Chemical Profile of Cell Cultures of Kalanchoë gastonis-bonnieri Transformed by Agrobacterium rhizogenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Induction of Transformed Roots
2.3. Morphological Description of In Vitro Cultures
2.4. DNA Extraction and PCR Analysis
2.5. Ultrasound Assisted Extraction (UAE)
2.6. Chemical Characterization of Kalanchoë Gastonis-Bonnieri Extracts
2.7. Statistical Analysis
3. Results
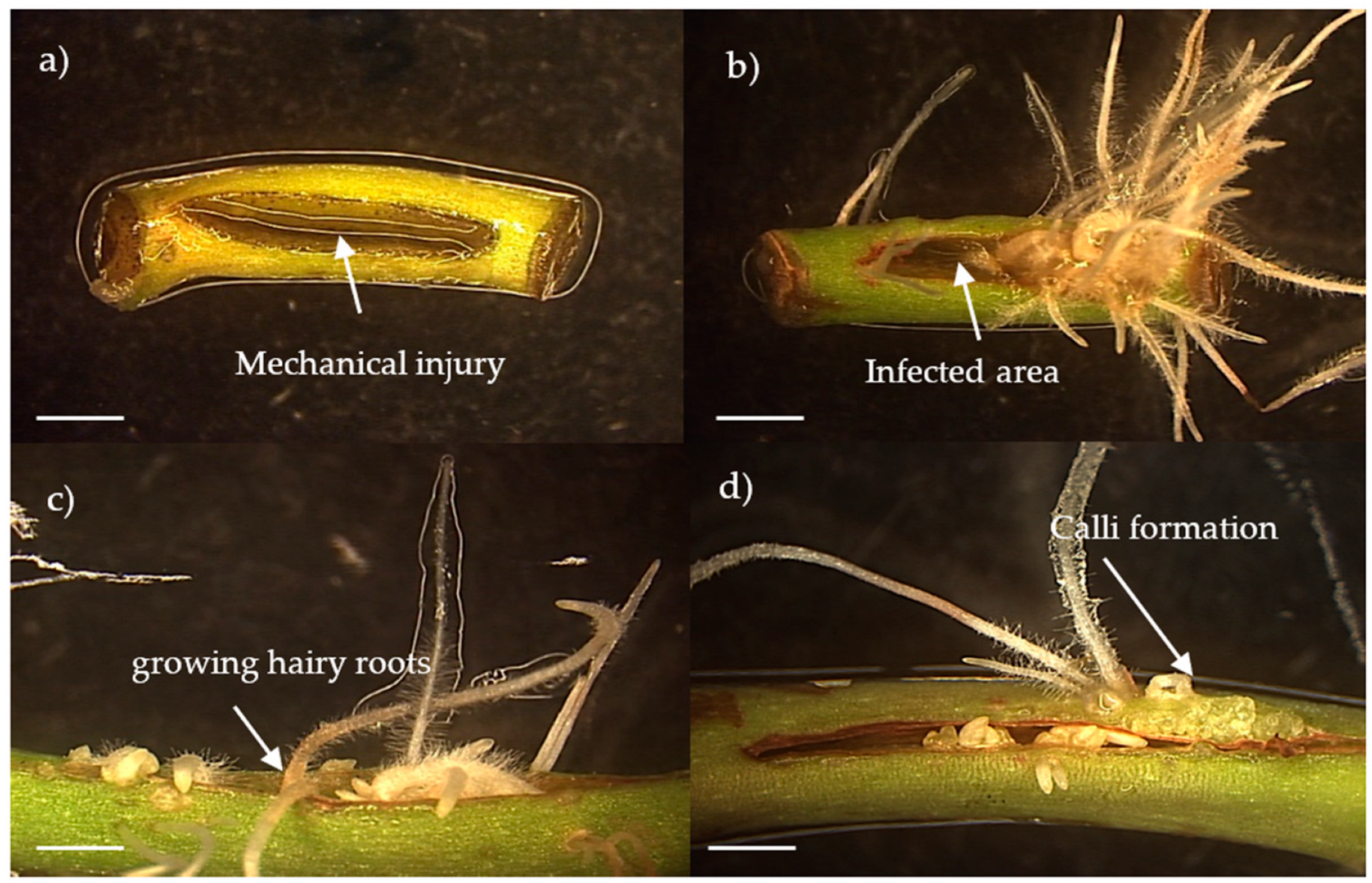
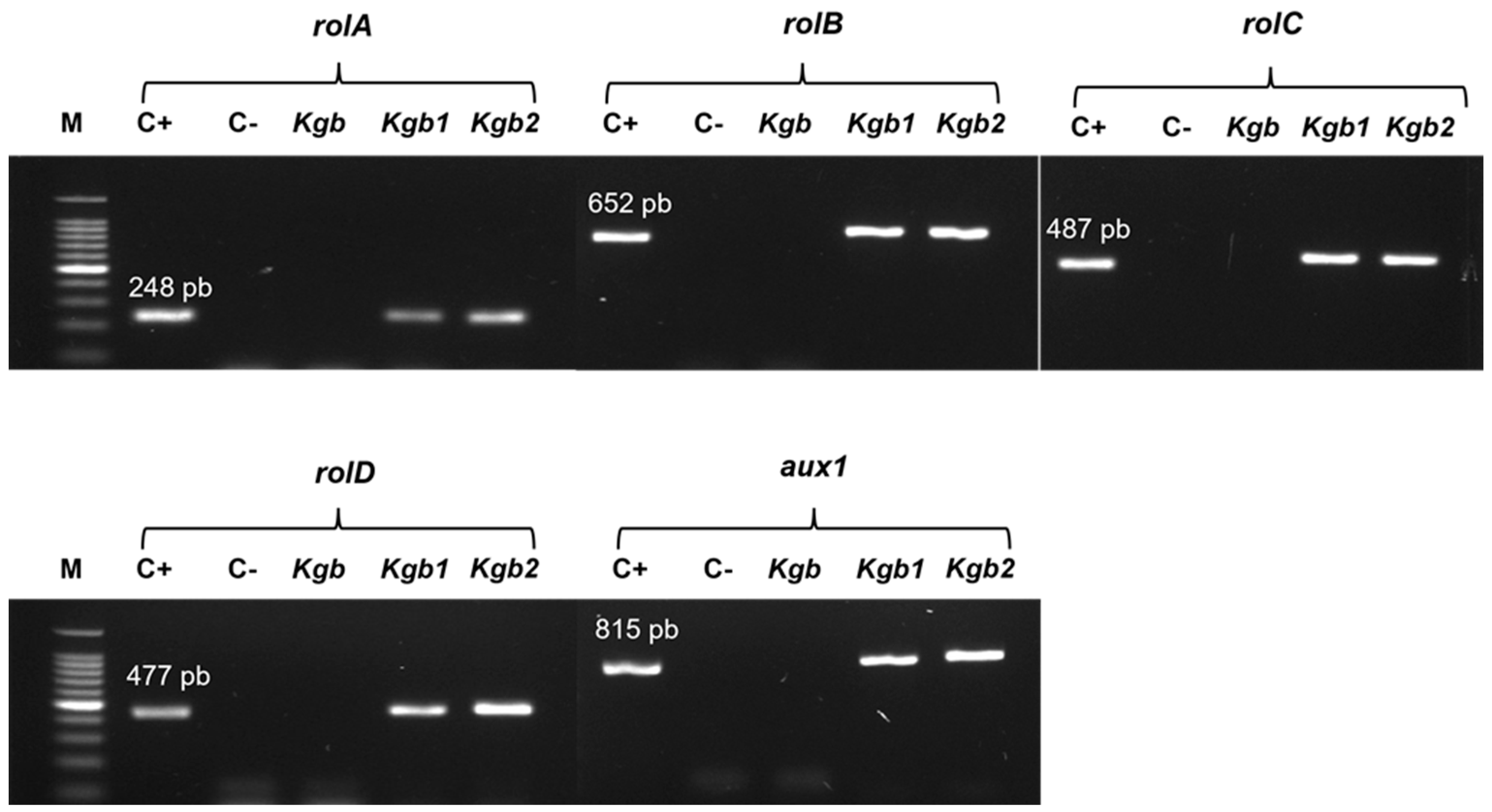
3.1. Morphology of the Kgb1 and Kgb2 Cultures and Molecular Analysis
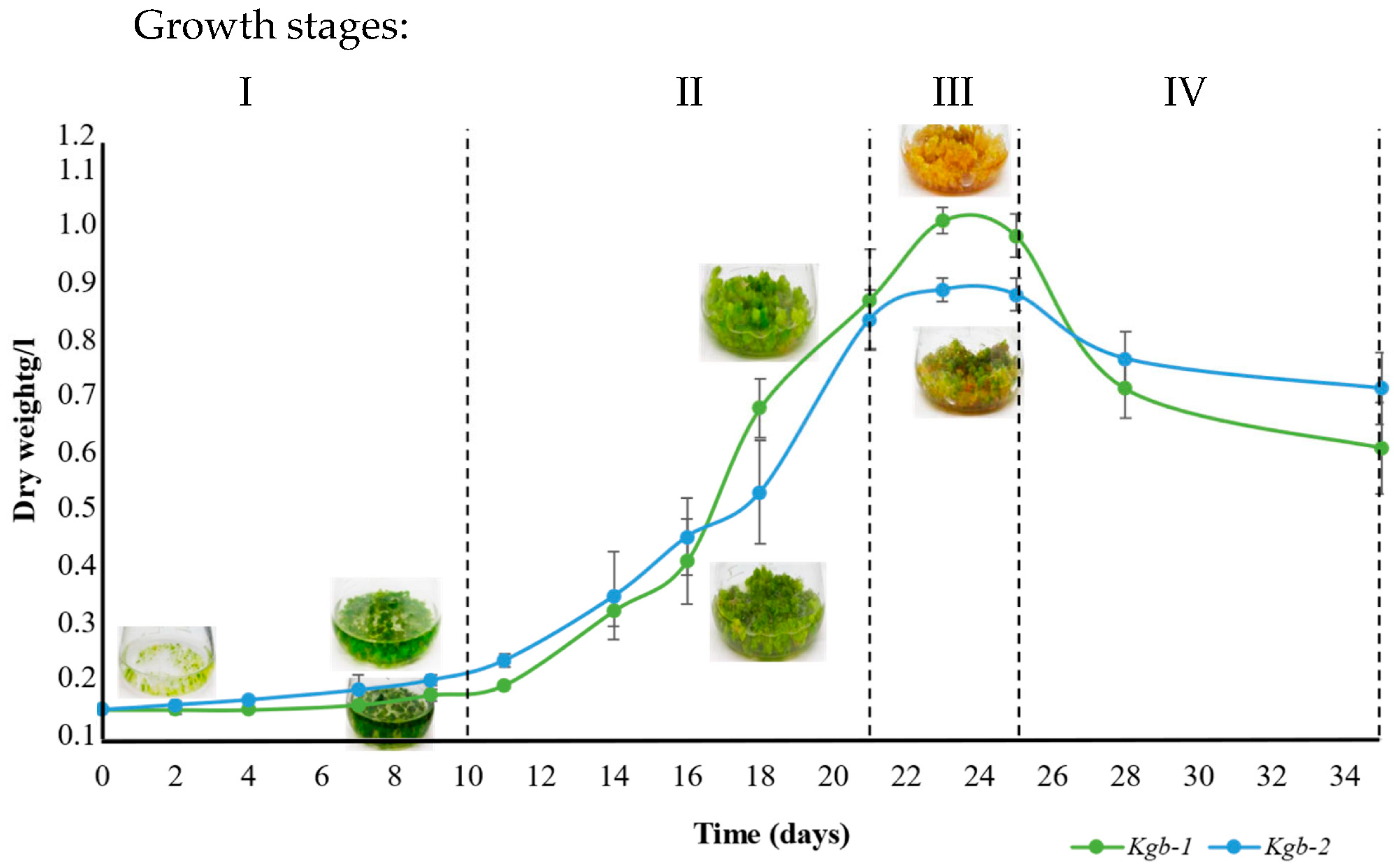
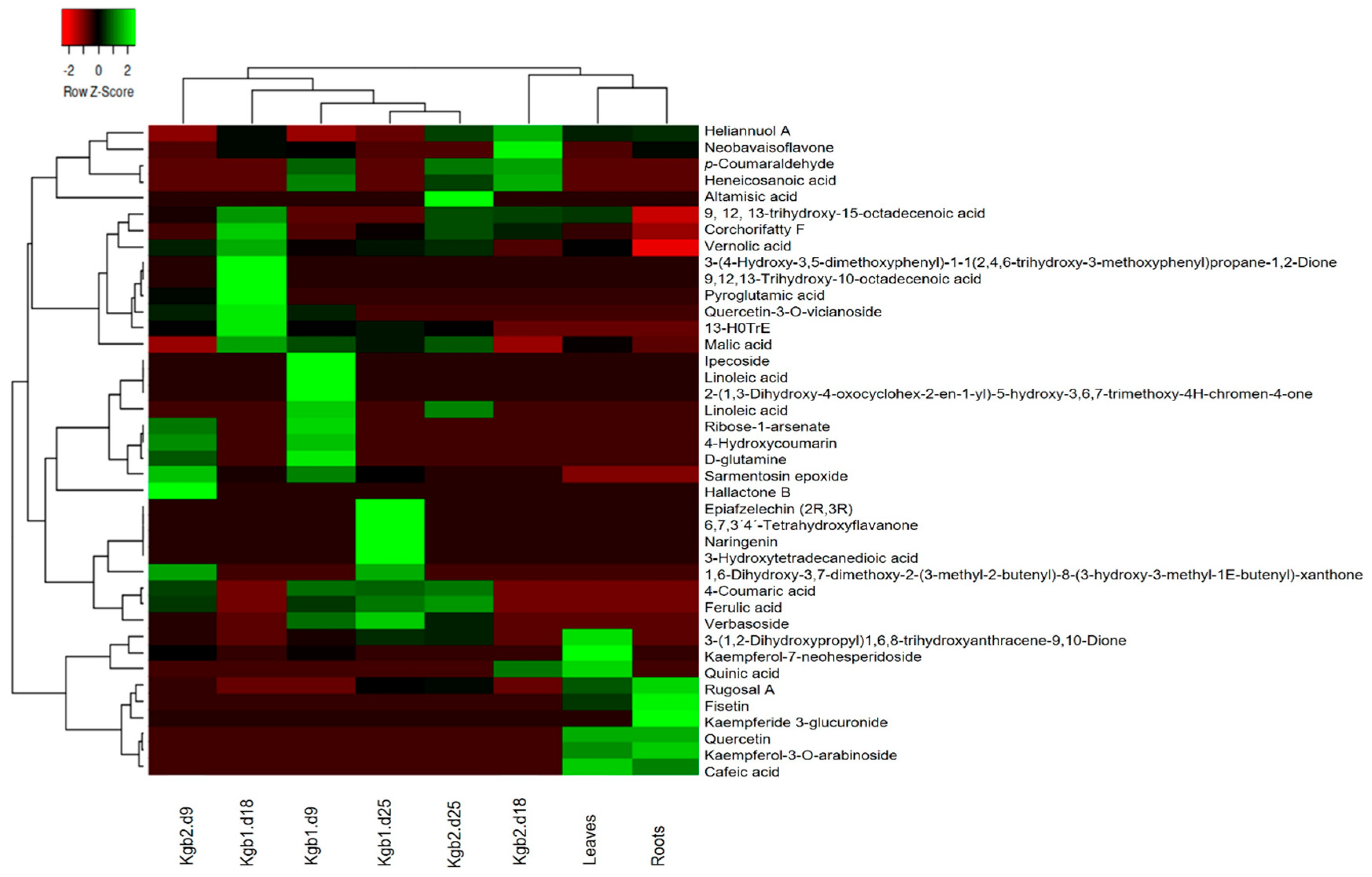
3.2. Analysis of the Chemical Profile of Kgb1 and Kgb2 Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, I.-M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Production of glucosinolates, phenolic compounds and associated gene expression profiles of hairy root cultures in turnip (Brassica rapa ssp. rapa). 3 Biotech 2016, 6, 175. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Abhyankar, G.; Suprasanna, P.; Pandey, B.N.; Mishra, K.P.; Rao, K.V.; Reddy, V.D. Hairy root extract of Phyllanthus amarus induces apoptotic cell death in human breast cancer cells. Innov. Food Sci. Emerg. Technol. 2010, 11, 526–532. [Google Scholar] [CrossRef]
- Rekha, K.; Thiruvengadam, M. Secondary metabolite production in transgenic hairy root cultures of cucurbits. In Transgenesis and Secondary Metabolism; Reference Series in, Phytochemistry; Jha, S., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Tusevski, O.; Stanoeva, J.P.; Stefova, M.; Pavokovic, D.; Simic, S.G. Identification and quantification of phenolic compounds in Hypericum perforatum L. transgenic shoots. Acta Physiol. Plant 2014, 36, 2555–2569. [Google Scholar] [CrossRef]
- Roychowdhury, D.; Halder, M.; Jha, S. Agrobacterium rhizogenes-mediated transformation in medicinal plants: Genetic stability in long-term culture. In Transgenesis and Secondary Metabolism; Reference Series in, Phytochemistry; Jha, S., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Vinterhalter, B.; Savić, J.; Zdravković-Korać, S.; Banjac, N.; Vinterhalter, D.; Krstić-Milošević, D. Agrobacterium rhizogenes-mediated transformation of Gentiana utriculosa L. and xanthones decussatin-1-O-primeveroside and decussatin accumulation in hairy roots and somatic embryo-derived transgenic plants. Ind. Crops Prod. 2019, 130, 216–229. [Google Scholar] [CrossRef]
- Hiebert-Giesbrecht, M.R.; Avilés-Berzunza, E.; Godoy-Hernández, G.; Peña-Rodriguez, L.M. Genetic transformation of the tropical vine Pentalinon andrieuxii (Apocynaceae) via Agrobacterium rhizogenes produces plants with an increased capacity of terpenoid production. In Vitro Cell Dev. Biol-Plant 2021, 57, 21–29. [Google Scholar] [CrossRef]
- Huang, S.H.; Vishwakarma, R.K.; Lee, T.T. Establishment of hairy root lines and analysis of iridoids and secoiridoids in the medicinal plant Gentiana scabra. Bot. Stud. 2014, 55, 17. [Google Scholar] [CrossRef] [PubMed]
