Abstract
Drought and high temperatures are among most dangerous attributes of climate change, which negatively affects the quantity and quality of sugar beet production. One of the most effective tools for eliminating unwanted effects is the application of biostimulants during the growing season. In this study, a 4 × 3 factorial scheme was adopted: Two biostimulant treatments, namely (i) pure extract from brown seaweed Ascophylum nodosum (B1) and (ii) concentrate from the seaweed Ascophylum nodosum and humus substances (B2), were compared to a control treatment (B0) in an experiment with four sugar beet varieties (Fischer, Fabius, Nicolaus, Lucius). The two-year research proved the significant influence of biostimulants on all monitored physiological and production parameters of sugar beet, with the exception of potassium content. Biostimulants positively influenced the results of root yield, polarized and white sugar yield, and the values of LAI (leaf area index), NDVI (normalized difference vegetation index), and PRI (photochemical reflectance index), while the positive effect on sugar content was only in the case of B1 treatment. The production potential fluctuated significantly depending on the observed interaction, but it can be concluded that the most limiting factor of production is the course of weather conditions. However, after treatment with biostimulants, an increased root yield (B2) and sugar content (B1) were found. Moreover, in this experiment, a strong positive relationship between root yield and physiological parameters (NDVI and PRI) and LAI was proven, while the relationship of sugar content to these parameters was weak. Monitoring of the physiological response to biostimulant application shows a high potential from the sustainability perspective in the context of sugar beet production. In addition, the impact on the height and quality of production was evident.
1. Introduction
Global climatic records of the past decades show a temperature increase and changes in rainfall, which have an effect on the environment and agriculture [1]. Furthermore, if the changes continue at the current rate, IPCC [2] forecasts a global warming of 1.5 °C between 2030 and 2052. Unfortunately, agriculture is exceedingly sensitive to climatic changes. Higher temperatures, changes in rainfall, and increasing frequency of dry periods decrease the yields of important crops and increase the probability of long-term decline of production [3,4].
In addition, these risks can be observed the most in the temperate zone. Sugar beet is the second most important crop used for industrial production of sucrose [5,6]. As reported by Zhang et al. [7], approximately 35% of global sugar production is supplied from this valuable crop. Based on the data from the Food and Agriculture Organization, the current global production of sugar beet is 270.2 million tons [8]. Although most of the primary production is used for sugar manufacturing, changes in global markets have forced European producers to seek alternative ways of utilizing obtained sucrose, such as for production of biodegradable polymers from dehydrated carbohydrates [9] or for use in pharmaceutical, beauty, and medical industries [10]. Furthermore, bioethanol production from sugar beet is becoming increasingly popular [11], and as reported by Panella [12], bioethanol manufactured from this crop reduces greenhouse gas emissions comparably to or more significantly than that from maize and sugar cane. However, drought stress is one of the most limiting factors of sugar beet production; it not only causes decrease in the harvest yield but also lowers the quality through accumulation of compatible solutes [13,14,15]. Trimpler et al. [16] also attributed the unfulfillment of the growth potential of the sugar beet to the weather conditions during the growth, which are often not optimal.
Moreover, for sugar beet to reach its production potential, an interaction of multiple factors is necessary, and the crop must be able to use them [17]. Sánchez-Sastre et al. [18] concluded that the production potential of sugar beet results from its ability to capture light for photosynthesis, that is, the ability to close its canopy as fast as possible for the longest period, thus optimizing the intake of light radiation. These processes can be quantified using spectral reflectance, which is used to monitor water capacity and physiological changes in the vegetation [19]. The normalized difference vegetation index (NDVI) is generally considered to be one of the most utilized vegetation indices [20]; its value is influenced especially by the size of the leaf area but also by the content of nitrogen, chlorophyll, and water in the plant tissue [21,22]. To determine the efficiency of light use, the photochemical reflectance index (PRI) can be used [23]; however, its use is conditioned upon full lighting of the leaves [24]. PRI can be also used as an indicator of water stress in early growth phases [25]. The depth of chlorophyll absorption can be measured using the modified chlorophyll absorption in reflectance index (MCARI), which is sensitive to changes in chlorophyll concentration, and the leaf area index (LAI) [26]. Moreover, Haboudane et al. [27] showed that the MCARI is sensitive to non-photosynthetic materials, especially at low chlorophyll concentrations.
Thus, the need to find new approaches to stimulate or protect plants is increasingly necessary. The application of plant biostimulants is becoming a common agricultural procedure to stimulate growth and protect against stress [28]. The global biostimulant market is one of the most rapidly developing areas in agriculture. In 2019, the revenue reached USD 2.50 billion, and it is expected to grow to USD 5.35 billion by 2027 [29]. Nardi et al. [30] reported that biostimulants can be of various origin, produced from diverse organic materials and their combinations. Any material or microorganism applied to plants with the aim of increasing nutrient efficiency, abiotic stress tolerance, and the quality of monitored crop parameters, regardless of its nutrient content, can be considered a biostimulant. Various biostimulants stimulate the growth of plants by increasing the metabolic activity of the plants, stimulating germination, improving photosynthesis, and increasing the absorption of soil nutrients, which improves the plant productivity [31]. The main categories of stimulants include (a) humic and fulvic acids, (b) protein hydrolysates and other compounds containing nitrogen, (c) seaweed extracts, (d) chitosan and other biopolymers, (e) inorganic compounds, (f) beneficial fungi, and (g) beneficial bacteria [32]. Brown seaweed and its products are among the most used in agriculture due to the presence of many nutrients that stimulate plant growth [33]. Seaweed extracts are used to support growth and to improve tolerance to salinity, heat, and drought [28]; one of the most used types is Ascophyllum nodosum [34]. Humic substances also represent organic materials present in the nature, and their positive effect on plant physiology, nutrient intake, and root growth has been confirmed [35]. A basic mechanism of the effect of humic substances is their interaction with auxins, jasmonic acid, and abscisic acid, which are known growth hormones present during stress caused by drought and high salinity, using phytohormonal regulation in the root [36,37].
Another factor that can decrease the negative influence of weather conditions is the choice of appropriate genetic material [38]. As reported by Curcic et al. [39], the available hybrids react differently to various growth conditions and do not have the same requirements or reactions to local environmental influences. A problem in breeding crops for drought tolerance is its time and economical cost [38]; however, the identification of morphophysiological features related to drought tolerance and high production potential proves a challenge when choosing sugar beet genotypes with high water-stress tolerance [40].
Thus, the objective and hypothesis of this study is to monitor the effect of biostimulants on the final production and quality of various sugar beet varieties using vegetation indices. Moreover, an important aim was to find interactions between the monitored factors and sugar beet parameters. All measurements were conducted in field conditions, which increases the applicability of the findings in practice.
2. Materials and Methods
The field experiment was conducted in 2021 and 2022 at the experimental station of Slovak University of Agriculture (SUA) in Nitra (E 18°09′, N 48°19′). This location belongs to a temperate area; the Zobor Mountains are in the near vicinity, and the river Nitra flows nearby. Based on its climatic conditions, it can be concluded that the location is warm and dry. Figure 1 shows the temperature and rainfall during the experiment as compared with a 30-year climatic normal. Data were recorded using hydro-meteorological station directly at the experimental base. The soil at the experimental plot was classified as haplic luvisol, and the soil texture was classified as silt loam.
