Effect of Simulated Grazing on Morphological Plasticity and Resource Allocation of Aeluropus lagopoides
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Plant Samples Collection
2.2. Establishment of Pot Experiment for Simulated Grazing
2.3. Measurement of Morphological Parameters
2.4. Estimation of Chlorophyll and Proline Contents
2.5. Statistical Analysis of the Data
3. Results
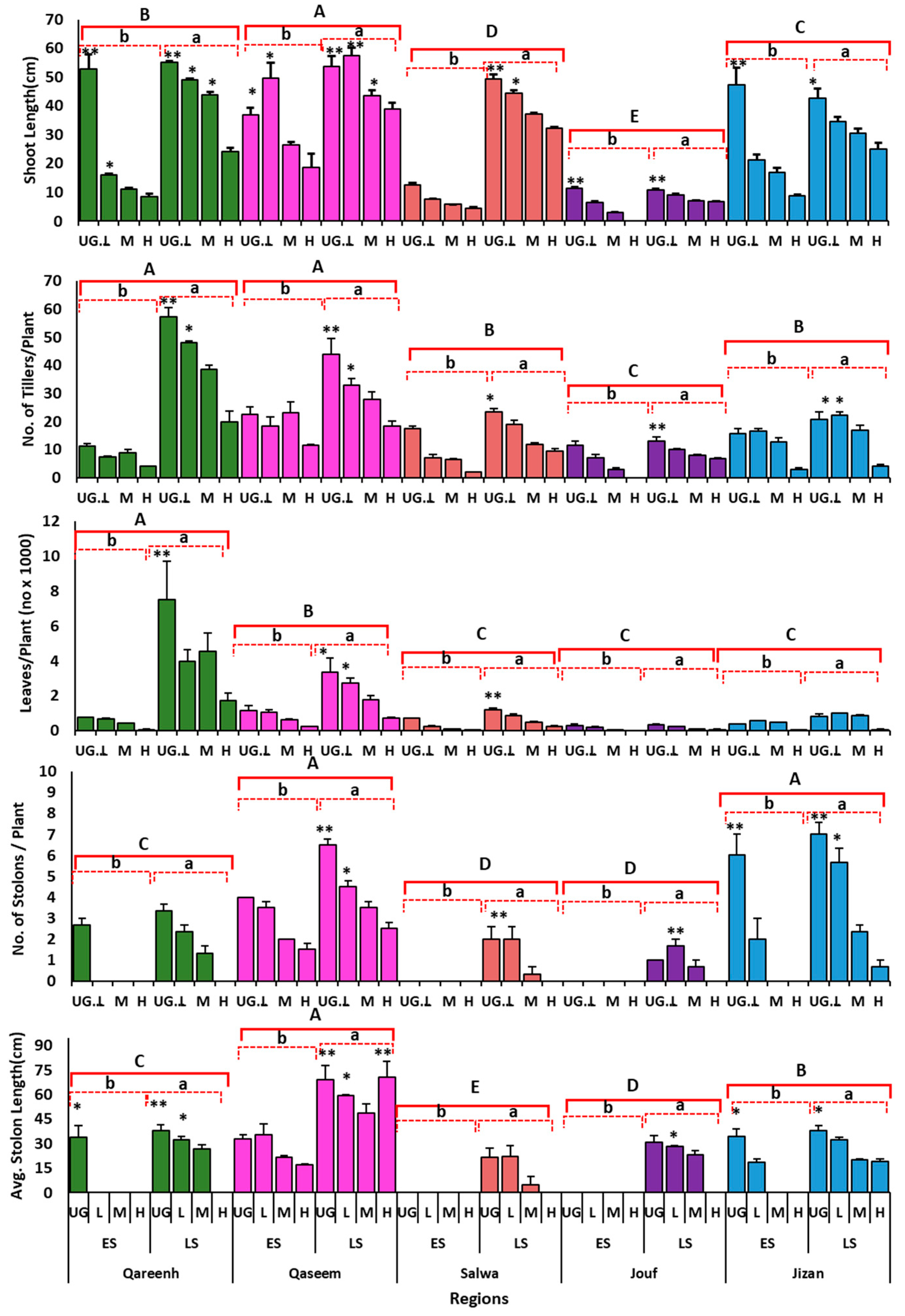
3.1. Effect of Grazing over Time on Shoot Morphological Traits
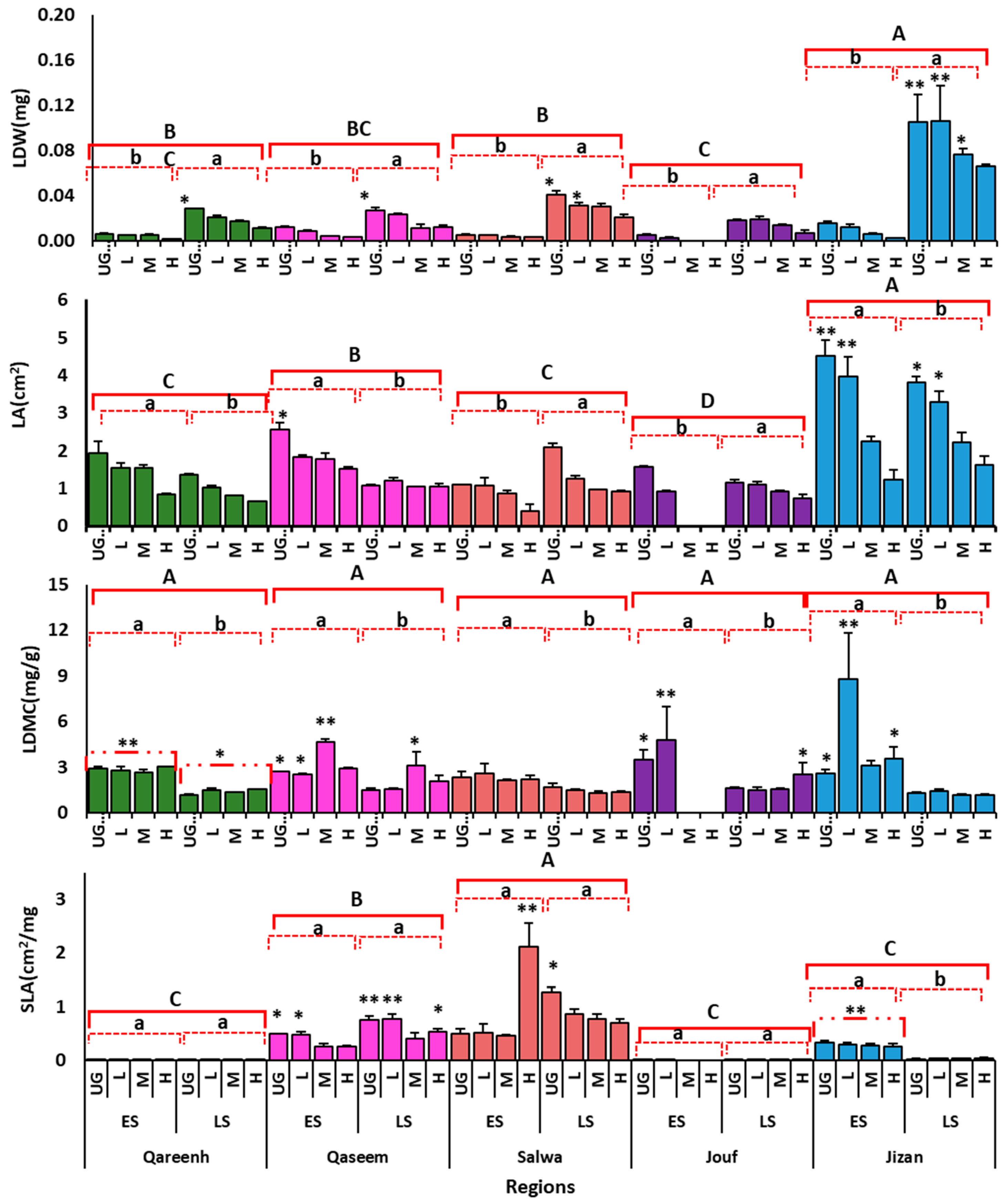
3.2. Grazing Effects on Leaf Morphological Traits
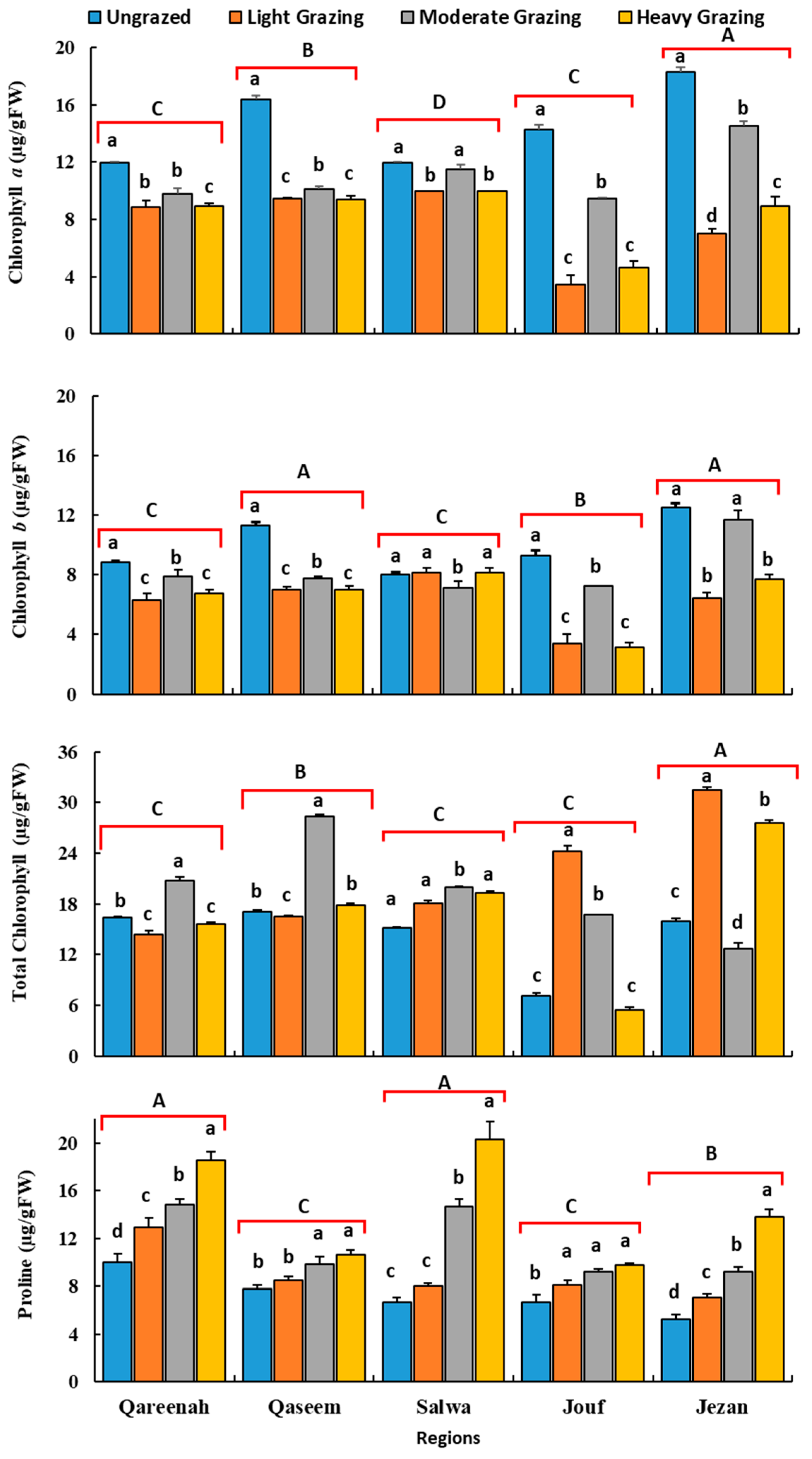
3.3. Effect of Grazing over Time Chlorophyll and Proline Content of A. lagopoides
3.4. Morphological Traits under Different Grazing Intensities at the Termination of Experiment
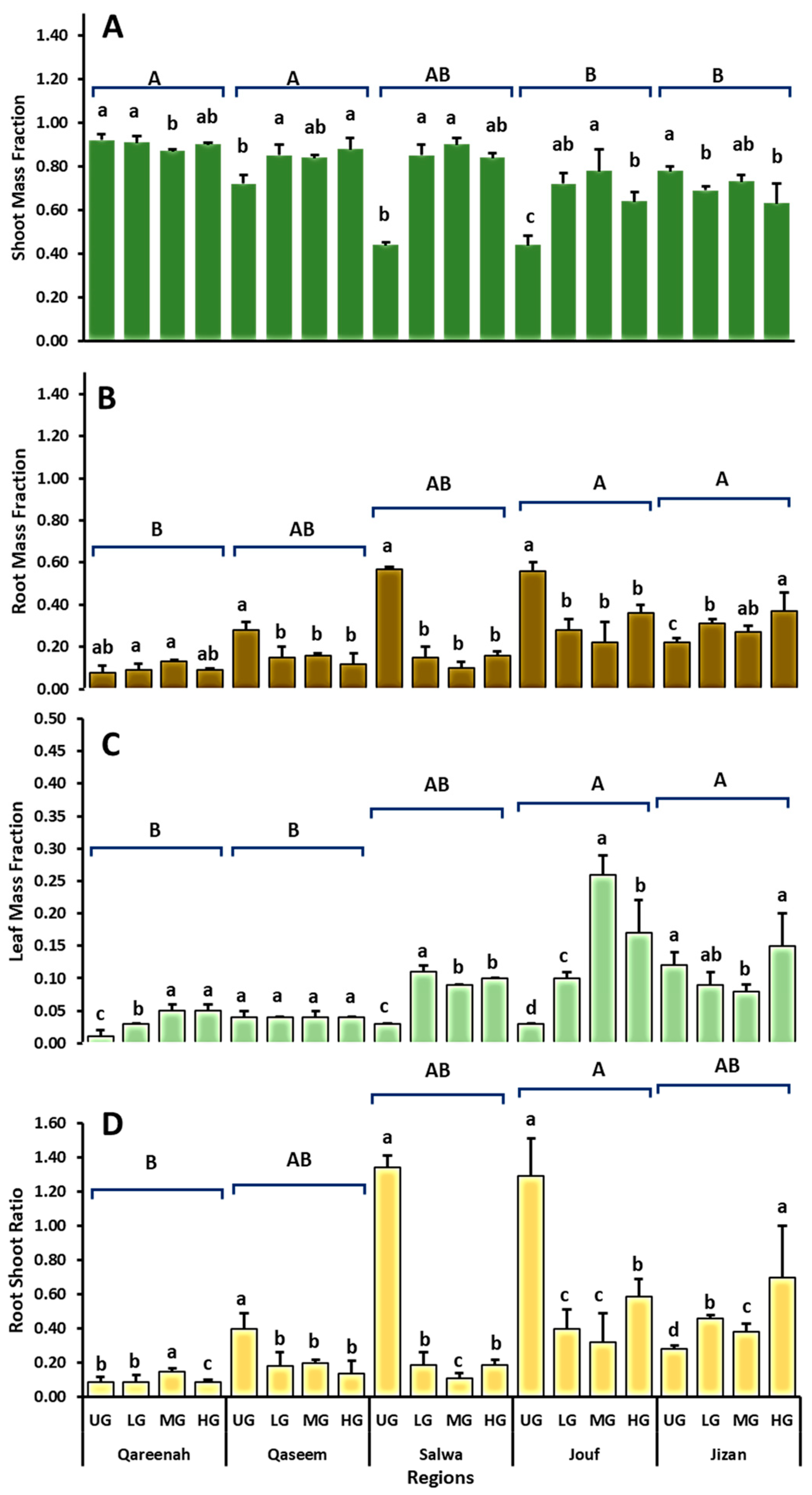
3.5. Comparisons of Shoot, Leaf, and Root Biomass Fractions of A. lagopoides under Different Grazing Intensities at the Termination of Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Houerou, H. An overview of vegetation and land degradation in world arid lands. In Degradation and Restoration of Arid Lands; Dregne, H.E., Ed.; International Center for Semi Arid Land Studies: Lubbock, TX, USA, 1992; pp. 127–163. [Google Scholar]
- Al-Tabini, R.; Al-Khalidi, K.; Al-Shudiefat, M. Livestock, medicinal plants and rangeland viability in Jordan’s Badia: Through the lens of traditional and local knowledge. Pastor. Res. Policy Pract. 2012, 2, 2041–7136. [Google Scholar] [CrossRef]
- Aidoud, A.; Le Floch, E.; Le Houérou, H.N. The arid steppe rangelands of Northern Africa. Sci. Et Chang. Planétaires/Sécheresse 2006, 17, 19–30. [Google Scholar]
- Squires, V.R. Overview of problems and prospects for utilizing halophytes as a resource for livestock and for rehabilitation of degraded lands. In Halophytes as A Resource for Livestock and for Rehabilitation of Degraded Lands; Springer: Berlin/Heidelberg, Germany, 1994; pp. 1–6. [Google Scholar]
- Zahran, M.A.; Murphy, K.J.; Mashaly, I.A.; Khedr, A.A. On the ecology of some halophytes and psammophytes in the Mediterranean coast of Egypt. Verhandlungen-Ges. Fur Okologie 1996, 25, 133–146. [Google Scholar]
- Loconsole, D.; Cristiano, G.; De Lucia, B. Glassworts: From wild salt marsh species to sustainable edible crops. Agriculture 2019, 9, 14. [Google Scholar] [CrossRef]
- Breckle, S.-W. Salinity, halophytes and salt affected natural ecosystems. In Salinity: Environment-Plants-Molecules; Springer: Dordrecht, The Netherlands, 2002; pp. 53–77. [Google Scholar]
- Basahi, R.A. Plant diversity of the coastal regions of Gulf of Aqaba, Saudi Arabia. Annu. Res. Rev. Biol. 2018, 26, 1–11. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Assaeed, A.M.; Al-Rowaily, S.L.; Dar, B.M.; Malik, J.A. Moisture and salinity drive the vegetation composition of Wadi Hargan, Riyadh, Saudi Arabia. Diversity 2021, 13, 587. [Google Scholar] [CrossRef]
