The Effects of Different Organic Materials and Cover Thicknesses on the Early Growth and Antioxidant Response of Direct-Seeded Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Details
3. Sampling and Measurements
3.1. Determination of the Germination Rate and Morphological Traits
3.2. Determination of the Antioxidant Response Parameters
3.3. Determination of the Contents of Chlorophyll and Carotenoids
3.4. Statistical Analyses
4. Results
4.1. Germination Attributes
4.2. Morphological Attributes
4.3. Attributes of the Antioxidant Response
4.4. Photosynthetic Pigments
4.5. Correlation Analysis
5. Discussion
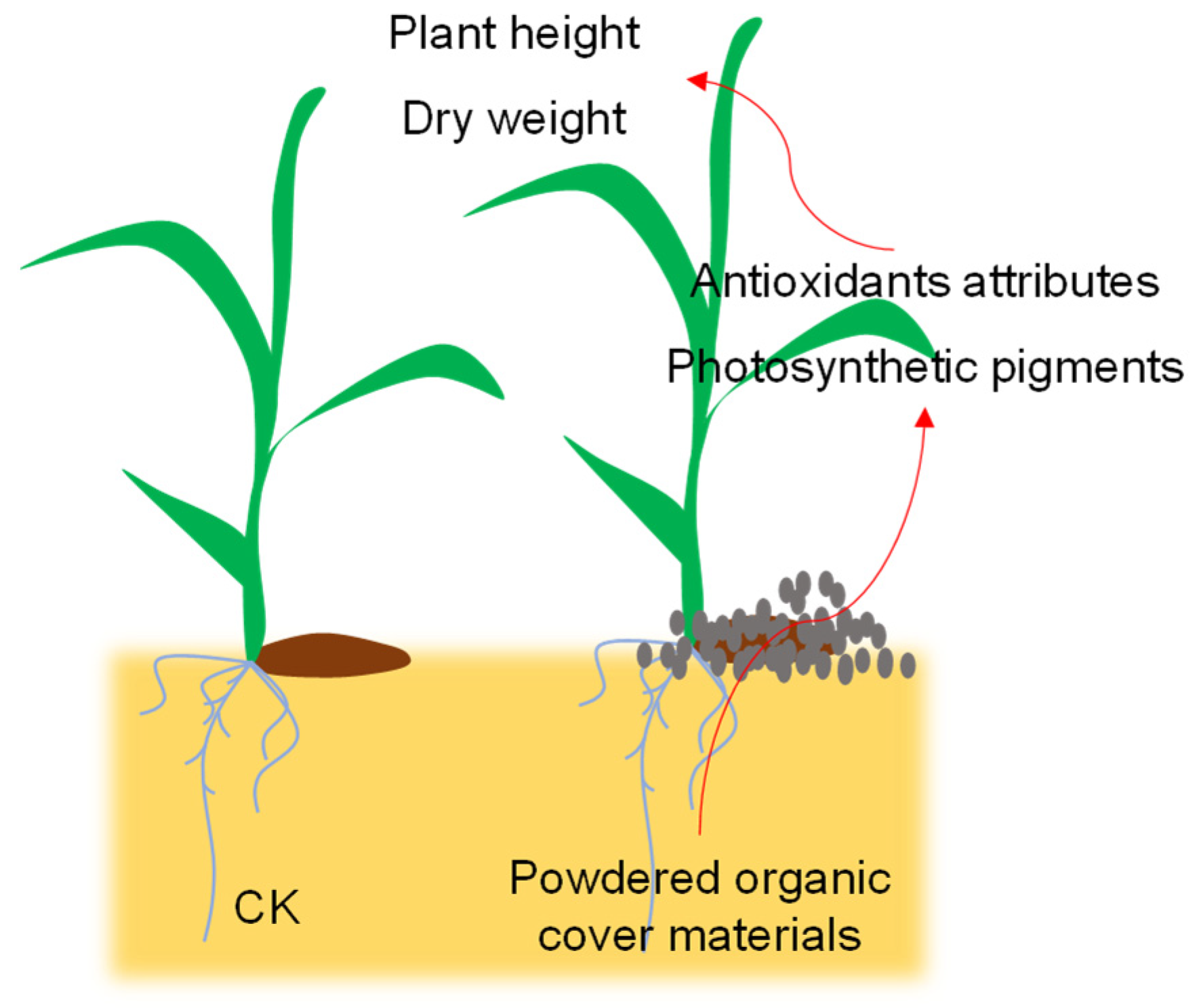
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.-J.; Dai, L.; Cheng, S.-R.; Ren, Y.; Deng, H.-Z.; Wang, X.-Y.; Li, Y.-Z.; Tang, X.-R.; Wang, Z.-M.; Mo, Z.-W. Regulation of 2-acetyl-1-pyrroline and grain quality of early-season indica fragrant rice by nitrogen–silicon fertilization under different plantation methods. J. Integr. Agric. 2023. [Google Scholar] [CrossRef]
- Farooq, M.; Siddique, K.H.M.; Rehman, H.; Aziz, T.; Lee, D.J.; Wahid, A. Rice direct seeding: Experiences, challenges and opportunities. Soil Tillage Res. 2011, 111, 87–98. [Google Scholar] [CrossRef]
- Van Nguyen, N.; Ferrero, A. Meeting the challenges of global rice production. Paddy Water Environ. 2006, 4, 1–9. [Google Scholar] [CrossRef]
- Prot, J.C.; Soriano, I.R.; Matias, D.M. Major root-parasitic nematodes associated with irrigated rice in the Philippines. Fundam. Appl. Nematol. 1994, 17, 75–78. [Google Scholar]
- Savary, S.; Castilla, N.P.; Elazegui, F.A.; Teng, P.S. Multiple effects of two drivers of agricultural change, labour shortage and water scarcity, on rice pest profiles in tropical Asia. Field Crops Res. 2005, 91, 263–271. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Yamane, K.; Garcia, R.; Imayoshi, K.; Mabesa-Telosa, R.C.; Kato, Y. Seed vigour contributes to yield improvement in dry direct-seeded rainfed lowland rice. Ann. Appl. Biol. 2018, 172, 100–110. [Google Scholar] [CrossRef]
- Hong, W.Y.; Chen, Y.J.; Huang, S.H.; Li, Y.Z.; Wang, Z.M.; Tang, X.R.; Pan, S.G.; Tian, H.; Mo, Z.W. Optimization of nitrogen-silicon (N-Si) fertilization for grain yield and lodging resistance of early-season indica fragrant rice under different planting methods. Eur. J. Agron. 2022, 136, 126508. [Google Scholar] [CrossRef]
- Mo, Z.W.; Ashraf, U.; Pan, S.G.; Kanu, A.S.; Li, W.; Duan, M.Y.; Tian, H.; Tang, X.R. Exogenous application of plant growth regulators induce chilling tolerance in direct seeded super and non-super rice seedlings through modulations in morpho-physiological attributes. Cereal Res. Commun. 2016, 44, 524–534. [Google Scholar] [CrossRef][Green Version]
- Chen, Q.; Peng, S.; Dong, H.; Wang, W.; Nie, L. Optimal nitrogen fertilizer management for direct seeding rice: A review. Int. J. Agric. Biol. 2018, 20, 1382–1390. [Google Scholar][Green Version]
- Mahajan, G.; Chauhan, B.S. Performance of dry direct-seeded rice in response to genotype and seeding rate. Agron. J. 2016, 108, 257. [Google Scholar] [CrossRef]