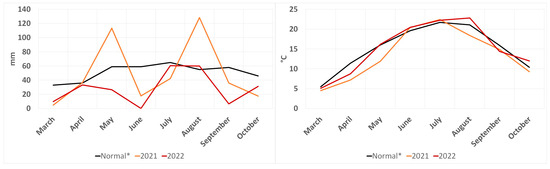
Figure 1.
Rainfall and temperature in experimental station during sugar beet vegetation in 2021 and 2022 (* represents climatic normal of experimental location from 1991–2020).
2.1. Experimental Setup and Design
The forecrop for sugar beet was common wheat (Triticum aestivum L.). After the forecrop harvest, the soil samples were taken from depth 0.30 m; subsequently, the following values were determined in a laboratory: phosphorus 26.30 and 31.30 mg kg−1, and potassium 200.00 and 275 mg kg−1, as well as humus content 2.08 and 1.65%, and soil pH 6.33 and 5.75 in 2021 and 2022, respectively. Total nitrogen content was determined in spring as 8.10 mg kg−1 for 2021 and 11.70 mg kg−1 for 2022. The ammonia form of nitrogen was determined calorimetrically using Nessler’s reagent [41] and the nitrate form calorimetrically using phenol 2.4-disulfonic acid, as in Pačuta et al. [42]. The Mehlich III test was used to determine the phosphorus and potassium content in the soil [43]. Furthermore, soil samples were collected to determine the soil reaction using a 1-molar solution of potassium chloride [44] and the humus content based on the Tjurin method [45].
In both years, prior to the establishment of the experiment, autumn deep plowing with PK fertilization was carried out using Zetor Forterra (Zetor Tractors, a.s. Brno, Czech Republic). Nitrogen fertilization was carried out in two doses: during pre-sowing preparation in the spring and during vegetation.
The experiment was established using the randomized complete block design [46] in three replications. Factorial scheme was as follows: 4 (varieties) × 3 (treatments). Sowing was conducted with a 12-row drill Monopill S (Kverneland group, Klepp, Norway) on 26 March 2021 and 31 March 2022, respectively. The seed distance in a row was 0.18 m, row spacing was 0.45 m, and each experimental plot had the following dimensions: 6 m length × 5.4 m width.
All agrotechnical operations during vegetation (e.g., application of herbicides and fungicides) was conducted according to the requirements and needs of the sugar beet vegetation.
2.2. Biostimulants and Plant Material
Two biostimulant treatments (B1 and B2) were applied in the experiment. The basis of both was an extract from brown seaweed Ascophyllum nodosum. Treatment B1 comprised purely natural product produced from double seaweed fermentation and applied in a dose of 1 L ha−1. Treatment B2 can be characterized as a super-concentrate, comprising seaweed extract (60%), organic humus substances (30%), and selected macro- and microelements (10%), which was applied in a dose of 1 L ha−1. The biostimulants were foliarly applied twice during the vegetation period in phases BBCH19 and BBCH33, with a dose of H2O 200 L ha−1. In the same phases, H2O in a dose of 200 L ha−1 was applied on the control plots.
Four commercial varieties of sugar beet, i.e., Fischer, Fabius, Nicolaus, and Lucius (Strube D&S GmbH, Söllingen, Germany), were used in the experiment. All varieties are diploid and tolerant to beet necrotic yellow vein virus a Cercospora beticola. Except for the Fischer (S-type) variety, all other varieties were of the transitional N/S type.
2.3. Analysis of Leaf Area Index and Physiological Parameters
Leaf area index (LAI) was measured three times during the vegetation period with SS1 SunScan Canopy Analysis System (Delta-T Devices Ltd., Cambridge, UK), as in Ariza-Carricondo et al. [47]. To ensure objective results, the measurements were carried out under a completely clear sky and full illumination of the leaves.
The PolyPen RP 410 device (Photon Systems Instruments Ltd., Brno, Czech Republic) was used to measure the following spectral vegetation indices: normalized difference vegetation index (NDVI), photochemical reflectance index (PRI), and modified chlorophyll absorption in reflectance index (MCARI). Similarly to LAI, the spectral indices were measured three times during vegetation. The measurements were performed between 10:00 and 13:00 in sunny and cloudless weather on the most developed leaves. Five representative plants were monitored on each experimental plot, with five measurements on each.
The equations used to calculate NDVI, PRI, and MCARI were as follows:
NDVI = (R740 − R660)/(R740 + R660);
PRI = (R531 − R570)/(R531 + R570);
MCARI = [(R700 − R670) − 0.2 × (R700 − R550)] × (R700/R670).
2.4. Harvest and Quality Analysis of Sugar Beet
The harvest was performed when the sugar beet was technologically ripe; two representative rows of each experimental plot were dug out, and subsequently, the epicotyl with the leaves were removed from the roots. The samples were weighed on mobile scales to determine the yield, which was later converted to yield per hectare. Subsequently, the samples (20 roots) were sent for a qualitative analysis to a sugar production facility (Považský cukor a.s., Trenčianska Teplá, Slovak Republic), where the Venema auto-analyzer IIIG device (Venema Consulting, Groningen, The Netherlands) was used, as in Barlog et al. [48].
Based on the obtained root yield and the quality analysis, the following parameters of white sugar content (WSC) [49], polarized sugar yield (PSY), and white sugar yield (WSY) were calculated according to Bajči et al. [50]:
WSC = SC − [(K + Na) × 0.343 + (0.094 × α-aminoN) + 0.29] (%);
PSY = 0.01 × (RY × SC) (t ha−1);
WSY = 0.01 × (RY × WSC) (t ha−1).
2.5. Statistical Analysis
The obtained data were analyzed and graphically represented using Statistica 10.0 software (StatSoft, Inc., Tulsa, OK, USA). A multifactor ANOVA was used, and to determine a within-factor significant level, post hoc analysis with Tukey test at the probability level of 0.05 was conducted. The conditions for the use of ANOVA were tested. Normal distribution was tested by Shapiro–Wilk test. Homogeneity of variances was determined by Bartlett’s test. Pearson correlation coefficient was used to analyze the relationships between the individual sugar beet traits.
3. Results
Based on the obtained meteorological data (Figure 1) and ANOVA results (Table 1 and Table S1) from the experimental years, it can be assumed that the fluctuation of rainfall and temperatures during the experimental years could have been one of the causes that significantly influenced the results of physiological and production parameters. It is possible to assume that the precipitation deficit in almost all months of the 2022 vegetation year and the above-average heat in July and August resulted in a decrease of the physiological activity and a subsequent decrease in sugar beet production. On the contrary, the average rainfall in May and August 2021 significantly affected the yield formation and the physiological functions of sugar beet.

Table 1.
Analysis of variance (ANOVA) for normalized difference vegetation index (NDVI), photochemical reflectance index (PRI), modified chlorophyll absorption in reflectance index (MCARI), and leaf area index (LAI).
