Abstract
Application of organic manure on farmland is one of the most important tasks in agricultural recycling. However, few studies have investigated the potential impact of different solid–liquid separation (SLS) technologies on soil porosity and greenhouse gas (GHG) emissions as a result of the application of liquid fractions (LFs). A microcosm experiment was conducted to track the emissions of ammonia (NH3), nitrous oxide (N2O), carbon dioxide (CO2), and methane (CH4) from soils (1) without liquid manure application (CK), (2) with a raw dairy slurry (RM), (3) with the liquid fractions of mechanical solid–liquid separation (MS) technologies, and (4) with the LF of an enhanced solid–liquid separation technology including a flocculant (tannic acid, Ta) (MS + Ta). Soil porosities of different treatments were measured using computed tomography (CT). The saturated water conductivities of the RM and MS treatments were 53.38% and 78.63%, respectively, lower than that of the CK. The application of raw slurry and LFs reduced the gas diffusion due to the strong decrease in pore sizes >500 μm and increased gas emissions compared to the CK. Compared with RM, MS had greater N2O and lower CH4 emissions, whereas MS + Ta had lower NH3 and N2O emissions. MS had the greatest CO2-e emissions, mainly owing to high N2O emissions, followed by RM and MS + Ta. The implementation of a simple SLS led to an increase in nitrogen (N) loss and GHG emissions when the resultant LFs were applied to farmlands, whereas high emissions were reduced when a simple SLS was combined with a flocculant, such as Ta. Further research is required to elucidate the reduction mechanism and its effectiveness under field conditions.
1. Introduction
Greenhouse gas (GHG) and ammonia (NH3) emissions during manure storage and farmland utilization in the integration of animal husbandry and cropping systems play important roles in national and global gas inventories [1,2,3]. Therefore, the ability to minimize gas emissions, optimize nutrient flows, and produce transportable, separated manure is a desirable feature of sustainable recycling agriculture. Currently, solid–liquid separation (SLS) technology is favored for manure management of various animal wastes on farms because it can meet the dual targets of reducing GHG emissions during manure storage and the transportation cost of manure [3,4,5,6]. For instance, high NH3 emissions from raw manure applied to fields occurred following application [7], but lower NH3 emissions were found when the liquid fraction (LF) from SLS technology was applied, and this can be attributed to the quick penetration of the LF into the soil, limiting NH3 emissions [8]. The nitrous oxide (N2O) emissions from LFs applied to the soil differed from soils with raw slurry, which might be due to the comprehensive impacts of the LFs on the availability of soil carbon(C), nitrogen (N), porosity, and gas diffusivity [9].
Studies have demonstrated that most of the solids (primarily organic matter) and nutrients in the LF are usually fine particles smaller than 125 μm [10], which are not easily separated by a simple mechanical sieve or press [11], resulting in a high content of solids in the separated LFs. The addition of flocculants is an effective measure to improve the efficiency of SLSs in cattle slurry [12,13]. For example, low-charge-density cationic polymers can be more effective in manure coagulation and flocculation, and high-charge-density cationic polymers can effectively reduce pathogen levels [14]. Traditional flocculants, such as Fe3+ and Al3+, are questionable [15], given their potential impact on public health and burden on the environment. Cationic polymers are used because of their poor ability to remove dissolved organic matter (DOC) [14]. Therefore, it is necessary to identify environmentally friendly flocculants for the recycling of various natural resources to enhance the separation efficiency of simple SLSs [16]. It has been demonstrated that tannic acid (Ta) can effectively reduce the turbidity of sewage through good flocculation [17] but with little secondary pollution [18]. Meanwhile, studies also showed that the addition of Ta to Corsican pine litter altered the rates of C mineralization and net N immobilization, as Ta can be a transient carbon source in the soil to stimulate many microbial processes [19,20,21]. However, few studies have investigated the use of Ta as a flocculant to improve the separation efficiency of SLS in animal slurry management.
Gas emissions from LF-applied fields are affected not only by the addition of substrates for N transformation but also by the resultant changes in soil structure. The latter may play an important role in the concurrent movement of soil microbes, air and water fluxes, and nutrient transport [22,23,24]. Thus, tracking changes in the soil structure after LF application is important to elucidate the underlying mechanisms for the production and emission of various gases. Computed tomography (CT) is a noninvasive imaging technique that allows high-resolution, three-dimensional, and nondestructive imaging of heterogeneous soils and reflects the actual, rather than inferred, characteristics of soil pores [25].
Therefore, the current study conducted a microcosm experiment to investigate (1) the changes in soil porosity, gas diffusivity, and water conductivity when daily LFs of simple mechanical and enhanced separation technologies were applied to soil; (2) the differences in NH3, N2O, methane (CH4), and carbon dioxide (CO2) emissions from soils with the application of LF from different SLSs; and (3) the driving factors of various gas emissions as a result of the quantified soil environmental variables. This study provides an insightful understanding of the evaluation and assessment of different SLS technologies on soil GHG emissions in a circular bioeconomy and is of great significance to environmentally friendly and healthy agricultural development.
2. Materials and Methods
2.1. Description of Soil and Liquid Manure
This study investigated the effects of raw slurry and separated liquid fractions on soil porosity and gas emissions using an indoor incubation method. The soil used in this study was obtained from an experimental farm at the Hebei Agricultural University, Baoding, China. The soil was characterized by a bulk density of 1.35 g cm−3, pH of 7.9, organic matter of 11.21 g kg−1, and total nitrogen (TN) of 0.89 g kg−1. The soil NH4+-N and NO3−-N contents were 3.86 mg kg−1 and 10.63 mg kg−1, respectively. The raw dairy slurry (RM) used in this study was obtained from a local dairy farm (approximately 1100 cows) in Baoding. Raw slurry in the feeding area of the feedlot was collected with a mechanical scraper and stored in an open pond without any previous treatments. A mechanical separator was used to prepare the LF (MS) from the RM. For the combined separation technology, a 5% (w/w) Ta solution was added to the RM at a rate of 3 mL L−1, and the mixture was subjected to the same mechanical separation to produce the corresponding LF (MS + Ta). The obtained LFs were stored for 2 months prior to the soil incubation. The main characteristics of the three types of liquid manure on wet-mass base right before the experiment are shown in Table 1.

Table 1.
Basic physical and chemical properties of raw slurry and separated liquid fractions.
The particle size distributions of the three liquid manures in each treatment were measured using a laser particle size analyzer (S3500, Microtrac Inc., Largo, FL, USA). In Figure 1, the particles of the RM were clustered from 100 to 1000 μm, whereas the particles of the MS and MS + Ta were clustered from 0 to 100 μm; more specifically, the particle distribution of the MS peaked at 20–50 μm, whereas the peak occurred at <10 μm for the MS + Ta. Generally, the nutrient content and particle distribution of the three liquid manures were effectively distinguishable from each other.