- Dar, B.A.; Assaeed, A.M.; Al-Rowaily, S.L.; Al-Doss, A.A.; Abd-ElGawad, A.M. Vegetation composition of the halophytic grass Aeluropus lagopoides communities within coastal and inland sabkhas of Saudi Arabia. Plants 2022, 11, 666. [Google Scholar] [CrossRef]
- Mohsenzadeh, S.; Malboobi, M.; Razavi, K.; Farrahi-Aschtiani, S. Physiological and molecular responses of Aeluropus lagopoides (Poaceae) to water deficit. Environ. Exp. Bot. 2006, 56, 314–322. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Lata, C.; Kumar, S. Eco-physiological responses of Aeluropus lagopoides (grass halophyte) and Suaeda nudiflora (non-grass halophyte) under individual and interactive sodic and salt stress. South Afr. J. Bot. 2016, 105, 36–44. [Google Scholar] [CrossRef]
- Chaudhary, S.A.; Le Houerou, H.N. The rangelands of the Arabian Peninsula. Sci. Chang. Planétaires/Sécheresse 2006, 17, 179–194. [Google Scholar]
- Torbatinezhad, N.; Maghsoodlowrad, H.; Gharahbash, A. Nutritive value of Aeluropus littoralis and Aeluropus logopoides in sheep. J. Agric. Sci. Nat. Resour. 2000, 7, 31–45. [Google Scholar]
- Huntly, N. Herbivores and the dynamics of communities and ecosystems. Annu. Rev. Ecol. Syst. 1991, 22, 477–503. [Google Scholar] [CrossRef]
- Briske, D. Strategies of plant survival in grazed systems: A functional interpretation. In The Ecology and Management of Grazing Systems; Hodgson, J., Illius, A.W., Eds.; CAB International: Wallingford, UK, 1996; pp. 37–67. [Google Scholar]
- Zheng, C.C.; Wang, Y.J.; Sun, H.; Wang, X.Y.; Gao, Y.Z. Effects of clipping on nitrogen allocation strategy and compensatory growth of Leymus chinensis under saline-alkali conditions. J. Appl. Ecol. 2017, 28, 2222–2230. [Google Scholar]
- Ma, H.; Zheng, C.; Gao, Y.; Baskin, C.C.; Sun, H.; Yang, H. Moderate clipping stimulates over-compensatory growth of Leymus chinensis under saline-alkali stress through high allocation of biomass and nitrogen to shoots. Plant Growth Regul. 2020, 92, 95–106. [Google Scholar] [CrossRef]
- Noy-Meir, I. Compensating growth of grazed plants and its relevance to the use of rangelands. Ecol. Appl. 1993, 3, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gong, J.; Yang, B.; Ding, Y.; Zhang, Z.; Wang, B.; Zhu, C.; Hou, X. Differences in the photosynthetic and physiological responses of Leymus chinensis to different levels of grazing intensity. BMC Plant Biol. 2019, 19, 558. [Google Scholar] [CrossRef]
- De Ryck, S.; Reheul, D.; De Riek, J.; De Keyser, E.; De Cauwer, B. Genetic and morphological variation of Belgian Cyperus esculentus L. clonal populations and their significance for integrated management. Agronomy 2023, 13, 572. [Google Scholar] [CrossRef]
