Abstract
In addressing the agricultural challenges posed by climate change, the use of biofertilizers, derived from living organisms, promotes environmentally friendly crop cultivation, and represents an adaptive strategy for sustainable agriculture in the face of climate uncertainty. Careful selection of the arbuscular mycorrhizal fungus (AMF) would represent a crucial step in mycorrhizal inoculation, considering the varying levels of compatibility between the AMF and the host plant. This study aimed to assess the impact of two AMF species that are prevalent in citrus soils of south-eastern Spain (Rhizophagus irregularis and Funneliformis mosseae) on the Citrus aurantium seedlings’ behavior. Sour-orange plants showed a high mycorrhizal dependence regardless of the specific AMF species. Both R. irregularis and F. mosseae fungi exhibited high colonization percentages, with R. irregularis outperforming F. mosseae in root colonization. Inoculation with both AMF yielded notable growth improvements, but R. irregularis exhibited higher positive effects in the long term. The heightened P nutrition and increased chlorophyll concentration significantly enhanced the performance of AMF-inoculated plants. With F. mosseae, plants showed more pronounced improvements in P nutrition and a stronger correlation of their dry mass with P concentration; however, in general, inoculation with R. irregularis produced a higher sour-orange-plant performance. Both R. irregularis and F. mosseae fungi produced strong positive effects in sour-orange growth, which positioned them as viable biofertilizer options. These results can contribute to enhancing understanding for the development of an improved design of biofertilizers used in regions that are vulnerable to climate change, such as south-eastern Spain. This promotes a shift towards more sustainable and environmentally friendly agricultural practices by reducing dependence on chemical fertilizers.
1. Introduction
In the coming decades of the 21st century, and due to factors such as climate change and the deterioration of biodiversity, agriculture will face significant obstacles in order to meet the growing demand for healthy foods [1]. By 2050, global food production must double, and to address the challenge of feeding the world’s growing population and ensuring food security amid climate change, there is an urgent need to transition towards agriculture that is sustainable and climate smart, as well as able to withstand environmental challenges, with agricultural intensification being considered the primary solution despite its associated environmental risks [2]. Hence, it is imperative to promote increased agricultural production while minimizing the strain on available arable lands.
In recent years, agriculture has shifted toward high-input, chemical-dependent practices that, in spite of having increased global food production, have also damaged soil and long-term crop yields, causing pollution and environmental degradation [3]. Agricultural intensification poses environmental threats, requiring a shift to environmentally friendly practices, as we reduce the use of inorganic fertilizers and increase the use of organic fertilizers and biofertilizers [4]. Thus, biofertilizers are explored as a sustainable alternative, using soil microorganisms to optimize production while preserving environmental health [5,6].
Biofertilizers are beneficial microbial inoculants derived from the rhizosphere that contain specific microorganisms. They are advocated as a secure partial alternative to replace chemical fertilizers and pesticides, offering cost effectiveness, eco friendliness, and easy farm-level production [7]. To maximize these advantages, it is recommended that we integrate them into the overall fertilization system alongside synthetic fertilizers, aiming to enhance soil properties and sustain horticultural crop productivity [8]. Biofertilizers represent a secure and eco-friendly alternative to chemical fertilizers and pesticides, reducing environmental pollution and offering direct benefits such as increased crop yields, fruit quality, nitrogen fixation, and phosphorus solubilization, improved plant growth, enhanced plant resistance to pests and diseases, reduced costs, and improved soil vitality and soil properties, as well as enhancing and conserving natural resources [7,9].
Arbuscular mycorrhizal fungi (AMF) have been known for establishing symbiotic relationships with approximately 80% of terrestrial plant species [10]. Due to their positive impact on soil organic-matter degradation and nutrient cycling, improving soil fertility, plant nutrition, water absorption, soil-aggregate stability, salinity, and drought-stress reduction, together with overall crop growth and productivity, can be employed as bioinoculants in sustainable agriculture [11,12,13,14]. Therefore, AMF can be used as an amendment to enhance long-term soil fertility, plant nutrition, crop productivity, and yield quality, contributing to protection in agriculture and revival of agro-ecosystems [15,16]. Moreover, mycorrhizal-based products are often more cost-effective than conventional fertilizers, particularly in regions where phosphorus depletion in soils is present [16].
Citrus is one of the most important horticultural crops in the world, being widely cultivated in south-eastern Spain, where climate conditions are those typical of a semi-arid area. On the other hand, in the northern hemisphere, fresh lemon (Citrus limon [L.] Osbeck) is mainly produced in the Mediterranean area. Specifically, in Spain, it is grown mainly in the south-eastern region, which concentrates almost 90% of its production [17]. In this area, the Intergovernmental Panel on Climate Change (IPCC) has proposed the use of desalinated seawater (DSW) as a potential option to adapt agriculture to the impacts of climate change [18]. In the design of new plantations of lemon, the choice of appropriate rootstock genotypes plays a fundamental role in plant health, especially when citrus trees are irrigated with DSW [19]. This is due to the fact that this water has high concentrations of Na+, Cl−, and boron [20], and citrus trees are sensitive to high concentrations of these elements [21,22]. Among commercial citrus rootstocks, sour orange (Citrus aurantium L.) is considered to be salt tolerant when compared with other rootstocks that are commonly used [23,24], which adds to a good tolerance to high B concentrations [25]. Moreover, sour orange is one of the rootstocks that are more frequently used in lemon-tree orchards [26], and, due to its low vigor compared with other more vigorous rootstocks, it confers a higher water-stress tolerance, and is recommended in regions where the availability of water is not assured [27]. In addition to its relevance as a rootstock in lemon plantations, sour orange is also important due to the bioactivity of its secondary metabolites, or phytochemicals, with properties that are of pivotal importance to human health, comprising, among others, anti-cancer, antiproliferative, hypolipidemic, and cardio-protective activities [28].
On the other hand, different studies indicate that citrus rootstocks show a broad variation in their mycorrhizal dependency [29,30], and the selection of AM fungi for a particular citrus rootstock under specific edaphic conditions may be necessary [31]. Although AM fungi are not host specific, previous studies have shown that mycorrhizal fungi vary widely in their effectiveness [31]. In the major citrus-growing regions of eastern Spain, F. mosseae and R. irregularis were the most prevalent AMF associated with citrus roots, with R. irregularis being the most efficient fungus in promoting growth due to its rapid colonization ability [32], which facilitates the formation of an extensive and effective network of external hyphae around roots [31]. The effectiveness of an AMF in stimulating growth and its infectivity in colonizing roots seem to vary depending on the specific fungus–host interaction. In this sense, the influence of the rootstock on mycorrhizal dependency has been demonstrated, with sour orange exhibiting a high mycorrhizal dependency and F. mosseae being the least infective and least effective in this rootstock [32].