3.1. Biostimulant Impact on Physiological Parameters and Leaf Area Index of Sugar Beet
In the average of the results, it can be definitively confirmed that the effect of biostimulants on physiological parameters was positive, and significant differences were found compared to the control treatment (Table 2). For NDVI, the significantly highest value was found in the B1 treatment, while for PRI, no significant difference was found between B1 and B2; however, compared to the control, these values were significantly higher. On the contrary, MCARI values were significantly highest in the control treatment. This can be evaluated positively, as high MCARI values indicate a low chlorophyll content in the leaves.

Table 2.
Evaluation of production and physiological parameters depending on biostimulant treatment.
The intensity of biomass growth, which was represented by LAI in this experiment, was significantly affected by biostimulant treatments (Table 1). As can be seen in Table 2, LAI values were significantly different in the trend B0 < B1 < B2. With regard to the above findings, a causal relationship between LAI under the influence of biostimulant treatment and the plant’s physiological response to this treatment is evident.
The direct influence on the course and progress of physiological parameters depending on the date of biostimulants treatment is shown in Figure 2. However, from the point of view of individual parameters, different trends were recorded. While for NDVI and PRI, an increase in values was recorded after both treatment dates, in the case of MCARI, a regression was recorded depending on the date of treatment. Moreover, in the case of NDVI, a continuous progress was detected in the B2 treatment after both treatment dates. On the other hand, in terms of PRI, after the second treatment with biostimulants, a significant decrease in values was detected compared to the previous measurements. The MCARI values were highest in the control treatment after the first two measurements, but after the second application, the trend reversed, and the highest MCARI value was found in the B1 treatment.
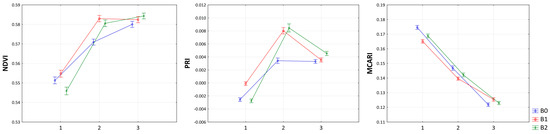
Figure 2.
Progress of normalized difference vegetation index (NDVI), photochemical reflectance index (PRI), and modified chlorophyll absorption in reflectance index (MCARI) according to date of biostimulant treatments. On the x-axis, 1 represents measurements before biostimulants treatment, 2 represents measurements after first biostimulants treatment, and 3 represents measurements after second biostimulants treatment.
3.2. Biostimulant Impact on Production Parameters of Sugar Beet
Regarding the production parameters investigated in this experiment, it can be concluded that the effect of the biostimulant treatment on almost all of them was significant, with the exception of the potassium content (Table S1). As can be seen in Table 2, the significantly highest root yield (RY), polarized sugar yield (PSY), and white sugar yield (WSY) were obtained in treatment B2. Regarding the sugar content (SC) as well as the white sugar content (WSC), significantly higher values compared to the control treatment were found in the B1 treatment. From the point of view of compatible solutes (K+, Na+, and α amino N), which reduce the yield of white sugar, reverse trends were recorded. In the case of Na+ content, the lowest value was found in the control, with a significant difference compared to B1. On the contrary, in the case of α amino N content, significantly lower values were detected in treatments with biostimulants compared to the control. Minimal, statistically insignificant differences among treatments in the K+ content results were found.
3.3. Evaluation of Interactions between Monitored Factors
3.3.1. Year × Treatment Interactions
As mentioned in the previous chapter, the weather conditions significantly affected the results of the monitored parameters, which is clearly demonstrated in Figure 3 and Figure 4. In the case of LAI and NDVI, the values did not reach statistical significance; nevertheless, the highest value of LAI was found in the interaction 2021 × B2 (Figure 3a), while NDVI and PRI were measured in the interaction of 2021 × B1, with PRI proving significant (Figure 3b,c). MCARI values were the highest in 2022 but without statistical significance depending on the treatment (Figure 3).
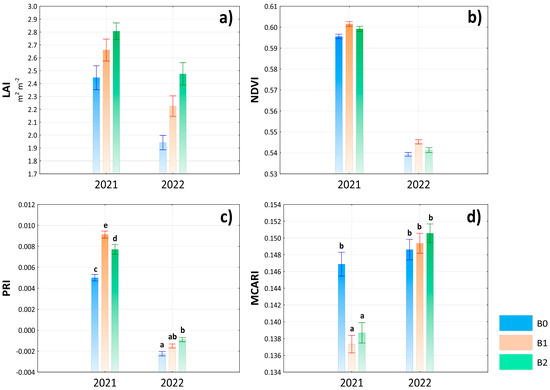
Figure 3.
Evaluation of leaf area index (LAI) (a), normalized difference vegetation index (NDVI) (b), photochemical reflectance index (PRI) (c) and modified chlorophyll absorption in reflectance index (MCARI) (d) depending on the interaction of year × treatment. Lowercase letters above bars denote a significance of difference at probability level α = 0.05. In bars without lowercase letters, there is no significance of difference. Vertical bars denote +/− standard errors.
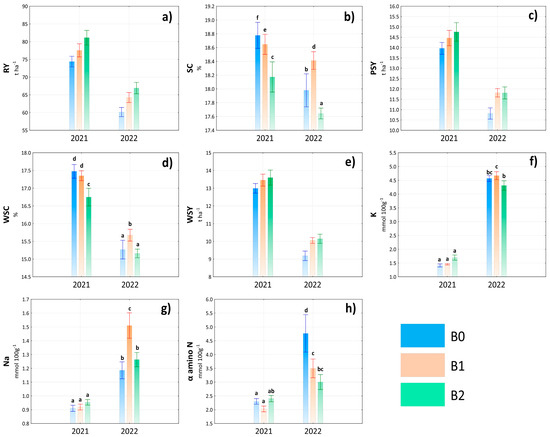
Figure 4.
Evaluation of root yield (RY) (a), sugar content (SC) (b), polarized sugar yield (PSY) (c), white sugar content (WSC) (d), white sugar yield (WSY) (e), potassium content (K) (f), sodium content (Na) (g), and α amino nitrogen content (α amino N) (h) depending on the interaction of year × treatment. Lowercase letters above bars denote a significance of difference at probability level α = 0.05. In bars without lowercase letters, there is no significance of difference. Vertical bars denote +/− standard errors.
Despite the fact that the interaction year × treatment did not have a significant effect on the root yield (Table S1), the results indicate that the application of biostimulants had a positive effect both in favorable and less favorable conditions throughout the studied years (Figure 4a). In both years, the highest RY values were achieved in treatment B2. However, an interesting finding was provided by the results of the sugar content depending on the year × treatment interaction (Figure 4b). Although the significantly highest value of SC was detected in the interaction 2021 × B0, an important finding was the increase of SC in treatment B1 in 2022, with a significant difference compared to the other treatments. This indicates a high potential for the use of pure Ascophyllum nodosum algae extract in sugar beet production under unfavorable conditions, while the mineral component in the B2 treatment probably resulted in a decrease in sugar content. The results of PSY and WSY largely followed the trend in RY, with a statistically insignificant effect of the interaction year × treatment. However, significant differences between treatments were found in terms of WSC, the highest value being found in the 2021 × B0 interaction (Figure 4d). In connection with the content of compatible solutes, a significant influence of the interaction year × treatment was found. However, significant variability was recorded especially in the year 2022, while in 2021, the differences were minimal (Figure 4f–h). In general, however, it can be concluded that treatments with biostimulants within the year × treatment interaction had a more positive effect on the content of α amino N, while increases in the content of K+ and Na+ were recorded especially in the case of B1 treatment.