- Yang, W.; Xu, D.; Li, S.; Tang, X.; Pan, S.; Chen, X.; Mo, Z. Emergence and Seedling Establishment of Rice Varieties at Different Sowing Depths. J. Plant Growth Regul. 2022, 41, 1672–1686. [Google Scholar] [CrossRef]
- Carrillo-Reche, J.; Vallejo-Marín, M.; Quilliam, R.S. Quantifying the potential of ‘on-farm’ seed priming to increase crop performance in developing countries. A meta-analysis. Agron. Sustain. Dev. 2018, 38, 64. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, M.; Niu, X.; Wang, C.; Xu, Q.; Feng, Y.; Wang, S.; Yuan, X.; Yu, H.; Wang, Y.; et al. Uncovering novel loci for mesocotyl elongation and shoot length in indica rice through genome-wide association mapping. Planta 2016, 243, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.R.; Zhang, G.H.; Wu, M.G.; Cao, L.Y.; Cheng, S.H. Genetic analysis of mesocotyl elongation in rice (Oryza sativa L. subsp. japonica). Acta Agron. Sin. 2006, 32, 249–252. [Google Scholar]
- Gealy, D.R.; Saldain, N.E.; Talbert, R.E. Emergence of Red Rice (Oryza sativa) Ecotypes Under Dry-Seeded Rice (Oryza sativa) Culture1. Weed Technol. 2000, 14, 406–412. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, W.; Jiang, C.; Wang, X.; Xiong, H.; Todorovska, E.G.; Yin, Z.; Chen, Y.; Wang, X.; Xie, J.; et al. Genetic Architecture and Candidate Genes for Deep-Sowing Tolerance in Rice Revealed by Non-syn GWAS. Front. Plant Sci. 2018, 9, 332. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Li, W.; Ashraf, U.; Ma, L.; Tang, X.; Pan, S.; Tian, H.; Mo, Z. ZnO nanoparticle-based seed priming modulates early growth and enhances physio-biochemical and metabolic profiles of fragrant rice against cadmium toxicity. J. Nanobiotechnol. 2021, 19, 75. [Google Scholar] [CrossRef]
- Cho, S.-C.; Chao, Y.-Y.; Hong, C.-Y.; Kao, C.H. The role of hydrogen peroxide in cadmium-inhibited root growth of rice seedlings. Plant Growth Regul. 2012, 66, 27–35. [Google Scholar] [CrossRef]
- Sun, F.; Chen, W.; Ren, Y.; Cheng, S.; Ashraf, U.; Zheng, J.; Lin, L.; Ma, Y.; Tang, X.; Mo, Z. Nano-Priming with La2O3 Improves Early Growth and Regulates Physio-Biochemical Mechanisms in Fragrant Rice Against Cadmium Toxicity. J. Soil. Sci. Plant Nutr. 2023, 23, 4004–4020. [Google Scholar] [CrossRef]
- Carpenter, J.R.; Mitchell, C.A. Root Respiration Characteristics of Flood-tolerant and Intolerant Tree Species1. J. Am. Soc. Hortic. Sci. 1980, 105, 684–687. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Su, P.-H.; Su, C.-H. Metabolic Responses of Luffa Roots to Long-term Flooding. J. Plant Physiol. 1996, 148, 735–740. [Google Scholar] [CrossRef]
- Wiedenroth, E.-M. Responses of roots to hypoxia: Their structural and energy relations with the whole plant. Environ. Exp. Bot. 1993, 33, 41–51. [Google Scholar] [CrossRef]
- Du, B.; Wang, W.; Jiang, S.; Xie, Y.; Cheng, Y.; Xu, J.; Xing, D. Silicon and selenium fertilizer management improved productivity and aroma of fragrant rice. Crop Sci. 2021, 61, 936–946. [Google Scholar] [CrossRef]
- Chen, W.; Liao, G.; Sun, F.; Ma, Y.; Chen, Z.; Chen, H.; Tang, X.; Mo, Z. Foliar spray of La2O3 nanoparticles regulates the growth, antioxidant parameters, and nitrogen metabolism of fragrant rice seedlings in wet and dry nurseries. Environ. Sci. Pollut. Res. 2023, 30, 80349–80363. [Google Scholar] [CrossRef]