- Vinterhalter, B.; Krstić-Milošević, D.; Janković, T.; Pljevljakusic, D.; Ninković, S.; Smigocki, A.; Vinterhalter, D. Gentiana dinarica Beck. hairy root cultures and evaluation of factors affecting growth and xanthone production. Plant Cell Tiss. Organ. Cult. 2015, 121, 667–679. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Asztemborska, M.; Krauze-Baranowska, M.; Godlewska, S.; Gucwa, M.; Moniuszko-Szajwaj, B.; Stochmal, A.; Ochocka, J.R. Identification of flavonoids and bufadienolides and cytotoxic effects of Kalanchoë daigremontiana extracts on human cancer cell lines. Planta Med. 2020, 86, 239–246. [Google Scholar] [CrossRef]
- Hernández-Caballero, M.E.; Sierra-Ramírez, J.A.; Villalobos Valencia, R.; Seseña-Méndez, E. Potential of Kalanchoe pinnata as a Cancer Treatment Adjuvant and an Epigenetic Regulator. Molecules 2022, 27, 6425. [Google Scholar] [CrossRef]
- Christensen, B.; Sriskandarajah, S.; Serek MMüller, R. Transformation of Kalanchoe blossfeldiana with rol-genes is useful in molecular breeding towards compact growth. Plant Cell Rep. 2008, 27, 1485–1495. [Google Scholar] [CrossRef]
- Fkiara, A.; Barba-Espín, G.; Bahij, R.; Müller, R.; Christensen, L.P.; Lütken, H. Elicitation of Flavonoids in Kalanchoe pinnata by Agrobacterium rhizogenes-mediated Transformation and UV-B Radiation. In Medicinal Plants: Biodiversity, Sustainable Utilization and Conservation; Khasim, S., Long, C., Thammasiri, K., Lütken, H., Eds.; Springer: Cham, Switzerland, 2020; pp. 395–403. [Google Scholar] [CrossRef]
- Ramasamy, M.; Dominguez, M.M.; Irigoyen, S.; Padilla, C.S.; Mandadi, K.K. Rhizobium rhizogenes-mediated hairy root induction and plant regeneration for bioengineering citrus. Plant Biotechnol. J. 2023, 21, 1728–1730. [Google Scholar] [CrossRef]
- Thirukkumaran, G.; Khan, R.S.; Chin, D.P.; Nakamura, I.; Mii, M. Isopentenyl transferase gene expression offers the positive selection of marker-free transgenic plant of Kalanchoe blossfeldiana. Plant Cell Tiss. Organ. Cult. 2009, 97, 237–242. [Google Scholar] [CrossRef]
- Cho, K.H.; Vieira, A.E.; Kim, J.; Clark, D.G.; Colquhoun, T.A. Transformation of Kalanchoe pinnata by Agrobacterium tumefaciens with ZsGreen1. Plant Cell Tissue Organ. Cult. 2021, 146, 401–407. [Google Scholar] [CrossRef]
- Yukes, J.; Balick, M. Dominican Medicinal Plants: A Guide for Health Care Providers, 2nd ed.; New York Botanical Garden: Bronx, NY, USA, 2010; pp. 7–14. [Google Scholar]
- Costa, S.S.; Corrêa, M.F.P.; Casanova, L.M. A new triglycosyl flavonoid isolated from leaf juice of Kalanchoë gastonis-bonnieri (Crassulaceae). Nat. Prod. Commun. 2015, 10, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, S.L.; Costa, S.S.; Gioso, M.A.; Casanova, L.M.; Coutinho, M.A.S.; Silva, M.F.A.; Botelho, M.C.D.S.N.; Díaz, R.S.G. Efficacy of a Kalanchoë gastonis-bonnieri extract to control bacterial biofilms and dental calculus in dogs. Pesqui. Vet. Bras. 2017, 37, 859–865. [Google Scholar] [CrossRef]
- Palumbo, A.; Casanova, L.M.; Corrêa, M.F.P.; Da Costa, N.M.; Nasciutti, L.E.; Costa, S.S. Potential therapeutic effects of underground parts of Kalanchoë gastonis-bonnieri on benign prostatic hyperplasia. Evid.-Based Complement. Altern. Med. 2019, 2019, 6340757. [Google Scholar] [CrossRef]