Therefore, selection of the AMF is a key factor before mycorrhizal inoculation, since there are different levels of compatibility between the AMF and the host plant. The objective of this work was to evaluate the effects of R. irregularis and F. mosseae, two of the AMF that are most commonly found in citrus soils of eastern Spain, on the growth of C. aurantium seedlings. This rootstock was selected considering that sour orange is one of the most common rootstocks used in lemon trees, being widely cultivated in the Spanish south-east, and its adoption has also been recommended in regions with water-scarcity problems; moreover, sour orange has shown a good relative response to irrigation with DSW, which is becoming increasingly prevalent each day in these areas. Understanding which mycorrhizal fungus produces a better plant response entails important advantages for its application as a biofertilizer. By identifying the most compatible fungus, we can enhance nutrient uptake, improve plant growth, and increase overall crop yields. This knowledge enables us to develop targeted and eco-friendly agricultural practices, fostering sustainable farming while reducing the need for chemical fertilizers.
2. Materials and Methods
2.1. Plant Culture and Experimental Design
The experiment was carried out in a walk-in controlled-environment room (3 m × 6.5 m) at the IMIDA under a 16 h photoperiod (07:00 a.m.–11:00 p.m.). Day–night variation caused fluctuations in temperature (20–24 °C) and relative humidity (65–85%). Seeds of C. aurantium, provided by the germplasm bank of the IMIDA, were surface sterilized for 10 min in 20% NaClO4, rinsed four times with sterile distilled water, and sown in plastic trays containing moistened vermiculite. Forty-day-old seedlings were inoculated with R. irregularis or F. mosseae at the moment of transplantation from the germination tray to 1.1-litre pots. Pot substrate was a mixture of silica sand and clay-loam soil (soil:sand 1:3, v/v), previously sterilized in an autoclave for 1 h at 100 °C for three times on alternate days.
The inoculum per plant consisted of 25 g of bulk inoculum of R. irregularis or F. mosseae (a mix of spores, mycorrhized roots, and substrate, with an average of 1200 total fungal propagules per g of inoculum), propagated with the hybrid of Sorghum bicolor (L.) Moench and Sorghum sudanense (Piper) Hitch (S. bicolor × sudanense) as a trap plant. The inoculum was supplied by the Mycology-Mycorrhizas Laboratory, Department of Plant Biology, University of Murcia (Spain).
Experimental design consisted of two treatments, inoculated plants (+AM) and non-inoculated plants (−AM), and two fungi, R. irregularis (Ri) and F. mosseae (Fm), combined in four different treatments. Eight mycorrhizal and eight non-mycorrhizal seedlings of uniform size were selected for each fungus, providing a total of 32 pots, whose positions were changed every week to eliminate environmental variation. Plants were watered to maintain relative humidity, and 250 mL of modified Hoagland’s solution [33] (6 mM KNO3, 4 mM Ca(NO3)2, 2 mM NH4H2PO4, 1 mM MgSO4, 25 µM H3BO3, 2 µM MnSO4, 2 µM ZnSO4, 0.5 µM CuSO4, 0.065 µM (NH4)6Mo7O24, and 20 µM Fe3+-masquolate) were applied weekly to −AM plants. Inoculated plants were also irrigated weekly using the same solution without P.
2.2. Growth and Plant Analysis
The experiment was ended 4 months after plants were inoculated. At the end of the experiment, leaf water potential (Ψleaf) was measured in mature fully expanded leaves with a Schölander-type pressure chamber (model 3000; Soil Moisture Equipment Corp., Santa Barbara, CA, USA), following Turner’s recommendations [34]. After that, plant roots were carefully separated from the substrate and washed with distilled water. Fresh weights of the stems, leaves, and roots; length and diameter of the stem; and number of leaves from each plant were independently measured and processed. After roots and stems were oven-dried at 60 °C for 48 h (until a constant weight was reached) and leaves were freeze-dried, their dry weights were determined. To evaluate the degree to which plants depended on the mycorrhizal condition to produce their maximum growth, mycorrhizal dependency (MD, [35]) and mycorrhizal growth response (MGR, [36]) were calculated as follows:
where Xi is the dry weight of the inoculated plant, and Xn is the dry weight of the non-inoculated plant.
MD (%) = 100 (Xi − Xn)/Xi
MGR = loge [Xi/Xn]
Freeze-dried leaves were ground and analyzed for their mineral and chlorophyll contents. Dried and ground plant tissues were digested, ashes were dissolved in HNO3, and Na+, K+, Mg2+, Ca2+, Fe, Cu, Mn, Zn, B, and P were analyzed using an inductively coupled plasma optical emission spectrometer (Varian MPX-OEX Vista). Nitrogen (N) content was determined using a LECO FP-528 elemental analyzer. Chlorophyll contents were estimated by extracting 20 mg of ground material with N,N-dimethylformamide, and the absorbance was measured at 664.5 and 647 nm in a Shimadzu UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) [37].
2.3. Determination of Mycorrhizal Colonization
For every plant, fragments of root were taken from the middle part of the root system to obtain an estimation of mycorrhizal colonization at the end of the experiment. One sample of each root system was cleaned and stained with trypan blue [38], but using lactic acid instead of lactophenol, and 100 root segments per plant were mounted on slides, squashed by pressing on the coverslips, and quantified for AM colonization [39].
2.4. Statistical Analysis
All data were analyzed using analysis of variance (ANOVA) procedures in Statgraphics Plus Version 5.1 (1994) software (Statistical Graphics Corporation, Warrenton, VA, USA). When there was a significant effect (value of p < 0.05), means were separated using Duncan’s multiple range test. The Pearson correlation coefficients between the leaf P and the plant growth were calculated using the same statistical software.
3. Results and Discussion
In the current study, all of the inoculated sour-orange roots that were examined were colonized with arbuscular mycorrhizal fungi (AMF) and displayed typical AMF structures, such as hyphae that were present both inside and between cells, arbuscules, and vesicles (Figure 1). Occasionally, intraradical spores were also observed, either individually or grouped together within root tissues. The presence of AMF hyphae in citrus roots was consistently observed in all of the samples, and there was an abundance of arbuscules.
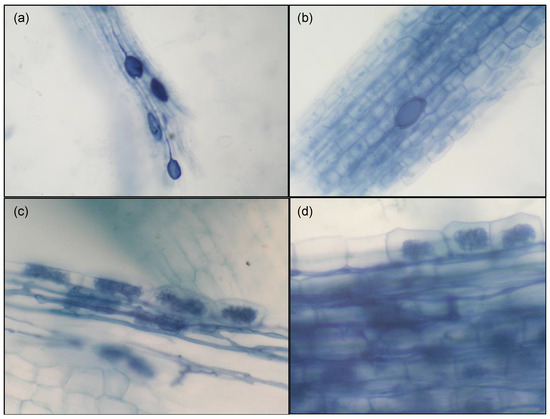
Figure 1.
Microscopic examinations of R. irregularis (Ri) and F. mosseae (Fm) colonization of C. aurantium roots 60 days after fungal inoculation. (a,b) Hyphae and vesicles formed by Fm and Ri in the roots of the host sour orange. (c,d) Arbuscules and hyphae formed by Fm and Ri in the root cortex cells of the host sour orange.