- Lantian, R.; Bing, H.; Xiang, Z.; Qin, S.; Shanjun, S.; Shoucheng, H.; Hong, W.; Li, Y.; Congjun, Z. Effects of press-formed crop residue rice seedling tray on the physiological characteristics of machine-transplanted rice seedlings. Pak. J. Bot. 2020, 52, 769–1775. [Google Scholar] [CrossRef]
- Qing, B.; Jiang, Y.; Chen, Y.; Chen, J.; Xie, H.; Mo, Z. Nitrogen modulates early growth and physio-biochemical attributes in fragrant rice grown under cadmium and multiwall carbon nanotubes stresses. Environ. Sci. Pollut. Res. 2022, 29, 67837–67855. [Google Scholar] [CrossRef]
- Niu, L.; Liao, W. Hydrogen Peroxide Signaling in Plant Development and Abiotic Responses: Crosstalk with Nitric Oxide and Calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, X.L.; Zhu, Y.; Yang, X.M.; Zhang, K.N.; Xiao, Z.Y.; Wang, H.; Zhao, J.H.; Zhang, L.L.; Li, G.B.; et al. Osa-miR398b boosts H2O2 production and rice blast disease-resistance via multiple superoxide dismutases. New Phytol. 2019, 222, 1507–1522. [Google Scholar] [CrossRef] [PubMed]
- Sariñana-Aldaco, O.; Sánchez-Chávez, E.; Troyo-Diéguez, E.; Tapia-Vargas, L.M.; Díaz-Pérez, J.C.; Preciado-Rangel, P. Foliar Aspersion of Salicylic Acid Improves Nutraceutical Quality and Fruit Yield in Tomato. Agriculture 2020, 10, 482. [Google Scholar] [CrossRef]
- Sardar, R.; Ahmed, S.; Yasin, N.A. Titanium dioxide nanoparticles mitigate cadmium toxicity in Coriandrum sativum L. through modulating antioxidant system, stress markers and reducing cadmium uptake. Environ. Pollut. 2022, 292 Pt A, 118373. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Shah, F.; Hussain, S.; Saud, S.; Hassan, S.; Tanveer, M.; Ihsan, M.Z.; Shah, A.N.; Ullah, A.; Nasrullah; Khan, F.; et al. A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol. Biochem. 2016, 103, 191–198. [Google Scholar]

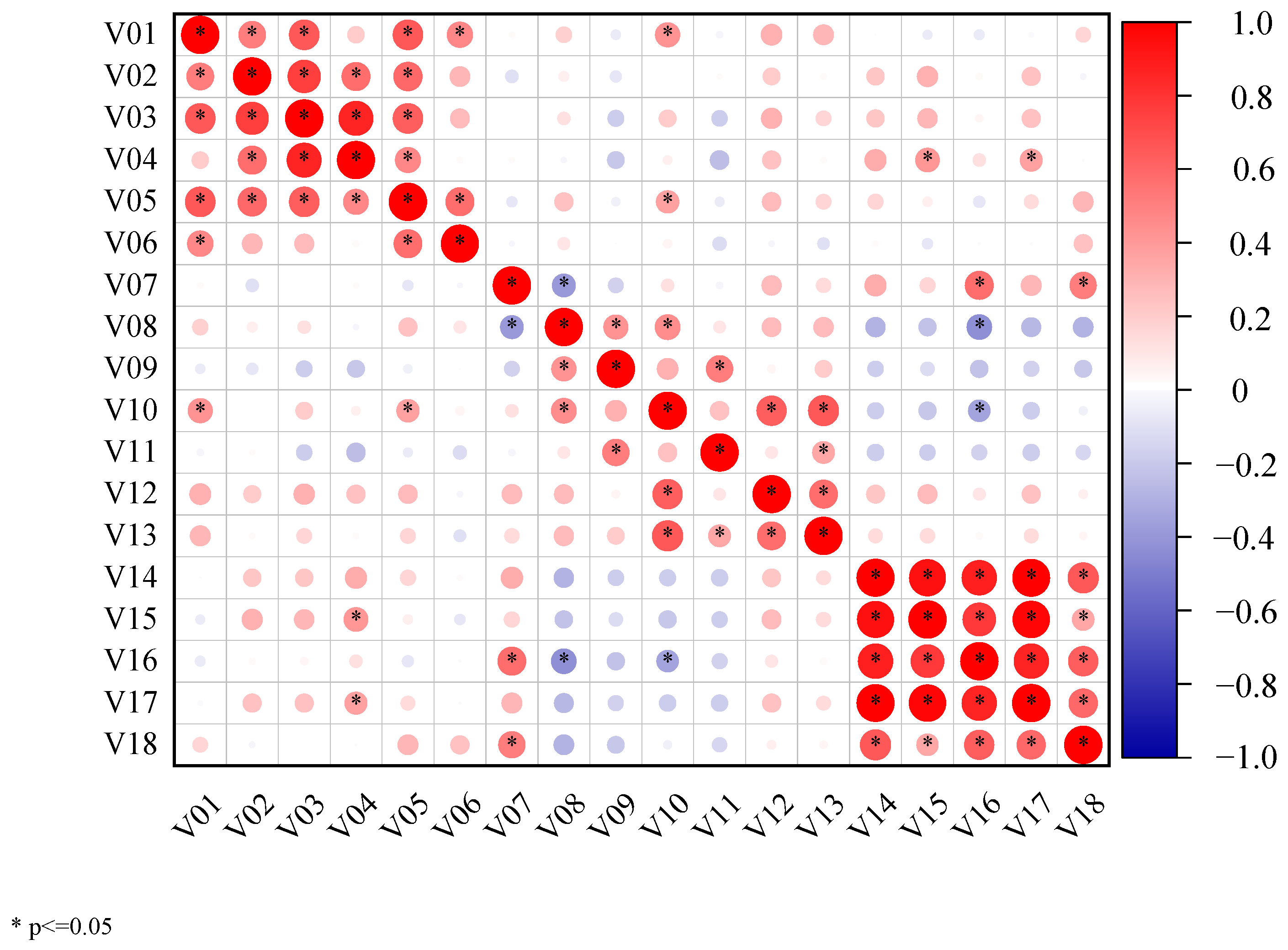
| Parameter | V | M | V × M | T | V × T | M × T | V × M × T |
|---|---|---|---|---|---|---|---|
| Plant height | ** | ** | ns | ** | ** | ** | ns |
| Dry weight of the seedlings | ns | ** | ** | ** | ** | ** | ** |
| Dry weight per unit seedling height | ns | ** | ** | ** | * | ns | * |
| Germination rate | * | ** | ns | ** | ns | ** | ns |
| Emergence rate | ns | * | ns | ** | ns | ns | ns |
| CAT activity | ** | ** | ** | ** | ** | ** | ** |
| SOD activity | ** | ** | ** | ** | ** | ** | ** |
| MDA contents | * | ** | ** | ** | ** | ** | ** |
| POD activity | ** | ** | ** | ** | ** | ** | ** |
| H2O2 content | ns | ** | ** | ** | ** | ** | ** |
| Soluble protein content | ** | ** | ** | ** | ** | ** | ** |
| Ascorbic acid content | * | ** | ** | ** | ** | ** | ** |
| Chlorophyll a content | * | ** | * | ** | ** | ** | ** |
| Chlorophyll b content | * | ** | ns | ** | ** | ** | ** |
| Carotenoids content | ** | ** | ** | ** | ** | ** | ** |
| Chlorophyll content | * | ** | * | ** | ** | ** | ** |
| Chlorophyll a:b | ns | ** | ns | ** | ** | ** | ns |
| Variety | Material | Treatment | Germination Rate (%) | Emergence Rate (%) |
|---|---|---|---|---|
| Wufengyou 286 | CK | 0 | 66.94 ± 6.84 de | 84.93 ± 3.44 f |
| O | 5 | 87.22 ± 6.05 ab | 92.50 ± 1.74 bcde | |