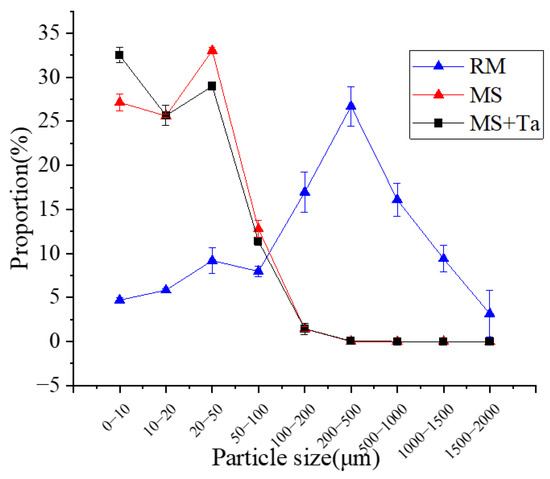
Figure 1.
Particle size distribution for raw slurry and separated liquid fractions.
2.2. Experimental Design
This study considered four treatments: (1) without liquid manure application (CK), (2) with raw dairy slurry (RM), (3) with the LF of mechanical SLS (MS), and (4) with the LF of an enhanced SLS including a flocculant Ta (MS + Ta). Each treatment had three replicates. The experiment lasted 2 weeks.
2.2.1. Preparation of Soil Incubation
The incubation facility consisted of a polyvinyl chloride (PVC) tube with an inner diameter of 16 cm and height of 30 cm. Soil was collected from the surface layer (0–20 cm depth) at the Experimental Farm of Hebei Agricultural University, Baoding, China. After the natural air-drying process and passing through a 2 mm sieve, approximately an amount of 5.5 kg for each soil column was mixed and filled into the incubation facility, leaving a head space with a height of 10 cm. This filling represented an equivalent soil bulk density of 1.25 g cm−3, which is similar to the actual soil bulk density. Prior to the application of liquid manure, two sequential wetting–drying processes were conducted to stabilize the soil structure and minimize the legacy impacts of existing microbes and nutrients on the following fertilization. Additionally, to track the dynamics of the soil properties during incubation, similar facilities with a thinner diameter (4.8 cm) and the same height (30 cm) were used for soil sampling.
2.2.2. Application of Liquid Manure
After the wetting—drying processes, raw dairy slurry (RW), LF from mechanical solid–liquid separation (MS), and LF from the combination of flocculation and mechanical separation (MS + Ta), representing an N application rate of 150 kg ha−1, were applied to the top of the soil columns (Table 2). Prior to application, the water contents of the raw slurry and the liquid fractions were adjusted to achieve a soil water content of 60% water holding capacity. It should be noted that the amounts of total ammoniacal nitrogen (TAN) and organic matter among treatments differed (Table 2). For the CK, the same amount of distilled water as for the other treatments was applied to the surface of the soil columns. The incubation facilities were kept in the laboratory with a constant room temperature of 25 ± 1 °C.

Table 2.
Inputs of nutrients per column during the incubation.
2.3. Gas Sampling and Measurement
For the measurement of NH3 emissions (Figure 2), a dynamic chamber was formed when port III on the lid was shut and the pump was switched on. The ambient air from the inlet flowed through the chamber via port I and carried the emitted NH3 to the sequent acid trap via port II and eventually released it to the ambient air. In the current study, the NH3 emission from the incubation facility was captured for 1 h every day using an acid trap (containing 200 mL of 0.05 mol L−1 dilute sulfuric acid). An automated discrete analyzer (SmartChem® 200, KPM Analytics, Guidonia, Italy) was used to determine the concentration of NH4+ in the acid absorption solutions.
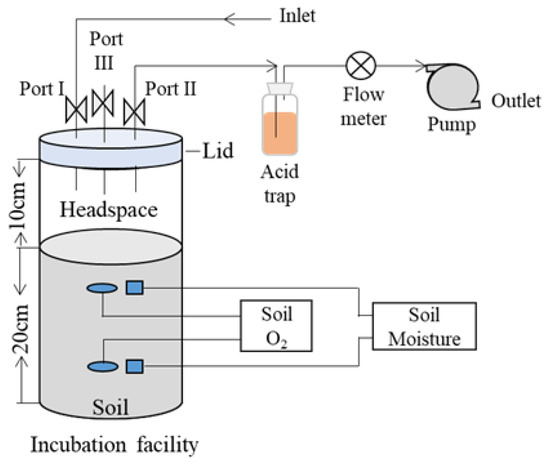
Figure 2.
Illustration of the incubation facility and gas sampling procedures.
To measure N2O, CO2, and CH4 emissions during incubation, a static chamber technique was used (Figure 2). A static, sealed chamber was formed when the incubation facility was covered with a lid. Port III was used for air sampling for N2O, CO2, and CH4 measurements when ports II and III were closed. When sampling air, the incubation facility was closed with a lid for 30 min. Air samples of approximately 20 mL were collected at 0 and 30 min and measured using a gas chromatography (Agilent 6820, Agilent Technologies, Inc., Santa Clara, CA, USA) over 24 h.
2.4. Measurement of Soil Properties
The oxygen (O2) content and water moisture at depths of 5 cm and 15 cm were measured online continuously; soil oxygen was monitored with soil O2 sensors (SO-110, Apogee Instruments, Logan, UT, USA) and recorded using a datalogger (CR1000X, Campbell Scientific, Logan, UT, USA), and soil moisture content was monitored using an automatic recording tachymeter (Saien Shandong, Jinan, China). A 10 cm surface soil sample was taken every other day to determine soil nutrients such as NO3−-N, NH4+-N, and DOC. Soil NH4+-N and NO3−-N were extracted using a 2 mol L–1 KCl solution at a soil–liquid ratio of 1:5 and determined using a chemical analyzer (AMS SmartChem200, Frépillon, France). The soil DOC was measured using the colorimetric method. This method is based on measuring the color loss of a Mn(III)-pyrophosphate complex (Mn(III) can be reduced by organic C in the presence of concentrated H2SO4 by determining the absorbance at 495 nm on a spectrophotometer [26].
After the application of liquid manure, soil cores with a diameter of 1 cm and a height of 2 cm were collected from the surface soil columns. The soil porosities were scanned using a Bruker Micro-CT Skyscan 1276 system (Bruker, Billerica, MA, USA). Scan settings were as follows: voxel 6.534165 μm, medium resolution, 70 kV, 200 uA, 1 mm Al filter, and integration time 350 ms. The cores were scanned using full 180° rotation of the sample. The soil column was divided into an average of three layers based on the longitudinal central axis of the soil sample, and the porosity of each layer was calculated. The water potential of the soils with liquid manure was measured using a tensiometer (Beirui Future, Tianjin, China) buried at a depth of 10 cm in the soil over a period of 15 days.