- Smith, S.E. Variation in response to defoliation between populations of Bouteloua curtipendula var. caespitosa (Poaceae) with different livestock grazing histories. Am. J. Bot. 1998, 85, 1266–1272. [Google Scholar]
- Kuijper, D.; Dubbeld, J.; Bakker, J. Competition between two grass species with and without grazing over a productivity gradient. Plant Ecol. 2005, 179, 237–246. [Google Scholar] [CrossRef]
- Wang, D.; Du, J.; Zhang, B.; Ba, L.; Hodgkinson, K.C. Grazing intensity and phenotypic plasticity in the clonal grass Leymus chinensis. Rangel. Ecol. Manag. 2017, 70, 740–747. [Google Scholar] [CrossRef]
- Bryant, R.H.; Matthew, C.; Hodgson, J. Growth strategy of rhizomatous and non-rhizomatous tall fescue populations in response to defoliation. Agriculture 2015, 5, 791–805. [Google Scholar] [CrossRef]
- Diaz, S.; Lavorel, S.; McIntyre, S.; Falczuk, V.; Casanoves, F.; Milchunas, D.G.; Skarpe, C.; Rusch, G.; Sternberg, M.; NOY-MEIR, I. Plant trait responses to grazing—A global synthesis. Glob. Chang. Biol. 2007, 13, 313–341. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Ren, H.; Jin, Y.; Han, M.; Wang, S.; Han, G. Plastic response of individual functional traits in Stipa breviflora to long-term grazing in a desert steppe. Acta Bot. Boreali Occident. Sin. Yangling 2017, 37, 1854–1863. [Google Scholar]
- Huhta, A.-P.; Lennartsson, T.; Tuomi, J.; Rautio, P.; Laine, K. Tolerance of Gentianella campestris in relation to damage intensity: An interplay between apical dominance and herbivory. Evol. Ecol. 2000, 14, 373–392. [Google Scholar] [CrossRef]
- Sun, J.; Zhan, T.; Liu, M.; Zhang, Z.; Wang, Y.; Liu, S.; Wu, G.-L.; Liu, G.; Tsunekawa, A. Verification of the biomass transfer hypothesis under moderate grazing across the Tibetan plateau: A meta-analysis. Plant Soil 2021, 458, 139–150. [Google Scholar] [CrossRef]
- Ilmarinen, K.; Mikola, J.; Vestberg, M. Do interactions with soil organisms mediate grass responses to defoliation? Soil Biol. Biochem. 2008, 40, 894–905. [Google Scholar] [CrossRef]
- Simms, E.L. Defining tolerance as a norm of reaction. Evol. Ecol. 2000, 14, 563–570. [Google Scholar] [CrossRef]
- Wang, L.; Gan, Y.; Wiesmeier, M.; Zhao, G.; Zhang, R.; Han, G.; Siddique, K.H.; Hou, F. Grazing exclusion—An effective approach for naturally restoring degraded grasslands in Northern China. Land Degrad. Dev. 2018, 29, 4439–4456. [Google Scholar] [CrossRef]
- Li, X.-L.; Liu, Z.-Y.; Ren, W.-B.; Yong, D.; Lei, J.; Guo, F.-H.; Hou, X.-Y. Linking nutrient strategies with plant size along a grazing gradient: Evidence from Leymus chinensis in a natural pasture. J. Integr. Agric. 2016, 15, 1132–1144. [Google Scholar] [CrossRef]
