Clonal Transgenerational Effects of Parental Grazing Environment on Offspring Shade Avoidance
Abstract
1. Introduction
2. Materials and Methods
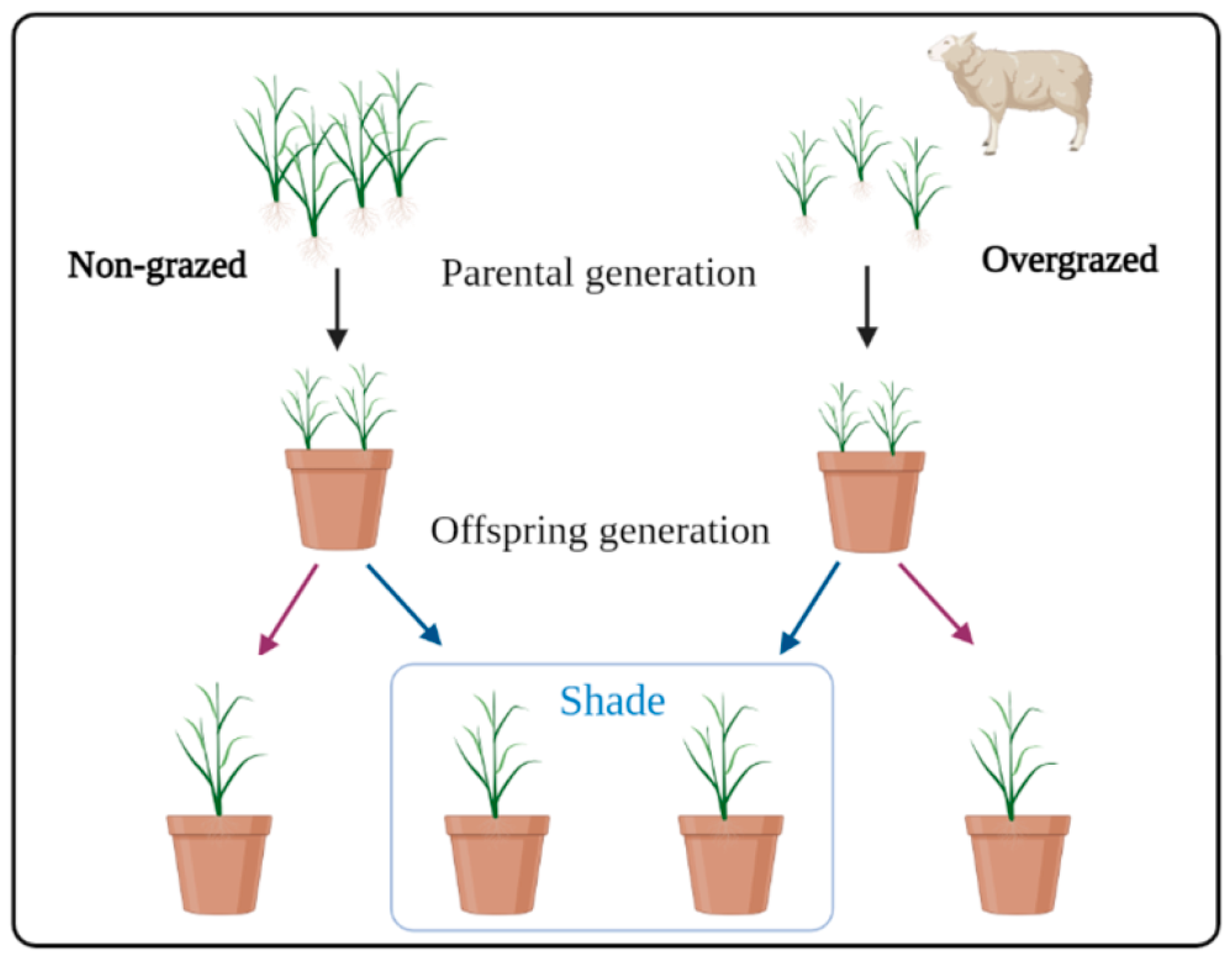
2.1. Field Site and Parental Generation Materials