- Siems, K.; Jas, G.; Arriaga-Giner, F.J.; Wollenweber, E.; Dörr, M. On the chemical nature of epicuticular waxes in some succulent Kalanchoë and Senecio species. Z. Naturforsch. 1995, C 50, 451–454. [Google Scholar] [CrossRef]
- Vanegas, P.E.; Cruz-Hernández, A.; Valverde, M.E.; Paredes-López, O. Plant regeneration via organogenesis in marigold. PCTOC 2002, 69, 279–283. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 472–497. [Google Scholar] [CrossRef]
- Hooykas, P.J.J.; Klapwijk, P.M.; Nuti, M.P.; Schilperoort, R.A.; Rörsch, A. Transfer of the Agrobacterium tumefaciens TI plasmid to avirulent Agrobacteria and to Rhizobium ex planta. J. Gen. Microbiol. 1977, 98, 477–484. [Google Scholar] [CrossRef]
- Sharifi, S.; Sattari, T.N.; Zebarjadi, A.; Majd, A.; Ghasempour, H. The influence of Agrobacterium rhizogenes on induction of hairy roots and ß-carboline alkaloids production in Tribulus terrestris L. Physiol. Mol. Biol. Plants 2014, 20, 69–80. [Google Scholar] [CrossRef][Green Version]
- Torres-García, B.E.; Morales-Domínguez, J.F.; Fraire-Velázquez, S.; Pérez-Molphe-Balch, E. Generación de cultivos de raíces transformadas de la planta medicinal Bidens odorata Cav (Compositae) y análisis fitoquímico preliminar. Polibotánica 2018, 46, 241–257. [Google Scholar] [CrossRef][Green Version]
- Tavassoli, P.; Safipour-Afshar, A. Influence of different Agrobacterium rhizogenes strains on hairy root induction and analysis of phenolic and flavonoid compounds in marshmallow (Althaea officinalis L.). 3 Biotech 2018, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Kieran, P.; MacLoughlin, P.; Malone, D. Plant cell suspension cultures: Someengineering considerations. J. Biotechnol. 1997, 59, 39–52. [Google Scholar] [CrossRef]
- Urquiza-López, A.; Álvarez-Rivera, G.; Ballesteros-Vivas, D.; Cifuentes, A.; Del Villar-Martínez, A.A. Metabolite profiling of rosemary cell lines with antiproliferative potential against human HT-29 colon cancer cells. Plant Foods Hum. Nutr. 2021, 76, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Petrova, M.; Zayova, E.; Vlahova, M. Induction of hairy roots in Arnica montana L. by Agrobacterium rhizogenes. Cent. Eur. J. Biol. 2013, 8, 470–479. [Google Scholar] [CrossRef]
- Savić, J.; Nikolić, R.; Banjac, N.; Zdravković-Korać, S.; Stupar, S.; Cingel, A.; Ćosić, T.; Raspor, M.; Smigocki, A.; Ninković, S. Beneficial implications of sugar beet proteinase inhibitor BvSTI on plant architecture and salt stress tolerance in Lotus corniculatus L. J. Plant Physiol. 2019, 243, 153055. [Google Scholar] [CrossRef]
- Rana, M.M.; Han, Z.-X.; Song, D.-P.; Liu, G.-F.; Li, D.-X.; Wan, X.-C.; Karthikeyan, A.; Wei, S. Effect of medium supplements on Agrobacterium rhizogenes mediated hairy root induction from the callus tissues of Camellia sinensis var. sinensis. Int. J. Mol. Sci. 2016, 17, 1132. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Misra, A.; Banerjee, N. Genetic transfection, hairy root induction and solasodine accumulation in elicited hairy root clone of Solanum erianthum D. Don. J. Biotechnol. 2020, 323, 238–245. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Owczarek, A.; Kiss, A.K.; Grąbkowska, R.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Establishment of hairy root cultures of Salvia bulleyana Diels for production of polyphenolic compounds. J. Biotechnol. 2020, 318, 10–19. [Google Scholar] [CrossRef]
