Effects of Different Proportions of Organic Fertilizer in Place of Chemical Fertilizer on Microbial Diversity and Community Structure of Pineapple Rhizosphere Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Storage
2.2. Experimental Design
2.3. DNA Extraction, PCR Amplification
2.4. 16SrRNA Gene Sequencing
2.5. Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
3.1. Sequencing Results of Different Fertilization Methods
3.2. Alpha Diversity of Bacteria in Different Fertilization Methods of Rhizosphere Soil
3.3. Bydiversity of Bacteria in Different Rhizosphere Soil Fertilization Methods
3.4. Composition of the Bacterial Community in the Soil of the Pineapple Rhizosphere under Different Fertilization Methods
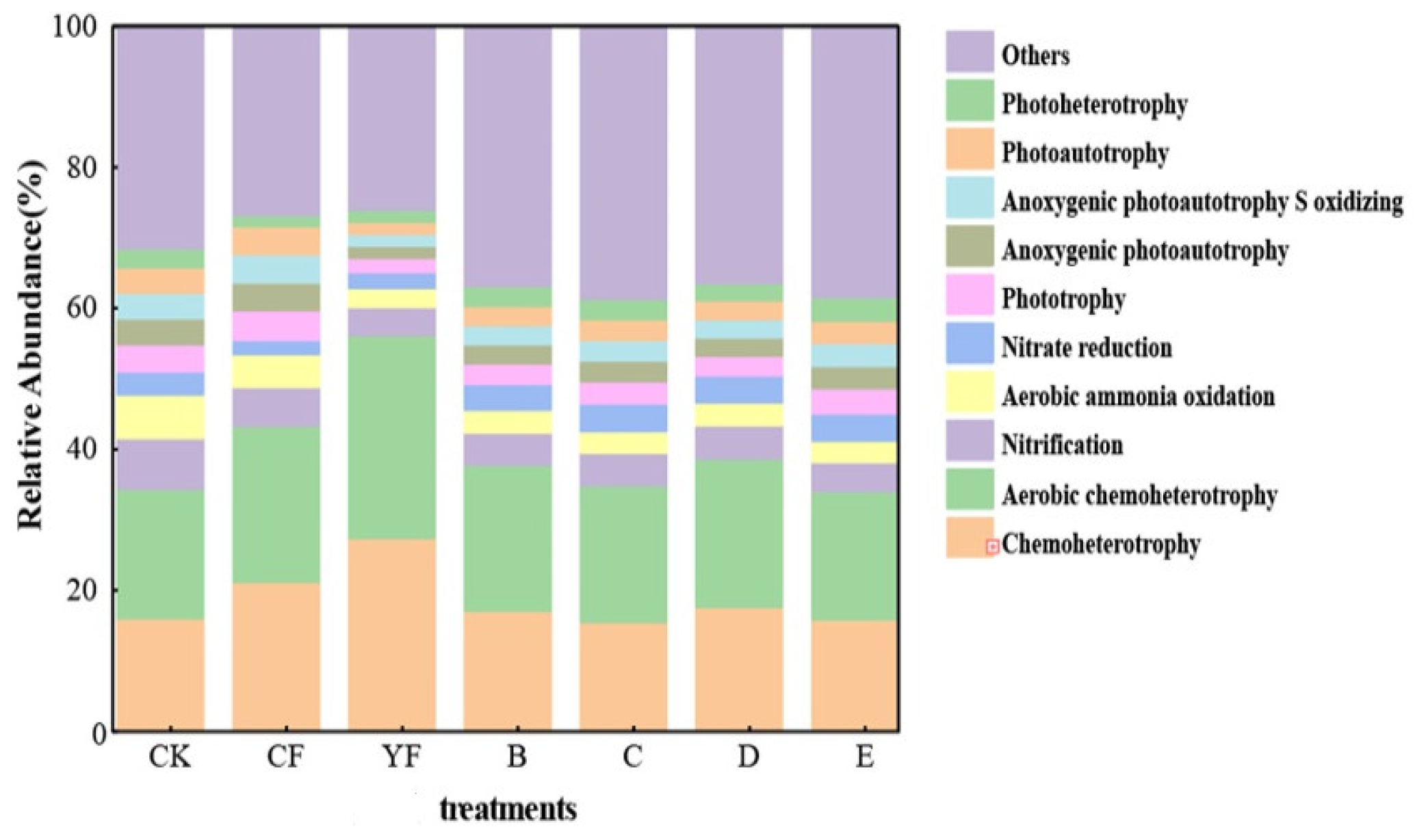
3.5. Function of Soil Bacteria in the Pineapple Rhizosphere under Different Fertilization Methods
3.6. Functional Differences of TOP 10 Bacteria in Pineapple Rhizosphere Soil under Different Fertilization Methods
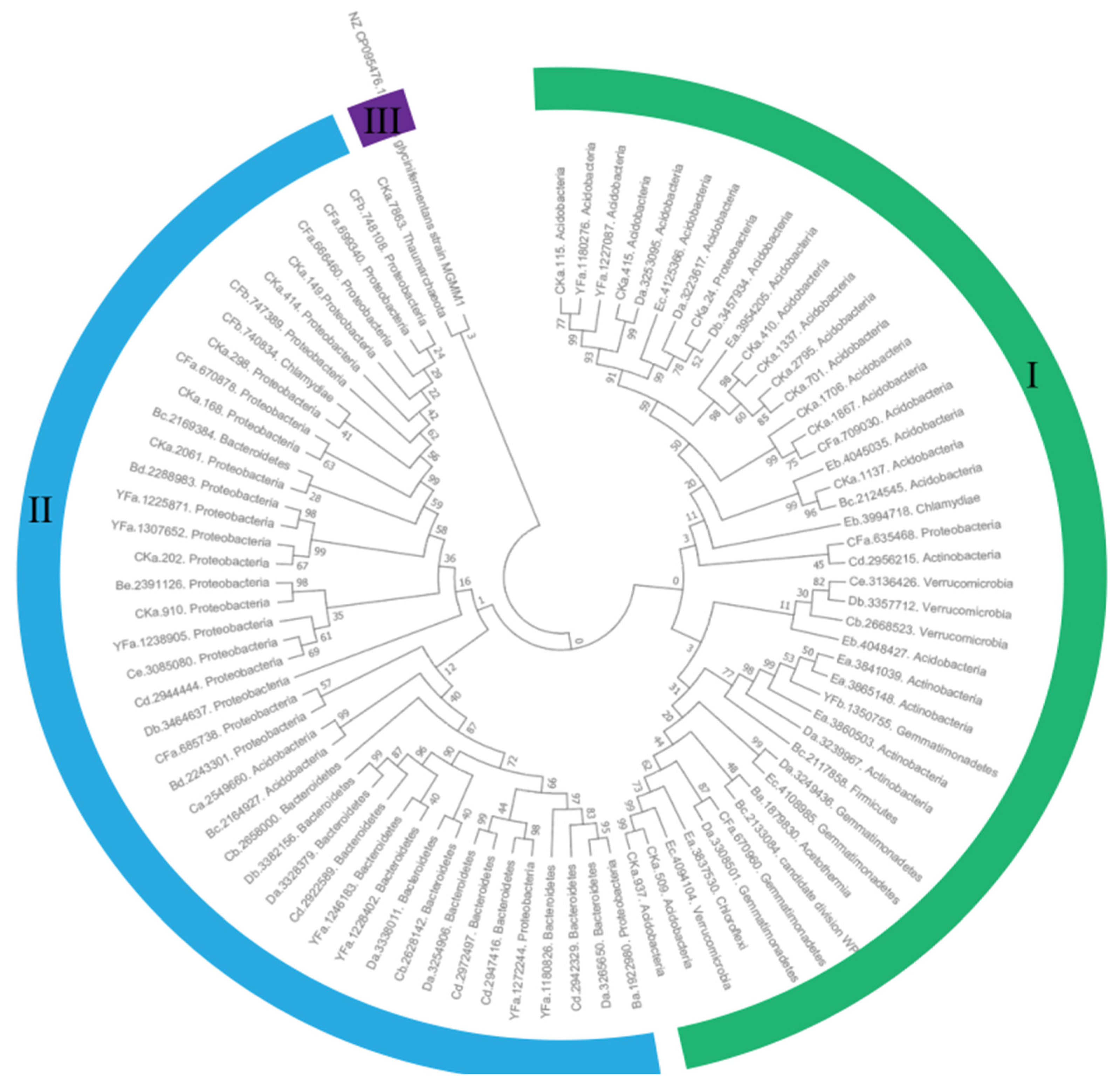
3.7. Bioinformatics Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.F.; Yuan, X.L.; Zhang, X.M. Research Progress and Prospects of the Quality Physiology of Pineapples. S. China Fruit Trees 2016, 45, 172–177. [Google Scholar]
- Deng, C.M.; Li, Y.P.; Liang, W.H.; Ye, L. State of the development and countermeasures of my Chinese pineapple industry. Shanxi Agric. Sci. 2018, 46, 1031–1034. [Google Scholar]
- Yan, L.; Yun, Z.; Chuanhe, L. Practical Technology for Pineapple Production; Guangdong Science and Technology Press: Guangzhou, China, 2008; pp. 19–20. [Google Scholar]
- Chen, M.Z.; Yang, Y.M.; Meng, S.R.; Zou, L.Y. Research on soil fertility decline in pineapple orchards with different planting years. Soil Environ. 2002, 363–366. [Google Scholar] [CrossRef]
- Gou, J.Y.; Zhang, H.Q.; Yang, Y.; Yang, J.M.; Tang, H.Z.; Deng, Y.; Ruan, Y.Z.; Zhao, Y. Evaluation of soil nutrient status in pineapple orchards based on factor-cluster analysis. Soil Bull. 2019, 50, 137–143. [Google Scholar]