2.5. Calculation of Gas Emissions
The emission rates of N2O, CH4, and CO2 were calculated using Equation (1):
where F is the emission rate of N2O, CH4, and CO2 (mg m−2 h−1), dCt/dt is the linear slope of gas concentrations in the headspace against time during the sampling period (mg m−3 h−1), H is the height of the headspace of the incubation facility (m); T0 is the absolute air temperature (K) under the standard state; and T is the actual air temperature (°C).
The NH3 emission rate of the soil column was calculated using Equation (2):
where F is the NH3 emission rate (mg m−2 h−1); C is the concentration of NH4+ absorbed by sulfuric acid (mmol L−1); V is the volume of acid absorption solution (L); t is the sampling period (h); M is the cross-sectional area of the incubation facility (m2); and 17 is the molecular weight of NH3 (g mol−1).
The cumulative N2O, CO2, CH4, and NH3 emissions were calculated using Equation (3).
where Q is the total cumulative emission of N2O, CO2, CH4, and NH3 during the experiment (mg m−2), n is the total number of sampling events during the test, i is the serial number of one sampling event, F is the emission rate of N2O, CO2, CH4, and NH3 (mg m−2 h−1), and t is the sampling period (h).
In addition, the CO2-e for the N2O and CH4 were calculated with the global warming potential over a 100-year horizon (IPCC, 2014):
where GHG is the sum of the CO2-e of the direct N2O and CH4 and the indirect N2O derived from the deposition of volatilized ammonia (NNH3) (mg m−2).
2.6. Calculation of Gas and Water Conductivities
This study calculated DP/DO as a function of air-filled and total porosities using the water-induced linear reduction (WLR) model by Moldrup et al. [27], which is written in a general form as [28]:
where DP is the gas diffusion coefficient in soil (cm3 air cm−1 soils−1), DO is the gas diffusion coefficient in free air (cm2 air s−1), ε is the soil-air content (cm3 soil-air cm−3 soil), is the soil total porosity (cm3 soil pore space cm−3 soil), and p, X, and Ta are model parameters. The WLR model developed by Moldrup et al. [27], which was suitable for sieved and repacked soils, was based on Ta = 1. Marshall [29] used p = 1 and X = 1.5.
The saturated water conductivity of the soil was calculated using Equation (6):
where K is the soil saturated water conductivity (mm h−1), Q is the seepage quantity (cm), L is the soil layer thickness (cm), t is the penetration time (h), S is the cross-sectional area of the penetration bucket (cm2), h is the water layer thickness (cm), and 10 is the conversion coefficient for cm to mm.
2.7. Statistical Analysis
SPSS24.0 was used for statistical analysis and the principal component analysis for the response of soil gas emissions to environmental variables, and the least significant difference (LSD) method (α = 0.05) was used to indicate the significance of differences between treatments.
3. Results
3.1. Effect of the Application of Liquid Manure on Physical Properties of Soil
3.1.1. Soil Porosity
To show the difference in soil porosities with the depth between different treatments, topsoil of 20.0 mm was divided into three layers of 0–6.6, 6.6–13.3, and 13.3–20.0 mm. The soil porosities of the three soil layers in the different treatments are shown in Figure 3. Generally, the soil porosities of the top two layers (0–13.3 mm) were in the order of CK > MS > RM. The soil porosities of the RM treatment in the 0–6.6, 6.6–13.3, and 13.3–20.0 mm layers were 23.5%, 16.9%, and 7.0% smaller than those in the CK, and weaker differences in the top two layers between the MS and CK were found. Thus, an apparent impact of liquid manure application on soil porosity is inferred, especially on the surface layer.
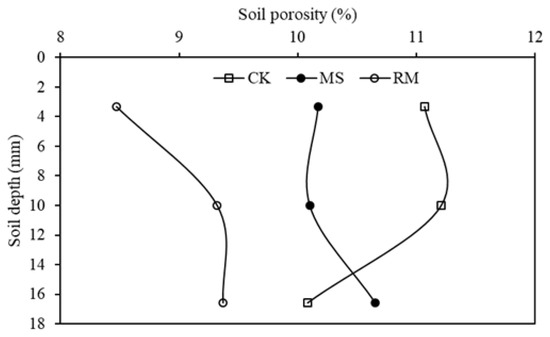
Figure 3.
Changes in soil porosities for soil depth 0–20 mm in the CK, RM, and MS treatments.
In addition to the effects of liquid manure on soil porosity, the application of the different liquid manures changed the composition of the soil pores (Figure 4). In the CK, the soil pores were dominated by three classes of pores with the sizes of 1000–2000, 500–1000, and 200–500 μm, respectively. However, in the MS treatment, the dominant pores shifted to those with sizes of 500–100 and 200–500 μm, indicating an apparent decrease in pores of 1000–2000 μm. In the 0–6.6 mm layer, the percentage of 1000–2000 μm pores in the CK was 34.64%, which was 14.53% and 32.56% higher than in the MS and RM treatments, respectively. A lower percentage (24.92%) of 200–500 μm pores was found in the CK treatment, approximately 13.23% and 30.99% lower than that in MS and RM treatments, respectively. Moreover, applying liquid manure had little effect on the proportion of fine pores (<50 μm) in soil (Figure 4).
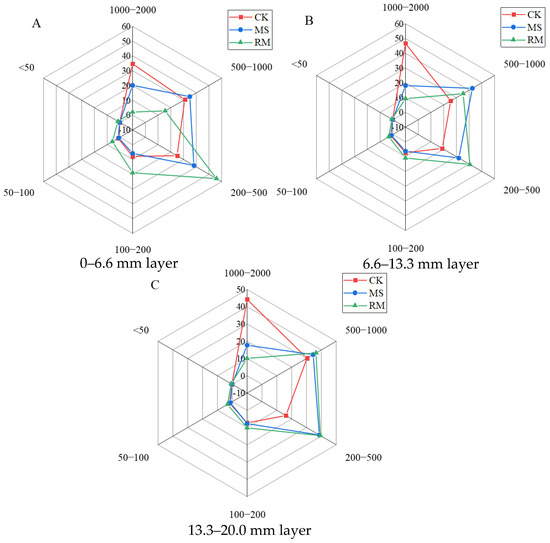
Figure 4.
Effects of the application of liquid manures on the proportions of soil pores with different sizes. (A) 0–6.6 mm layer; (B) 6.6–13.3 mm layer; and (C) 13.3–20.0 mm layer.
3.1.2. Soil Water Potential and Saturated Water Conductivity
Consistent differences in the soil moisture between the CK, RM, and MS existed during the measurement period of 15 days (Figure 5A), during which the soil moisture of the CK was consistently lower than that of RM and MS. The water potential of the CK was higher than that of the treatments with liquid manure, an opposite trend to that of soil moisture (Figure 5A). In addition, the saturated water conductivity of the CK treatment was determined to be 1.08 mm h−1 (Figure 5B); however, the saturated water conductivities of the RM and MS treatments with liquid manures were 53.38% and 78.63%, respectively, lower than that of the CK. Thus, the application of raw slurry and separated LF had an important impact on reducing water movement in the surface soil layer.