- Chaudhuri, K.N.; Ghosh, B.; Tepfer, D.; Jha, S. Spontaneous plant regeneration in transformed roots and calli from Tylophora indica: Changes in morphological phenotype and tylophorine accumulation associated with transformation by Agrobacterium Rhizogenes. Plant Cell Rep. 2006, 25, 1059–1066. [Google Scholar] [CrossRef]
- Bahramnejad, B.; Naji, M.; Bose, R.; Jha, S. A critical review on use of Agrobacterium rhizogenes and their associated binary vectors for plant transformation. Biotechnol. Adv. 2019, 37, 107405. [Google Scholar] [CrossRef] [PubMed]
- Ozyigit, I.I.; Dogan, I.; Artam Tarhan, E. Agrobacterium rhizogenes-mediated transformation and its biotechnological applications in crops. In Crop improvement; Springer: Boston, MA, USA, 2013; pp. 1–48. [Google Scholar]
- Sarkar, S.; Ghosh, I.; Roychowdhury, D.; Jha, S. The effects of rol genes of Agrobacterium rhizogenes on morphogenesis and secondary metabolite accumulation in medicinal plants. In Biotechnological Approaches for Medicinal and Aromatic Plants; Kumar, N., Ed.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Rattan, S.; Kumar, D.; Warghat, A.R. Growth kinetics, metabolite yield, and expression analysis of biosynthetic pathway genes in friable callus cell lines of Rhodiola imbricata (Edgew). Plant Cell Tiss. Organ Cult. 2021, 146, 149–160. [Google Scholar] [CrossRef]
- Chiavegatto, R.B.; Castro, A.H.F.; Marçal, M.G.; Padua, M.S.; Alves, E.; Techio, V.H. Cell Viability, Mitotic Indexand Callus Morphology of Byrsonima verbascifolia (Malpighiaceae). TropicalPlant Biol. 2015, 8, 87–97. [Google Scholar] [CrossRef]
- Sathish, S.; Venkatesh, R.; Safia, N.; Sathishkumar, R. Studies on growth dynamics ofembryogenic cell suspension cultures of commercially important Indica rice cultivars ASD16 and Pusa basmati. 3 Biotech 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Pan, Y.; Li, L.; Xiao, S.; Chen, Z.; Sarsaiya, S.; Zhang, S.; ShangGuan, Y.; Liu, H.; Xu, D. Callus growth kinetics and accumulation of secondary metabolites of Bletilla striata Rchb.f. using a callus suspension culture. PLoS ONE 2020, 15, e0220084. [Google Scholar] [CrossRef] [PubMed]
- Partap, M.; Kumar, P.; Ashrita; Kumar, P.; Kumar, D.; Warghat, A.R. Growth kinetics, metabolites production and expression profiling of picrosides biosynthetic pathway genes in friable callus culture of Picrorhiza kurroa Royle ex Benth. Appl. Biochem. Biotechnol. 2020, 192, 1298–1317. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, D.K.; Rahayu, S.; Zaidan, A.H.; Ekasari, W.; Prasongsuk, S.; Purnobasuki, H. Growth, secondary metabolite production, and in vitro antiplasmodial activity of Sonchus arvensis L. callus under dolomite [CaMg(CO3)2] treatment. PLoS ONE 2021, 16, e0254804. [Google Scholar] [CrossRef] [PubMed]