- Hodgkinson, K. Influence of partial defoliation on photosynthesis, photorespiration and transpiration by lucerne leaves of different ages. Funct. Plant Biol. 1974, 1, 561–578. [Google Scholar] [CrossRef]
- Tiffin, P. Mechanisms of tolerance to herbivore damage: What do we know? Evol. Ecol. 2000, 14, 523–536. [Google Scholar] [CrossRef]
- Li, X.; Png, G.K.; Li, Y.; Jimoh, S.O.; Ding, Y.; Li, F.; Sun, S. Leaf plasticity contributes to plant anti-herbivore defenses and indicates selective foraging: Implications for sustainable grazing. Ecol. Indic. 2021, 122, 107273. [Google Scholar] [CrossRef]
- Maurya, J.P.; Bhalerao, R.P. Photoperiod-and temperature-mediated control of growth cessation and dormancy in trees: A molecular perspective. Ann. Bot. 2017, 120, 351–360. [Google Scholar] [CrossRef]
- Li, S.L.; Yu, F.H.; Werger, M.J.; Dong, M.; Ramula, S.; Zuidema, P.A. Understanding the effects of a new grazing policy: The impact of seasonal grazing on shrub demography in the Inner Mongolian steppe. J. Appl. Ecol. 2013, 50, 1377–1386. [Google Scholar] [CrossRef]
- Leffler, A.J.; Beard, K.H.; Kelsey, K.C.; Choi, R.T.; Schmutz, J.A.; Welker, J.M. Delayed herbivory by migratory geese increases summer-long CO2 uptake in coastal western Alaska. Glob. Chang. Biol. 2019, 25, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Pan, Y.; Gong, J.; Li, X.; Liu, M.; Yang, B.; Zhang, Z.; Baoyin, T. Physiology of Leymus chinensis under seasonal grazing: Implications for the development of sustainable grazing in a temperate grassland of Inner Mongolia. J. Environ. Manag. 2020, 271, 110984. [Google Scholar] [CrossRef]
- Freitag, M.; Kamp, J.; Dara, A.; Kuemmerle, T.; Sidorova, T.V.; Stirnemann, I.A.; Velbert, F.; Hölzel, N. Post-soviet shifts in grazing and fire regimes changed the functional plant community composition on the Eurasian steppe. Glob. Chang. Biol. 2021, 27, 388–401. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, D.; Zhang, M.; Tong, S.; Wang, W.; An, Y. Spatial distribution of soil organic carbon and total nitrogen in disturbed Carex tussock wetland. Ecol. Indic. 2021, 120, 106930. [Google Scholar] [CrossRef]
- Assaeed, A.M.; Dar, B.A.; Al-Doss, A.A.; Al-Rowaily, S.L.; Malik, J.A.; Abd-ElGawad, A.M. Phenotypic plasticity strategy of Aeluropus lagopoides grass in response to heterogenous saline habitats. Biology 2023, 12, 553. [Google Scholar] [CrossRef]
- Phondani, P.C.; Bhatt, A.; Elsarrag, E.; Alhorr, Y.M.; El-Keblawy, A. Criteria and indicator approach of global sustainability assessment system for sustainable landscaping using native plants in Qatar. Ecol. Indic. 2016, 69, 381–389. [Google Scholar] [CrossRef]