Sour-orange roots inoculated with AMF were colonized with R. irregularis besides F. mosseae, showing very good colonization 120 days after inoculation. No colonization was found on non-inoculated plants. Among two microbial inoculations, a significantly higher root fungal colonization was found with R. irregularis than with F. mosseae, with colonization percentages of 87% and 64%, respectively (Table 1). This lower colonization of Fm in sour orange plants had been previously described by other authors, who found that Fm was not as effective or infective in sour orange compared with other rootstocks such as Troyer citrange or Cleopatra mandarin [32]. However, the colonization capability of citrus roots with Fm is highly variable, and it depends on the host genotype [40]; additionally, studies with Poncirus trifoliata have shown higher colonization percentages of Fm with regard to Ri [41,42]. These different colonization responses of both AMF species in other genotypes reveal the significant influence of host-plant species on the composition of the root-colonizing AMF community, as has been demonstrated in several studies [43,44].

Table 1.
Root AM colonization of C. aurantium seedlings 120 days after inoculation with R. irregularis (Ri) and F. mosseae (Fm). −AM: non-inoculated plants; +AM: inoculated plants.
In this study, a high root infection with AMF significantly increased sour-orange growth from the early stages of the experiment. However, it is not clear whether R. irregularis or F. mosseae colonized the root of sour orange first, since 60 days after fungal inoculation, a large number of mycorrhizal intraradical hyphae, vesicles, and arbuscules were found in both R. irregularis and F. mosseae-colonized plants (Figure 1). Eighty days after inoculation, the size of sour orange plants inoculated with Fm was higher than that of Ri-inoculated plants (Figure 2a,b). However, at the end of the experiment, Ri-inoculated plants showed a higher height than Fm-inoculated plants (Figure 2c and Figure 3). Seemingly, over a brief period, Fm demonstrated a greater proneness to fostering positive outcomes in plant growth. Nonetheless, Ri exhibited superior positive effects compared with Fm over an extended period of time.

Figure 2.
Effect of the two AM fungi, R. irregularis (Ri) and F. mosseae (Fm), on Citrus aurantium seedling growth 80 days after fungal inoculation (a,b) and at the end of the experiment, 120 days after fungal inoculation (c,d). −AM: non-inoculated plants; +AM: inoculated plants.
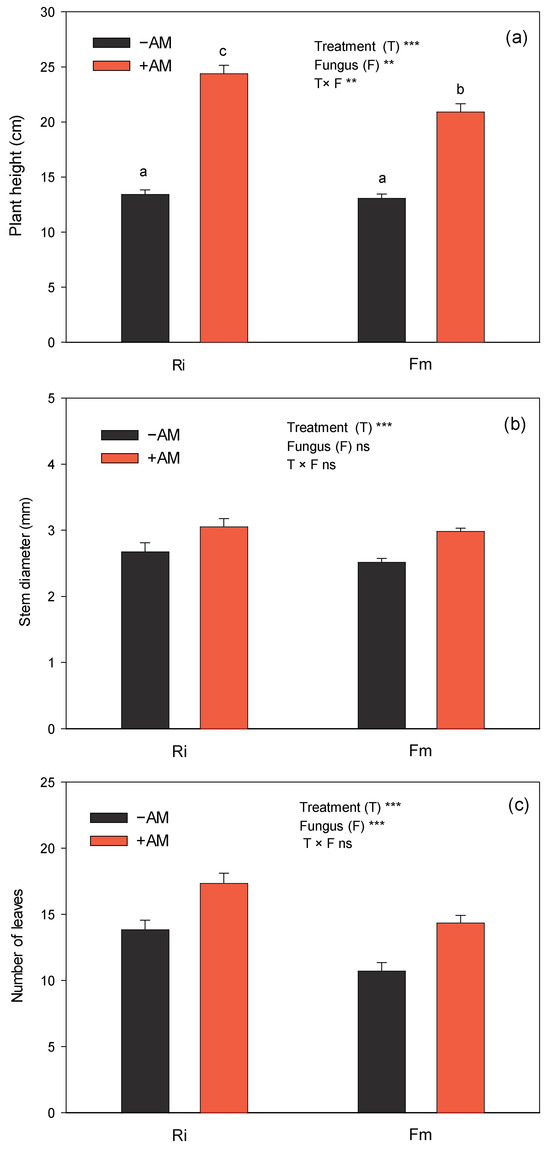
Figure 3.
Effect of the two AM fungi, R. irregularis (Ri) and F. mosseae (Fm), on plant height (a), stem diameter (b), and number of leaves (c) of C. aurantium seedlings 120 days after fungal inoculation. −AM: non-inoculated plants; +AM: inoculated plants. Data are means ± SE (n = 8). ** and *** indicate significant differences at the 0.01 and 0.001 levels of probability, respectively. Non-significant differences at the 0.05 level of probability are indicated as ns. Different letters indicate significant differences according to Duncan’s multiple range test at the 95% confidence level.
Regardless of the species of fungus, the effects of colonization with the two AMF on seedling growth in this experiment were very clear 120 days after inoculation, with large visible differences between non-inoculated and inoculated plants, mainly in the shoot growth (Figure 2 and Figure 3). On the whole, plant height, number of leaves, and stem diameter were significantly greater in +AM plants (63%, 29%, and 16%, respectively) than in −AM plants, showing the positive effect of AMF on the growth of sour-orange plants. Growth enhancement due to AMF has been described in different culture conditions for C. aurantium [45,46,47], but also for other citrus species [48,49,50,51]. On the other hand, as it was found with plant height, sour-orange plants inoculated with Ri were the plants with the greatest number of leaves at the end of the experiment (Figure 3).
With regard to the rest of the growth parameters that were analyzed, after 4 months of culture, sour-orange plants inoculated with AMF had significantly higher fresh and dry weights compared with non-inoculated plants (Table 2). The highest increase in fresh weight due to AMF inoculation was observed in the leaves, where this amounted to 113% compared with that registered by −AM plants. Moreover, with AMF inoculation, the increase of weight was higher in the shoots than in the roots, given the lower root/shoot ratios, both in fresh and dry weights, with regard to non-inoculated plants. The whole plant had a significantly better growth response (higher number of leaves and fresh weight of leaves and shoots) when it was inoculated with Ri (Table 2). However, although Ri had the best positive effect on plant-growth performance, no differences were found between the two studied AMF regarding the root and plant dry weight.

Table 2.
Effect of the two AM fungi, R. irregularis (Ri) and F. mosseae (Fm), on the fresh and dry weights of C. aurantium seedlings 120 days after fungal inoculation. −AM: non-inoculated plants; +AM: inoculated plants.
Moreover, AMF inoculation increased plant dry weight to a greater extent than fresh weight (180% more dry weight on the leaves of +AM plants than in −AM plants, versus the 115% of fresh weight, Table 2). In fact, the H2O percentage of +AM plants was significantly lower than that of −AM plants, mainly in the roots and leaves (Figure 4); therefore, AMF promoted the dry weight increase in plants, primarily in the leaves, but also, to a lower extent, in the roots. On the other hand, Fm emerged as the fungus that facilitated the greatest accumulation of dry weight in both leaves and roots. This was evident because plants inoculated with Fm exhibited the lowest water percentages in these tissues (Figure 4).