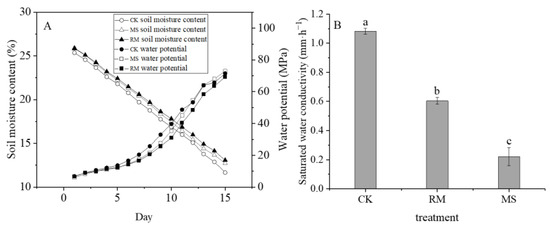
Figure 5.
Effects of liquid manure on soil water potential and saturated water conductivity. (A) Soil moisture content and water potential; and (B) soil saturated water conductivity. The small letters in the bars indicate the significant difference among treatments; α = 0.05.
3.2. Gaseous Nitrogen Emissions
3.2.1. NH3 Emissions
This study indicated different NH3 emission patterns among the four treatments (Figure 6A). For example, in addition to the higher emission rates of the RM, MS, and MS + Ta treatments compared to the CK, the NH3 emissions of the MS and MS + Ta declined gradually during the measurement period, whereas the NH3 emissions of the RM showed an emission peak reaching 14.86 mg m−2 h−1 on days 4–6. This difference may be attributed to the difference in N forms in the different liquid manures.
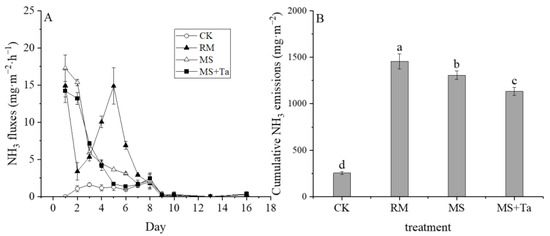
Figure 6.
Ammonia emissions from soil under different types of liquid fractions. (A) NH3 emission rate; and (B) cumulative NH3 emission. The small letters in the bars indicate the significant difference among treatments; α = 0.05.
Figure 6B shows the cumulative emissions of NH3 from the different treatments. During the measurement period, the cumulative emission of NH3 in the RM treatment was 1456 mg m−2, which was substantially higher than in the other two treatments. Following that was the MS treatment with an NH3 emission of 1307 mg m−2; the MS + Ta treatment had a 13.19% lower NH3 emission than the MS treatment. The LF derived from simple SLS had a lower NH3 emission potential than the raw slurry; NH3 reduction was enhanced by the flocculation process (i.e., the use of Ta).
3.2.2. N2O Emissions
The application of liquid manure markedly increased N2O emission rates compared to CK (Figure 7A). A slight difference was observed in the N2O emission patterns of the treatments with liquid manure. For instance, the N2O emission rates of the MS and MS + Ta treatments increased faster than those in the RM treatment before day 6; greater emissions in the MS treatment were observed from days 6–10 than in the MS + Ta treatment. A sharp emission peak was observed in the RM treatment on day 7.
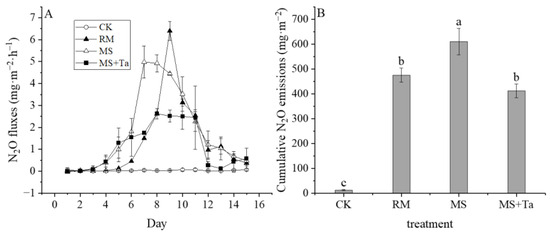
Figure 7.
Nitrous oxide emissions from soil under different types of liquid fractions. (A) N2O emission rate; and (B) cumulative N2O emission. Small letters on the bars indicate the significant difference among treatments; α = 0.05.
The cumulative emission of N2O in the RM treatment reached 475.61 mg m−2, whereas that in the MS treatment reached 610.19 mg m−2 (Figure 7B). However, the emission of N2O in the MS + Ta treatment was 32.46% lower than that in the MS treatment. Therefore, this study showed that the application of LF from simple SLS led to higher N2O emissions in comparison to the raw slurry treatment, and such an increase in N2O emissions was reduced once the LF from the SLS was flocculated with Ta.
3.2.3. Total Gaseous Nitrogen Losses
Table 3 shows the proportions of NH3-N and N2O-N losses from the total N input in each treatment. The loss of NH3-N accounted for 7.96% of N input in the RM treatment, which was 13.39% higher than that in the MS treatment. However, the MS + Ta treatment showed a lower NH3-N loss, at 5.87%, which was 16.38% lower than that of the MS treatment. The percentage of N2O-N loss in the RM treatment was 3.08%, 22.61% lower than that in the MS treatment, whereas the N2O-N loss in the MS + Ta treatment accounted for 2.66% of the N input, which was 33.17% lower than that of the MS treatment. The total N losses of (NH3 + N2O)-N in the RM and MS treatments were similar at 11.04% and 10.10%, respectively, which were substantially higher than those in the MS + Ta treatment (8.53%) (Table 3). Therefore, this study emphasized the role of enhanced SLS using Ta in reducing N losses from LF applied to soils.

Table 3.
Percentages of NH3-N and N2O-N losses from the total N inputs in different treatments.
3.3. CO2 Emissions
The CO2 emissions of CK were consistently lower than those of liquid manure (Figure 8A). Moreover, the dynamics of CO2 emissions among these manure-applied treatments differed. The CO2 emission rates of the RM treatment were lower than those of MS and MS + Ta during the first 4 d but were higher in the following period. The difference in CO2 emissions between MS and MS + Ta occurred mainly in the latter period, i.e., 8–15 d.
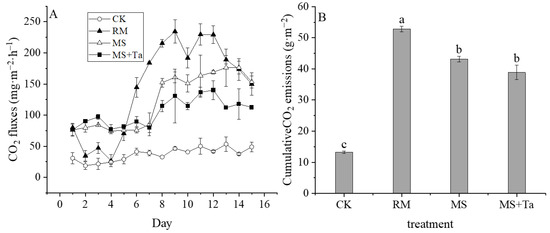
Figure 8.
Carbon dioxide emissions from soil under different types of liquid fractions. (A) CO2 emission rate; and (B) cumulative CO2. The small letters in the bars indicate the significant difference among treatments; α = 0.05.
The cumulative CO2 emissions of all treatments are shown in Figure 8B. The RM treatment had the highest cumulative CO2 emission of 52.80 g m−2, which was markedly higher than that of the MS and MS + Ta treatments. The cumulative CO2 emissions of the MS and MS + Ta treatments were similar (43.13 g m−2 and 38.87 g m−2, respectively), which were 2.26 and 1.93 times higher than that of the CK treatment, respectively. Therefore, the application of LF from simple SLS had lower soil CO2 emissions compared to the soil treated with raw slurry, and the addition of Ta had little effect on soil CO2 emissions.