| O | 10 | 33.61 ± 6.13 g | 91.10 ± 1.84 de | |
| O | 15 | 40.56 ± 3.06 fg | 95.10 ± 0.93 abcd | |
| O | 20 | 49.72 ± 15.92 f | 93.27 ± 1.82 abcde | |
| S | 5 | 95.83 ± 0.83 a | 96.83 ± 0.23 ab | |
| S | 10 | 94.72 ± 3.20 a | 98.03 ± 1.58 a | |
| S | 15 | 81.67 ± 0.48 abcd | 94.57 ± 1.72 abcd | |
| S | 20 | 85.00 ± 3.00 abc | 95.63 ± 2.03 abcd | |
| NA | 5 | 93.89 ± 1.69 a | 97.97 ± 0.73 a | |
| NA | 10 | 87.22 ± 2.17 ab | 98.07 ± 0.58 a | |
| NA | 15 | 71.67 ± 1.44 bcd | 96.50 ± 0.75 abc | |
| NA | 20 | 50.28 ± 5.62 f | 88.63 ± 1.74 ef | |
| NB | 5 | 86.11 ± 5.30 abc | 97.40 ± 0.80 ab | |
| NB | 10 | 68.06 ± 4.84 de | 95.70 ± 1.46 abcd | |
| NB | 15 | 70.56 ± 3.09 cde | 96.43 ± 1.45 abc | |
| NB | 20 | 55.00 ± 4.74 ef | 91.70 ± 3.49 cde | |
| Zhongjiazao 17 | CK | 0 | 56.11 ± 4.72 c | 87.93 ± 3.34 d |
| O | 5 | 80.00 ± 3.63 a | 93.77 ± 0.47 abc | |
| O | 10 | 26.11 ± 5.70 gh | 91.77 ± 2.19 bcd | |
| O | 15 | 19.17 ± 2.41 h | 88.23 ± 3.80 cd | |
| O | 20 | 32.78 ± 2.78 fg | 95.53 ± 1.13 ab | |
| S | 5 | 77.78 ± 2.17 a | 93.20 ± 0.74 abcd | |
| S | 10 | 76.11 ± 3.09 a | 97.47 ± 0.30 a | |
| S | 15 | 60.00 ± 2.41 bc | 94.07 ± 1.59 ab | |
| S | 20 | 48.06 ± 3.92 cde | 92.23 ± 1.91 abcd | |
| NA | 5 | 78.61 ± 2.27 a | 96.43 ± 1.07 ab | |
| NA | 10 | 68.33 ± 2.50 ab | 96.00 ± 0.98 ab | |
| NA | 15 | 35.00 ± 1.73 fg | 93.50 ± 1.91 abcd | |
| NA | 20 | 39.44 ± 5.53 def | 90.90 ± 2.92 bcd | |
| NB | 5 | 70.28 ± 8.66 ab | 95.17 ± 1.22 ab | |
| NB | 10 | 50.00 ± 5.42 cd | 93.67 ± 1.69 abc | |
| NB | 15 | 50.83 ± 5.46 cd | 94.10 ± 2.57 ab | |
| NB | 20 | 36.94 ± 2.27 efg | 91.63 ± 1.10 bcd |
| Variety | Material | Treatment | Plant Height (cm) | Leaf Age |
|---|---|---|---|---|
| Wufengyou 286 | CK | 0 | 13.94 ± 0.30 g | 3.23 ± 0.14 d |
| O | 5 | 17.19 ± 0.56 ab | 3.94 ± 0.10 a | |
| O | 10 | 13.96 ± 0.46 g | 3.19 ± 0.12 d | |
| O | 15 | 15.07 ± 0.40 def | 3.28 ± 0.15 d | |
| O | 20 | 16.12 ± 0.38 cd | 3.31 ± 0.13 d | |
| S | 5 | 15.99 ± 0.36 cd | 3.95 ± 0.05 a | |
| S | 10 | 16.15 ± 0.31 bc | 3.67 ± 0.12 abc | |
| S | 15 | 17.59 ± 0.26 a | 3.40 ± 0.01 cd | |
| S | 20 | 17.81 ± 0.46 a | 3.21 ± 0.08 d | |
| NA | 5 | 15.36 ± 0.42 cde | 3.73 ± 0.11 ab | |
| NA | 10 | 15.61 ± 0.41 cde | 3.43 ± 0.13 bcd | |
| NA | 15 | 15.78 ± 0.37 cde | 3.14 ± 0.08 d | |
| NA | 20 | 14.12 ± 0.38 fg | 3.45 ± 0.07 bcd | |
| NB | 5 | 15.48 ± 0.23 cde | 3.35 ± 0.13 d | |
| NB | 10 | 15.32 ± 0.40 cde | 3.37 ± 0.15 cd | |
| NB | 15 | 14.81 ± 0.40 efg | 3.18 ± 0.11 d | |
| NB | 20 | 15.69 ± 0.25 cde | 3.37 ± 0.07 cd | |
| Zhongjiazao 17 | CK | 0 | 13.96 ± 0.50 de | 3.21 ± 0.11 defgh |
| O | 5 | 15.72 ± 0.22 ab | 3.76 ± 0.12 ab | |
| O | 10 | 13.62 ± 0.47 de | 3.09 ± 0.08 gh | |
| O | 15 | 13.51 ± 0.55 de | 3.15 ± 0.13 efgh | |
| O | 20 | 15.62 ± 0.39 ab | 3.57 ± 0.12 bc | |
| S | 5 | 16.05 ± 0.29 ab | 3.95 ± 0.11 a | |
| S | 10 | 16.06 ± 0.29 ab | 3.51 ± 0.13 bcd | |
| S | 15 | 16.25 ± 0.26 ab | 3.62 ± 0.16 bc | |
| S | 20 | 16.47 ± 0.26 a | 3.38 ± 0.07 cdefg | |
| NA | 5 | 15.31 ± 0.32 bc | 3.55 ± 0.16 bc | |
| NA | 10 | 15.37 ± 0.20 bc | 3.56 ± 0.13 bc | |
| NA | 15 | 13.33 ± 0.44 e | 2.95 ± 0.04 h | |
| NA | 20 | 14.12 ± 0.38 de | 3.45 ± 0.07 bcde | |