3.3.2. Variety × Treatment Interactions
In the context of the obtained results, it can be stated that the influence of the variety × treatment interaction on all physiological parameters (NDVI, PRI, and MCARI) was highly significant. On the contrary, there was an insignificant effect in the case of LAI, although, as shown in Figure 5a, remarkable values of LAI were achieved in the interaction of all varieties and B2 treatment. Positive responses to treatments with biostimulants but with different statistical significances were recorded on all sugar beet varieties in relation to NDVI and PRI (Figure 5b,c). The exception was the NDVI value of the Lucius variety in the B2 treatment, but the difference compared to the control was insignificant. The absolute highest NDVI value was found in the Fabius × B2 interaction and the highest PRI value in the Fischer × B1 interaction. In the case of MCARI, two different trends were found from the point of view of the variety × treatment interaction. While the Fabius and Nicolaus varieties achieved the highest MCARI values in combination with the control treatment, the Fischer and Lucius varieties achieved the lowest values of that physiological parameter under these conditions (Figure 5d).
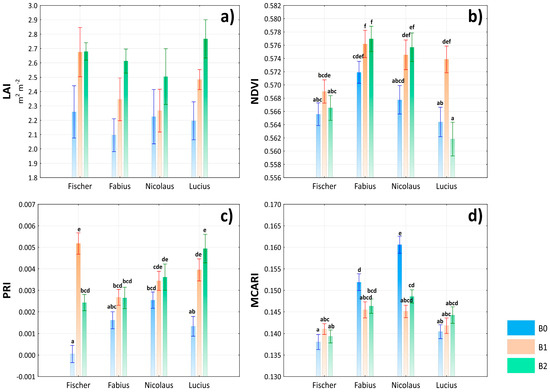
Figure 5.
Evaluation of leaf area index (LAI) (a), normalized difference vegetation index (NDVI) (b), photochemical reflectance index (PRI) (c) and modified chlorophyll absorption in reflectance index (MCARI) (d) depending on the interaction of variety × treatment. Lowercase letters above bars denote a significance of difference at probability level α = 0.05. In bars without lowercase letters, there is no significance of difference. Vertical bars denote +/− standard errors.
The production potential of varieties is one of the basic limits of total production, but this factor can be significantly influenced by the application of biostimulants. In this study, the interaction of variety × treatment had a significant effect on all production parameters, with the exception of RY (Table S1). Nevertheless, even in the case of RY, an increase in values can be found for all varieties in combination with biostimulation treatment. The highest degree of variability within the variety × treatment interaction was manifested in the sugar content parameter. The significantly highest SC and WSC were found in the Nicolaus × B0 interaction and, conversely, the lowest values of these parameters in the Fabius × B2 interaction (Figure 6b). However, an important finding was the responses of Fabius and Lucius cultivars to the B1 treatment, where a significant increase in SC was found compared to the other treatments on the same cultivars. From the point of view of PSY and WSY, it can be concluded that the highest values were found in the interaction of Lucius variety and treatments B1 and B2, respectively. The reactions of the monitored varieties to treatment with biostimulants in relation to the content of compatible solutes were very different (Figure 6f–h). While for α amino N, a slightly positive but insignificant effect of B2 treatment was noted on all sugar beet varieties, a negative effect was recorded in the Fabius and Nicolaus varieties in the case of K+ and Fabius and Fischer varieties in the case of Na+.
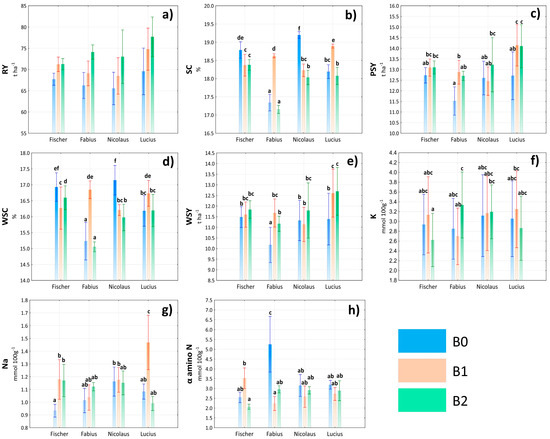
Figure 6.
Evaluation of root yield (RY) (a), sugar content (SC) (b), polarized sugar yield (PSY) (c), white sugar content (WSC) (d), white sugar yield (WSY) (e), potassium content (K) (f), sodium content (Na) (g), and α amino nitrogen content (α amino N) (h) depending on the interaction of variety × treatment. Lowercase letters above bars denote a significance of difference at probability level α = 0.05. In bars without lowercase letters, there is no significance of difference. Vertical bars denote +/− standard errors.
3.4. Analysis of Relationships between Production and Physiological Parameters of Sugar Beet
The dependence of the key indicators of sugar beet production is considered to be the basic limit for the successful cultivation of this crop. The negative dependence between root yield and sugar content has been known for a long time, and one of the goals of this experiment was to reverse this trend. Based on the results, it can be concluded that a positive albeit very weak dependence was detected in this experiment (Figure 7).
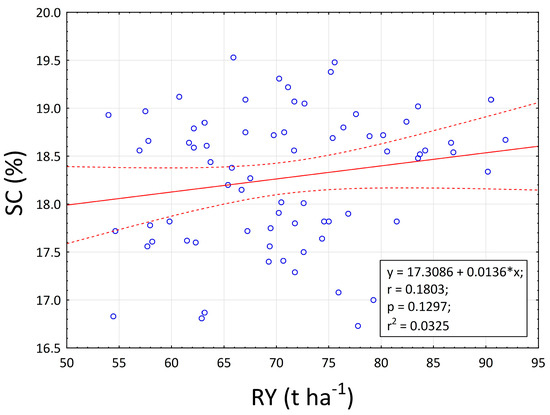
Figure 7.
Relationship between root yield (RY) and sugar content (SC). The linear relationship between parameters is represent by solid lines. The confidence band at 95% is representing by dot lines. The circles in the figure representing data points. Linear equations, correlation coefficient, probability, and regression are inserted inside figures.
An important hypothesis in this study was that leaf area index and physiological parameters measured using spectral indices are closely correlated with production of sugar beet. As can be seen in Figure 8, the results need to be evaluated from the point of view of production quantity and, on the other hand, from the point of view of quality. While the relationship of RY to LAI and PRI is strongly positive and that to NDVI even very strong, the relationship of SC to LAI is very weak and that to NDVI and PRI weak. In the relationship of RY and SC to MCARI, only a very weak dependence was found, which was even negative in the case of SC. These findings confirm the fact that treatment with biostimulants positively affects the course of physiological parameters and LAI, especially in relation to the amount of production and not quality.