- Pang, G.S.; Tan, S.B.; Wu, H.; Xi, J.G. Investigation on soil nutrient status in the main pineapple producing areas of Guangdong, Guangxi and Hainan. Guangdong Agric. Sci. 2013, 40, 40–42. [Google Scholar]
- Wang, Q.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B.; Ma, M.; Chen, S.; Li, L.; Cao, F.; Li, J. Long-term fertilization changes bacterial diversity and bacterial communities in the maize rhizosphere of Chinese mollisols. Appl. Soil Ecol. 2018, 125, 88–96. [Google Scholar] [CrossRef]
- Min, J.S.; Kong, X.Z. Research progress on agricultural non-point source pollution in my country. J. Huazhong Agric. Univ. (Soc. Sci. Ed.) 2016, 122, 59–66. [Google Scholar]
- Ju, H.L. Research on the Mechanism of Pepper-Banana Rotation Combined with Bio-Organic Fertilizer in Reducing Continuous Cropping Obstacles in Banana Orchards with High Incidence of Fusarium Wilt. Ph.D. Thesis, Hainan University, Haikou, China, 2017. [Google Scholar]
- Hou, H.; Dong, K.; Yang, Z.X.; Dong, Y.; Tang, L.; Zheng, Y. Research progress on the occurrence mechanism of continuous cropping obstacles. Soil 2016, 48, 1068–1076. [Google Scholar]
- Liu, Y.N.; Ma, H.Y.; Zhang, J.Z.; Yan, C.M.; Si, W.Q. Effects of different fertilization levels in continuous cropping soil on pineapple yield and quality. Guangdong Agric. Sci. 2014, 41, 71–74. [Google Scholar]
- Jin, X.T.; Ma, J.Y.; Zou, Y.S.; Chen, L.J.; Li, T.; Zhao, H.W. Soil bacterial diversity and community structure characteristics of mango orchards under chemical fertilizer reduction and organic fertilizer application. J. Trop. Crops 2019, 40, 1205–1212. [Google Scholar]
- Li, L. Effects of Reduction of Chemical Fertilizers on Soil Nutrients and Microbial Activity under Crop Rotation. Ph.D. Thesis, Northeast Agricultural University, Harbin, China, 2018. [Google Scholar]