- Ang, H.; Mak, K.; Lum, M. Liquid chromatography mass spectrometry based high-throughput, unbiased profiling of upland and lowland rice varieties cultivated in Sabah. Trans. Sci. Technol. 2020, 7, 137–146. [Google Scholar]
- Nahrstedt, A.; Walther, A.; Wray, V. Sarmentosin epoxide, a new cyanogenic compound from Sedum cepaea. Phytochemistry 1982, 21, 107–110. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Maszczyk, M.; Rzepka, Z.; Rok, J.; Beberok, A.; Wrześniok, D. Neobavaisoflavone may modulate the activity of topoisomerase inhibitors towards U-87 MG cells: An in vitro study. Molecules 2021, 26, 4516. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.-X.; Guo, S.-D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef] [PubMed]

| RT (min) | Tentative Identification | Match Factor | Monoisotopic Mass | Main Fragments (m/z) | Parent Ion [M-H]- | Kgb1 | Kgb2 | Wild Type Plants | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days of Culture | Leaves | Roots | |||||||||||
| 9 | 18 | 25 | 9 | 18 | 25 | ||||||||
| Flavonoids | |||||||||||||
| 5.9 | 3,7-Dihydroxy-3’,4’-dimethoxyflavone | 79 | 314.0863 | 637.1386 | 313.0684 | - | - | - | - | - | - | + | - |
| 638.1423 | |||||||||||||
| 653.1331 | |||||||||||||
| 6.1 | Syringetin-3-O-glucoside | 78 | 508.1272 | 477.1005 | 507.1111 | - | - | - | - | - | - | + | - |
| 508.1177 | |||||||||||||
| 535.2139 | |||||||||||||
| 6.2 | Quercetin-3-O-pentosyl (1-2) acetilpentosida | 80 | 608.1295 | 327.0844 | 607.1259 | - | - | - | - | - | - | + | - |
| 623.1247 | |||||||||||||
| 623.2683 | |||||||||||||
| 6.2 | 3,4-dimethoxy-myricetin-3-O-dideoxyhexosyl(1-2)-dideoxyhexoside | 80 | 638.1445 | 521.2012 | 637.1383 | - | - | - | - | - | - | + | - |
| 623.1227 | |||||||||||||
| 638.1445 | |||||||||||||
| 6.3 | Guaijaverin | 79 | 434.0762 | 434.0762 | 433.0762 | - | - | - | - | - | - | + | - |
| 491.0744 | |||||||||||||
| 519.2203 | |||||||||||||
| 6.5 | Apigenin-6-C-glucoside-7-O-glucoside | 90 | 594.1500 | 461.1064 | 593.1466 | - | - | - | - | - | - | + | - |
| 506.0978 | |||||||||||||
| 594.1500 | |||||||||||||
| 6.6 | 6,7,3’,4’-Tetrahydroxyflavanone | 86 | 288.0149 | 146.9666 | 287.1465 | - | - | + | - | - | - | - | - |
| 288.1511 | |||||||||||||
| 309.1295 | |||||||||||||
| 6.7 | Kaempferol-3-O-arabinoside | 95 | 418.0852 | 418.0852 | 417.0791 | - | - | - | - | - | - | + | + |
| 491.1174 | |||||||||||||
| 607.2728 | |||||||||||||
| 6.9 | Epiafzelechin (2R,3R)(-) | 80 | 274.1671 | 187.0955 | 273.1671 | - | - | + | - | - | - | - | - |
| 289.1635 | |||||||||||||
| 607.2757 | |||||||||||||
| 7.0 | Naringenin | 80 | 272.1627 | 112.9843 | 271.1528 | - | - | + | - | - | - | - | - |
| 289.1627 | |||||||||||||
| 607.2727 | |||||||||||||
| 7.1 | Kaempferide 3-glucuronide | 95 | 476.0914 | 597.2469 | 475.0873 | - | - | - | - | - | - | - | + |
| 607.2738 | |||||||||||||
| 608.2776 | |||||||||||||
| 7.2 | Diosmine | 89 | 608.1664 | 475.0857 | 607.1641 | - | - | - | - | - | - | + | - |
| 608.1683 | |||||||||||||
| 643.1410 | |||||||||||||