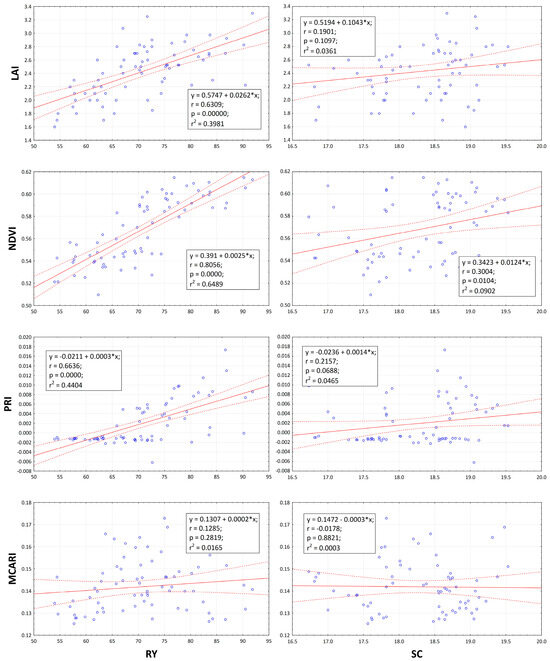
Figure 8.
Root yield (RY; t ha−1) and sugar content (SC; %) relationships towards leaf area index (LAI m2 m−2), normalized difference vegetation index (NDVI), photochemical reflectance index (PRI), and modified chlorophyll absorption in reflectance index (MCARI). The linear relationship between parameters is represent by solid lines. The confidence band at 95% is representing by dot lines. The circles in the figure representing data points. Linear equations, correlation coefficient, probability, and regression are inserted inside figures.
4. Discussion
Sufficient production and supply of food is at risk due to climatic changes represented by irreversible weather variations [51]. Furthermore, research shows a correlation between drought and yield reduction in the recent decades [52]. Thus, we conducted this experiment of biostimulant application to sugar beet with the objective to determine ways to mitigate the negative effect of drought and heat on physiological parameters, which are represented by spectral vegetation indices, production parameters, and their mutual relationships.
4.1. Biostimulants Impact on Physiological Activity of Sugar Beet
Efficient methods of remote sensing are necessary to evaluate the effect of climatic change on physiological reactions and seasonal photosynthetic activity [53]. Spectral reflectance, which is used to monitor water capacity and physiological changes in the vegetation, is among the newer and nondestructive methods of hyperspectral remote sensing. The responses of spectral reflectance caused by water stress reflect the interaction between and bonds of carbon, nitrogen, and water cycles [29]. These investigated vegetation indices are relatively simple and effective algorithms used for quantitative and qualitative evaluation of the vegetation cover, vitality, and plant growth dynamics [54].
However, the currently available information about the effect of biostimulants on the values of crop vegetation indices is limited. Some studies have found justification for this research: increase in the NDVI of common buckwheat after application of daminozide [55] and increase in the NDVI of tomato plants treated with tannin-based biostimulants [56]. In this experiment, a significant effect of treatment on the NDVI, PRI, and MCARI values was shown, while the highest NDVI and PRI values and the lowest MCARI value were found primarily for treatment B1. This is consistent with the claim of Guillard, Inguagiato [57], who indicated that one of the benefits of the application of biostimulant from seaweed extract in dry conditions is particularly the increase in NDVI. The significantly higher MCARI values on the control variant were probably caused by the lower chlorophyll content in the leaves, which supports the claim of Nagler et al. [58] about the direct reaction of the MCARI to chlorophyll concentration in the leaves and reflectance of the soil. Furthermore, using correlation analysis, a positive correlation between the NDVI and root yield as well as PRI and root yield was confirmed. Similar conclusions were drawn in cereal studies by Panek, Gozdowski [59] and Chandel et al. [60].
Interesting results were also obtained from the perspective of variability of spectral vegetation indices depending on the measurement date. As can be clearly seen in Figure 2, the NDVI and PRI had significantly higher values than the control in second measurements performed after first biostimulant application. This contradicts the claim of Cordon et al. [61] about the negative correlation between NDVI and PRI. Furthermore, an increase in temperature may lead to increase in PRI values; however, the subsequent drought decreases the NDVI values [62]. However, our results suggest that biostimulants significantly affect the increase of chlorophyll pigments in sugar beet as well as the overall photosynthetic intensity. Several authors were consistent in reporting that the NDVI value is directly correlated with the photosynthetic activity of the monitored vegetation [63] and is positively correlated with the chlorophyll content in the leaves [64]. In the case of the MCARI, the tendencies were in the opposite direction, as the control yielded higher values than the biostimulant treatments at the first and second measurements, although at the third measurement, control was found to have the lowest value of MCARI.
In this study, it was shown that biostimulant application has a significant effect on LAI in field conditions. This is undoubtedly an important finding, as LAI is an important parameter used in vegetation structure monitoring [65] and is a key biophysical parameter connected with the functional processes of the vegetation, such as photosynthesis and respiration [66]. Both treatments with biostimulants had significantly higher values than the control, although the overall highest value was found for treatment B2. It has been often observed that biostimulant application increases the leaf area and chlorophyll content in leaves [67]. Wadas, Dziugiel [68] reported that the tuber yield, LAI, and abiotic stress tolerance of potatoes were significantly increased with the application of biostimulants during vegetation. Similarly, Kim et al. [69] reported that drought relatively limits the growth of crops and carbon dioxide absorption and that LAI can be used to evaluate this reaction. A significant increase in the value of the leaf area index after biostimulant application to salad leaves was found by Di Mola et al. [70]. As reported by Przybysz et al. [71], the use of the foliarly applied biostimulant Atonik led to the development of a more efficient photosynthetic apparatus in many species of field crops, which led to increases in LAI values, chlorophyll content, photosynthetic intensity, and other important parameters.
4.2. Biostimulants Impact on Production Parameters of Sugar Beet
Under our field conditions, biostimulants had a significant effect on all production traits except for potassium content. However, different trends were found in comparing the effect of biostimulants on quantitative and qualitative traits of sugar beet. Whereas treatment B2 increased the quantitative traits RY, PSY, and WSY compared with the control, it negatively affected the quality, primarily represented by SC. The significantly highest SC and WSC values were found for the B1 treatment. This is undoubtedly an important finding, as it negated the well-known negative correlation between the quantity and quality of the sugar beet yield [72,73]. Recently, studies have been conducted that indicate the possibility of influencing the production quality using biostimulant application [74,75,76]. Very different trends were found in the accumulation of compatible solutes in the root. The significantly highest accumulation of sodium content was observed for the B1 treatment, whereas the significantly highest α amino N content was found for treatment B0. These fluctuations might have been caused by the weather conditions, as confirmed by Hoffmann [77]. Bloch et al. [17] further reported that adjusting to low water availability during sugar beet vegetation involves the accumulation of compatible solutes that are important for its technical quality. The effect of compatible solutes especially involves a decrease in white sugar yield because they increase the solubility of sucrose and concurrently prevent its crystallization [78].