- Huang, X.Y.; Huang, X.S.; Han, H.D. Research progress on soil the structure of the microbial community of the soil in tea gardens and prospects for the application of “tea-grass-bacteria” technology. J. Tea 2021, 62, 94–99. [Google Scholar]
- Cai, J.; Zhang, J.; Yu, S.; Lin, H.X.; Li, K.M.; Chen, S.B.; Ou, W.J. Effects of fertilization methods on bacterial diversity and community structure characteristics of cassava rhizosphere soil. J. Fujian Univ. Agric. (Nat. Sci. Ed.) 2022, 51, 15–20. [Google Scholar]
- Garbeva, P.; van Veen, J.A.; van Elsas, J.D. Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease prevalence. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; Liu, J.J.; Xu, Y.X.; Zhang, W.; Mi, G.; Yao, Q.; Wang, G.H. Effects of different continuous cropping years of soybeans on the structure of the bacterial community of black soil. Acta Ecol. Sin. 2019, 39, 4337–4346. [Google Scholar]
- Chen, J.; Zeng, H. Effects of continuous and rotational cropping practices on soil fungal communities in pineapple cultivation. PeerJ 2022, 10, 13937. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Sun, M.; Yang, J.M.; Shen, Z.Z.; Ou, Y.N.; Fu, L.; Zhao, Y.; Li, R.; Ruan, Y.; Shen, Q. Inducing suppression of banana fusarium wilt disease through reshaping the soil by pineapple–banana rotation combined with the application of biofertilizer. Soil 2022, 8, 17–29. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Q.; Wang, Y.; Chen, X.; Gao, W.; Zhao, Y.; Wang, B.; Ruan, Y. Suppression of banana fusarium wilt disease with soil microbial mechanisms via pineapple rotation and residue amendment. Agronomy 2013, 13, 377. [Google Scholar] [CrossRef]
- Hu, Y.H.; Zhao, Y.; Ren, Z.G.; Yang, S.Y.; Tang, H.Z.; Zhang, X.B.; Wang, B.B.; Lv, L.W. Effects of bioorganic fertilizer on the fungal community of the pineapple rhizosphere and incidence of heart rot. J. Fruit Sci. 2022, 39, 1678–1690. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Tang, H.Z.; Hu, Y.H.; Ren, Z.G.; Yang, S.Y.; Zhao, Y.; Wang, B.B.; Zhang, X.B.; Ruan, Y.Z. Effects of different bioorganic fertilizers on the growth and control of heart rot in continuous cropping pineapple. Chin. J. Microbiol. 2019, 48, 4156–4166. [Google Scholar]