| NB | 5 | 14.43 ± 0.37 cd | 3.63 ± 0.15 abc | |
| NB | 10 | 13.64 ± 0.31 de | 3.12 ± 0.11 fgh | |
| NB | 15 | 13.61 ± 0.22 de | 3.12 ± 0.07 fgh | |
| NB | 20 | 13.92 ± 0.24 de | 3.43 ± 0.13 cdef |
| Variety | Material | Treatment | Seedling Dry Weight | Dry Weight Per Unit Seedling Height |
|---|---|---|---|---|
| Wufengyou 286 | CK | 0 | 0.27 ± 0.01 efgh | 0.021 ± 0.001 b |
| O | 5 | 0.37 ± 0.01 b | 0.021 ± 0.002 bc | |
| O | 10 | 0.26 ± 0.02 fghi | 0.019 ± 0.002 bcd | |
| O | 15 | 0.24 ± 0.01 ij | 0.015 ± 0.000 fg | |
| O | 20 | 0.25 ± 0.01 ghij | 0.015 ± 0.001 g | |
| S | 5 | 0.41 ± 0.02 a | 0.025 ± 0.000 a | |
| S | 10 | 0.28 ± 0.01 def | 0.018 ± 0.001 cdefg | |
| S | 15 | 0.33 ± 0.01 c | 0.019 ± 0.001 bcd | |
| S | 20 | 0.31 ± 0.01 cd | 0.018 ± 0.001 cdefg | |
| NA | 5 | 0.29 ± 0.01 de | 0.019 ± 0.002 bcd | |
| NA | 10 | 0.25 ± 0.01 hij | 0.015 ± 0.001 fg | |
| NA | 15 | 0.28 ± 0.01 defg | 0.018 ± 0.001 bcdef | |
| NA | 20 | 0.25 ± 0.00 hij | 0.017 ± 0.001 defg | |
| NB | 5 | 0.28 ± 0.00 defg | 0.019 ± 0.000 bcde | |
| NB | 10 | 0.23 ± 0.01 j | 0.016 ± 0.001 efg | |
| NB | 15 | 0.25 ± 0.01 ghij | 0.016 ± 0.001 defg | |
| NB | 20 | 0.27 ± 0.01 efgh | 0.017 ± 0.000 cdefg | |
| Zhongjiazao 17 | CK | 0 | 0.27 ± 0.01 def | 0.020 ± 0.000 bc |
| O | 5 | 0.38 ± 0.00 a | 0.023 ± 0.000 a | |
| O | 10 | 0.26 ± 0.02 def | 0.018 ± 0.000 ab | |
| O | 15 | 0.22 ± 0.01 ef | 0.016 ± 0.001 c | |
| O | 20 | 0.30 ± 0.02 def | 0.019 ± 0.002 bc | |
| S | 5 | 0.32 ± 0.01 ab | 0.020 ± 0.002 abc | |
| S | 10 | 0.29 ± 0.01 abcde | 0.018 ± 0.001 ab | |
| S | 15 | 0.30 ± 0.01 abc | 0.018 ± 0.001 bc | |
| S | 20 | 0.28 ± 0.00 f | 0.017 ± 0.000 bc | |
| NA | 5 | 0.31 ± 0.01 def | 0.021 ± 0.001 bc | |
| NA | 10 | 0.29 ± 0.00 bcdef | 0.019 ± 0.001 ab | |
| NA | 15 | 0.23 ± 0.00 bcdef | 0.016 ± 0.001 bc | |
| NA | 20 | 0.23 ± 0.01 abcd | 0.016 ± 0.001 c | |
| NB | 5 | 0.29 ± 0.01 bcdef | 0.019 ± 0.001 bc | |
| NB | 10 | 0.23 ± 0.00 bcdef | 0.018 ± 0.001 bc | |
| NB | 15 | 0.23 ± 0.01 cdef | 0.017 ± 0.001 bc | |
| NB | 20 | 0.24 ± 0.01 bcdef | 0.018 ± 0.001 bc |
| CAT Activity | SOD Activity | POD Activity | |||
|---|---|---|---|---|---|
| Variety | Material | Treatment | (mmol·min−1·mg−1·FW) | (U·mg−1·FW) | (U·min−1·g−1·FW) |
| Wufengyou 286 | CK | 0 | 431.52 ± 4.94 d | 0.79 ± 0.09 d | 259.88 ± 1.67 cd |
| O | 5 | 410.45 ± 12.53 de | 0.77 ± 0.02 d | 274.07 ± 2.55 b | |
| O | 10 | 416.03 ± 14.62 de | 0.77 ± 0.02 d | 274.00 ± 1.28 b | |
| O | 15 | 375.98 ± 12.40 efg | 0.77 ± 0.00 d | 166.83 ± 7.67 f | |
| O | 20 | 499.38 ± 12.45 c | 0.58 ± 0.04 e | 281.08 ± 4.11 b | |
| S | 5 | 589.39 ± 12.42 b | 0.76 ± 0.02 d | 269.66 ± 1.43 bc | |
| S | 10 | 408.40 ± 12.47 de | 1.22 ± 0.01 a | 298.67 ± 4.32 a | |
| S | 15 | 482.05 ± 28.93 c | 0.91 ± 0.05 bc | 253.71 ± 3.96 d | |
| S | 20 | 497.51 ± 12.40 c | 0.97 ± 0.03 b | 281.70 ± 2.98 b | |
| NA | 5 | 210.71 ± 18.77 h | 0.64 ± 0.03 e | 137.85 ± 6.62 h | |
| NA | 10 | 355.29 ± 7.15 fg | 1.21 ± 0.00 a | 305.74 ± 3.99 a | |
| NA | 15 | 641.41 ± 8.87 a | 0.76 ± 0.08 d | 307.92 ± 5.75 a | |
| NA | 20 | 398.22 ± 7.20 de | 0.79 ± 0.02 d | 198.67 ± 7.01 e | |
| NB | 5 | 133.09 ± 12.42 i | 0.64 ± 0.03 e | 145.57 ± 2.86 gh | |