4.3. Interactions between the Experimental Factors
The monitoring of interactions between varieties and biostimulant treatment is one of the most important tools for selecting reasonable genetic material and stimulants for the specific environment. However, few studies have focused on this objective [79]. Based on the results of this experiment, it can be confirmed that the variety × treatment interaction had a significant effect on nearly all monitored sugar beet traits except for the LAI and RY. Similar results were found across three-way interaction, where, in addition, the significant influence on NDVI was not proven. Several studies are consistent in concluding that the crop yield and quality are the result of the variety × environment interaction [39,80,81], although currently, a metabolomic approach to investigate interactions between biostimulants and plants and to clarify their operating processes in different environments is becoming more widespread [82]. This is also supported by our results, where it is clearly seen that individual varieties reacted differently to the biostimulant application in different weather conditions, which might have had a considerable effect on the overall yield and quality of the sugar beet.
5. Conclusions
The unpredictable course of weather conditions in the recent years has increased the need to perform protective and supporting measures in the system of field crop cultivation. One such measure is the application of biostimulants of various origins. In this study, a significant and, in nearly all cases, positive effect of biostimulants on production and physiological traits of sugar beet was found. From the treatments, the highest effect on the production quantity and leaf area index was observed by the application of the concentrate of seaweed Ascophylum nodosum and humus substances (B2). However, from the perspective of spectral vegetation indices, it can be concluded that the significantly highest values were determined for the treatment with pure extract of Ascophylum nodosum (B1). Moreover, the B1 treatment significantly affected the sugar content of sugar beet. An important finding of this study is that not only the varieties themselves have different production potential, but this can be significantly influenced by the application of biostimulants. Furthermore, a correlation analysis showed a positive relationship between the yield and quality of the sugar beet. In addition, a strong positive dependence was found between the root yield and the vegetation indices LAI, NDVI, and PRI. Our results confirm the need to investigate physiological and production parameters under the influence of biostimulant treatment as well as mutual interactions between genetic material, the environment, and the biostimulant treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14010062/s1; Table S1: Analysis of variance (ANOVA) for sugar beet production traits; Table S2: Evaluation of production and physiological parameters depending on the interaction of year × variety × treatment.
Author Contributions
V.P. prepared the topic of the experiment; M.R. and N.B. conducted the experiment and realized experimental measurements and statistical analysis in STATISTICA 10 software; L.D., A.Z. and D.L. prepared the formal analysis, tables, and figures; V.P. and M.R. wrote the original manuscript and conducted the investigation and discussion. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by European Regional Development Fund via Operational program Integrated infrastructure within the projects: Sustainable smart farming system taking into account the future challenges 313011W112 (100%).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Füssel, H.-M.; Heinke, J.; Popp, A.; Gerten, D. Food security in a changing climate. In Climate Change, Justice and Sustainability: Linking Climate and Development Policy; Edenhofer, O., Wallacher, J., Campen, H.L., Reder, M., Knopf, B., Müller, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 33–43. [Google Scholar]
- IPCC. Summary for Policymakers, Global Warming of 1.5 °C. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018; 32p. [Google Scholar]
- Nelson, G.C.; Rosegrant, M.W.; Koo, J.; Robertson, R.; Sulser, T.; Zhu, T.; Ringler, C.; Msangi, S.; Palazzo, A.; Batka, M.; et al. Climate Change Impact on Agriculture and Costs of Adaptation; International Food Policy Research Institute: Washington, DC, USA, 2009. [Google Scholar] [CrossRef]
- Straffelini, E.; Tarolli, P. Climate change-induced aridity is affecting agriculture in Northeast Italy. Agric. Syst. 2023, 208, 103647. [Google Scholar] [CrossRef]
- OECD; FAO. OECD-FAO Agricultural Outlook 2019–2028; OECD Publishing: Paris, France, 2019; 326p, ISBN 978-92-64-31246-3. [Google Scholar]
- Leilah, A.; Badawi, M.; Said, E.; Ghonema, M.; Abdou, M.A. Effect of Planting Dates, Plant Population and Nitrogen Fertilization on Sugar Beet Productivity Under the Newly Reclaimed Sandy Soils in Egypt. Sci. J. King Faisal Univ. 2005, 6, 95–110. [Google Scholar]
- Zhang, Y.; Nan, J.; Yu, B. OMICS Technologies and Applications in Sugar Beet. OMICS Technologies and Applications in Sugar Beet. Front. Plant Sci. 2016, 7, 900. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAOSTAT Database. Food and Agriculture Organization of the United Nations. [Online]. 2023. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 17 May 2023).
- Tomaszewska, J.; Bielinski, D.; Binczarski, M.; Berlowska, J.; Dziugan, P.; Piotrowski, J.; Stanishevsky, A.; Witońska, I.A. Products of sugar beet processing as raw materials for chemicals and biodegradable polymers. RSC Adv. 2018, 8, 3161. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk-Bator, K.; Blaszczyk, A.; Czyzniejewski, M.; Kachlicki, P. Identification of saponins from sugar beet (Beta vulgaris) by low andhigh-resolution HPLC–MS/MS. J. Chromatogr. B 2016, 1029–1030, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Erdal, G.; Esengun, K.; Erdal, H.; Gunduz, O. Energy use and economical analysis of sugar beet production in Tokat province of Turkey. Energy 2007, 32, 35–41. [Google Scholar] [CrossRef]
- Panella, L. Sugar Beet as an Energy Crop. Sugar Tech 2010, 12, 288–293. [Google Scholar] [CrossRef]
- Monreal, J.A.; Jiménez, E.T.; Remesal, E.; Morillo-Velarde, R.; García-Maurino, S.; Echevarría, C. Proline content of sugar beet 447 storage roots: Response to water deficit and nitrogen fertilization at field conditions. Environ. Expr. Bot. 2006, 448, 257–267. [Google Scholar]