| 7.7 | Quercetin | 90 | 302.0368 | 146.9683 | 301.0317 | - | - | - | - | - | - | + | + |
| 157.0095 | |||||||||||||
| 302.0331 | |||||||||||||
| 8.2 | 2’,7-Dihydroxy-4’-methoxy-8-prenylflavan 2’,7-diglucoside | 83 | 664.2705 | 653.2342 | 663.2626 | - | - | - | - | - | - | + | - |
| 680.2472 | |||||||||||||
| 707.2874 | |||||||||||||
| 8.6 | Fisetin | 80 | 286.0392 | 112.9833 | 285.0360 | - | - | - | - | - | - | + | + |
| 286.0392 | |||||||||||||
| 315.0477 | |||||||||||||
| 10.5 | Kaempferol-7-neohesperidoside | 93 | 594.2728 | 197.9606 | 593.2716 | + | - | - | + | - | - | + | - |
| 201.0350 | |||||||||||||
| 594.2728 | |||||||||||||
| 11.1 | Quercetin-3-O-vicianoside | 94 | 596.2922 | 197.9612 | 595.2873 | + | + | - | + | - | - | - | - |
| 596.2922 | |||||||||||||
| 723.3794 | |||||||||||||
| 11.9 | Neobavaisoflavone | 90 | 322.1733 | 322.1762 | 321.1724 | + | + | - | - | +++ | - | - | + |
| 406.1516 | |||||||||||||
| 421.0987 | |||||||||||||
| Fatty acids | |||||||||||||
| 6.3 | Deacetoxy (7)-7-Oxokhivorinic acid | 78 | 520.2216 | 509.1912 | 519.2210 | - | - | - | - | - | - | + | - |
| 520.2216 | |||||||||||||
| 536.2101 | |||||||||||||
| 7.6 | 3-Hydroxytetradecanedioic acid | 83 | 274.171 | 146.9682 | 273.1676 | - | - | + | - | - | - | - | - |
| 173.9999 | |||||||||||||
| 274.1710 | |||||||||||||
| 8.4 | (9S,10E,12S,13S)-9,12,13-Trihydroxy-10-octadecenoic acid | 81 | 330.2366 | 174.0007 | 329.2320 | - | + | - | - | - | - | - | - |
| 201.0377 | |||||||||||||
| 330.2366 | |||||||||||||
| 8.7 | Corchorifatty acid F | 83 | 328.2277 | 201.0351 | 327.2141 | + | + | + | + | + | + | + | - |
| 263.1309 | |||||||||||||
| 328.2158 | |||||||||||||
| 9.2 | (Z)-9,12,13-trihydroxyoctadec-15-enoic acid | 80 | 330.9998 | 157.0100 | 329.2300 | + | ++ | + | + | + | + | + | - |
| 330.2365 | |||||||||||||
| 397.2198 | |||||||||||||
| 11.3 | 13-HOTrE | 81 | 294.2131 | 275.2000 | 293.2095 | + | + | + | + | - | + | - | - |
| 294.2131 | |||||||||||||
| 613.9943 | |||||||||||||
| 11.7 | Vernolic acid | 83 | 296.2289 | 116.9269 | 295.2255 | + | + | + | + | + | + | + | - |
| 296.2289 | |||||||||||||
| 363.2136 | |||||||||||||
| 12.4 | Altamisic acid | 80 | 112.9851 | 279.1628 | - | - | - | - | - | + | - | - | |
| 135.9701 | |||||||||||||
| 309.1725 | |||||||||||||
| 12.8 | α-Linolenic acid | 80 | 278.7812 | 135.9699 | 277.2160 | + | - | - | - | - | + | - | - |
| 278.2192 | |||||||||||||
| 400.2108 | |||||||||||||
| 13.3 | Linoleic acid | 80 | 280.2351 | 278.7270 | 279.2322 | + | - | - | - | - | - | - | - |
| 280.2351 | |||||||||||||
| 325.1854 | |||||||||||||
| 14.9 | Heneicosanoic acid | 90 | 326.1831 | 326.1873 | 325.1829 | +++ | - | - | - | +++ | ++ | - | - |
| 339.2004 | |||||||||||||
| 340.2043 | |||||||||||||
| Coumarins | |||||||||||||
| 1.4 | 4-Hydroxycoumarin | 83 | 162.0495 | 117.0193 | 161.0451 | + | - | - | + | - | - | - | - |
| 128.0353 | |||||||||||||
| 292.1378 | |||||||||||||
| 4.9 | 3,4,5-Trihydroxy-6-{[2-oxo-6-(3-oxobutyl)-2H-chromen-7-yl]oxy}oxane-2-carboxylate | 85 | 408.0981 | 341.0851 | 407.0945 | - | - | - | - | - | - | + | - |
| 408.0981 | |||||||||||||