- Li, L.; Zhu, Z.M.; Wang, X.; Wu, X.; Fan, L.Q.; Ji, L.D. Effects of straw returning on soil fertility indexes and bacterial community diversity in Yinbei Saline-alkali land. J. Northwest Agric. Sci. 2019, 32, 1603–1615. [Google Scholar]
- Faith, D.P. Conservation Evaluation and Phylogenetic Diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Roesch, L.F.; Fulthorpe, R.R.; Riva, A.; Casella, G.; Hadwin, A.K.; Kent, A.D.; Daroub, S.H.; Camargo, F.A.O.; Farmerie, W.G.; Triplett, E.W. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007, 1, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, J.; Wang, W.; Zhou, J.; Wang, D.; Fan, B.; Zhang, X.; Sun, D.; Gong, G.; Suolang, S.; et al. Next-Generation Sequencing Reveals Four Novel Viruses Associated with Calf Diarrhea. Viruses 2021, 13, 1907. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.; Jannoura, R.; Beuschel, R.; Pfeiffer, B.; Dyckmans, J.; Murugan, R.; Chavannavar, S.; Wachendorf, C.; Joergensen, R.G. Carbon use efficiency and microbial functional diversity in a temperate Luvisol and a tropical Nitisol after millet litter and N addition. Biol. Fertil. Soils 2020, 56, 1139–1150. [Google Scholar] [CrossRef]
- Xu, H.D.; Yu, M.K.; Cheng, X.R. Abundant fungal and rare bacterial taxa jointly reveal soil nutrient cycling and multifunctionality in uneven-aged mixed plantations. Ecol. Indic. 2021, 129, 107932. [Google Scholar] [CrossRef]
- Fan, X.G.; Jin, K.; Li, Z.M.; Rong, X.N. Research progress on soil microbial diversity under different fertilization and farming systems. J. Plant Nutr. Fertil. 2010, 16, 744–751. [Google Scholar]
- Xu, Y.C.; Shen, Q.R.; Ran, W. Effects of long-term no-tillage and application of organic fertilizer on soil microbial biomass carbon, nitrogen and phosphorus. Acta Pedol. Sin. 2002, 39, 83–90. [Google Scholar]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.J. The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]
