening techniques for heat and drought tolerance 1. Crop Sci. 1984, 24, 933–937. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Correa, S.; Sevilla, F. Identification of Superoxide Dismutase (SOD) Isozymes in Plant Tissues. Methods Mol. Biol. 2024, 2798, 205–212. [Google Scholar] [PubMed]
- Freitas, C.D.T.; Costa, J.H.; Germano, T.A.; de Rocha, R.O.; Ramos, M.V.; Bezerra, L.P. Class III plant peroxidases: From classification to physiological functions. Int. J. Biol. Macromol. 2024, 263 Pt 1, 130306. [Google Scholar] [CrossRef]
- González-Gordo, S.; Rodríguez-Ruiz, M.; Palma, J.M.; Corpas, F.J. Comparative Analysis of Catalase Activity in Plants: Spectrophotometry and Native PAGE Approaches. Methods Mol. Biol. 2024, 2798, 213–221. [Google Scholar]
- Zhou, L.; Zhou, L.; Wu, H.; Jing, T.; Li, T.; Li, J.; Kong, L.; Zhu, F. Application of Chlorophyll Fluorescence Analysis Technique in Studying the Response of Lettuce (Lactuca sativa L.) to Cadmium Stress. Sensors 2024, 24, 1501. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Munné-Bosch, S. Malondialdehyde Assays in Higher Plants. Methods Mol. Biol. 2024, 2798, 79–100. [Google Scholar]
- Zhang, K.; Lan, Y.; Wu, M.; Wang, L.; Liu, H.; Xiang, Y. PhePLATZ1, a PLATZ transcription factor in moso bamboo (Phyllostachys edulis), improves drought resistance of transgenic Arabidopsis thaliana. Plant Physiol. Biochem. PPB 2022, 186, 121–134. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Zhao, H.; Deng, X.; Su, Y.; Li, R.; Chen, B. Overexpression of Sugarcane ScDIR Genes Enhances Drought Tolerance in Nicotiana benthamiana. Int. J. Mol. Sci. 2022, 23, 5340. [Google Scholar] [CrossRef]
- Zhu, K.; Huang, C.; Phan, T.T.; Yang, L.T.; Zhang, B.Q.; Xing, Y.X.; Li, Y.R. Overexpression of SoACLA-1 gene confers drought tolerance improvement in sugarcane. Plant Mol. Biol. Report. 2021, 39, 489–500. [Google Scholar] [CrossRef]
- Liu, W.; Liang, X.; Cai, W.; Wang, H.; Liu, X.; Cheng, L.; Song, P.; Luo, G.; Han, D. Isolation and Functional Analysis of VvWRKY28, a Vitis vinifera WRKY Transcription Factor Gene, with Functions in Tolerance to Cold and Salt Stress in Transgenic Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 13418. [Google Scholar] [CrossRef]
- Xian, P.; Yang, Y.; Xiong, C.; Guo, Z.; Alam, I.; He, Z.; Zhang, Y.; Cai, Z.; Nian, H. Overexpression of GmWRKY172 enhances cadmium tolerance in plants and reduces cadmium accumulation in soybean seeds. Front. Plant Sci. 2023, 14, 1133892. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, W.; Jia, J.; Zhao, P.; Qi, D.; Chen, S.; Cheng, L.; Cheng, L.; Liu, G. Ectopic Expression of a Salt-Inducible Gene, LcSAIN3, from Sheepgrass Improves Seed Germination and Seedling Growth under Salt Stress in Arabidopsis. Genes 2021, 12, 1994. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.K.; Verma, S.; Tripathi, R.D.; Pandey, N.; Chakrabarty, D. CC-type glutaredoxin, OsGrx_C7 plays a crucial role in enhancing protection against salt stress in rice. J. Biotechnol. 2021, 329, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, A.; Alfatih, A.; Jan, S.U.; Sun, L.; Zhao, P.; Xiang, C. Transcription elongation factor AtSPT4-2 positively modulates salt tolerance in Arabidopsis thaliana. BMC Plant Biol. 2023, 23, 49. [Google Scholar] [CrossRef]