- Nause, N.; Meier, T.; Hoffmann, C. Tissue composition and arrangement in sugar beet genotypes of different tissue strength 450 with regard to damage and pathogen infestation. Sugarindustry 2020, 145, 114–123. [Google Scholar]
- Bloch, D.; Ch Hoffman, M.; Märländer, B. Solute Accumulation as a Cause for Quality Losses in Sugar Beet Submitted to Con-452 tinuous and Temporary Drought Stress. J. Agron. Crop Sci. 2006, 192, 17–24. [Google Scholar] [CrossRef]
- Trimpler, K.; Stockfish, N.; Märländer, B. Efficiency in sugar beet cultivation related to field history. Eur. J. Agron. 2017, 91, 1–9. [Google Scholar] [CrossRef]
- Hoffmann, C.M.; Kenter, C. Yield Potential of Sugar Beet–Have We Hit the Ceiling? Front. Plant Sci. 2018, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sastre, L.F.; Martín-Ramos, P.; Navas-Gracia, L.M.; Hernández-Navarro, S.; Martín-Gil, J. Impact of Climatic Variables on Carbon Content in Sugar Beet Root. Agronomy 2018, 8, 147. [Google Scholar] [CrossRef]
- Chang, L.; Peng-Sen, S.; Shi-Rong, L. A review of plant spectral reflectance response to water physiological changes. Chin. J. Plant Ecol. 2016, 40, 80–91. [Google Scholar] [CrossRef]
- Karnieli, A.; Agam, N.; Pinker, R.T.; Anderson, M.; Imhoff, M.L.; Gutman, G.G.; Panov, N.; Goldberg, A. Use of NDVI and Land Surface Temperature for Drought Assessment: Merits and Limitations. J. Clim. 2010, 23, 618–633. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.; Zhao, C.; Liu, S.; Tong, X. The spatial continuity study of NDVI based on Kriging and BPNN algorithm. Math. Comput. Model. 2011, 54, 1138–1144. [Google Scholar] [CrossRef]
- Din, M.; Zheng, W.; Rashid, M.; Wang, S.; Shi, Z. Evaluating Hyperspectral Vegetation Indices for Leaf Area Index Estimation of Oryza sativa L. at Diverse Phenological Stages. Front. Plant Sci. 2017, 8, 820. [Google Scholar] [CrossRef]
- Gamon, J.A.; Penuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Sarlikioti, V.; Driever, S.M.; Marcelis, L.F.M. Photochemical reflectance index as a mean of monitoring early water stress. Ann. Appl. Biol. 2010, 157, 81–89. [Google Scholar] [CrossRef]
- Bernář, M.; Šarapatka, B.; Netopil, P.; Zeidler, M.; Hanousek, T.; Homolová, L. The Use of Spectral Indices to Recognize Waterlogged Agricultural Land in South Moravia, Czech Republic. Agriculture 2023, 13, 287. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated Narrow-Band Vegetation Indices for Prediction of Crop Chlorophyll Content for Application to Precision Agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- FBIR. Biostimulants Market Size, Share & COVID-19 Impact Analysis, by Source (Microbial and Non-Microbial), Active Ingredients (Seaweed Extracts, Humic Substances, Vitamins & Amino Acids, Microbial Amendments, and Others), Application (Foliar Application, Soil Treatment, and Seed Treatment), Crop, and Regional Forecast, 2020–2027. 2020. Available online: https://www.fortunebusinessinsights.com/industry-reports/biostimulants-market-100414 (accessed on 19 May 2023).
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolysed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O´Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 12, 63–73. [Google Scholar] [CrossRef]
- Trevisan, S.; Francioso, O.; Quaggioti, S.; Nardi, S. Humic substances biological activity at the plant-soil interface. Plant Signal. Behav. 2010, 5, 635–643. [Google Scholar] [CrossRef]
- De Hita, D.; Fuentes, M.; García, A.C.; Olaetxea, M.; Baigorri, R.; Zamarreno, A.M.; Berbara, R.; Garcia-Mina, J.M. Humic substances: A valuable agronomic tool for improving crop adaptation to saline water irrigation. Water Supply 2019, 19, 1735–1740. [Google Scholar] [CrossRef]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Nimir, N.E.A.; Jiao, X.; Zhu, G.; Elsiddig, A.M.I.; Suliman, M.S.E.; Elradi, S.B.M.; Yue, W. Exogenous jasmonic acid and humic acid increased salinity tolerance of sorghum. Agron. J. 2020, 112, 871–884. [Google Scholar] [CrossRef]
- Pidgeon, J.D.; Ober, E.S.; Qi, A.; Clark, C.J.A.; Royal, A.; Jaggard, K.W. Using multi-environment sugar beet variety trials 459 to screen for drought tolerance. Field Crops Res. 2005, 95, 268–279. [Google Scholar] [CrossRef]
- Curcic, Z.; Ciric, M.; Nagl, N.; Taski-Ajdukovic, K. Effect of Sugar Beet Genotype, Planting and Harvesting Dates and Their Interaction on Sugar Yield. Front. Plant Sci. 2018, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Sorgoná, A.; Lupini, A.; Araniti, F.; Stevanato, P.; Cacco, G.; Abenavoli, M.R. Morpho-physiological responses of sugar beet (Beta vulgaris L.) genotypes to drought stress. Acta Physiol. Plant. 2013, 35, 853–865. [Google Scholar] [CrossRef]
- Koch, F.C.; McMeekin, T.L. A new direct nesslerization Micro-Kjeldahl method and a modification of the Nessler-folin reagent for ammonia. J. Am. Chem. Soc. 1924, 46, 2066–2069. [Google Scholar] [CrossRef]
- Pačuta, V.; Rašovský, M.; Michalska-Klimczak, B.; Wyszyńsky, Z. Impact of Superabsorbent Polymers and Variety on Yield, Quality and Physiological Parameters of the Sugar Beet (Beta vulgaris prov. Altissima Doell). Plants 2021, 10, 757. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Kabala, C.; Musztyfaga, E.; Galka, B.; Labuńska, D.; Mańczyńska, P. Conversion of Soil pH 1,2.5 KCl and 1,2.5 H2O to 1,5 H2O: Conclusions for Soil Management, Environmental Monitoring, and International Soil Databases. Pol. J. Environ. Stud. 2016, 25, 647–653. [Google Scholar] [CrossRef]
- Kononova, M.M. Humus of Virgin and Cultivated Soils. In Soil Components; Gieseking, J.E., Ed.; Springer: Berlin/Heidelberg, Germany, 1975. [Google Scholar] [CrossRef]
- Salkind, N.J. Encyclopedia of Research Design; SAGE Publications: Thousand Oaks, CA, USA, 2010; pp. 1399–1432. ISBN 978-1-4129-6127-1. [Google Scholar]
- Ariza-Carricondo, C.; Di Mauro, F.; Op de Beeck, M.; Roland, M.; Gielen, B.; Vitale, D.; Ceulemans, R.; Papale, D. A comparison of different methods for assessing leaf area index in four canopy types. Cent. Eur. For. J. 2019, 65, 67–80. [Google Scholar] [CrossRef]
- Barlog, P.; Szczepaniak, W.; Grzebisz, W.; Poglodzinski, R. Sugar beet response to different K, Na and Mg ratios in applied fertilizers. Plant Soil Environ. 2018, 64, 173–179. [Google Scholar] [CrossRef]
- Reinefeld, E.; Emmerich, A.; Baumgarten, G.; Winner, C.; Beiss, U. Zur Voraussage des Melassezuckers aus Rübenanalysen. Zucker 1974, 27, 2–15. [Google Scholar]
- Bajči, P.; Pačuta, V.; Černý, I. Cukrová Repa; ÚVTIP-NOI: Nitra, Slovak, 1993; pp. 99–100. ISBN 80-85330-35-0. [Google Scholar]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, J. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef] [PubMed]