- Li, C.Y.; Li, X.L.; Zhang, Y.X.; Sun, H.F.; Li, C.Y.; Jing, L.Q.; Yang, X.G.; Liu, K. Response of soil microbial community structure to different degradation in alpine swamp wetland in the source region of the Yellow River. J. Environ. Sci. 2018, 42, 3971–3984. [Google Scholar]
- Chen, Z.G.; Cui, H.H.; Zhang, Q.; Li, J.; Zhang, Q.; Zhang, J.; Zhao, C.P. Effects of combined application of light-leaf sweet potato and reduced chemical fertilizer on soil fertility and corn yield. J. Jiangxi Agric. Univ. 2015, 411–416. [Google Scholar] [CrossRef]
- Huang, Y.H.; Qiu, Q.; Xu, Y.Z. Effects of nitrogen fertilizer reduction on rice production and soil fertility. Shanghai Agric. Sci. Technol. 2006, 39–40. [Google Scholar] [CrossRef]
- Yao, C.X.; Chen, Z.L.; Qiu, Q.; Huang, Y.H.; Yin, J.; Zhang, J.X.; Li, C.H.; Mao, G.F. Effects of chemical fertilizer reduction on watermelon yield and quality in facility cultivation. J. N. China Agric. Sci. 2005, 20, 76–79. [Google Scholar]
- Zhang, X.T.; Zhang, Y.D.; Pu, Z.X.; Wu, L.Q.; Yang, W.H. Effects of different fertilization treatments on soil acidity and nutrients in pomelo orchard. China Soil Fertil. 2023, 81–89. [Google Scholar] [CrossRef]
- Wang, E.Y.; Hao, S.; Li, W.R.; Zhang, X.; Zhang, Y.; Gao, Y.Y.; Li, H.; Wei, C.H.; Yang, J.Q.; Ma, J.X. Effects of optimized fertilization combined with vermicompost organic fertilizer on the yield and quality of grafted watermelon. Chin. Melon Veg. 2021, 34, 30–35. [Google Scholar]
- Yang, L.T.; Shang, W.Y.; Wan, Z.L.; Yang, H.X.; Zhang, G.B. Effects of fertilizer reduction combined with bio-organic fertilizer on yield, quality and nutrient distribution of open field zucchini. J. Gansu Agric. Univ. 2023, 12, 1–12. [Google Scholar]