- Chun, H.J.; Baek, D.; Jin, B.J.; Cho, H.M.; Park, M.S.; Lee, S.H.; Lim, L.H.; Cha, Y.J.; Bae, D.W.; Kim, S.T.; et al. Microtubule Dynamics Plays a Vital Role in Plant Adaptation and Tolerance to Salt Stress. Int. J. Mol. Sci. 2021, 22, 5957. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, Y.; Li, Y.; Ren, G.; Chen, C.; Jin, X. SLs signal transduction gene CsMAX2 of cucumber positively regulated to salt, drought and ABA stress in Arabidopsis thaliana L. Gene 2023, 864, 147282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Qiu, Z.; Zeng, B.; Zhang, Y.; Wang, X.; Chen, J.; Zhong, C.; Deng, R.; Fan, C. Transcriptome and structure analysis in root of Casuarina equisetifolia under NaCl treatment. PeerJ 2021, 9, e12133. [Google Scholar] [CrossRef]
- Guo, X.; Ahmad, N.; Zhao, S.; Zhao, C.; Zhong, W.; Wang, X.; Li, G. Effect of Salt Stress on Growth and Physiological Properties of Asparagus Seedlings. Plants 2022, 11, 2836. [Google Scholar] [CrossRef]
- Du, N.; Xue, L.; Xue, D.; Dong, X.; Yang, Q.; Shah Jahan, M.; Guo, H.; Fu, R.; Wang, Y.; Piao, F. The transcription factor SlNAP1 increases salt tolerance by modulating ion homeostasis and ROS metabolism in Solanum lycopersicum. Gene 2023, 849, 146906. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Q.; Zong, J.; Guo, H.; Liu, J.; Chen, J. Effects of Supplemental Potassium on the Growth, Photosynthetic Characteristics, and Ion Content of Zoysia matrella under Salt Stress. Horticulturae 2023, 10, 31. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, H.; Wang, B.; Wu, X.; Lan, R.; Huang, X.; Chen, B.; Chen, G.; Jiang, C.; Wang, J.; et al. Exogenous melatonin improves the growth of rice seedlings by regulating redox balance and ion homeostasis under salt stress. J. Plant Growth Regul. 2022, 41, 2108–2121. [Google Scholar] [CrossRef]
- Hu, F.; Fang, D.; Zhang, W.; Dong, K.; Ye, Z.; Cao, J. Lateral root primordium: Formation, influencing factors and regulation. Plant Physiol. Biochem. PPB 2024, 207, 108429. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, Y.; Datla, R.; Ren, M. Auxin and Target of Rapamycin Spatiotemporally Regulate Root Organogenesis. Int. J. Mol. Sci. 2021, 22, 11357. [Google Scholar] [CrossRef]
- García-González, J.; Lacek, J.; Retzer, K. Dissecting Hierarchies between Light, Sugar and Auxin Action Underpinning Root and Root Hair Growth. Plants 2021, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Zhang, F.; Wang, J.; Wang, F.; Liu, J.; Xie, C.; Hu, Y.; Shani, E.; Kong, X.; Ding, Z.; et al. Cell-type action specificity of auxin on Arabidopsis root growth. Plant J. Cell Mol. Biol. 2021, 106, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Gong, J.; Zhang, Z.; Meng, Z.; Wang, J.; Wang, S.; Sun, J.; Gu, X.; Jin, Y.; Wu, T.; et al. The Arabidopsis thaliana trehalose-6-phosphate phosphatase gene AtTPPI regulates primary root growth and lateral root elongation. Front. Plant Sci. 2023, 13, 1088278. [Google Scholar] [CrossRef]
- Xu, J.; Yang, C.; Ji, S.; Ma, H.; Lin, J.; Li, H.; Chen, S.; Xu, H.; Zhong, M. Heterologous expression of MirMAN enhances root development and salt tolerance in Arabidopsis. Front. Plant Sci. 2023, 14, 1118548. [Google Scholar] [CrossRef]

| Functionality | Name of Material | Source | Appliance | References | |
|---|---|---|---|---|---|
| Plant resistance | Drought stress | XTH11, XTH29 and XTH33 | Arabidopsis | Participation in the response of Arabidopsis thaliana to drought stress | [11] |
| TaXTH12.5a | Wheat | Regulation of wheat response to drought stress | [12] | ||
| GmXTH1 | Soybean | Enhancing drought tolerance in soybean | [13] | ||
| Salt stress | PtrXTH | Poplar | Important role in salt stress response | [14] | |
| XTH30 | Arabidopsis | Reduced tolerance to salt stress | [15] | ||