- Vile, D.; Garnier, E.; Shipley, B.; Laurent, G.; Navas, M.-L.; Roumet, C.; Lavorel, S.; Díaz, S.; Hodgson, J.G.; Lloret, F. Specific leaf area and dry matter content estimate thickness in laminar leaves. Ann. Bot. 2005, 96, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.; Cornwell, W.; Craine, J.; Gurvich, D. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 603, 591–592. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Principal and Procedure of Statistics: A Biochemical Approach, 3rd ed.; Mcgraco-Hill book Company: New York, NY, USA, 1986. [Google Scholar]
- Cahill, J.F., Jr.; McNickle, G.G.; Haag, J.J.; Lamb, E.G.; Nyanumba, S.M.; St. Clair, C.C. Plants integrate information about nutrients and neighbors. Science 2010, 328, 1657. [Google Scholar] [CrossRef]
- Chai, J.; Yu, X.; Xu, C.; Xiao, H.; Zhang, J.; Yang, H.; Pan, T.J.A.S.E. Effects of yak and Tibetan sheep trampling on soil properties in the northeastern Qinghai-Tibetan Plateau. J. Appl. Soil Ecol. 2019, 144, 147–154. [Google Scholar] [CrossRef]
- Tuomi, J.; Nilsson, P.; Astrom, M. Plant compensatory responses: Bud dormancy as an adaptation to herbivory. Ecology 1994, 75, 1429–1436. [Google Scholar] [CrossRef]
- Mocelin, N.G.; Schmitt, D.; Zanini, G.D.; Camacho, P.A.G.; Sbrissia, A.F. Grazing management targets for tangolagrass pastures. Agriculture 2022, 12, 279. [Google Scholar] [CrossRef]
- Hoque, M.A.; Okuma, E.; Banu, M.N.A.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. J. Plant Physiol. 2007, 164, 553–561. [Google Scholar] [CrossRef]
- Qian, L.; Qi, S.; Cao, F.; Zhang, J.; Zhao, F.; Li, C.; Wang, C. Toxic effects of boscalid on the growth, photosynthesis, antioxidant system and metabolism of Chlorella vulgaris. Environ. Pollut. 2018, 242, 171–181. [Google Scholar] [CrossRef]
- Chen, Y.; Hung, Y.-C.; Chen, M.; Lin, M.; Lin, H. Enhanced storability of blueberries by acidic electrolyzed oxidizing water application may be mediated by regulating ROS metabolism. Food Chem. 2019, 270, 229–235. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Mohammadi, H.; Ghorbanpour, M.; Brestic, M. Exogenous putrescine changes redox regulations and essential oil constituents in field-grown Thymus vulgaris L. under well-watered and drought stress conditions. Ind. Crops Prod. 2018, 122, 119–132. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Ivanov, A.G.; Zaman, M.; Pharis, R.P.; Allakhverdiev, S.I.; Hurry, V.; Huener, N. Stress-related hormones and glycinebetaine interplay in protection of photosynthesis under abiotic stress conditions. Photosynth. Res. 2015, 126, 221–235. [Google Scholar] [CrossRef]
- Qu, L.; Liu, J.; Yang, J.; Bai, L.; Huang, Y.; Lu, N.; Yu, H.; Wang, Z.; Li, Z. Soil saline-alkali heterogeneity is an important factor driving the spatial expansion of clonal plant in grassland. Front. Environ. Sci. 2023, 10, 2750. [Google Scholar] [CrossRef]
- Liu, H.-D.; Yu, F.-H.; He, W.-M.; Chu, Y.; Dong, M. Clonal integration improves compensatory growth in heavily grazed ramet populations of two inland-dune grasses. Flora 2009, 204, 298–305. [Google Scholar] [CrossRef]