2.2. Common Garden Experiment of Offspring Generation
2.3. Gas Exchange Measurements
2.4. Determination of Hormone Contents
2.5. RNA Sequencing and Analysis
2.5.1. RNA Extraction, Library Construction, and Sequencing
2.5.2. RNA-Seq Data Processing and Functional Annotation
2.6. Quantitative Real-Time PCR Validation
2.7. Statistical Analysis
3. Results
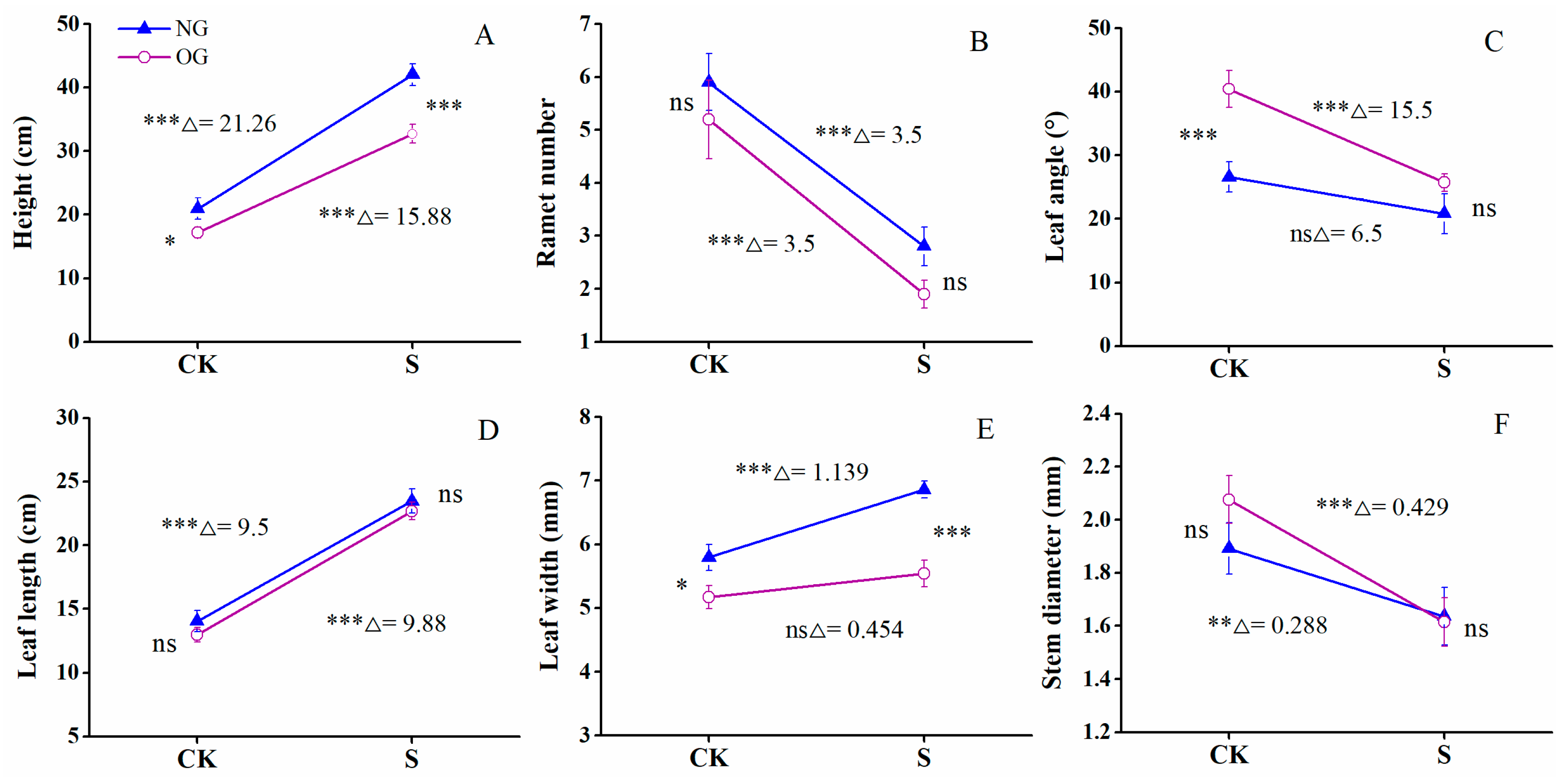
3.1. Morphological Changes Induced by Shade in Offspring