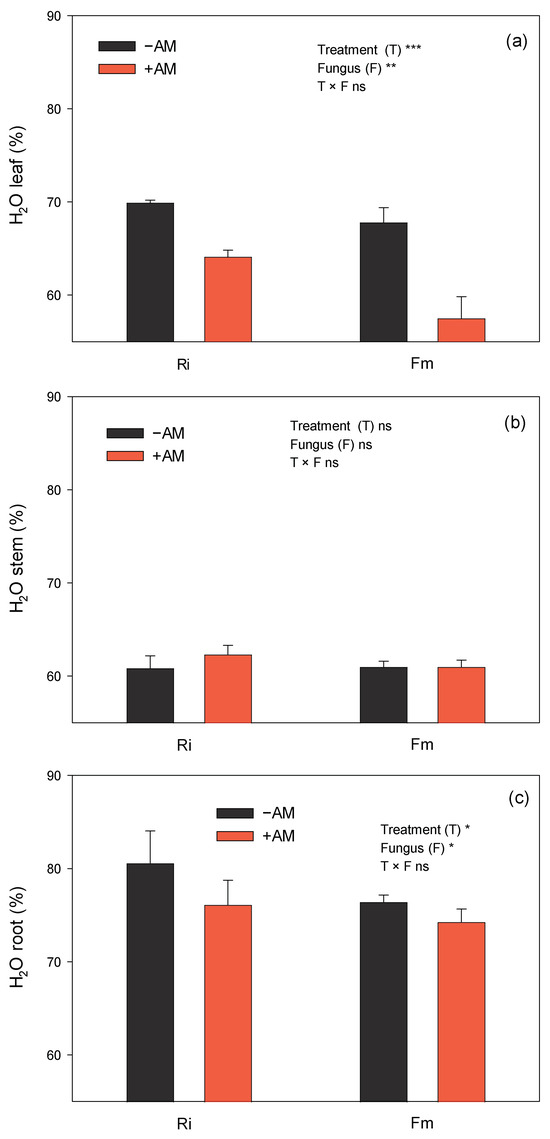
Figure 4.
Effect of the two AM fungi, R. irregularis (Ri) and F. mosseae (Fm), on water percentage in leaves (a), stems (b), and roots (c) of C. aurantium seedlings 120 days after fungal inoculation. −AM: non-inoculated plants; +AM: inoculated plants. Data are means ± SE (n = 8). *, **, and *** indicate significant differences at the 0.05, 0.01, and 0.001 levels of probability, respectively. Non-significant differences at the 0.05 level of probability are indicated as ns.
One of the important implications of mycorrhiza inoculation on plant growth and nutrient uptake is mycorrhizal dependency (MD) [52], which represents the degree to which a plant species is dependent on the mycorrhizal condition to produce its maximum growth or yield at a given level of soil fertility [53]. MD and mycorrhizal growth response (MGR) were calculated, and the results confirmed the high dependency of citrus species on AMF. They revealed that sour-orange seedlings are highly responsive to MD and MGR, since they increased the mycorrhizal dependency of sour orange over 50% for both Ri and Fm (Figure 5). Due to shallow root systems and underdeveloped root hairs, citrus is heavily dependent on arbuscular mycorrhizal fungi [54,55]. Thus, AM symbiosis partly replaces root hairs for the absorption of water and nutrients from the soil [56,57]. Moreover, citrus plants showed a higher root-hair density when they were inoculated, especially with F. mosseae [56].
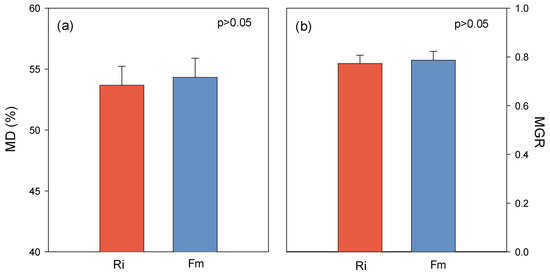
Figure 5.
Mycorrhizal dependency (MD, (a) and mycorrhizal growth response (MGR, (b) of C. aurantium seedlings to the two AM fungi, R. irregularis (Ri) and F. mosseae (Fm), 120 days after fungal inoculation.
As AMF are the completely symbiotic fungi, and their growth and development depend on the photosynthetic products of the host plant [57], higher chlorophyll concentrations in +AM plants would help the host to enhance photosynthesis. The results of this study showed that, at the end of the experiment, chlorophyll a, chlorophyll b, and total chlorophyll concentrations were significantly higher in +AM than in non-AM seedlings, irrespective of AM species (Figure 6), which is consistent with previous results found for citrus plants [41,58]. However, although plant growth of the seedlings was greater with Ri (Table 2), no differences between Fm and Ri were found, and both fungi presented similar chlorophyll a, chlorophyll b, and total chlorophyll concentrations.
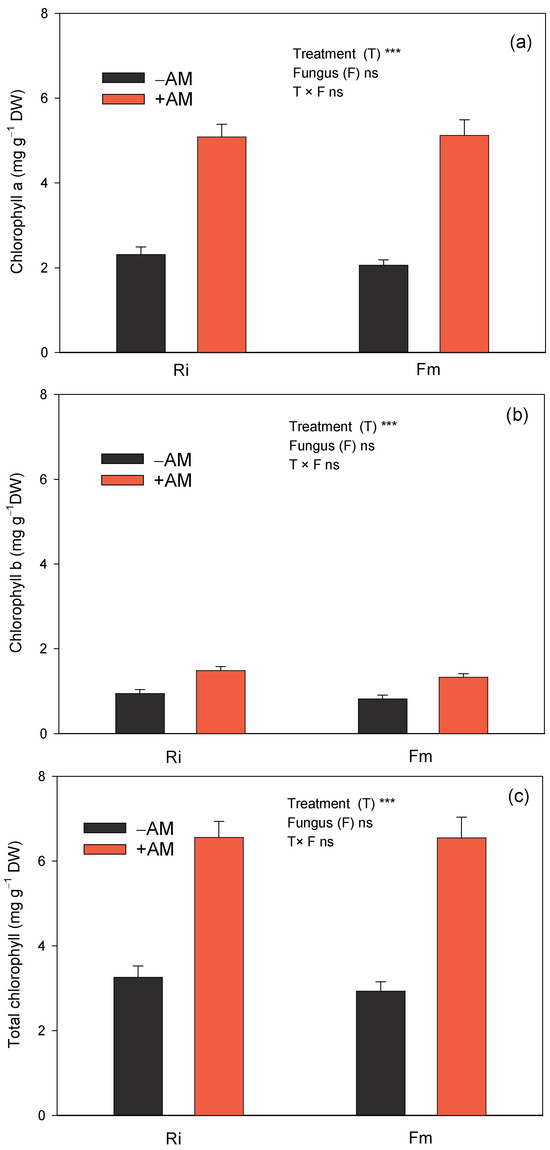
Figure 6.
Effect of the two AM fungi, R. irregularis (Ri) and F. mosseae (Fm), on chlorophyll a (a), chlorophyll b (b), and total chlorophyll (c) in leaves of C. aurantium seedlings 120 days after fungal inoculation. −AM: non-inoculated plants; +AM: inoculated plants. Data are means ± SE (n = 8). *** indicates significant differences at the 0.001 level of probability. Non-significant differences at the 0.05 level of probability are indicated as ns.