| 443.0710 | |||||||||||||
| 6.3 | Hallactone B | 80 | 440.1090 | 112.9848 | 439.1031 | - | - | - | + | - | - | - | - |
| 174.0002 | |||||||||||||
| 440.1090 | |||||||||||||
| 11.4 | Rugosal A | 87 | 266.1482 | 134.8628 | 265.1446 | - | - | + | + | - | + | ++ | +++ |
| 135.9684 | |||||||||||||
| 817.1497 | |||||||||||||
| 11.8 | Corylifol A | 85 | 390.2340 | 321.1705 | 389.2295 | - | - | - | - | - | - | + | - |
| 390.2340 | |||||||||||||
| 411.2125 | |||||||||||||
| Phenolic acids | |||||||||||||
| 5.4 | Caffeic acid | 93 | 180.0365 | 135.0425 | 179.0325 | - | - | - | - | - | - | + | + |
| 180.0365 | |||||||||||||
| 755.1999 | |||||||||||||
| 5.7 | 1-Caffeoyl-4-deoxyquinic acid | 90 | 338.0910 | 191.0536 | 337.0887 | - | - | - | - | - | - | + | - |
| 338.0910 | |||||||||||||
| 359.0706 | |||||||||||||
| 5.9 | 4-Coumaric acid | 81 | 164.0467 | 164.0467 | 163.0378 | + | - | + | + | - | + | - | - |
| 279.0519 | |||||||||||||
| 475.1830 | |||||||||||||
| 6.0 | Ferulic acid | 86 | 194.0537 | 194.0537 | 193.0486 | + | - | ++ | + | - | ++ | - | - |
| 309.0612 | |||||||||||||
| 311.1114 | |||||||||||||
| Phenolic compounds | |||||||||||||
| 5.2 | Syringate | 81 | 198.0453 | 198.0453 | 197.0428 | - | - | - | - | - | - | + | - |
| 313.0536 | |||||||||||||
| 431.1863 | |||||||||||||
| 5.5 | Verbasoside | 83 | 462.1709 | 415.1603 | 461.1640 | + | - | + | + | - | + | - | - |
| 451.1389 | |||||||||||||
| 462.1709 | |||||||||||||
| 15.2 | p-Coumaraldehyde | 81 | 147.8759 | 178.8796 | 146.9643 | + | - | - | - | + | + | - | - |
| 220.9484 | |||||||||||||
| 231.9439 | |||||||||||||
| Terpenes | |||||||||||||
| 6.4 | Kanokoside D | 89 | 624.2753 | 577.2622 | 623.2694 | - | - | - | + | - | - | - | + |
| 613.2428 | |||||||||||||
| 624.2753 | |||||||||||||
| 10.3 | Heliannuol A | 94 | 250.1536 | 248.9578 | 249.1497 | + | ++ | ++ | + | +++ | +++ | ++ | ++ |
| 250.1536 | |||||||||||||
| 251.1476 | |||||||||||||
| 12.3 | Ipecoside | 80 | 565.3214 | 116.9282 | 564.3191 | + | - | - | - | - | - | - | - |
| 554.2898 | |||||||||||||
| 581.3077 | |||||||||||||
| Carboxylic acids | |||||||||||||
| 1.0 | Malic acid | 80 | 134.0215 | 128.0336 | 133.0119 | +++ | +++ | +++ | - | - | +++ | ++ | + |
| 341.1061 | |||||||||||||
| 377.0831 | |||||||||||||
| 1.0 | DL-Pyroglutamic acid | 80 | 129.0345 | 290.0858 | 128.0336 | - | + | - | + | - | - | - | - |
| 310.0687 | |||||||||||||
| 403.1352 | |||||||||||||
| 1.2 | Citrate | 90 | 192.0301 | 111.0072 | 191.0175 | - | - | - | - | - | - | + | - |
| 128.0334 | |||||||||||||
| 173.0081 | |||||||||||||
| 1.3 | D-(-)-Quinic acid | 91 | 192.0579 | 157.0341 | 191.0520 | - | - | - | - | ++ | - | +++ | - |
| 377.0813 | |||||||||||||
| 379.0805 | |||||||||||||
| Alkaloids | |||||||||||||
| 6.1 | Voacristine | 80 | 384.0108 | 384.0108 | 383.0066 | - | - | - | - | - | - | - | + |
| 481.1331 | |||||||||||||
| 563.2139 | |||||||||||||
| Amino acids | |||||||||||||
| 0.8 | D-Glutamine | 87 | 146.0324 | 179.0556 | 145.0598 | + | - | - | + | - | - | - | - |
| 215.0324 | |||||||||||||
| 307.1131 | |||||||||||||