| NB | 10 | 346.03 ± 12.42 g | 0.65 ± 0.04 e | 143.46 ± 3.80 h | |
| NB | 15 | 395.75 ± 18.92 def | 0.84 ± 0.02 cd | 194.12 ± 6.94 e | |
| NB | 20 | 565.17 ± 25.91 b | 0.78 ± 0.02 d | 158.12 ± 0.31 fg | |
| Zhongjiazao 17 | CK | 0 | 513.98 ± 26.38 ef | 0.55 ± 0.02 de | 196.40 ± 11.13 ab |
| O | 5 | 292.80 ± 19.14 h | 0.99 ± 0.11 a | 137.26 ± 3.08 defg | |
| O | 10 | 293.89 ± 19.21 h | 0.99 ± 0.11 a | 127.33 ± 2.46 fgh | |
| O | 15 | 477.26 ± 14.32 f | 0.68 ± 0.03 bcd | 172.47 ± 3.79 c | |
| O | 20 | 387.43 ± 14.69 g | 0.70 ± 0.04 bc | 150.84 ± 5.04 d | |
| S | 5 | 661.13 ± 19.23 cd | 0.69 ± 0.02 bc | 187.35 ± 3.87 bc | |
| S | 10 | 759.88 ± 7.15 a | 0.77 ± 0.03 b | 208.03 ± 1.08 a | |
| S | 15 | 632.32 ± 19.04 d | 0.36 ± 0.07 fg | 144.89 ± 1.57 de | |
| S | 20 | 625.26 ± 28.01 d | 0.57 ± 0.03 cde | 185.26 ± 11.78 bc | |
| NA | 5 | 687.50 ± 21.73 bc | 0.63 ± 0.03 cd | 141.78 ± 6.94 def | |
| NA | 10 | 533.87 ± 17.74 e | 0.26 ± 0.03 g | 131.04 ± 3.35 efgh | |
| NA | 15 | 658.73 ± 7.15 cd | 0.47 ± 0.05 ef | 132.06 ± 2.44 efgh | |
| NA | 20 | 397.23 ± 18.99 g | 0.97 ± 0.02 a | 142.98 ± 4.75 def | |
| NB | 5 | 385.50 ± 14.62 g | 0.70 ± 0.04 bc | 119.07 ± 6.73 h | |
| NB | 10 | 740.57 ± 21.48 ab | 0.59 ± 0.01 cde | 180.20 ± 2.93 c | |
| NB | 15 | 743.35 ± 21.56 a | 0.59 ± 0.01 cde | 178.89 ± 6.80 c | |
| NB | 20 | 383.58 ± 14.54 g | 0.70 ± 0.04 bc | 124.09 ± 1.08 gh |
| Variety | Material | Treatment | MDA Contents | H2O2 Contents | Soluble Protein Contents | Ascorbic Acid Contents |
|---|---|---|---|---|---|---|
| (μmol·g−1·FW) | (μmol·g−1·FW) | (mg·g−1·FW) | (μg·g−1·FW) | |||
| Wufengyou 286 | CK | 0 | 22.84 ± 0.62 c | 6.63 ± 0.16 d | 7.98 ± 0.17 ab | 0.63 ± 0.01 abc |
| O | 5 | 19.09 ± 0.67 efg | 5.26 ± 0.21 h | 8.21 ± 0.69 a | 0.63 ± 0.01 abc | |
| O | 10 | 16.61 ± 0.33 ghi | 5.53 ± 0.10 gh | 8.52 ± 0.28 a | 0.62 ± 0.04 abcd | |
| O | 15 | 15.89 ± 0.58 hi | 5.63 ± 0.16 fgh | 2.43 ± 0.11 f | 0.56 ± 0.05 d | |
| O | 20 | 38.47 ± 0.52 b | 11.89 ± 0.23 a | 4.50 ± 0.05 e | 0.67 ± 0.01 a | |
| S | 5 | 17.95 ± 1.84 fgh | 6.00 ± 0.00 efg | 8.33 ± 0.39 a | 0.62 ± 0.02 abcd | |
| S | 10 | 50.99 ± 1.46 a | 7.82 ± 0.37 c | 7.35 ± 0.20 bc | 0.60 ± 0.02 abcd | |
| S | 15 | 17.29 ± 0.69 fgh | 6.17 ± 0.16 def | 7.05 ± 0.21 cd | 0.59 ± 0.02 bcd | |
| S | 20 | 22.55 ± 1.67 cd | 6.23 ± 0.10 de | 8.10 ± 0.14 a | 0.67 ± 0.02 a | |
| NA | 5 | 11.48 ± 1.14 j | 5.64 ± 0.06 fgh | 4.90 ± 0.22 e | 0.43 ± 0.01 e | |
| NA | 10 | 20.14 ± 1.37 cdef | 8.20 ± 0.24 c | 7.10 ± 0.10 cd | 0.58 ± 0.02 cd | |
| NA | 15 | 21.31 ± 1.73 cde | 6.18 ± 0.31 def | 8.18 ± 0.32 a | 0.59 ± 0.02 bcd | |
| NA | 20 | 19.75 ± 0.40 def | 11.31 ± 0.14 b | 6.59 ± 0.16 d | 0.65 ± 0.01 abc | |
| NB | 5 | 18.74 ± 0.95 efgh | 5.45 ± 0.12 gh | 2.93 ± 0.18 f | 0.43 ± 0.01 e | |
| NB | 10 | 19.64 ± 1.08 defg | 5.33 ± 0.12 h | 2.80 ± 0.11 f | 0.44 ± 0.04 e | |
| NB | 15 | 13.71 ± 0.27 ij | 5.56 ± 0.32 gh | 4.56 ± 0.22 e | 0.58 ± 0.04 bcd | |
| NB | 20 | 11.92 ± 0.91 j | 5.50 ± 0.14 gh | 6.42 ± 0.15 d | 0.65 ± 0.02 ab | |
| Zhongjiazao 17 | CK | 0 | 18.30 ± 0.92 de | 6.60 ± 0.41 bc | 4.94 ± 0.62 c | 0.58 ± 0.05 a |
| O | 5 | 10.72 ± 0.42 h | 5.63 ± 0.23 de | 1.88 ± 0.02 f | 0.41 ± 0.05 f | |