Several studies have shown that mycorrhizal citrus seedlings present higher root hydraulic conductivity, stomatal conductance, and transpiration rates than non-mycorrhizal seedlings [45,48,58]. Due to their greater stomatal openness, and, therefore, due to their higher transpiration rates, mycorrhizal plants show higher levels of water uptake through their roots and are able to lower the water potential of the soil in pots more than non-mycorrhizal plants [48]. Moreover, since root systems are constrained to a relatively low soil volume, the leaf-water potential declines more quickly in +AM than in −AM plants [48], since +AM plants are larger (Table 2) and deplete soil moisture reserves more quickly. Hence, both the greater plant size and the higher transpiration rates could be the reasons behind the lower Ψx of +AM when compared with −AM plants (Figure 7). In spite of the lower values of Ψx found in +AM plants, these did not show any visual symptoms of water stress, since watering was sufficient to satisfy the water requirements of the largest plants. For this reason, the greater size of plants inoculated with Ri with regard to those inoculated with Fm did not produce any differences to plant water status (Figure 7).
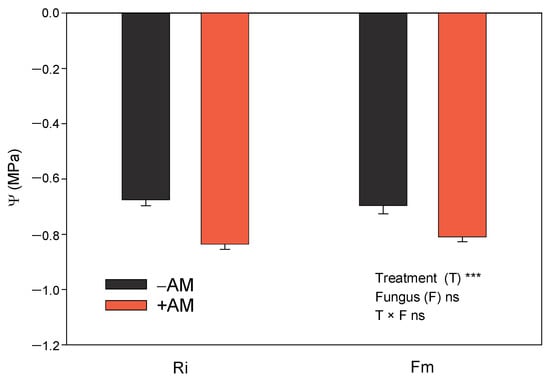
Figure 7.
Effect of the two AM fungi, R. irregularis (Ri) and F. mosseae (Fm), on leaf water potential in leaves of Citrus aurantium seedlings 120 days after fungal inoculation. −AM: non-inoculated plants; +AM: inoculated plants. Data are means ± SE (n = 8). *** indicates significant differences at the 0.001 level of probability. Non-significant differences at the 0.05 level of probability are indicated as ns.
In our experiment, mycorrhizal inoculation significantly improved plant phosphorus acquisition (it increased by approximately 100%) compared with non-inoculated plants. F. mosseae showed a higher acquisition efficiency than R. irregularis (92% and 122%, respectively; Figure 8). Inoculation with F. mosseae also improved leaf phosphorus acquisition in licorice plants with regard to non-inoculated plants [59]. It is widely established that one of the primary benefits of AMF is the improved P uptake conferred on symbiotic plants, and the higher uptake of phosphorus by host plants is primarily due to extraradical hyphae and elevated acid phosphatase activity [60]. Moreover, the distribution of hyphae in soil zones from which roots are absent and the greater contact of the hyphae with the soil have a large contribution to the increased nutrient uptake [61]. In any case, foliar P concentrations found in the experiment in both +AM and −AM plants were below the optimum range of P for adult citrus trees [62]. Nonetheless, previous results showed that these values were sufficient to sustain the proper growth of young citrus seedlings without deficiency symptoms [48,63].
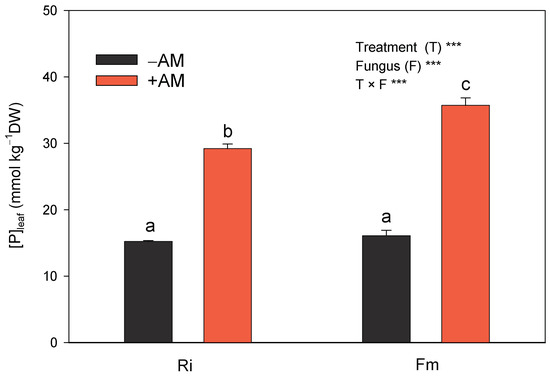
Figure 8.
Effect of the two AM fungi, R. irregularis (Ri) and F. mosseae (Fm), on phosphorus concentration in leaves of C. aurantium seedlings 120 days after fungal inoculation. −AM: non-inoculated plants; +AM: inoculated plants. Data are means ± SE (n = 8). *** indicates significant differences at the 0.001 level of probability. Different letters indicate significant differences according to Duncan’s multiple range test at the 95% confidence level.
As a consequence of the higher phosphorus uptake in mycorrhizal plants, their growth is increased, and the great effect of mycorrhizal infection on plant growth has been related to the higher uptake of soil P by the extraradical mycorrhizal mycelium [60,64,65]. In this regard, leaf P has been positively correlated with root dry weight or root length in Citrus volkameriana inoculated plants [66]. In our experiment, positive and linear correlations were found between leaf P and root and leaf dry weights at the end of the experiment for both Ri and Fm fungi species (Figure 9). Moreover, although sour-orange growth was positively correlated with the leaf P status regardless of fungi species, this correlation was stronger when Fm fungus was used.
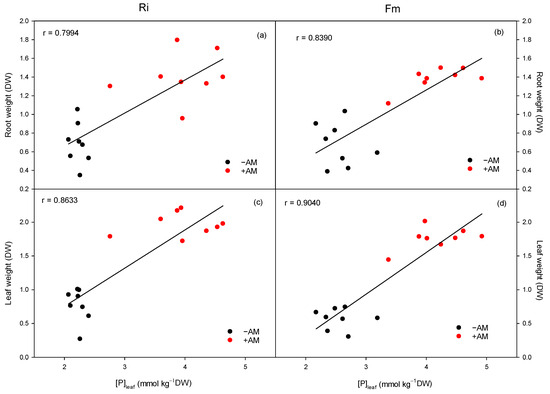
Figure 9.
Effect of leaf phosphorus concentration on the growth of the root (a,b) and leaves (c,d) of C. aurantium seedlings for the two AM fungi, R. irregularis (Ri) and F. mosseae. −AM: non-inoculated plants; +AM: inoculated plants.
In general, AMF contributes to an accelerated acquisition of nutrients in the citrus plants [47,55,67,68]. However, in our experiment, mycorrhizal inoculation decreased foliar nitrogen levels (Table 3). Some results obtained with other citrus species show no effect of inoculation on nitrogen content [69]. Moreover, similarly to the results that we attained, it has been found that growth stimulation in inoculated plants resulting from the increased P acquisition can produce a reduction in N concentrations due to growth dilution [45]. This dilution effect caused by growth stimulation, a consequence of the better phosphorus nutrition, could also be the reason for the lower concentrations of foliar Ca2+, K+, Na+, and Mg2+ found in mycorrhizal seedlings (Table 3). A similar effect on foliar K+ levels was found in other citrus studies [45], and even higher levels of K+ and Ca2+ were observed in non-inoculated plants with regard to R. irregularis-inoculated plants in trifoliate orange [70].

Table 3.
Effect of the two AM fungi, R. irregularis (Ri) and F. mosseae (Fm), on the N, K, Ca, Mg, Na, B, Fe, Cu, Mn, and Zn concentrations of C. aurantium leaves 120 days after fungal inoculation. Concentrations are expressed as mmol kg−1 DW for all of the nutrients, except for N, which is expressed as % of DW. −AM: non-inoculated plants; +AM: inoculated plants.