| 4.3 | Tryptophan | 82 | 204.0841 | 204.0841 | 203.0800 | - | - | - | - | - | - | + | - |
| 261.0370 | |||||||||||||
| 271.0673 | |||||||||||||
| Carbohydrate | |||||||||||||
| 1.1 | Sarmentosin epoxide | 90 | 291.0908 | 133.0134 | 290.0859 | +++ | ++ | ++ | +++ | ++ | ++ | - | - |
| 200.0561 | |||||||||||||
| 632.2048 | |||||||||||||
| Others | |||||||||||||
| 0.7 | Ribose-1-arsenate | 89 | 273.9598 | 158.9785 | 272.9565 | ++ | - | - | + | - | - | - | - |
| 273.9598 | |||||||||||||
| 274.9575 | |||||||||||||
| 0.9 | 3-(4-hydroxy-3,5-dimethoxyphenyl)-1-(2,4,6-trihydroxy-3-methoxyphenyl)propane-1,2-dione | 90 | 378.0889 | 341.1077 | 377.0854 | - | +++ | - | - | - | - | - | - |
| 379.0830 | |||||||||||||
| 404.1046 | |||||||||||||
| 1.0 | 2-(1,3-dihydroxy-4-oxocyclohex-2-en-1-yl)-5-hydroxy-3,6,7-trimethoxy-4H-chromen-4-one | 79 | 378.0822 | 341.1083 | 377.0846 | + | - | - | - | - | - | - | - |
| 404.1043 | |||||||||||||
| 470.1521 | |||||||||||||
| 4.4 | Cusparine | 82 | 307.1179 | 112.9835 | 306.1163 | - | - | - | - | - | - | + | - |
| 296.0879 | |||||||||||||
| 350.1422 | |||||||||||||
| 5.1 | 1,6-Dihydroxy-3,7-dimethoxy-2-(3-methyl-2-butenyl)-8-(3-hydroxy-3-methyl-1E-butenyl)-xanthone | 86 | 440.1819 | 393.1746 | 439.1785 | - | - | + | + | - | - | - | - |
| 429.1495 | |||||||||||||
| 440.1819 | |||||||||||||
| 5.8 | 1-(2H-1,3-benzodioxol-5-yl)-2-[2,6-dimethoxy-4-(prop-2-en-1-yl)phenoxy]propyl benzoate | 83 | 476.1831 | 429.1758 | 475.1800 | - | - | - | - | - | - | + | - |
| 476.1831 | |||||||||||||
| 521.1996 | |||||||||||||
| 8.5 | 3-(1,2-dihydroxypropyl)-1,6,8-trihydroxyanthracene-9,10-dione | 83 | 330.2334 | 285.0364 | 329.2298 | + | - | + | + | - | + | + | - |
| 286.0402 | |||||||||||||
| 330.2334 | |||||||||||||
| 8.7 | Isocyclocalamin | 85 | 502.2151 | 491.1836 | 501.2109 | - | - | - | - | - | - | - | + |
| 493.1828 | |||||||||||||
| 518.2009 | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrera Núñez, M.G.; Bueno, M.; Molina-Montiel, M.Á.; Reyes-Vaquero, L.; Ibáñez, E.; Del Villar-Martínez, A.A. Chemical Profile of Cell Cultures of Kalanchoë gastonis-bonnieri Transformed by Agrobacterium rhizogenes. Agronomy 2024, 14, 189. https://doi.org/10.3390/agronomy14010189
Barrera Núñez MG, Bueno M, Molina-Montiel MÁ, Reyes-Vaquero L, Ibáñez E, Del Villar-Martínez AA. Chemical Profile of Cell Cultures of Kalanchoë gastonis-bonnieri Transformed by Agrobacterium rhizogenes. Agronomy. 2024; 14(1):189. https://doi.org/10.3390/agronomy14010189
Chicago/Turabian StyleBarrera Núñez, María Guadalupe, Mónica Bueno, Miguel Ángel Molina-Montiel, Lorena Reyes-Vaquero, Elena Ibáñez, and Alma Angélica Del Villar-Martínez. 2024. "Chemical Profile of Cell Cultures of Kalanchoë gastonis-bonnieri Transformed by Agrobacterium rhizogenes" Agronomy 14, no. 1: 189. https://doi.org/10.3390/agronomy14010189
APA StyleBarrera Núñez, M. G., Bueno, M., Molina-Montiel, M. Á., Reyes-Vaquero, L., Ibáñez, E., & Del Villar-Martínez, A. A. (2024). Chemical Profile of Cell Cultures of Kalanchoë gastonis-bonnieri Transformed by Agrobacterium rhizogenes. Agronomy, 14(1), 189. https://doi.org/10.3390/agronomy14010189