3.4. CH4 Emissions
Substantial CH4 emissions were observed from the treatment with liquid manure, whereas they were negligible for CK without liquid manure application (Figure 9A). The dynamics of CH4 emission rates differed among the treatments with liquid manure. For example, CH4 from MS and MS + Ta declined quickly from day 1 and then peaked on day 3 for MS + Ta and on Day 8 for MS + Ta. For the RM, CH4 emissions showed one sharp peak (with the highest emission rate of 3.00 mg m−2 h−1) on day 5. The application of liquid manure increased the CH4 emissions compared to the CK (1.18 mg m−2), and a large difference in CH4 among the RM, MS, and MS + Ta was observed (Figure 9B). More specifically, the RM treatment had the greatest CH4 emission of 176.53 mg m−2, whereas the CH4 emissions of the MS treatment were approximately 44.59% lower than those of the RM treatment. This study demonstrated that the application of LF from simple SLS had lower soil CH4 emissions than RM, and enhanced SLS had little effect on CH4 emissions.
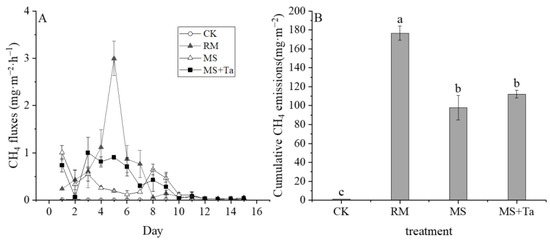
Figure 9.
Methane emissions from soil under different types of liquid fractions. (A) CH4 emission rate; and (B) cumulative CH4 emission. The small letters in the bars indicate the significant difference among treatments; α = 0.05.
3.5. Dynamics of Soil Properties
3.5.1. O2 and Gas Diffusion
The soil O2 in the MS treatment showed a faster decrease close to zero than the CK, RM, and MS + Ta treatments, which had similar dynamics in O2 changes (Figure 10A). This study also measured the gas diffusion scaled with the DP/DO, and evident differences in the DP/DO between treatments were observed. The application of raw slurry showed the largest decrease in DP/DO, and moderate impacts of the MS and MS + Ta on DP/DO were observed (Figure 10B). The low O2 and poor DP/DO of the soil treated with liquid manure, especially when soil O2 and DP/DO were less than 5% and 0.006, respectively, may have provided favorable conditions for N2O production and emission during the measurement period.
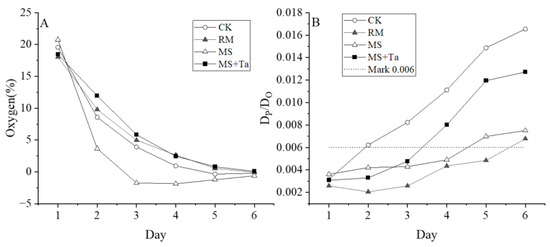
Figure 10.
Soil oxygen and free diffusion rates. (A) O2 content; and (B) average soil DP/DO modeled using the SWLR model [28], where the dashed line marks 0.006.
3.5.2. Dynamics of Soil NO3−-N, NH4+-N, and DOC
Compared to the relatively stable NH4+-N in the CK, the soil NH4+-N in the MS and MS + Ta declined quickly and stabilized after day 9 (Figure 11A). However, the NH4+-N in the RM increased and peaked (186.17 mg kg−1) on day 5 and then approached that of the other treatments after day 11. The soil NO3−-N of all treatments gradually increased, except for CK, and was ranked as MS > RM > MS + Ta > CK (Figure 11B). The NO3−-N of MS and RM behaved similarly, although it was slightly lower in RM. However, NO3−-N in the MS + Ta treatment was consistently lower than that in the RM and MS treatments. The soil DOC content in the MS + Ta treatment was higher than in the other treatments during most of the measurement period, reaching the highest value of 8.12 mg kg−1 on Day 5 (Figure 11C). The RM treatment had a lower DOC during the first 7 days and approached the other treatments thereafter. This study demonstrated that the type of liquid manure played an important role in soil nutrients and dissolvable organic carbon.
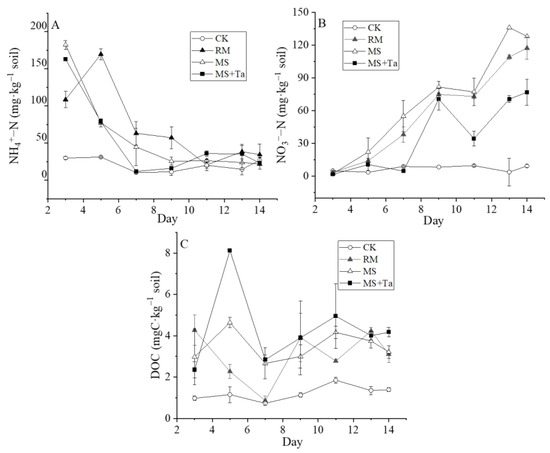
Figure 11.
Changes in NH4+-N, NO3−-N, and DOC in soil under different treatments. (A) NH4+-N content in soil; (B) NO3−-N content in soil; and (C) DOC content in soil.
3.6. Integral Impacts of the Soil Environmental Variables on N2O Emissions
To indicate the relationship between the soil N2O emissions and environmental variables, such as soil NO3−, NH4+, DP/DO, DOC, and water-filled pores pace (WFPS), principal component analysis (PCA) was conducted (Figure 12). The average N2O emission rate during days 7–8 (representing the high emission period according to Figure 7) and the aforementioned soil variables in the same period in different treatments were used. The N2O emissions of different treatments during the high emission periods had strong positive correlations with soil NO3− (r = 0.998) and WFPS (r = 0.965) and moderate correlations with soil NH4+ (r = 0.575) and DOC (r = 0.316) (Figure 12). A weak yet negative correlation between the N2O and the soil DP/DO (r = −0.199) was observed. Of the environmental variables, strong negative correlations between soil DOC and DP/DO were observed, implicitly demonstrating weak decomposition of DOC under poor gas diffusivity conditions with low DP/DO.
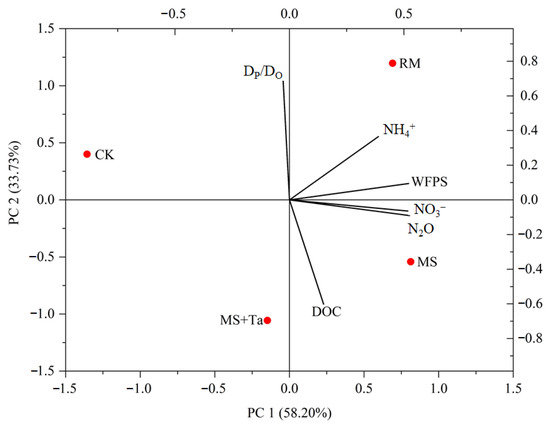
Figure 12.