On the other hand, the effect of AM fungi in the micronutrients B, Fe, Cu, Mn, and Zn was similar, showing a lower concentration in +AM plants compared with −AM plants (Table 3). Again, a dilution effect due to the greater growth of inoculated plants could have taken place. Other authors found that AM symbiosis does not affect plant Cu concentrations or even reduces Mn concentration [48,71]. This decrease in Mn in mycorrhizal plants has been explained by changes in the physiology of the host, with reflections on the biological processes of Mn oxidation and alterations of Mn4+ reducing potential in the rhizosphere of mycorrhizal plants, probably due to a lower population of Mn-reducing organisms [72]. In general, AMF increase the Fe uptake and translocation in mycorrhizal plants, since the higher amount of hyphae and the larger surface area of roots could help the host plant obtain more Fe from the soil [73]. However, a lower Fe concentration was observed in the leaves of +AM than in −AM plants (Table 3); although, all of the plants had optimal leaf Fe concentrations regardless of the treatments. The literature describes the different impacts of AM fungi on Fe nutrition, from an increase in Fe uptake and translocation in mycorrhizal plants [73] to no response to mycorrhizal inoculation in citrus seedlings [74].
Anyhow, regardless of the differences found in the nutrition of mycorrhizal and non-mycorrhizal plants, the type of fungus had little influence on the nutritional status of the plants. In addition to the differences described in the nutrition of P, only N, Cu, and Zn concentrations were significantly higher in plants inoculated with Ri compared with Fm-inoculated plants (Table 3).
4. Conclusions
Sour-orange plants exhibit a strong positive response to arbuscular mycorrhizal fungi, showing a high mycorrhizal dependency regardless of the AMF species. Both R. irregularis and F. mosseae fungi displayed high colonization percentages, and yet R. irregularis exhibited a higher root colonization compared with F. mosseae.
Inoculation resulted in a significant growth improvement, with F. mosseae leading to higher plant-growth stimulation in the short term. However, R. irregularis demonstrated superior positive effects compared with F. mosseae in the long term. The improvement in P nutrition was more pronounced with F. mosseae, and the stimulation of plant growth was also higher in plants inoculated with F. mosseae. In spite of this, R. irregularis showed a better overall plant-growth performance, indicating that factors beyond P nutrition, such as overall plant nutrition, could contribute to an improved plant performance with this fungus.
In summary, both R. irregularis and F. mosseae fungi caused a positive response in sour-orange plants. Therefore, the combination of both fungal species could establish a more comprehensive and balanced symbiosis, mutually reinforcing each other and resulting in a more robust and healthier growth of citrus trees. All these findings position both fungi as viable options for use as biofertilizers in the cultivation of citrus trees grafted with sour-orange rootstock. This knowledge can help to promote the use of biofertilizers for citrus-species cultivation, particularly in areas that are especially vulnerable to climate change, promoting the reduction of chemical fertilizers for the sake of a more sustainable and environmentally friendly agriculture.
Author Contributions
Conceptualization, J.M.N. and A.M.; methodology, J.M.N. and A.M.; formal analysis, J.M.N.; investigation, J.M.N. and A.M.; data curation, J.M.N.; writing—original draft preparation, J.M.N.; writing—review and editing, J.M.N. and A.M.; funding acquisition, J.M.N. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by 15369/PI/10 Fundación Séneca (Región de Murcia, Spain) and POI07-12 (Instituto Murciano de Investigación y Desarrollo Agrario y Medioambiental), as well as the FEDER Funds.
Data Availability Statement
Data will be made available upon request.
Acknowledgments
To Eva Arques for her help with the analyses. We also want to thank Andrés Paredes for his assistance with the correction of the written English.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO (Food and Agriculture Organization of the United Nations). Crop Prospects and Food Situation—Quarterly Global Report No. 1; FAO: Rome, Italy, 2020. [Google Scholar]
- GSDR (Global Sustainable Development Report) 2019. The Future Is Now—Science for Achieving Sustainable Development, Independent Group of Scientists Appointed by the Secretary-General; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Suhag, M. Potential of biofertilizers to replace chemical fertilizers. Int. Adv. Res. J. Sci. Eng. Technol. 2016, 3, 163–167. [Google Scholar]
- Kuila, D.; Ghosh, S. Aspects, problems and utilization of Arbuscular Mycorrhizal (AM) application as bio-fertilizer in sustainable agriculture. Curr. Res. Microb. Sci. 2022, 3, 100107. [Google Scholar] [CrossRef] [PubMed]
- Raei, Y.; Shariati, J.; Weisany, W. Effect of biological fertilizers on seed oil, yield and yield components of safflower (Carthamus tinctorius L.) at different irrigation levels. J. Agric. Sci. Sustain. Prod. 2015, 25, 65–84. [Google Scholar]
- Khalediyan, N.; Weisany, W.; Schenk, P.M. Arbuscular mycorrhizae and rhizobacteria improve growth, nutritional status and essential oil production in Ocimum basilicum and Satureja hortensis. Ind. Crops Prod. 2021, 160, 113163. [Google Scholar] [CrossRef]
- Abobatta, W.F. Biofertilizers and citrus cultivation. MOJ Ecol. Environ. Sci. 2020, 5, 171–176. [Google Scholar] [CrossRef]
- Pathak, D.V.; Kumar, M.; Rani, K. Biofertilizer application in horticultural crops. In Microorganisms for Green Revolution. Microorganisms for Sustainability; Panpatte, D., Jhala, Y., Vyas, R., Shelat, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 215–227. [Google Scholar]
- Hazarika, T.K.; Aheibam, B. Soil nutrient status, yield and quality of lemon (Citrus limon Burm.) cv. ‘Assam lemon’ as influenced by bio-fertilizers, organics and inorganic fertilizers. J. Plant Nutr. 2019, 42, 853–863. [Google Scholar] [CrossRef]
- Kottke, I.; Nebel, M. The evolution of mycorrhiza-like associations in liverworts: An update. New Phytol. 2005, 167, 330–334. [Google Scholar] [CrossRef]
- Ortas, I.; Rafique, M. The mechanisms of nutrient uptake by arbuscular mycorrhizae. In Mycorrhiza—Nutrient Uptake, Biocontrol, Ecorestoration; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–19. [Google Scholar]
- Makarov, M. The role of mycorrhiza in transformation of nitrogen compounds in soil and nitrogen nutrition of plants: A review. Eurasian Soil Sci. 2019, 52, 193–205. [Google Scholar] [CrossRef]
- Anand, K.; Pandey, G.K.; Kaur, T.; Pericak, O.; Olson, C.; Mohan, R.; Akansha, K.; Yadav, A.; Devi, R.; Kour, D.; et al. Arbuscular mycorrhizal fungi as a potential biofertilizers for agricultural sustainability. J. Appl. Biol. Biotechnol. 2022, 10 (Suppl. 1), 90–107. [Google Scholar] [CrossRef]
- Jansa, J.; Forczek, S.T.; Rozmos, M.; Puschel, D.; Bukovska, P.; Hrselova, H. Arbuscular mycorrhiza and soil organic nitrogen: Network of players and interactions. Chem. Biol. Technol. Agric. 2019, 6, 10. [Google Scholar] [CrossRef]
- Lu, F.; Lee, C.; Wang, C. The influence of arbuscular mycorrhizal fungi inoculation on yam (Dioscorea spp.) tuber weights and secondary metabolite content. Peer J. 2015, 3, 12–66. [Google Scholar] [CrossRef] [PubMed]
- Basiru, S.; Mwanza, H.P.; Hijri, M. Analysis of arbuscular mycorrhizal fungal inoculant benchmarks. Microorganisms 2021, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- MAGRAMA. Anuario de Estadística Agraria 2021; Ministerio de Agricultura, Pesca y Alimentación, Madrid, Spain. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadistica-diqital/ (accessed on 10 October 2023).