| O | 10 | 33.69 ± 4.14 a | 6.32 ± 0.12 bcde | 2.48 ± 0.04 ef | 0.43 ± 0.00 ef | |
| O | 15 | 22.81 ± 0.19 c | 5.93 ± 0.15 cde | 4.77 ± 0.24 c | 0.44 ± 0.02 def | |
| O | 20 | 22.12 ± 0.43 c | 6.73 ± 0.36 bc | 7.27 ± 0.21 b | 0.59 ± 0.00 a | |
| S | 5 | 14.82 ± 0.87 fg | 6.33 ± 0.27 bcde | 7.62 ± 0.19 ab | 0.58 ± 0.05 a | |
| S | 10 | 14.12 ± 1.05 fg | 6.34 ± 0.57 bcde | 8.32 ± 0.13 a | 0.58 ± 0.05 a | |
| S | 15 | 10.64 ± 0.11 h | 6.02 ± 0.36 cde | 3.76 ± 0.29 d | 0.38 ± 0.03 f | |
| S | 20 | 12.91 ± 0.50 gh | 5.93 ± 0.29 cde | 7.20 ± 0.15 b | 0.46 ± 0.02 bcdef | |
| NA | 5 | 18.45 ± 0.40 de | 6.36 ± 0.12 bcde | 5.36 ± 0.11 c | 0.55 ± 0.05 ab | |
| NA | 10 | 15.26 ± 0.82 efg | 6.60 ± 0.25 bc | 1.99 ± 0.10 f | 0.52 ± 0.02 abcd | |
| NA | 15 | 16.87 ± 0.40 def | 5.56 ± 0.23 e | 3.90 ± 0.22 d | 0.59 ± 0.02 a | |
| NA | 20 | 30.56 ± 0.84 ab | 6.90 ± 0.27 b | 3.00 ± 0.03 e | 0.53 ± 0.02 abc | |
| NB | 5 | 28.02 ± 0.73 b | 8.03 ± 0.24 a | 7.39 ± 0.03 b | 0.50 ± 0.02 abcde | |
| NB | 10 | 18.66 ± 0.57 d | 6.49 ± 0.49 bc | 5.03 ± 0.39 c | 0.44 ± 0.01 cdef | |
| NB | 15 | 22.16 ± 0.46 c | 6.01 ± 0.17 cde | 4.90 ± 0.52 c | 0.44 ± 0.02 cdef | |
| NB | 20 | 16.17 ± 0.41 defg | 6.47 ± 0.06 bcd | 7.12 ± 0.03 b | 0.46 ± 0.01 cdef |
| Chlorophyll a Contents | Chlorophyll b Contents | Carotenoid Contents | Chlorophyll Contents | Chlorophyll a:b | |||
|---|---|---|---|---|---|---|---|
| Variety | Material | Treatment | (mg·g−1·FW) | (mg·g−1·FW) | (mg·g−1·FW) | (mg·g−1·FW) | |
| Wufengyou 286 | CK | 0 | 0.40 ± 0.012 ab | 0.17 ± 0.004 ab | 0.05 ± 0.003 bc | 0.57 ± 0.016 ab | 2.32 ± 0.024 bcd |
| O | 5 | 0.39 ± 0.012 abcd | 0.18 ± 0.004 a | 0.05 ± 0.002 bc | 0.57 ± 0.016 ab | 2.14 ± 0.016 e | |
| O | 10 | 0.28 ± 0.005 hi | 0.14 ± 0.002 def | 0.04 ± 0.000 cdefg | 0.43 ± 0.008 gh | 2.01 ± 0.005 f | |
| O | 15 | 0.28 ± 0.008 hi | 0.13 ± 0.003 fg | 0.03 ± 0.002 fg | 0.41 ± 0.010 h | 2.24 ± 0.021 bcde | |
| O | 20 | 0.27 ± 0.023 i | 0.13 ± 0.008 fg | 0.04 ± 0.005 defg | 0.40 ± 0.030 h | 2.13 ± 0.056 ef | |
| S | 5 | 0.36 ± 0.010 bcdef | 0.16 ± 0.003 bc | 0.04 ± 0.003 cde | 0.52 ± 0.013 bcde | 2.26 ± 0.022 bcde | |
| S | 10 | 0.30 ± 0.020 ghi | 0.14 ± 0.007 efg | 0.04 ± 0.004 defg | 0.44 ± 0.027 fgh | 2.22 ± 0.053 cde | |
| S | 15 | 0.32 ± 0.017 fghi | 0.14 ± 0.005 efg | 0.04 ± 0.005 cdef | 0.45 ± 0.022 fgh | 2.32 ± 0.066 bcd | |
| S | 20 | 0.32 ± 0.008 fgh | 0.14 ± 0.003 efg | 0.04 ± 0.002 cde | 0.46 ± 0.011 efgh | 2.34 ± 0.021 bc | |
| NA | 5 | 0.39 ± 0.013 abc | 0.17 ± 0.005 abc | 0.05 ± 0.003 bc | 0.56 ± 0.019 abc | 2.34 ± 0.024 bc | |
| NA | 10 | 0.28 ± 0.043 hi | 0.13 ± 0.012 g | 0.03 ± 0.009 g | 0.41 ± 0.055 h | 2.20 ± 0.135 de | |
| NA | 15 | 0.43 ± 0.013 a | 0.17 ± 0.003 ab | 0.06 ± 0.002 a | 0.60 ± 0.017 a | 2.49 ± 0.031 a | |
| NA | 20 | 0.35 ± 0.017 cdefg | 0.15 ± 0.006 cd | 0.05 ± 0.004 cd | 0.50 ± 0.023 cdef | 2.25 ± 0.034 bcde | |
| NB | 5 | 0.34 ± 0.011 defg | 0.15 ± 0.006 cd | 0.04 ± 0.001 cde | 0.50 ± 0.016 def | 2.21 ± 0.014 cde | |
| NB | 10 | 0.34 ± 0.008 efg | 0.14 ± 0.003 de | 0.04 ± 0.002 cde | 0.48 ± 0.011 defg | 2.36 ± 0.021 b | |
| NB | 15 | 0.31 ± 0.006 ghi | 0.13 ± 0.002 efg | 0.03 ± 0.001 efg | 0.44 ± 0.007 fgh | 2.28 ± 0.034 bcd | |