- Mahlooji, M.; Seyed Sharifi, R.; Razmjoo, J.; Sabzalian, M.; Sedghi, M. Effect of salt stress on photosynthesis and physiological parameters of three contrasting barley genotypes. Photosynthetica 2018, 56, 549–556. [Google Scholar] [CrossRef]
- Cingolani, A.M.; Posse, G.; Collantes, M.B. Plant functional traits, herbivore selectivity and response to sheep grazing in Patagonian steppe grasslands. Appl. Ecol. 2005, 42, 50–59. [Google Scholar] [CrossRef]
- Rusch, G.; Skarpe, C.; Halley, D. Plant traits link hypothesis about resource-use and response to herbivory. Basic Appl. Ecol. 2009, 10, 466–474. [Google Scholar] [CrossRef]
- Cai, Y.; Yan, Y.; Xu, D.; Xu, X.; Wang, C.; Wang, X.; Chen, J.; Xin, X.; Eldridge, D.J. The fertile island effect collapses under extreme overgrazing: Evidence from a shrub-encroached grassland. Plant Soil 2020, 448, 201–212. [Google Scholar] [CrossRef]
- Fedrigo, J.K.; Ataide, P.F.; Filho, J.A.; Oliveira, L.V.; Jaurena, M.; Laca, E.A.; Overbeck, G.E.; Nabinger, C. Temporary grazing exclusion promotes rapid recovery of species richness and productivity in a long-term overgrazed Campos grassland. Restor. Ecol. 2018, 26, 677–685. [Google Scholar] [CrossRef]
- Gao, Y.Z.; Giese, M.; Lin, S.; Sattelmacher, B.; Zhao, Y.; Brueck, H. Belowground net primary productivity and biomass allocation of a grassland in Inner Mongolia is affected by grazing intensity. Plant Soil 2008, 307, 41–50. [Google Scholar] [CrossRef]
- Wang, L.; Niu, K.; Yang, Y.; Zhou, P. Patterns of above-and belowground biomass allocation in China’s grasslands: Evidence from individual-level observations. Sci. China Life Sci. 2010, 53, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Han, G.; Zhou, H.; Zhao, M.; Snyman, H.A.; Shan, D.; Havstad, K.M. Grazing intensity on vegetation dynamics of a typical steppe in northeast Inner Mongolia. Rangel. Ecol. Manag. 2009, 62, 328–336. [Google Scholar] [CrossRef]

| Region | pH | EC (dS·m−1) |
|---|---|---|
| Qareenah | 8.32 ± 0.18 | 11.37 ± 1.03 |
| Qaseem | 8.30 ± 0.14 | 10.28 ± 1.87 |
| Salwa | 8.49 ± 0.61 | 31.35 ± 4.41 |
| Jouf | 8.14 ± 0.11 | 8.78 ± 1.43 |
| Jizan | 8.04 ± 0.22 | 3.58 ± 1.76 |
| Source of Variation | DF | SS | MS | F-Value | p-Value | SS | MS | F-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Shoot length (cm) | Number of Tillers per Plant (no.) | ||||||||
| Region (R) | 4 | 15,162.7 | 3790.67 | 269.79 | <0.0001 *** | 5788.6 | 1447.15 | 134.62 | <0.0001 *** |
| Season (S) | 1 | 8167.7 | 8167.67 | 581.3 | <0.0001 *** | 4416.5 | 4416.53 | 410.84 | <0.0001 *** |
| Grazing (G) | 3 | 7079.9 | 2359.98 | 167.96 | <0.0001 *** | 4029.2 | 1343.06 | 124.94 | <0.0001 *** |
| R × S | 4 | 3131.2 | 782.79 | 55.71 | <0.0001 *** | 3514.1 | 878.51 | 81.72 | <0.0001 *** |