Principal component analysis of the impact of different environmental variables on N2O emissions.
3.7. CO2-e Emissions of Different Treatments
This study evaluated the GHG emissions from different treatments in terms of CO2-e. N2O emissions were the dominant contributor to GHG emissions in all treatments (Table 4). This was followed by indirect CO2-e from NH3 emissions and CH4, indicating a relatively large contribution of indirect emissions to CH4. The cumulative CO2-e emissions of the four treatments were ranked MS > RM > MS + Ta > CK; more specifically, the application of LF from the simple SLS increased the GHG emissions by 24.0% compared to the RM, mainly due to the increased N2O emissions, whereas this was alleviated by 14.2% in MS + Ta due to the decrease in N2O emissions, i.e., the flocculant in the SLS reduced GHG emissions by 31.1%. Therefore, the use of enhanced SLS may reduce GHG emissions in soils subjected to LFs.

Table 4.
Contributions of the direct CH4 and N2O emissions and indirect N2O from NH3 emissions to the CO2-e emissions in different treatments.
4. Discussion
This study identified the impacts of simple and enhanced SLS technologies on the resulting composition of LFs and subsequent gas emissions from LF-treated soils. Potential mechanisms of N losses and GHG emissions are discussed from different perspectives regarding the issue of liquid manure application.
4.1. Impact on NH3 Emissions
The cumulative NH3 emissions from the MS treatment were lower than those from the RM treatment, which is consistent with the results reported by Johanna [5]. This may be due to the low dry matter content in LFs after solid–liquid separation (Table 1). Usually, the liquid fraction with enriched NH4+-N after SLS can penetrate the soil well [30,31,32], which is conducive to the absorption and utilization of nitrogen by plants [33]. In addition, NH3 emissions peaked on day 5 in the RM treatment, a major contribution to the total NH3 emissions, which might have been derived from the large amount of organic nitrogen mineralization and the resulting increase in soil NH4+ concentrations (Figure 11A). From this aspect, SLS played an important role in the NH3 emissions from slurry-applied soils [34]. However, the MS + Ta treatment applied with LF from the enhanced SLS showed lower NH3 emissions than the MS treatment, even though the former was characterized with a higher TAN input (Table 2). This might be attributable to the inhibitory effect of Ta in the LF from the enhanced SLS technology on urease activity and microbial abundance [35], which can be confirmed by the lower level of NH4+ in MS + Ta (Figure 11A).
4.2. Impact on N2O Emissions
The MS treatment had higher N2O emissions than the RM treatment, which is consistent with the findings of Meng et al. [9]. This can be attributed to several factors: (1) the greater contact between the liquid fraction and soil [36] and (2) the higher input of TAN and DOC in the LF [8,37]. Meanwhile, this study also observed that the application of raw slurry or the separated LF reduced the saturated water conductivity, O2, and gas diffusion rate, owing to the blocking of soil pores (mainly macropores with sizes 1000–2000 μm) by organic particles in the liquid manure [38,39,40]. This might have favored a gradual development of anoxia [41] that facilitates an increase in the abundance of ammonia-oxidizing bacteria (AOB) and nirS and a decrease in the abundance of nosZ during denitrification [42,43,44,45], thus contributing to the higher N2O emission in the MS [9].
4.3. Impact on Soil Respiration
The CO2 emissions of the different treatments in the early stage were in the order of MS + Ta > MS > RM, followed by RM > MS > MS + Ta in the latter stage. Fangueiro et al. studied the effect of soil CO2 emissions by classifying manure particle size. They showed that the soil CO2 emission was higher at the early stage of the experiment with a smaller particle size, whereas it was highest in the treatment with a coarse particle size at the late stage [46], which is consistent with the results of the current study. The cumulative emissions of CO2 in the soil treated with the raw slurry were higher than those treated with the separated LF because the organic matter content of the raw slurry was higher than that of the LFs (Table 1). Therefore, SLSs with different separation efficiencies for organic particles may play important roles in soil respiration during liquid manure recycling [47]. The addition of Ta as a flocculant more effectively removed organic matter from liquid manure but did not significantly reduce the soil CO2 and CH4 emissions; this might be caused by the fact that tannin could be decomposed to be microbial carbon source for methanogens [48,49].
4.4. Implications
This study demonstrated that the emission of CO2-e under the MS treatment was higher than that under the RM treatment, mainly because the N2O emissions of the MS treatment were higher than those of the RM treatment [31,50]. Similarly, Dinuccio et al. found that CO2-e emissions from LFs during on-farm storage and field application were 11% higher than those from raw slurry [51], which may, to some extent, limit the application of SLS technologies on animal farms. Plausibly, this study demonstrated lower NH3, N2O, and CO2-e emissions from liquid fraction-applied soil when the enhanced SLS using flocculant Ta was employed for solid–liquid separation. Such emissions reductions were partly attributed to the Ta residue in the LF from the enhanced SLS, which may have inhibited the nitrification owing to the complexation effect of Ta on proteins [52], leading to low soil NH4+, NO3− concentration, and soil N2O emission [35].
Overall, this study demonstrates the promising use of Ta as a flocculant in SLS to decrease CO2-e emission from fields where LFs from SLS are applied, which could likely further prompt the wider application of SLSs. However, it is necessary to carry out in-depth research to understand the microbial production and emission mechanisms of soil N2O and CH4 in this situation and to carry out lifecycle assessments using this technique to reveal the reductions in GHG emissions under field conditions.
5. Conclusions
This study investigated the GHG emissions from soil applied with liquid fractions from two solid–liquid separation technologies. Compared to the raw slurry application, a simple mechanical separation for slurry may increase the N loss and greenhouse gas emissions from farmlands applied with the obtained liquid fraction due to the high ammoniacal N, poor porosity, and gas diffusion in soil. However, the enhanced solid–liquid separation of slurry with the use of a flocculant like tannic acid substantially reduced the N losses and GHG emissions from the liquid fraction-applied soil, mainly through minimizing N2O emissions. Therefore, the enhanced separation technology with the use of tannic acid is considered useful for reducing greenhouse gas emissions from soils applied with separated liquid fractions. However, more efforts are required to elucidate the reduction mechanism and verify the mitigation potential of the enhanced solid–liquid separation technologies under field conditions.
Author Contributions
Conceptualization, S.W., Z.G. and W.L.; Methodology, S.W., C.L., W.L. and Z.G.; Investigation, S.W., W.W. and Y.C.; Writing-original draft preparation, S.W. and Y.B.; Writing-review and editing, C.L., W.L. and Z.G.; Funding acquisition, W.L. and Z.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC: Grant nos. 42375170), and the Key Research and Development Program of Hebei Province (21327303D and 20327306D).