- Zhao, Z.J.; Sun, J.P.; Dai, X.L.; Liu, Y.H. Effects of straw returning combined with reduced fertilization on rice yield and soil nutrients. Jiangsu Agric. Sci. 2022, 50, 66–71. [Google Scholar]
- Liu, Y.Y.; Lu, L.L.; Xie, S.Y.; Chen, S.T.; Wang, Y.P.; Yang, W.B. Microbial diversity and community structure in rhizosphere soil of different varieties of coconut under intercropping model of pineapple. Fruit Trees S. China. 2023, 52, 84–93. (In Chinese) [Google Scholar] [CrossRef]
- Han, Y.F.; Yi, W.H.; Wang, W.B.; Wang, Y.P.; Wang, H.T. Study of soil bacterial diversity in continuous poplar plantation based on high-throughput sequencing technology. Shandong Univ. J. (Sci. Ed.) 2014, 49, 1–6. [Google Scholar]
- Zhang, Y.J.; Zhang, S.; LI, S.; Jin, B.H.; Quan, Y.J.; Wang, W.P.; Yang, K.; He, H.X. Effects of Panax notoginseng bionic planting on rhizosphere soil microbial diversity in Shilin County. J. Yunnan Agric. Univ. (Nat. Sci. Ed.) 2021, 36, 1–7. [Google Scholar]
- Sha, Y.X.; Huang, Z.Y.; Li, Y.X.; Zhao, P. Effects of biological inoculants on the structure and function of soil microbial communities. J. Agric. Environ. Sci. 2022, 41, 2752–2762. [Google Scholar]
- Ding, X.J.; Huang, Y.L.; Jin, R.Y.; Ma, F.Y.; An, R.; Tian, Q.; Chen, B.J. Study on the structure and diversity of soil bacteria in four plantations in the Yellow River Delta based on high-throughput sequencing. Acta Ecol. Sin. 2018, 38, 5857–5864. [Google Scholar]
- Li, Y.; Lei, S.N.; Chen, Z.Q.; Zhang, T.; Jing, L.Y.; Shu, L.; Lin, C.M.; Zhao, K.; Tian, B.Y. Bacterial community structure composition and characteristics of red soil covered by five types of vegetation. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2020, 49, 231–239. [Google Scholar]
- Chen, J.; Liu, Z.B.; Ou, L.J. Comparison of microbial diversity before and after pepper planting in different types of soil. Chin. Agric. Sci. Bull. 2021, 37, 84–93. [Google Scholar]