- Bates, B.C.; Kundzewicz, Z.W.; Wu, S.; Palutikof, J.P. Climate Change and Water; Technical paper of the Intergovernmental Panel on Climate Change; IPCC Secretariat: Geneva, Switzerland, 2008; p. 210. [Google Scholar]
- Navarro, J.M.; Antolinos, V.; Robles, J.M.; Botía, P. Citrus irrigation with desalinated seawater under a climate change scenario. Front. Plant Sci. 2022, 13, 909083. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alvarez, V.; González-Ortega, M.J.; Martin-Gorriz, B.; Soto-García, M.; Maestre-Valero, J.F. The use of desalinated seawater for crop irrigation in the Segura River Basin (south-eastern Spain). Desalination 2017, 422, 153–164. [Google Scholar] [CrossRef]
- Maas, E.V. Salinity and citriculture. Tree Physiol. 1993, 12, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Grattan, S.R.; Díaz, F.J.; Pedrero, F.; Vivaldi, G.A. Assessing the suitability of saline wastewaters for irrigation of Citrus spp.: Emphasis on boron and specific-ion interactions. Agric. Water Manag. 2015, 157, 48–58. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Garcia-Sanchez, F. Multiple abiotic stresses occurring with salinity stress in citrus. Environ. Exp. Bot. 2014, 103, 128–137. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Espinoza-Núñez, E.; Junior, J.P.; Filho, F.A.A.M.; Machado, E.C. Citrus rootstocks for improving the horticultural performance and physiological responses under constraining environments. In Improvement of Crops in the Era of Climatic Changes; Ahmad, P., Wani, M., Azooz, M., Tran, L.S., Eds.; Springer: London, UK, 2014; pp. 1–37. [Google Scholar]
- Simón-Grao, S.; Nieves, M.; Martínez-Nicolás, J.J.; Cámara-Zapata, J.M.; Alfosea-Simón, M.; García-Sánchez, F. Response of three citrus genotypes used as rootstocks grown under boron excess conditions. Ecotoxicol. Environ. Saf. 2018, 159, 10–19. [Google Scholar] [CrossRef]
- Pérez-Pérez, J.G.; Porras, I.; Garcia-Lidon, A.; Botía, P.; Garcia-Sanchez, F. ‘Fino’ lemon clones compared with two other lemon varieties on two rootstocks in Murcia (Spain). Sci. Hortic. 2005, 106, 530–538. [Google Scholar] [CrossRef]
- Robles, J.M.; Botía, P.; Pérez-Pérez, J.G. Sour orange rootstock increases water productivity in deficit irrigated ‘Verna’ lemon trees compared with Citrus macrophylla. Agric. Water Manag. 2017, 186, 98–107. [Google Scholar] [CrossRef]
- Maksoud, S.; Abdel-Massih, R.M.; Rajha, H.N.; Louka, N.; Chemat, F.; Barba, F.J.; Debs, E. Citrus aurantium L. active constituents, biological effects and extraction methods. An updated review. Molecules 2021, 26, 5832. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Syvertsen, J.P. Host determinants of mycorrhizal dependency of citrus rootstock seedlings. New Phytol. 1985, 101, 667–676. [Google Scholar] [CrossRef]
- Nemec, S. Response of six citrus rootstocks to three species of Glomus, a mycorrhizal fungus. Proc. Florida. State Hortic. Soc. 1978, 91, 10–14. [Google Scholar]
- Graham, J.H.; Linderman, R.G.; Menge, J.A. Development of external hyphae by different isolates of mycorrhizal Glomus sp. in relation to root colonization and growth of Troyer citrange. New Phytol. 1982, 91, 683. [Google Scholar] [CrossRef]
- Camprubí, A.; Calvet, C. Isolation and screening of mycorrhizal fungi from citrus nurseries and orchards and inoculation studies. HortScience 1996, 31, 366–369. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- Turner, N.C. Measurements of plant water status by pressure chamber technique. Irrig. Sci. 1988, 9, 289–308. [Google Scholar] [CrossRef]
- Plenchette, C.; Fortin, J.A.; Furlan, V. Growth response of several plant species to mycorrhiza in a soil of moderate P fertility. I. Mycorrhizal dependency under field conditions. Plant Soil 1983, 70, 191–209. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C.; et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef]
- Inskeep, W.P.; Bloom, P.R. Extinction coefficient of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol. 1985, 77, 483–485. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Quatrini, P.; Gentile, M.; Carimi, F.; De Pasquale, F.; Puglia, A.M. Effect of native arbuscular mycorrhizal fungi and Glomus mosseae on acclimatization ad development of micropropagated Citrus limon (L.) Burm. J. Hortic. Sci. Biotechnol. 2003, 78, 39–45. [Google Scholar] [CrossRef]
- Wu, Q.S.; Srivastava, A.K.; Li, Y. Effects of mycorrhizal symbiosis on growth behavior and carbohydrate metabolism of trifoliate orange under different substrate P levels. J. Plant Growth Regul. 2015, 34, 499–508. [Google Scholar] [CrossRef]
- Liu, C.Y.; Srivastava, A.; Wu, Q.S. Mycorrhizal fungi regulate root responses and leaf physiological activities in trifoliate orange. Not. Bot. Horti Agrobot. 2017, 45, 17–21. [Google Scholar] [CrossRef][Green Version]
- Martínez-García, L.B.; Richardson, S.J.; Tylianakis, J.M.; Peltzer, D.A.; Dickie, I.A. Host identity is a dominant driver of mycorrhizal fungal community composition during ecosystem development. New Phytol. 2015, 205, 1565–1576. [Google Scholar] [CrossRef]
- Krüger, C.; Kohout, P.; Janoušková, M.; Püschel, D.; Frouz, J.; Rydlová, J. Plant communities rather than soil properties structure arbuscular mycorrhizal fungal communities along primary succession on a mine spoil. Front. Microbiol. 2017, 8, 719. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Graham, J.H. Phosphorus supply and arbuscular mycorrhizas increase growth and net gas exchange responses of two Citrus spp. grown at elevated CO2. Plant Soil 1999, 208, 209–219. [Google Scholar] [CrossRef]
- Satir, N.Y.; Ortas, I.; Satir, O. The influence of mycorrhizal species on sour orange (Citrus aurantium L.) growth under saline soil conditions. Pak. J. Agric. Sci. 2016, 53, 399–406. [Google Scholar]
- Ortas, I.; Akpinar, C.; Demirbas, A. Sour orange (Citrus aurantium L.) growth is strongly mycorrhizal dependent in terms of phosphorus (P) nutrition rather than zinc (Zn). Commun. Soil Sci. Plant Anal. 2016, 22, 2514–2527. [Google Scholar] [CrossRef]