Data Availability Statement
Data will be available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Veltman, K.; Rotz, C.A.; Chase, L.; Cooper, J.; Ingraham, P.; Izaurralde, R.C.; Jones, C.D.; Gaillard, R.; Larson, R.A. A quantitative assessment of beneficial management practices to reduce carbon and reactive nitrogen footprints and phosphorus losses on dairy farms in the us great lakes region. Agric. Syst. 2018, 166, 10–25. [Google Scholar] [CrossRef]
- Zervas, G.; Tsiplakou, E. An assessment of GHG emissions from small ruminants in comparison with GHG emissions from large ruminants and monogastric livestock. Atmos. Environ. 2012, 49, 13–23. [Google Scholar] [CrossRef]
- Abelenda, A.M.; Semple, K.T.; Aggidis, G.; Aiouache, F. Dataset on the solid-liquid separation of anaerobic digestate by means of wood ash-based treatment. Data Brief 2022, 44, 108536. [Google Scholar] [CrossRef]
- Aguirre-Villegas, H.A.; Larson, R.A. Evaluating greenhouse gas emissions from dairy manure management practices using survey data and lifecycle tools. J. Clean. Prod. 2017, 143, 169–179. [Google Scholar] [CrossRef]
- Pedersen, J.; Hafner, S.D.; Adamsen, A.P.S. Effectiveness of mechanical separation for reducing ammonia loss from field-applied slurry: Assessment through literature review and model calculations. J. Environ. Manag. 2022, 323, 116196. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Wu, S. Nutrient reduction of dairy manure through solid-liquid separation with flocculation and subsequent microalgal treatment. Appl. Biochem. Biotechnol. 2020, 190, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Vanderzaag, A.C.; Sokolov, V.; Baldé, H.; Macdonald, D.; Wagner-Riddle, C.; Gordon, R. Ammonia emissions from the field application of liquid dairy manure after anaerobic digestion or mechanical separation in ontario, canada. Agric. For. Meteorol. 2018, 258, 89–95. [Google Scholar] [CrossRef]
- Guilayn, F.; Jimenez, J.; Rouez, M.; Crest, M. Digestate mechanical separation: Efficiency profiles based on anaerobic digestion feedstock and equipment choice. Bioresour. Technol. 2019, 274, 180–189. [Google Scholar] [CrossRef]
- Meng, X.; Ma, C.; Petersen, S.O. Sensitive control of N2O emissions and microbial community dynamics by organic fertilizer and soil interactions. Biol. Fertil. Soils 2022, 58, 771–788. [Google Scholar] [CrossRef]
- Meyer, D.; Ristow, P.; Lie, M. Particle size and nutrient distribution in fresh dairy manure. Appl. Eng. Agric. 2007, 23, 113–118. [Google Scholar] [CrossRef]
- Peters, K.; Hjorth, M.; Jensen, L.S.; Magid, J. Carbon, nitrogen, and phosphorus distribution in particle size–fractionated separated pig and cattle slurry. Environ. Qual. 2011, 40, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Grell, T.; Marchuk, S.; William, I.; Mccabe, B.K.; Tait, S. Resource recovery for environmental management of dilute livestock manure using a solid-liquid separation approach. J. Environ. Manag. 2023, 325, 116254. [Google Scholar] [CrossRef] [PubMed]
- Vanotti, M.; Rashash, D.; Hunt, P. Solid–liquid separation of flushed swine manure with pam: Effect of wastewater strength. Trans. ASAE 2002, 45, 1959–1969. [Google Scholar] [CrossRef]
- Liu, Z.; Carroll, Z.S.; Long, S.C.; Gunasekaran, S. Use of cationic polymers to reduce pathogen levels during dairy manure separation. J. Environ. Manag. 2016, 166, 260–266. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Liu, D.; Li, J.; Wang, X.L.; Song, B.Y.; Yue, B.; Zhao, K.H.; Song, Y. Hydrolysis of polyaluminum chloride prior to coagulation: Effects on coagulation behavior and implications for improving coagulation performance. Environ. Sci. 2017, 57, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Aljuboori AH, R.; Idris, A.; Abdullah, N.; Mohamad, R. Production and characterization of a bioflocculant produced by aspergillus flavus. Bioresour. Technol. 2013, 127, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumar, S.; Garg, M. Domestic wastewater treatment using tanfloc: A tannin based coagulant. Geostat. Geospat. Approaches Charact. Nat. Resour. Environ. Chall. Process. Strateg. 2016, 53, 349–354. [Google Scholar]
- Hameed, Y.T.; Idris, A.; Hussain, S.A.; Abdullah, N.; Cheman, H. Effect of pre-treatment with a tannin-based coagulant and flocculant on a biofilm bacterial community and the nitrification process in a municipal wastewater biofilm treatment unit. Environ. Chem. Eng. 2020, 8, 103679. [Google Scholar] [CrossRef]
- Nierop, K.G.; Preston, C.M.; Verstraten, J.M. Linking the b ring hydroxylation pattern of condensed tannins to c, n and p mineralization. A case study using four tannins. Soil Biol. Biochem. 2006, 38, 2794–2802. [Google Scholar] [CrossRef]
- Nierop, K.G.; Verstraten, J.M.; Tietema, A.; Westerveld, J.W.; Wartenbergh, P.E. Short-and long-term tannin induced carbon, nitrogen and phosphorus dynamics in corsican pine litter. Biogeochemistry 2006, 79, 275–296. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Mitigation of Climate Change; Technical Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Negassa, W.C.; Guber, A.K.; Kravchenko, A.N.; Marsh, T.L.; Hildebrandt, B.; Rivers, M.L. Properties of soil pore space regulate pathways of plant residue decomposition and community structure of associated bacteria. PLoS ONE 2015, 10, e0123999. [Google Scholar] [CrossRef] [PubMed]
- De Gryze, S.; Jassogne, L.; Six, J.; Bossuyt, H.; Wevers, M.; Merckx, R. Pore structure changes during decomposition of fresh residue: X-ray tomography analyses. Geoderma 2006, 134, 82–96. [Google Scholar] [CrossRef]
- Young, I.M.; Crawford, J.W. Interactions and self-organization in the soil-microbe complex. Science 2004, 304, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Cortina-Januchs, M.; Quintanilla-Dominguez, J.; Vega-Corona, A.; Tarquis, A.; Andina, D. Detection of pore space in ct soil images using artificial neural networks. Biogeosciences 2011, 8, 279–288. [Google Scholar] [CrossRef]
- Bartlett, R.J.; Ross, D.S. Colorimetric Determination of Oxidizable Carbon in Acid Soil Solutions. Soil Sci. Soc. Am. J. 1988, 52, 1191–1192. [Google Scholar] [CrossRef]
- Moldrup, P.; Olesen, T.; Gamst, J.; Schjønning, P.; Yamaguchi, T.; Rolston, D. Predicting the gas diffusion coefficient in repacked soil water-induced linear reduction model. Soil Sci. Soc. 2000, 64, 1588–1594. [Google Scholar] [CrossRef]