- Xiao, L.T.; Yang, H.L.; Huang, W.X.; Fu, X.Q. Effects of grass cultivation on the structure and functional characteristics of soil microbial communities in Nanfeng orange orchards. J. Nucl. Agric. 2022, 36, 190–200. [Google Scholar]
- Yan, C.B.; Zhao, Y.; Hu, F.C.; Rui, K.; Chen, Z.; Zhang, S.Q.; Fan, H.Y. Study on the structure of the soil bacterial community and the diversity of Pintuo peanuts intercropped in the betel nut forest. China Soil Fertil. 2022, 2, 42–53. [Google Scholar]
- Podosokorskaya, O.A.; Kadnikov, V.V.; Gavrilov, S.N.; Mardanov, A.V.; Merkel, A.Y.; Karnachuk, O.V.; Ravin, N.V.; Bonch-Osmolovskaya, E.A.; Kublanov, I.V. Characterization of Melioribacter roseus gen. nov., sp. nov., a new facultatively anaerobic thermophilic cellulolytic bacterium from class Ignavibacteria, and a proposal of a new bacterial phylum Ignavibacteriae. Environ. Microbiol. 2013, 15, 1759–1771. [Google Scholar] [CrossRef]
- Lian, J.S.; Wang, H.Y.; Xu, M.G.; Wei, W.L.; Duan, Y.H.; Liu, S.T. Bacterial diversity and function prediction of long-term application of organic fertilizer in tidal soil. Plant Nutr. Fertil. 2021, 27, 2073–2082. (In Chinese) [Google Scholar]
- Liang, J.; Zou, R.; Huang, Y.; Qin, H.; Tang, J.; Wei, X.; Liang, Y.; Chai, S. Structure and diversity of mycorrhizal fungi communities of different part of Bulbophyllum tianguii in three terrestrial environments. Front. Plant Sci. 2022, 13, 992184. [Google Scholar] [CrossRef]
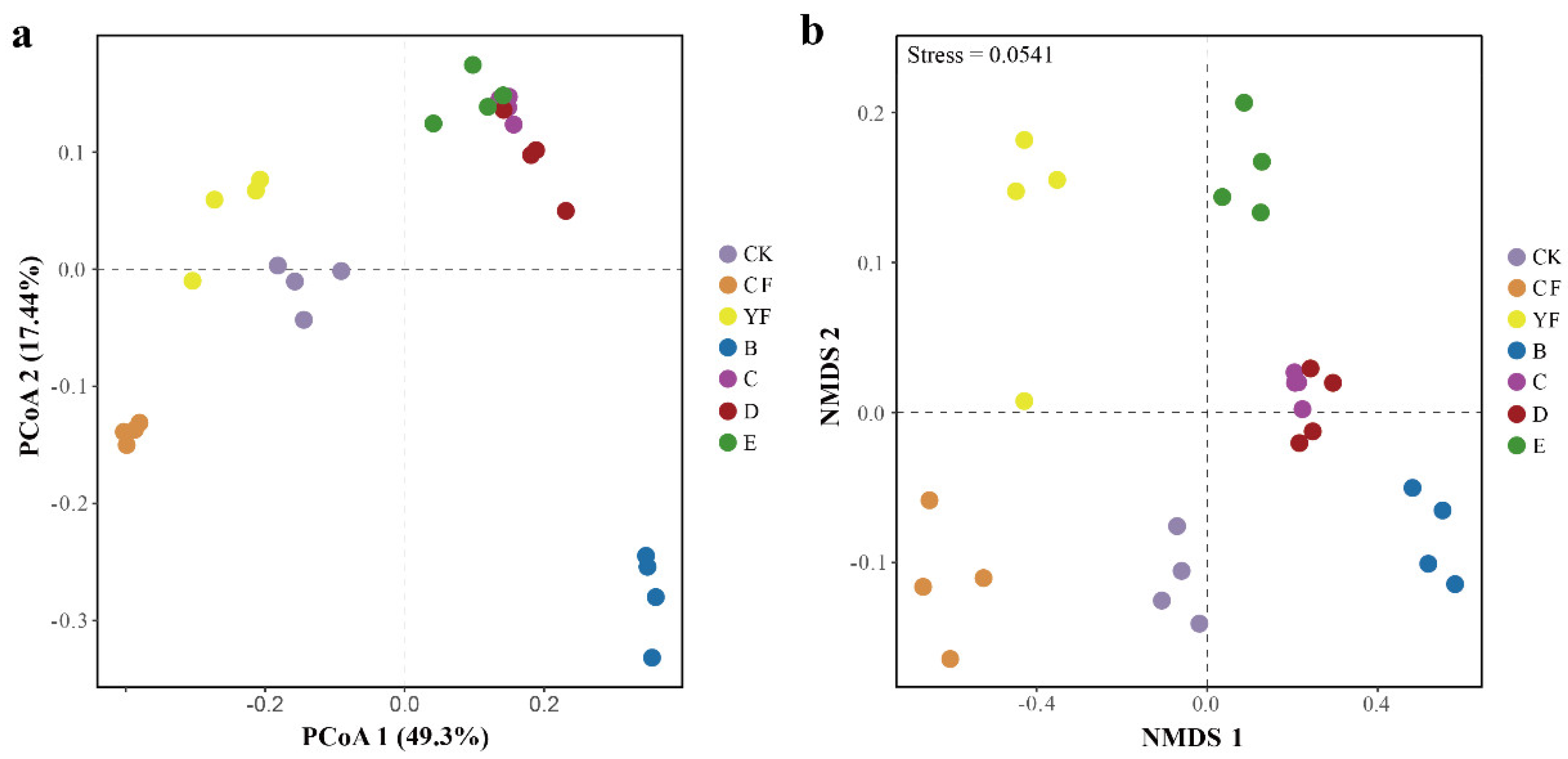
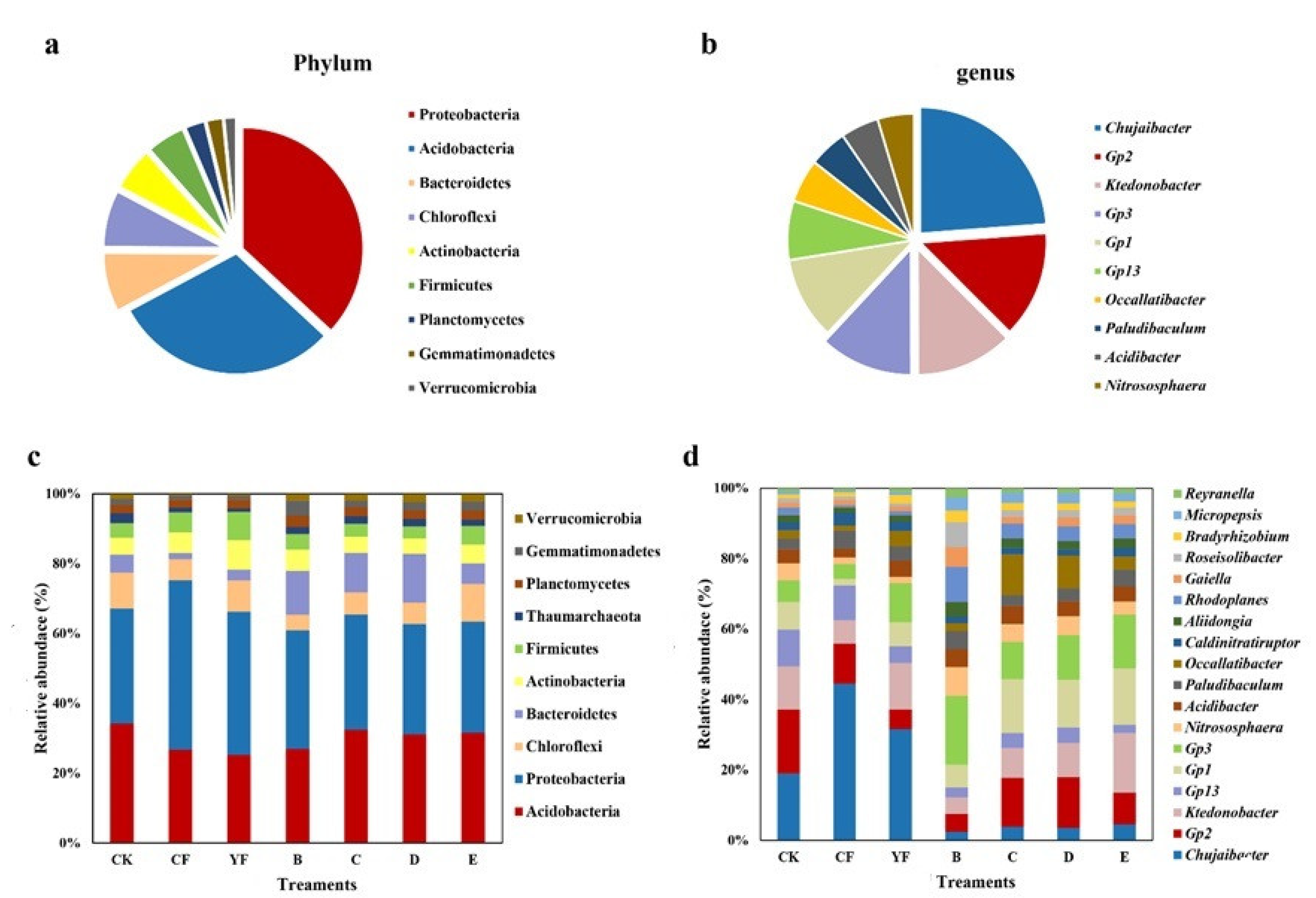
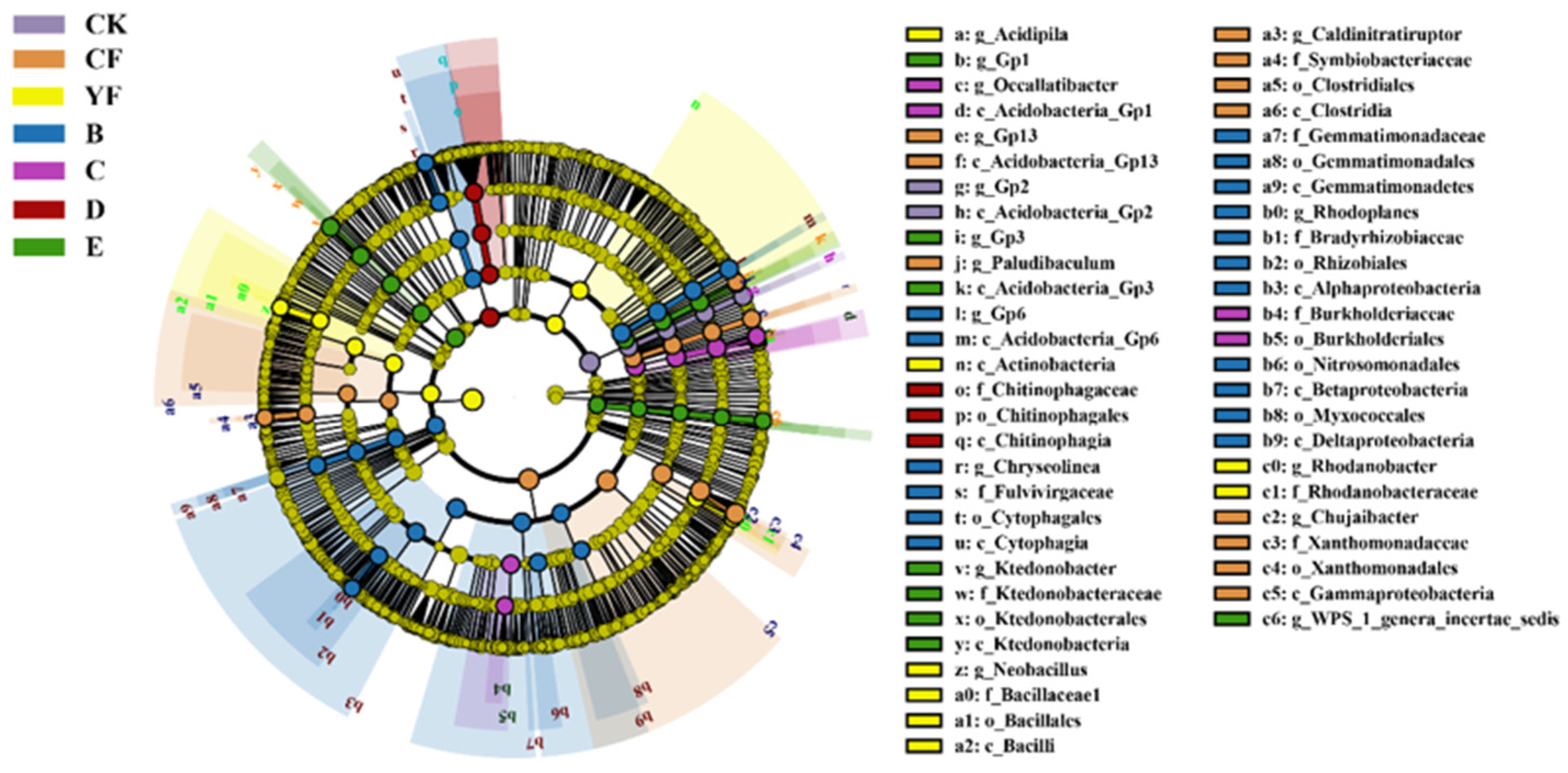
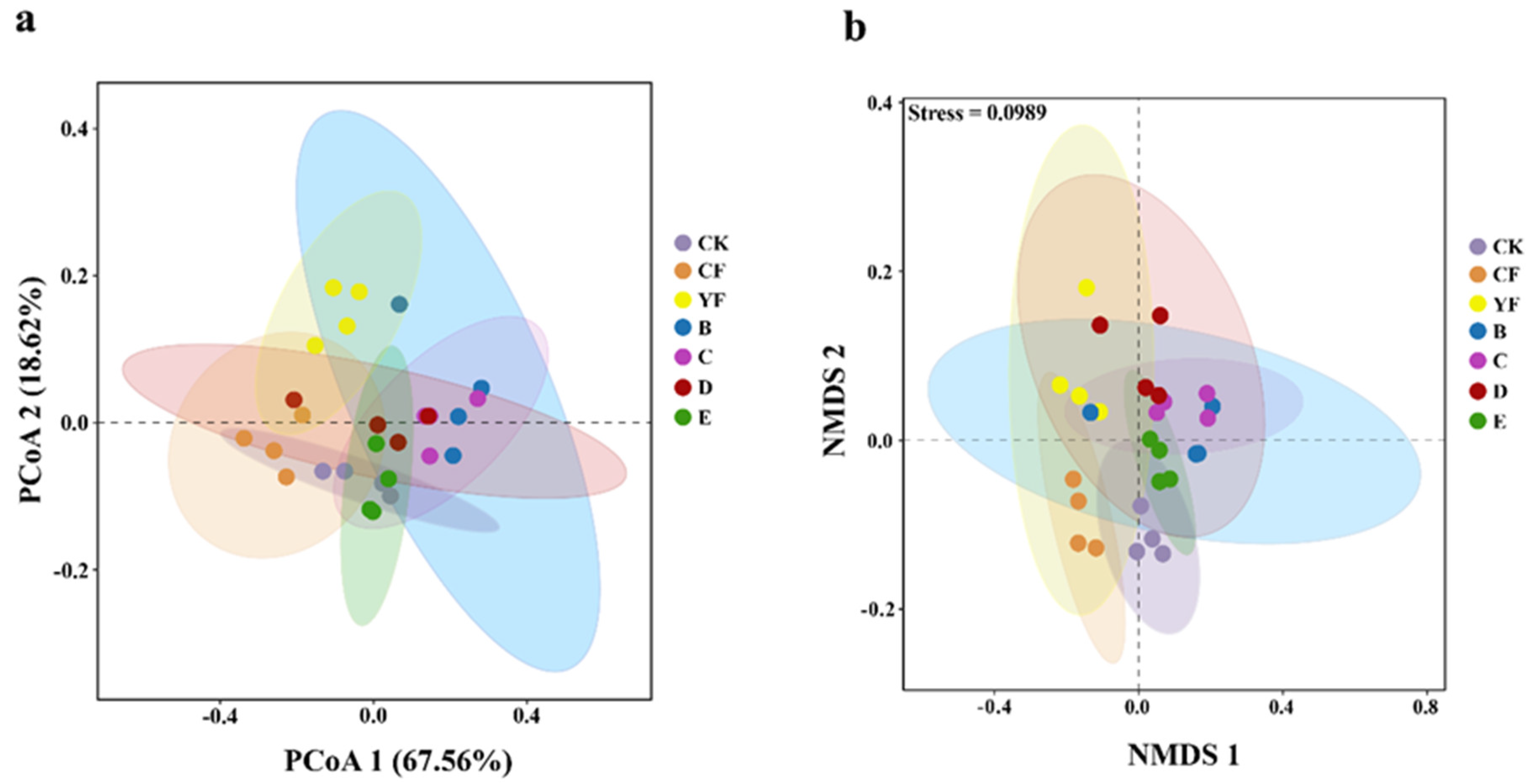
| Treatment | Ace Index | Chao1 Index | InvSimpson Index |
|---|---|---|---|
| CK | 2508.335(2397.782,2699.087) | 2512.196(2416.731,2692.541) | 62.002(41.116,108.520) |
| CF | 1960.327(1718.570,2490.319) | 1976.111(1681.807,2517.021) | 19.555(5.350,62.816) |
| YF | 2061.438(1885.417,2228.642) | 2083.692(1916.148,2228.130) | 23.850(6.185,37.074) |
| B | 2336.544(2081.459,2483.687) | 2357.377(2094.644,2496.876) | 90.544(15.853,157.123) |