- Leng, G.; Hall, J. Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future. Sci. Total Environ. 2018, 654, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Filella, I.; Liu, D.; Ogaya, R.; Llusiá, J.; Asensio, D.; Penuelas, J. Photochemical Reflectance Index (PRI) for Detecting Responses of Diurnal and Seasonal Photosynthetic Activity to Experimental Drought and Warming in a Mediterranean Shrubland. Remote Sens. 2017, 9, 1189. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Krupa, M.; Witkowicz, R. Biostimulants as a Response to the Negative Impact of Agricultural Chemicals on Vegetation Indices and Yield of Common Buckwheat (Fagopyrum esculentum Moench). Agriculture 2023, 13, 825. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Mannino, G.; Beekwilder, J.; Contartese, V.; Karlova, R.; Bertea, C.M. The application of a biostimulant based on tannins affects root architecture and improves tolerance to salinity in tomato plants. Sci. Rep. 2021, 11, 354. [Google Scholar] [CrossRef]
- Guillard, K.; Inguagiato, J.C. Normalized Difference Vegetative Index Response of Nonirrigated Kentucky Bluegrass and Tall Fescue Lawn Turf Receiving Seaweed Extracts. HortScience 2017, 11, 1615–1620. [Google Scholar] [CrossRef]
- Nagler, P.L.; Daughtry, C.S.T.; Goward, S.N. Plant Litter and Soil Reflectance. Remote Sens. Environ. 2000, 71, 207–215. [Google Scholar] [CrossRef]
- Panek, E.; Gozdowski, D. Analysis of relationship between cereal yield and NDVI for selected regions of Central Europe based on MODIS satellite data. Remote Sens. Appl. Soc. Environ. 2020, 17, 100286. [Google Scholar] [CrossRef]
- Chandel, N.S.; Tiwari, P.S.; Singh, K.P.; Jat, D.; Gailwad, B.; Tripathi, H.; Golhani, K. Yield Prediction in Wheat (Triticum aestivum L.) using Spectral Reflectance Indices. Curr. Sci. 2019, 116, 272–278. [Google Scholar] [CrossRef]
- Cordon, G.; Lagorio, M.G.; Paruelo, J.M. Chlorophyll fluorescence, photochemical reflective index andnormalized difference vegetative index during plant senescence. J. Plant Physiol. 2016, 199, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Mänd, P.; Hallik, L.; Penuelas, J.; Nilson, T.; Duce, P.; Emmett, B.A.; Beier, C.; Estiarte, M.; Garadnai, J.; Kalapos, T.; et al. Responses of the reflectance indices PRI and NDVI to experimental warming and drought in European shrublands along a north–south climatic gradient. Remote Sens. Environ. 2010, 114, 626–636. [Google Scholar] [CrossRef]
- Tucker, C.J.; Pinzon, J.E.; Brown, M.E.; Slayback, D.A.; Pak, E.W.; Mahoney, R.; El Saleous, N. An extended AVHRR 8-km NDVI dataset compatible with MODIS and SPOT vegetation NDVI data. Int. J. Remote Sens. 2005, 26, 4485–4498. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Chen, D.; Huang, Y.; Kong, W.; Yuan, L.; Ye, H.; Huang, W. Assessment of Leaf Chlorophyll Content Models for Winter Wheat Using Landsat-8 Multispectral Remote Sensing Data. Remote Sens. 2020, 2, 2574. [Google Scholar] [CrossRef]
- Cao, Y.; Li, G.L.; Luo, Y.K.; Pan, Q.; Zhang, S.Y. Monitoring of sugar beet growth indicators using wide-dynamic-range vegetation index (WDRVI) derived from UAV multispectral images. Comput. Electron. Agric. 2020, 171, 105331. [Google Scholar] [CrossRef]
- Casa, R.; Varella, H.; Buis, S.; Guérif, M.; De Solan, B.; Baret, F. Forcing a wheat crop model with LAI data to access agronomic variables: Evaluation of the impact of model and LAI uncertainties and comparison with an empirical approach. Eur. J. Agron. 2012, 37, 1–10. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Wadas, W.; Dziugiel, T. Changes in Assimilation Area and Chlorophyll Content of Very Early Potato (Solanum tuberosum L.) Cultivars as Influenced by Biostimulants. Agronomy 2020, 10, 387. [Google Scholar] [CrossRef]
- Kim, K.; Wang, M.; Ranjitkar, S.; Liu, S.; Xu, J.; Zomer, R.J. Using leaf area index (LAI) to assess vegetation response to drought in Yunnan province of China. J. Mt. Sci. 2017, 14, 1863–1872. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mori, M. Plant-Based Biostimulants Influence the Agronomical, Physiological, and Qualitative Responses of Baby Rocket Leaves under Diverse Nitrogen Conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Przybysz, A.; Gawrónska, H.; Gajc-Wolska, J. Biological mode of action of a nitrophenolates-based biostimulant: Case study. Front. Plant Sci. 2014, 5, 713. [Google Scholar] [CrossRef] [PubMed]
- Gartland, J.S.; Fowler, M.R.; Slater, A.; Scott, N.W.; Gartland, K.M.A.; Elliott, M.C. Enhancement of Sugar Yield: A Molecular Biological Approach. In Progress in Plant Cellular and Molecular Biology: Proceedings of the VIIth International Congress on Plant Tissue and Cell Culture, Amsterdam, The Netherlands, 24–29 June 1990; Springer: Dordrecht, The Netherlands, 1990; pp. 50–55. [Google Scholar] [CrossRef]
- Lee, J.; O´Connor, L.J. Sugar-Beet Yields in Ireland with Special Reference to Spatial Patterns. Ir. J. Agric. Res. 1976, 15, 25–37. [Google Scholar]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Drobek, M.; Frac, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Artyszak, A.; Gozdowski, D. Influence of Various Forms of Foliar Application on Root Yield and Technological Quality of Sugar Beet. Agriculture 2021, 11, 693. [Google Scholar] [CrossRef]
- Hoffmann, C.M. Sucrose Accumulation in Sugar Beet Under Drought Stress. J. Agron. Crop Sci. 2010, 196, 243–252. [Google Scholar] [CrossRef]
- Schneider, F.; Emmerich, A.; Reinefeld, E.; Walter, E.; Kelm, W. Auswirkung der Nichtzuckerstoffe der Rube, insbesondere auf die Melassebildung. Tech. Wert Zuckerrübe 1961, 14, 208–212. [Google Scholar]
- Rašovský, M.; Pačuta, V.; Ducsay, L.; Lenická, D. Quantity and Quality changes in Sugar Beet (Beta vulgaris provar. Altissima Doel) Induced by Different Sources of Biostimulants. Plants 2022, 11, 2222. [Google Scholar] [CrossRef]
- Ndhlela, T.; Herselman, L.; Magorokosho, C.; Setimela, P.; Mutimamba, C.; Labuschagne, M. Genotype × environment interaction of maize grain yield using AMMI biplots. Crop Sci. 2014, 54, 1992–1999. [Google Scholar] [CrossRef]
- Studnicki, M.; Lenartowicz, T.; Noras, K.; Wójcik-Gront, E.; Wyszyński, Z. Assessment of Stability and Adaptation Patterns of White Sugar Yield from Sugar Beet Cultivars in Temperate Climate Environments. Agronomy 2019, 9, 405. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on Crops: Their Impact under Abiotic Stress Conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).