| R × G | 12 | 2861.8 | 238.49 | 16.97 | <0.0001 *** | 563.6 | 46.97 | 4.37 | <0.0001 *** |
| S × G | 3 | 427.5 | 142.49 | 10.14 | <0.0001 *** | 383.1 | 127.69 | 11.88 | <0.0001 *** |
| R × S × G | 12 | 1239.7 | 103.31 | 7.35 | <0.0001 *** | 743.4 | 61.95 | 5.76 | <0.0001 *** |
| Error | 80 | 1124 | 14.05 | 860 | 10.75 | ||||
| Number of Stolons (no.) | Average Stolon Length (cm) | ||||||||
| Region (R) | 4 | 204.167 | 51.0417 | 133.15 | <0.0001 *** | 21,528 | 5381.9 | 147.75 | <0.0001 *** |
| Season (S) | 1 | 50.7 | 50.7 | 132.26 | <0.0001 *** | 11,570 | 11,570.3 | 317.65 | <0.0001 *** |
| Grazing (G) | 3 | 137.367 | 45.7889 | 119.45 | <0.0001 *** | 6661.8 | 2220.6 | 60.96 | <0.0001 *** |
| R × S | 4 | 4.633 | 1.1583 | 3.02 | 0.022 * | 2098.1 | 524.5 | 14.4 | <0.0001 *** |
| R × G | 12 | 75.967 | 6.3306 | 16.51 | <0.0001 *** | 2613.3 | 217.8 | 5.98 | <0.0001 *** |
| S × G | 3 | 12.433 | 4.1444 | 10.81 | <0.0001 *** | 345.4 | 115.1 | 3.16 | 0.0291 * |
| R × S × G | 12 | 10.567 | 0.8806 | 2.3 | 0.014 * | 3402.9 | 283.6 | 7.79 | <0.0001 *** |
| Error | 80 | 30.667 | 0.383 | 2914 | 36.4 | ||||
| Specific Leaf Area (cm2) | Leaf Dry Matter Content | ||||||||
| Region (R) | 4 | 13.9595 | 3.48988 | 21.03 | <0.0001 *** | 18.853 | 4.7133 | 1.39 | 0.2453 ns |
| Season (S) | 1 | 0.001 | 0.00096 | 0.01 | 0.9395 ns | 58.186 | 58.1856 | 17.14 | 0.0001 *** |
| Grazing (G) | 3 | 0.4644 | 0.15478 | 0.93 | 0.4289 ns | 14.707 | 4.9024 | 1.44 | 0.2361 ns |
| R × S | 4 | 0.7583 | 0.18957 | 1.14 | 0.3428 ns | 30.12 | 7.5301 | 2.22 | 0.0743 * |
| R × G | 12 | 2.244 | 0.187 | 1.13 | 0.351 ns | 60.151 | 5.0125 | 1.48 | 0.1504 ns |
| S × G | 3 | 0.7821 | 0.26071 | 1.57 | 0.2029 ns | 22.362 | 7.454 | 2.2 | 0.0949 * |
| R × S × G | 12 | 3.4177 | 0.28481 | 1.72 | 0.0786 * | 47.763 | 3.9803 | 1.17 | 0.3168 ns |
| Error | 80 | 13.2774 | 0.16597 | 271.51 | 3.398 | ||||
| Variation Source | DF | SS | MS | F-Value | p-Value | SS | MS | F-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Shoot length (cm) | Root length (cm) | ||||||||
| Region (R) | 4 | 11,814.60 | 2953.60 | 169.73 | <0.0001 *** | 2657.60 | 664.40 | 29.33 | <0.0001 *** |
| Grazing (G) | 3 | 2497.20 | 832.39 | 158.60 | <0.0001 *** | 2475.92 | 825.30 | 34.48 | <0.0001 *** |
| R × G | 12 | 814.80 | 67.90 | 12.94 | 0.0001 *** | 1598.67 | 133.22 | 5.57 | <0.0001 *** |
| Error | 40 | 331.80 | 8.29 | 331.8 | 8.29 | ||||
| Shoot dry weight (g) | Root dry weight (g) | ||||||||
| Region (R) | 4 | 3443.46 | 860.86 | 137.98 | <0.0001 *** | 76.10 | 19.02 | 17.22 | 0.0005 *** |
| Grazing (G) | 3 | 1439.88 | 479.95 | 129.14 | <0.0001 *** | 448.99 | 149.66 | 50.66 | <0.0001 *** |
| R × G | 12 | 731.35 | 60.94 | 16.40 | <0.0001 *** | 215.08 | 17.92 | 6.07 | <0.0001 *** |