| C | 2515.378(2195.118,2839.768) | 2524.132(2174.560,2850.696) | 113.373(18.255,173.424) |
| D | 2452.182(2061.470,2726.632) | 2471.458(2075.504,2739.894) | 135.448(20.890,215.097) |
| E | 2526.409(2197.966,2706.195) | 2529.136(2170.520,2742.050) | 154.237(34.164,260.080) |
| Treatment | Shannon Index | Sobs Index | Coverage |
| CK | 5.668(5.527,5.953) | 2208.2(2090,2406) | 0.993(0.992,0.993) |
| CF | 4.492(3.729,5.728) | 1644.2(1460,2153) | 0.994(0.993,0.995) |
| YF | 4.894(4.055,5.334) | 1769.6(1605,1939) | 0.994(0.993,0.994) |
| B | 5.594(4.844,6.078) | 2074(1826,2207) | 0.994(0.993,0.994) |
| C | 5.848(5.116,6.225) | 2234.8(1975,2558) | 0.993(0.992,0.995) |
| D | 5.860(5.110,6.204) | 2178(1810,2464) | 0.993(0.992,0.994) |
| E | 5.966(5.204,6.388) | 2215(1895,2379) | 0.993(0.992,0.993) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhang, X.; Hu, Y.; Zhao, Y. Effects of Different Proportions of Organic Fertilizer in Place of Chemical Fertilizer on Microbial Diversity and Community Structure of Pineapple Rhizosphere Soil. Agronomy 2024, 14, 59. https://doi.org/10.3390/agronomy14010059
Chen W, Zhang X, Hu Y, Zhao Y. Effects of Different Proportions of Organic Fertilizer in Place of Chemical Fertilizer on Microbial Diversity and Community Structure of Pineapple Rhizosphere Soil. Agronomy. 2024; 14(1):59. https://doi.org/10.3390/agronomy14010059
Chicago/Turabian StyleChen, Wanying, Xiaobo Zhang, Yinghong Hu, and Yan Zhao. 2024. "Effects of Different Proportions of Organic Fertilizer in Place of Chemical Fertilizer on Microbial Diversity and Community Structure of Pineapple Rhizosphere Soil" Agronomy 14, no. 1: 59. https://doi.org/10.3390/agronomy14010059
APA StyleChen, W., Zhang, X., Hu, Y., & Zhao, Y. (2024). Effects of Different Proportions of Organic Fertilizer in Place of Chemical Fertilizer on Microbial Diversity and Community Structure of Pineapple Rhizosphere Soil. Agronomy, 14(1), 59. https://doi.org/10.3390/agronomy14010059