| NB | 20 | 0.37 ± 0.008 bcde | 0.16 ± 0.002 bc | 0.06 ± 0.002 ab | 0.53 ± 0.010 bcd | 2.30 ± 0.032 bcd | |
| Zhongjiazao 17 | CK | 0 | 0.34 ± 0.010 efgh | 0.16 ± 0.004 defg | 0.04 ± 0.002 fghi | 0.50 ± 0.014 cde | 2.19 ± 0.008 defg |
| O | 5 | 0.29 ± 0.011 i | 0.14 ± 0.002 gh | 0.04 ± 0.004 i | 0.43 ± 0.012 f | 2.10 ± 0.077 efg | |
| O | 10 | 0.31 ± 0.031 hi | 0.15 ± 0.010 efgh | 0.04 ± 0.005 hi | 0.46 ± 0.041 ef | 2.06 ± 0.078 fg | |
| O | 15 | 0.31 ± 0.016 hi | 0.14 ± 0.004 h | 0.04 ± 0.004 ghi | 0.44 ± 0.020 ef | 2.25 ± 0.067 cde | |
| O | 20 | 0.40 ± 0.013 bcd | 0.19 ± 0.015 ab | 0.06 ± 0.001 cd | 0.59 ± 0.028 ab | 2.21 ± 0.101 def | |
| S | 5 | 0.42 ± 0.010 abc | 0.17 ± 0.002 abc | 0.06 ± 0.004 bcd | 0.60 ± 0.012 ab | 2.41 ± 0.029 ab | |
| S | 10 | 0.38 ± 0.013 cdef | 0.16 ± 0.007 cde | 0.06 ± 0.003 bc | 0.54 ± 0.019 bc | 2.30 ± 0.055 abcd | |
| S | 15 | 0.35 ± 0.010 efgh | 0.15 ± 0.003 fgh | 0.05 ± 0.002 def | 0.49 ± 0.012 cdef | 2.38 ± 0.023 abc | |
| S | 20 | 0.32 ± 0.013 hi | 0.14 ± 0.005 gh | 0.05 ± 0.003 efgh | 0.46 ± 0.017 ef | 2.26 ± 0.040 bcde | |
| NA | 5 | 0.46 ± 0.011 a | 0.19 ± 0.003 a | 0.07 ± 0.003 ab | 0.65 ± 0.015 a | 2.45 ± 0.017 a | |
| NA | 10 | 0.34 ± 0.027 efgh | 0.15 ± 0.010 efgh | 0.05 ± 0.005 defg | 0.49 ± 0.037 cdef | 2.28 ± 0.049 bcd | |
| NA | 15 | 0.42 ± 0.021 ab | 0.17 ± 0.004 abcd | 0.07 ± 0.005 a | 0.60 ± 0.025 ab | 2.46 ± 0.066 a | |
| NA | 20 | 0.38 ± 0.011 bcde | 0.17 ± 0.005 bcd | 0.06 ± 0.002 cde | 0.55 ± 0.016 bc | 2.24 ± 0.045 cde | |
| NB | 5 | 0.37 ± 0.008 defg | 0.16 ± 0.003 cdef | 0.05 ± 0.002 defg | 0.53 ± 0.011 cd | 2.31 ± 0.018 abcd | |
| NB | 10 | 0.33 ± 0.017 fghi | 0.14 ± 0.004 gh | 0.05 ± 0.003 defg | 0.48 ± 0.020 def | 2.35 ± 0.075 abcd | |
| NB | 15 | 0.33 ± 0.010 ghi | 0.15 ± 0.005 efgh | 0.05 ± 0.002 fghi | 0.48 ± 0.013 def | 2.25 ± 0.082 cde | |
| NB | 20 | 0.32 ± 0.015 hi | 0.15 ± 0.004 defg | 0.04 ± 0.003 fghi | 0.47 ± 0.019 def | 2.04 ± 0.042 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, B.; Yu, J.; Liu, M.; Liu, J.; Yu, G.; Liu, Z.; Xiao, L.; Wang, X.; Mo, Z.; Chen, X. The Effects of Different Organic Materials and Cover Thicknesses on the Early Growth and Antioxidant Response of Direct-Seeded Rice. Agronomy 2024, 14, 98. https://doi.org/10.3390/agronomy14010098
Zeng B, Yu J, Liu M, Liu J, Yu G, Liu Z, Xiao L, Wang X, Mo Z, Chen X. The Effects of Different Organic Materials and Cover Thicknesses on the Early Growth and Antioxidant Response of Direct-Seeded Rice. Agronomy. 2024; 14(1):98. https://doi.org/10.3390/agronomy14010098
Chicago/Turabian StyleZeng, Bohan, Jiajia Yu, Muhua Liu, Junan Liu, Guodong Yu, Zhaopeng Liu, Liping Xiao, Xiao Wang, Zhaowen Mo, and Xiongfei Chen. 2024. "The Effects of Different Organic Materials and Cover Thicknesses on the Early Growth and Antioxidant Response of Direct-Seeded Rice" Agronomy 14, no. 1: 98. https://doi.org/10.3390/agronomy14010098
APA StyleZeng, B., Yu, J., Liu, M., Liu, J., Yu, G., Liu, Z., Xiao, L., Wang, X., Mo, Z., & Chen, X. (2024). The Effects of Different Organic Materials and Cover Thicknesses on the Early Growth and Antioxidant Response of Direct-Seeded Rice. Agronomy, 14(1), 98. https://doi.org/10.3390/agronomy14010098