| Error | 40 | 197.13 | 4.92 | 98.46 | 2.46 | ||||
| Shoot mass (g) | Root mass (g) | ||||||||
| Region (R) | 4 | 0.49 | 0.12 | 44.14 | <0.0001 ** | 0.4860 | 0.12 | 44.14 | <0.0001 *** |
| Grazing (G) | 3 | 3.02 | 0.08 | 11.75 | <0.0001 *** | 0.2500 | 0.08 | 11.75 | <0.0001 *** |
| R × G | 12 | 12.04 | 0.04 | 5.59 | 0.0001 *** | 0.4750 | 0.04 | 5.59 | 0.0001 *** |
| Error | 40 | 0.23 | 0.01 | 0.02 | 0.01 | ||||
| Leaf mass (g) | Chlorophyll a (µg/gFW) | ||||||||
| Region (R) | 4 | 0.10 | 0.03 | 27.41 | 0.0001 *** | 141.92 | 35.48 | 164.10 | <0.0001 *** |
| Grazing (G) | 3 | 0.03 | 0.01 | 8.42 | 0.0003 *** | 139.17 | 46.39 | 160.20 | <0.0001 *** |
| R × G | 12 | 0.08 | 0.01 | 5.53 | 0.0001 *** | 557.44 | 46.45 | 164.92 | <0.0001 *** |
| Error | 40 | 0.03 | 0.01 | 1.03 | 0.03 | ||||
| Chlorophyll b (µg/g FW) | Total chlorophyll (µg/g FW) | ||||||||
| Region (R) | 4 | 107.91 | 26.97 | 510.28 | <0.0001 *** | 493.33 | 123.32 | 937.94 | <0.0001 *** |
| Grazing (G) | 3 | 44.89 | 14.96 | 296.17 | <0.0001 *** | 341.79 | 113.93 | 866.45 | <0.0001 *** |
| R × G | 12 | 227.89 | 18.99 | 375.85 | <0.0001 *** | 1473.08 | 122.75 | 933.57 | <0.0001 *** |
| Error | 40 | 1.96 | 0.05 | 5.26 | 0.13 | ||||
| Proline (µg/g FW) | Root/shoot ratio | ||||||||
| Region (R) | 4 | 303.92 | 75.97 | 189.50 | <0.0001 *** | 2.18 | 0.54 | 47.63 | <0.0001 *** |
| Grazing (G) | 3 | 233.72 | 77.90 | 156.90 | <0.0001 *** | 1.91 | 0.64 | 15.66 | <0.0001 *** |
| R × G | 12 | 578.56 | 48.21 | 97.14 | <0.0001 *** | 3.40 | 0.28 | 7.00 | <0.0001 *** |
| Error | 40 | 1.86 | 0.05 | 1.37 | 0.03 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dar, B.A.; Assaeed, A.M.; Al-Rowaily, S.L.; Al-Doss, A.A.; Habib, M.M.; Malik, J.A.; Abd-ElGawad, A.M. Effect of Simulated Grazing on Morphological Plasticity and Resource Allocation of Aeluropus lagopoides. Agronomy 2024, 14, 144. https://doi.org/10.3390/agronomy14010144
Dar BA, Assaeed AM, Al-Rowaily SL, Al-Doss AA, Habib MM, Malik JA, Abd-ElGawad AM. Effect of Simulated Grazing on Morphological Plasticity and Resource Allocation of Aeluropus lagopoides. Agronomy. 2024; 14(1):144. https://doi.org/10.3390/agronomy14010144
Chicago/Turabian StyleDar, Basharat A., Abdulaziz M. Assaeed, Saud L. Al-Rowaily, Abdullah A. Al-Doss, Muhammad M. Habib, Jahangir A. Malik, and Ahmed M. Abd-ElGawad. 2024. "Effect of Simulated Grazing on Morphological Plasticity and Resource Allocation of Aeluropus lagopoides" Agronomy 14, no. 1: 144. https://doi.org/10.3390/agronomy14010144
APA StyleDar, B. A., Assaeed, A. M., Al-Rowaily, S. L., Al-Doss, A. A., Habib, M. M., Malik, J. A., & Abd-ElGawad, A. M. (2024). Effect of Simulated Grazing on Morphological Plasticity and Resource Allocation of Aeluropus lagopoides. Agronomy, 14(1), 144. https://doi.org/10.3390/agronomy14010144