- Moldrup, P.; Chamindu Deepagoda TK, K.; Hamamoto, S.; Komatsu, T.; Kawamoto, K.; Rolston, D.; Jonge, L.W. Structure-dependent water-induced linear reduction model for predicting gas diffusivity and tortuosity in repacked and intact soil. Vadose Zone J. 2013, 12, 1–11. [Google Scholar] [CrossRef]
- Marshall, T. The diffusion of gases through porous media. Soil Sci. 1959, 10, 79–82. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, X. Relationship between the particle size and nutrient distribution of feces and nutrients in pigs and dairy cows and the efficiency of solid-liquid separation. IOP Conference Series: Earth and Environmental Science. IOP Conf. Ser. Earth Environ. Sci. 2020, 514, 052035. [Google Scholar] [CrossRef]
- Baral, K.R.; Guillaume, J.; Barbara, A.; Chantigny, M.; Olesen, J.; Petersen, S. Greenhouse gas emissions during storage of manure and digestates: Key role of methane for prediction and mitigation. Agric. Syst. 2018, 166, 26–35. [Google Scholar] [CrossRef]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S. Solid–liquid separation of animal slurry in theory and practice. Sustain. Agric. 2011, 2, 953–986. [Google Scholar]
- Morris, D.R.; Lathwell, D.J. Anaerobically digested dairy manure as fertilizer for maize in acid and alkaline soils. Commun. Soil Sci. Plant Anal. 2004, 35, 1757–1771. [Google Scholar] [CrossRef]
- Pedersen, J.; Nyord, T.; Feilberg, A.; Labouriau, R.; Hunt, D.; Bittman, S. Effect of reduced exposed surface area and enhanced infiltration on ammonia emission from untreated and separated cattle slurry. Biosyst. Eng. 2021, 211, 141–151. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C.; Aktas, N.; Turk, M. Biocompatible and biodegradable poly (tannic acid) hydrogel with antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2016, 82, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Bhandral, R.; Bolan, N.S.; Saggar, S.; Hedley, M. Nitrogen transformation and nitrous oxide emissions from various types of farm effluents. Nutr. Cycl. Agroecosyst. 2007, 79, 193–208. [Google Scholar] [CrossRef]
- Fangueiro, D.; Senbayran, M.; Trindade, H.; Chadwick, D. Cattle slurry treatment by screw press separation and chemically enhanced settling: Effect on greenhouse gas emissions after land spreading and grass yield. Bioresour. Technol. 2008, 99, 7132–7142. [Google Scholar] [CrossRef]
- Wen, Y.C.; Li, H.Y.; Lin, Z.A.; Zhao, B.Q.; Sun, Z.B.; Yuan, L.; Xu, J.K.; Li, Y.Q. Long-term fertilization alters soil properties and fungal community composition in fluvo-aquic soil of the north China Plain. Sci. Rep. 2020, 10, 7198. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and soil physical properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Wen, T.; Wang, P.; Shao, L.; Guo, X.X. Experimental investigations of soil shrinkage characteristics and their effects on the soil water characteristic curve. Eng. Geol. 2021, 284, 106035. [Google Scholar] [CrossRef]
- Zhu, K.; Bruun, S.; Larsen, M.; Glud, R.N.; Jensen, L.S. Spatial oxygen distribution and nitrous oxide emissions from soil after manure application: A novel approach using planar optodes. J. Environ. Qual. 2014, 43, 1809–1812. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Philippot, L.; Peyrard, C.; Bru, D.; Breuil, M.; Bizouard, F.; Justes, E.; Mary, B.; Léonard, J. Peaks of in situ N2O emissions are influenced by N2O-producing and reducing microbial communities across arable soils. Glob. Chang. Biol. 2018, 24, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Blagodatskaya, E.; Zheng, X.; Blagodatsky, S.; Wiegl, R.; Dannenmann, M.; Butterbach-Bahl, K. Oxygen and substrate availability interactively control the temperature sensitivity of CO2 and N2O emission from soil. Biol. Fertil. Soils 2014, 50, 775–783. [Google Scholar] [CrossRef]
- Charles, A.; Rochette, P.; Whalen, J.K.; Angers, D.A.; Chantigny, M.H.; Bertrand, N. Global nitrous oxide emission factors from agricultural soils after addition of organic amendments: A meta-analysis. Agric. Ecosyst. Environ. 2017, 236, 88–98. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Singh, B.P.; Yang, Q.; Zhang, Q.; Wang, H.; Xia, Z.; Di, H.; Singh, B.K.; Xu, J.M. Nosz clade ii rather than clade i determine in situ N2O emissions with different fertilizer types under simulated climate change and its legacy. Soil Biol. Biochem. 2020, 150, 107974. [Google Scholar] [CrossRef]
- Fangueiro, D.; Chadwick, D.; Dixon, L.; Bol, R. Quantification of priming and CO2 emission sources following the application of different slurry particle size fractions to a grassland soil. Soil Biol. Biochem. 2007, 39, 2608–2620. [Google Scholar] [CrossRef]
- Masse, L.; Massé, D.; Beaudette, V.; Muir, M. Size distribution and composition of particles in raw and anaerobically digested swine manure. Trans. ASAE 2005, 48, 1943–1949. [Google Scholar] [CrossRef]
- Dalby, F.R.; Hansen, M.J.; Feilberg, A.; Kuemmel, S.; Nikolausz, M. Effect of tannic acid combined with fluoride and lignosulfonic acid on anaerobic digestion in the agricultural waste management chain. Bioresour. Technol. 2020, 307, 123171. [Google Scholar] [CrossRef]
- Dalby, F.R.; Svane, S.; Sigurdarson, J.J.; Sorensen, M.K.; Hansen, M.J.; Karring, H.; Feilberg, A. Synergistic tannic acid-fluoride inhibition of ammonia emissions and simultaneous reduction of methane and odor emissions from livestock waste. Environ. Sci. Technol. 2020, 54, 7639–7650. [Google Scholar] [CrossRef]
- Aguerre, M.; Wattiaux, M.; Powell, J. Emissions of ammonia, nitrous oxide, methane, and carbon dioxide during storage of dairy cow manure as affected by dietary forage-to-concentrate ratio and crust formation. J. Dairy Sci. 2012, 95, 7409–7416. [Google Scholar] [CrossRef]
- Dinuccio, E.; Berg, W.; Balsari, P. Effects of mechanical separation on ghg and ammonia emissions from cattle slurry under winter conditions. Anim. Feed Sci. Technol. 2011, 166, 532–538. [Google Scholar] [CrossRef]
- Adamczyk, S.; Kiikkilä, O.; Kitunen, V.; Smolander, A. Potential response of soil processes to diterpenes, triterpenes and tannins: Nitrification, growth of microorganisms and precipitation of proteins. Appl. Soil Ecol. 2013, 67, 47–52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).