| VvXTHs | Vitis vinifera L. | Involved in response to salt stress | [16] | ||
| Metal ion stress | BnXTH1 | Boehmeria nivea | Involved in mediating cadmium tolerance in plants | [17] | |
| MaXTH | Morus alba L. | Possible involvement in plant response to magnesium stress | [18] | ||
| BnXTHs | Brassica napus L. | Involved in response to aluminum stress | [19] | ||
| Heat stress | LsXTH43 | Lactuca sativa L. | Promotion of seed germination under high-temperature conditions | [20] | |
| Cold stress | XTH19 | Arabidopsis | Improving cold tolerance in Arabidopsis thaliana | [21] | |
| Fruit softening | AcXTH1 and AcXTH2 | Kiwifruit | Regulation of fruit ripening and softening | [22] | |
| PavXTHs | Sweet Cherry | Reduces fruit firmness | [23] | ||
| MdXTH3, MdXTH25 and MdXTH26 | Apple | Involved in regulation fruit softening | [24] | ||
| Ad-XTH7 | Kiwifruit | Participate in fruit softening | [25] | ||
| Growth and development | XTH20 | Arabidopsis | Damaged repair of inflorescence stems in Arabidopsis thaliana | [26] | |
| Cs-XTH1 and Cs-XTH3 | Cucumbers | Promotes root elongation | [27] | ||
| AtXTH4 and AtXTH9 | Arabidopsis | Involved in xylem cell production and regulates secondary wall thickening | [28] | ||
| AtXTH10 | Arabidopsis | Promotes root growth | [29] | ||
| XTH19 and XTH23 | Arabidopsis | Promotes root growth in Arabidopsis thaliana | [30] | ||
| IbXTHs | Sweet Potato | Critical for root specificity | [31] | ||
| Name of Primer | Sequence Information (5′→3′) |
|---|---|
| β-actin-F | CGGTGGTTCTATCTTGGCATC |
| β-actin-R | GTCTTTCGCTTCAATAACCCTA |
| GmXTH1-F | AGGCAAGGGTGATAGAGAGCAAAG |
| GmXTH1-R | CCTCGTCCACAAAGAACACAATCTG |
| Processing Group | Material | Germination Rate | Germination Potential | Germination Index |
|---|---|---|---|---|
| 0 mmol | M18 | 1.00 ± 0.00 | 0.60 ± 0.10 | 25.24 ± 0.64 |
| OEA1 | 1.00 ± 0.00 | 0.73 ± 0.07 | 29.41 ± 1.46 | |
| OEA2 | 1.00 ± 0.00 | 0.63 ± 0.08 | 26.52 ± 1.12 | |
| OEA3 | 0.98 ± 0.02 | 0.58 ± 0.06 | 26.04 ± 1.00 | |
| IEA2 | 0.98 ± 0.02 | 0.65 ± 0.08 | 27.68 ± 2.03 | |
| 100 mmol | M18 | 0.60 ± 0.03 | 0.07 ± 0.02 | 5.41 ± 0.52 |
| OEA1 | 0.83 ± 0.03 ** | 0.23 ± 0.02 ** | 11.49 ± 0.67 ** | |
| OEA2 | 0.78 ± 0.03 ** | 0.18 ± 0.02 ** | 10.30 ± 0.37 ** | |
| OEA3 | 0.82 ± 0.03 ** | 0.15 ± 0.00 ** | 9.11 ± 0.50 ** | |
| IEA2 | 0.42 ± 0.03 ** | 0.02 ± 0.02 * | 3.31 ± 0.06 ** | |
| 150 mmol | M18 | 0.43 ± 0.03 | 0.00 ± 0.00 | 3.38 ± 0.49 |
| OEA1 | 0.65 ± 0.03 ** | 0.03 ± 0.02 | 6.26 ± 0.04 ** | |
| OEA2 | 0.53 ± 0.02 * | 0.02 ± 0.02 | 4.75 ± 0.06 ** | |
| OEA3 | 0.57 ± 0.02 ** | 0.03 ± 0.02 | 5.53 ± 0.15 ** | |
| IEA2 | 0.35 ± 0.03 * | 0.00 ± 0.00 | 2.58 ± 0.12 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Wang, K.; Yao, D.; Zhang, Q.; Yuan, B.; Wang, P. Overexpression of GmXTH1 Enhances Salt Stress Tolerance in Soybean. Agronomy 2024, 14, 2276. https://doi.org/10.3390/agronomy14102276
Song Y, Wang K, Yao D, Zhang Q, Yuan B, Wang P. Overexpression of GmXTH1 Enhances Salt Stress Tolerance in Soybean. Agronomy. 2024; 14(10):2276. https://doi.org/10.3390/agronomy14102276
Chicago/Turabian StyleSong, Yang, Kun Wang, Dan Yao, Qi Zhang, Boran Yuan, and Piwu Wang. 2024. "Overexpression of GmXTH1 Enhances Salt Stress Tolerance in Soybean" Agronomy 14, no. 10: 2276. https://doi.org/10.3390/agronomy14102276
APA StyleSong, Y., Wang, K., Yao, D., Zhang, Q., Yuan, B., & Wang, P. (2024). Overexpression of GmXTH1 Enhances Salt Stress Tolerance in Soybean. Agronomy, 14(10), 2276. https://doi.org/10.3390/agronomy14102276