- Navarro, J.M.; Morte, A. Mycorrhizal effectiveness in Citrus macrophylla at low phosphorus fertilization. J. Plant Physiol. 2019, 232, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Guo, X.N.; Wu, X.L.; Dai, F.J.; Wu, Q.S. The comprehensive effects of Rhizophagus intraradices and P on root system architecture and P transportation in Citrus limon L. Agriculture 2022, 12, 317. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Wu, Q.H.; Zou, Y.; Wu, Q.S. Arbuscular mycorrhizas improve plant growth and soil structure in trifoliate orange under salt stress. Arch. Agron. Soil Sci. 2017, 63, 491–500. [Google Scholar] [CrossRef]
- Ding, Y.E.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhizal fungi regulate daily rhythm of circadian clock in trifoliate orange under drought stress. Tree Physiol. 2022, 42, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Ortas, I. Do maize and pepper plants depend on mycorrhizae in terms of phosphorus and zinc uptake? J. Plant Nutr. 2012, 35, 1639–1656. [Google Scholar] [CrossRef]
- Gerdemann, J.W. Vesicular-arbuscular mycorrhiza. In The Development and Function of Roots; Torrey, J.G., Clarkson, D.T., Eds.; Academic Press: New York, NY, USA, 1975; pp. 575–591. [Google Scholar]
- Wu, Q.S.; He, X.H.; Zou, Y.N.; Liu, C.Y.; Xiao, J.; Li, Y. Arbuscular mycorrhizas alter root system architecture of Citrus tangerine through regulating metabolism of endogenous polyamines. Plant Growth Regul. 2012, 68, 27–35. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Z.; Yu, L.; Li, Y. Research progress of arbuscular mycorrhizal fungi promoting citrus growth. Horticulturae 2023, 9, 1162. [Google Scholar] [CrossRef]
- Wu, Q.S.; Liu, C.Y.; Zhang, D.J.; Zou, Y.N.; He, X.H.; Wu, Q.H. Mycorrhiza alters the profile of root hairs in trifoliate orange. Mycorrhiza 2016, 26, 237–247. [Google Scholar] [CrossRef]
- Hodge, A.; Campbell, C.D.; Fitter, A.H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 2001, 413, 297–299. [Google Scholar] [CrossRef]
- Wu, Q.S.; Xia, R.X. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J. Plant Physiol. 2006, 163, 417–425. [Google Scholar] [CrossRef]
- Liu, J.; Wu, L.; Wei, S.; Xiao, X.; Su, C.; Jiang, P.; Song, Z.; Wang, T.; Yu, Z. Effects of arbuscular mycorrhizal fungi on the growth, nutrient uptake and glycyrrhizin production of licorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul. 2007, 52, 29–39. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; He, X.H. Differences of hyphal and soil phosphatase activities in drought-stressed mycorrhizal trifoliate orange (Poncirus trifoliata) seedlings. Sci. Hortic. 2011, 129, 294–298. [Google Scholar] [CrossRef]
- Joubert, S.; Archer, E. The Influence of Mycorrhiza on Vines. Wynboer A Tech. Guide Wine Prod. 2000, 130, 86–88. [Google Scholar]
- Sivrastava, A.K.; Singh, S. Leaf and soil nutrient guide in citrus. A review. Agric. Rev. 2004, 25, 235–251. [Google Scholar]
- Navarro, J.M.; Pérez-Tornero, O.; Morte, A. Alleviation of salt stress in citrus seedlings inoculated with arbuscular mycorrhizal fungi depends on the rootstock salt tolerance. J. Plant Physiol. 2014, 171, 76–85. [Google Scholar] [CrossRef]
- Grimoldi, A.A.; Kavanova, M.; Lattanzi, F.A.; Schnyder, H. Phosphorus nutrition-mediated effects of arbuscular mycorrhiza on leaf morphology and carbon allocation in perennial ryegrass. New Phytol. 2005, 168, 435–444. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: San Diego, CA, USA, 2008; 800p. [Google Scholar]
- Fidelibus, M.W.; Martin, C.A.; Stutz, J.C. Geographic isolates of Glomus increase root growth and whole-plant transpiration of Citrus seedlings grown with high phosphorus. Mycorrhiza 2001, 10, 231–236. [Google Scholar] [CrossRef]
- Wu, Q.S.; Gao, W.Q.; Srivastava, A.K.; Zhang, F.; Zou, Y.N. Nutrient acquisition and fruit quality of Ponkan mandarin in response to AMF inoculation. Indian J. Agric. Sci. 2020, 90, 1563–1567. [Google Scholar] [CrossRef]
- Khanum, S.; Al-Tawaha, A.R.M.; Al-Tawaha, A.R.; Alatrash, H.; Rauf, A.; Karnwal, A.; Dey, A.; Alimad, N.; Thangadurai, D.; Sangeetha, J.; et al. Arbuscular mycorrhiza in citrus. In Mycorrhizal Technology: Managing Plant Stress and Mitigating Climate Change Using Mycorrhizae; Sangeetha, J., Al-Tawaha, A.R.M., Thangadurai, D., Eds.; Apple Academic Press Inc.: Palm Bay, FL, USA; Burlington, ON, Canada; CRC Press: Boca Raton, FL, USA; Abingdon, Oxon, UK, 2023; pp. 65–83. [Google Scholar]
- Dutra, P.V.; Abad, M.; Almela, V.; Agustí, M. Auxin interaction with the vesicular-arbuscular mycorrhizal fungus Glomus intraradices Schenck & Smith improves vegetative growth of citrus rootstocks. Sci. Hortic. 1996, 66, 77–83. [Google Scholar]
- Xiao, J.X.; Hu, C.Y.; Chen, Y.Y.; Hua, J.; Yang, B. Growth and nutrient content of trifoliate orange seedlings influenced by arbuscular mycorrhizal fungi inoculation in low magnesium soil. J. Plant Nutr. 2015, 38, 1516–1529. [Google Scholar] [CrossRef]
- Wu, Q.S.; Levy, Y.; Zou, Y.N. Arbuscular mycorrhizae and water relations in citrus. In Tree and Forestry Science and Biotechnology; Wu, Q.S., Levy, Y., Zou, Y.N., Eds.; University of the West Indies: Kingston, Jamaica, 2009; Volume 3, pp. 105–112. [Google Scholar]
- Kothari, S.K.; Marschner, H.; Romheld, V. Contribution of the VA mycorrhizal hyphae in acquisition of phosphorus and zinc by maize grown in a calcareous soil. Plant Soil 1991, 131, 177–185. [Google Scholar] [CrossRef]
- Caris, C.; Hoerdt, W.; Hawkins, H.J.; Roemheld, V.; George, E. Studies on the iron transport by arbuscular mycorrhizal hyphae from soil to peanut and sorghum plants. Mycorrhiza 1998, 8, 35–39. [Google Scholar] [CrossRef]
- Khalil, H.A.; Eissa, A.M.; El-Shazly, S.M.; Aboul Nasr, A.M. Improved growth of salinity-stressed citrus after inoculation with mycorrhizal fungi. Sci. Hortic. 2011, 130, 624–632. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).