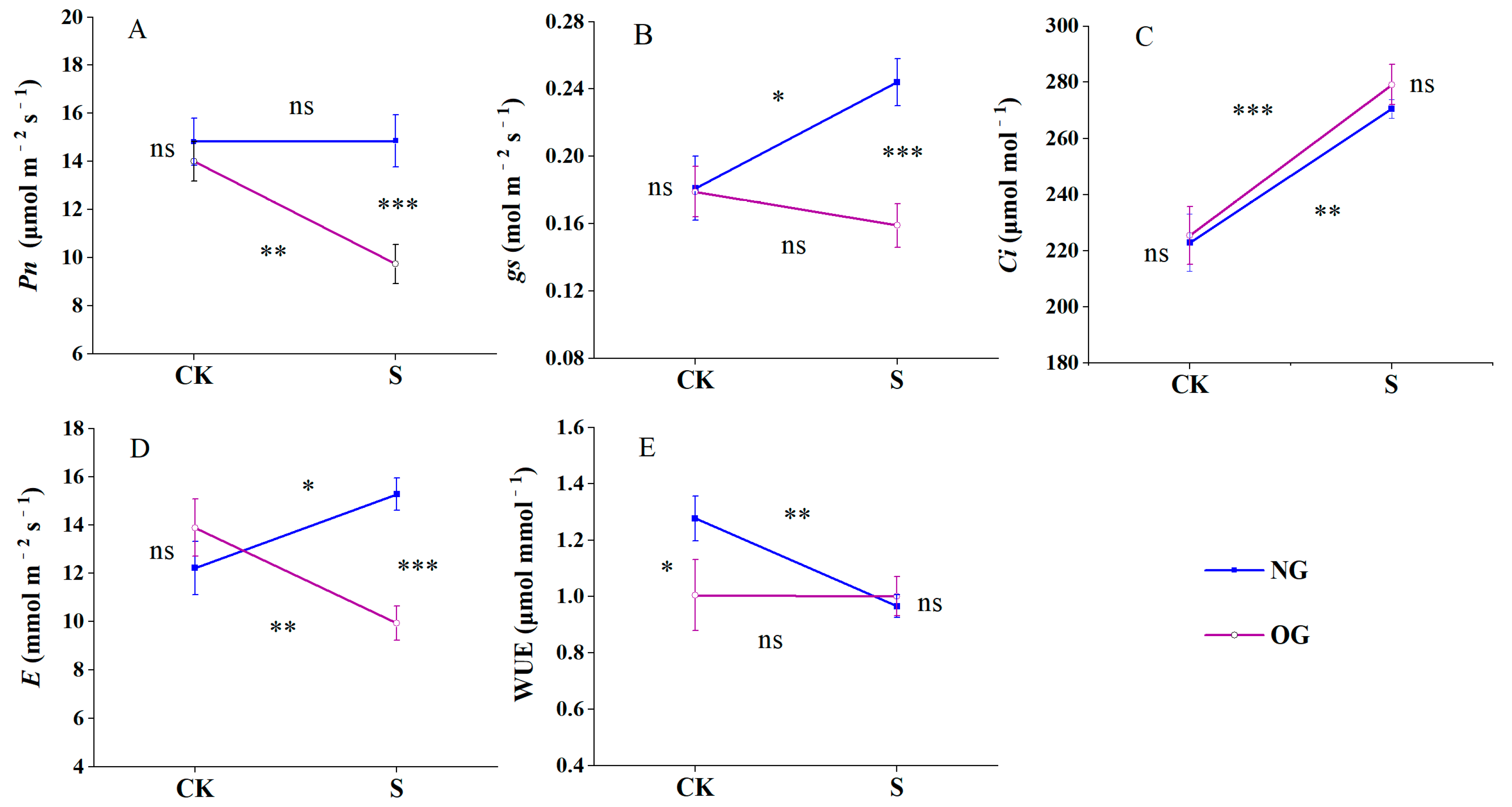
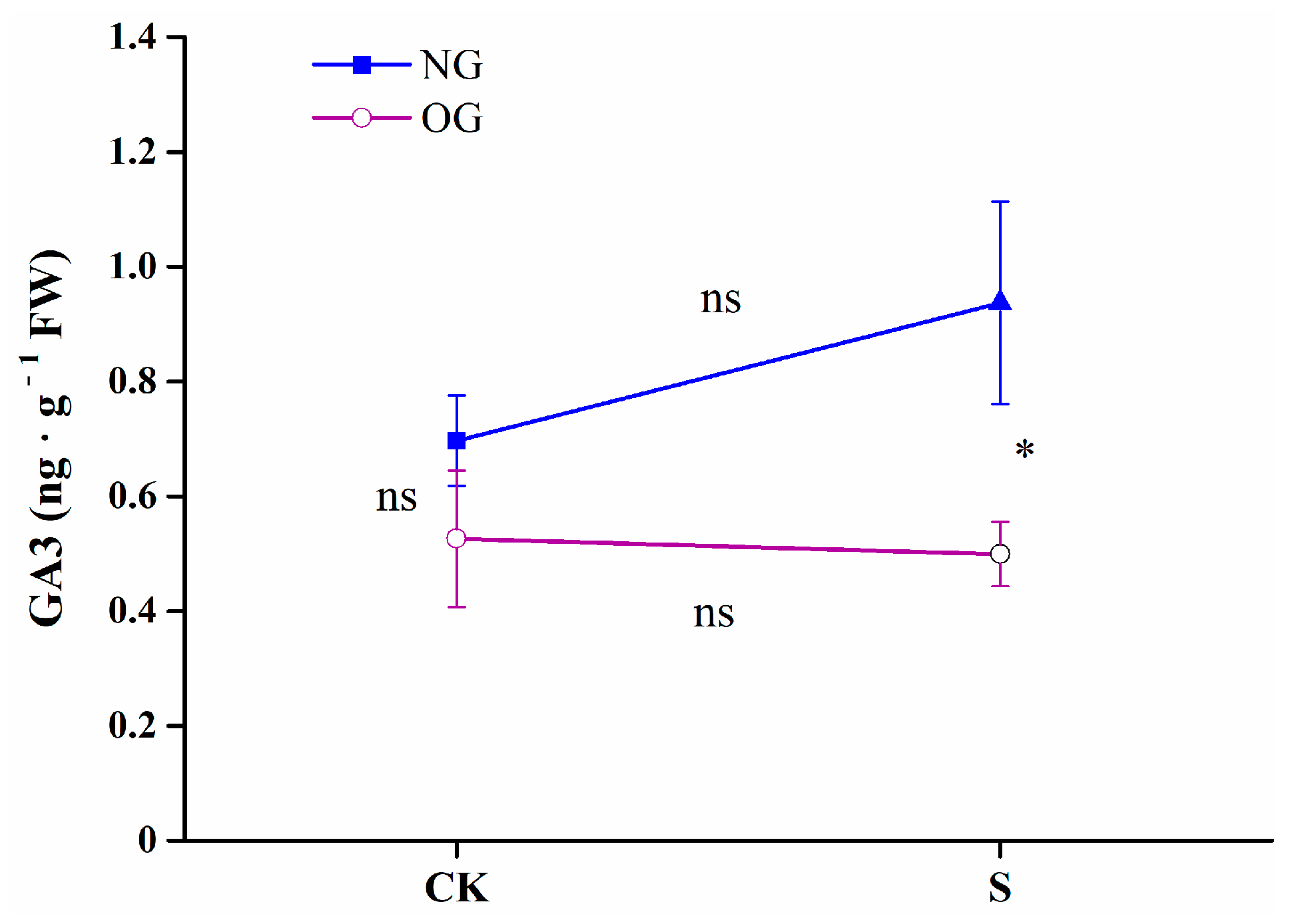
3.2. Shade Alters Leaf Photosynthesis and GA3 Content in Offspring
3.3. Transcriptional Responses in Offspring Leaves
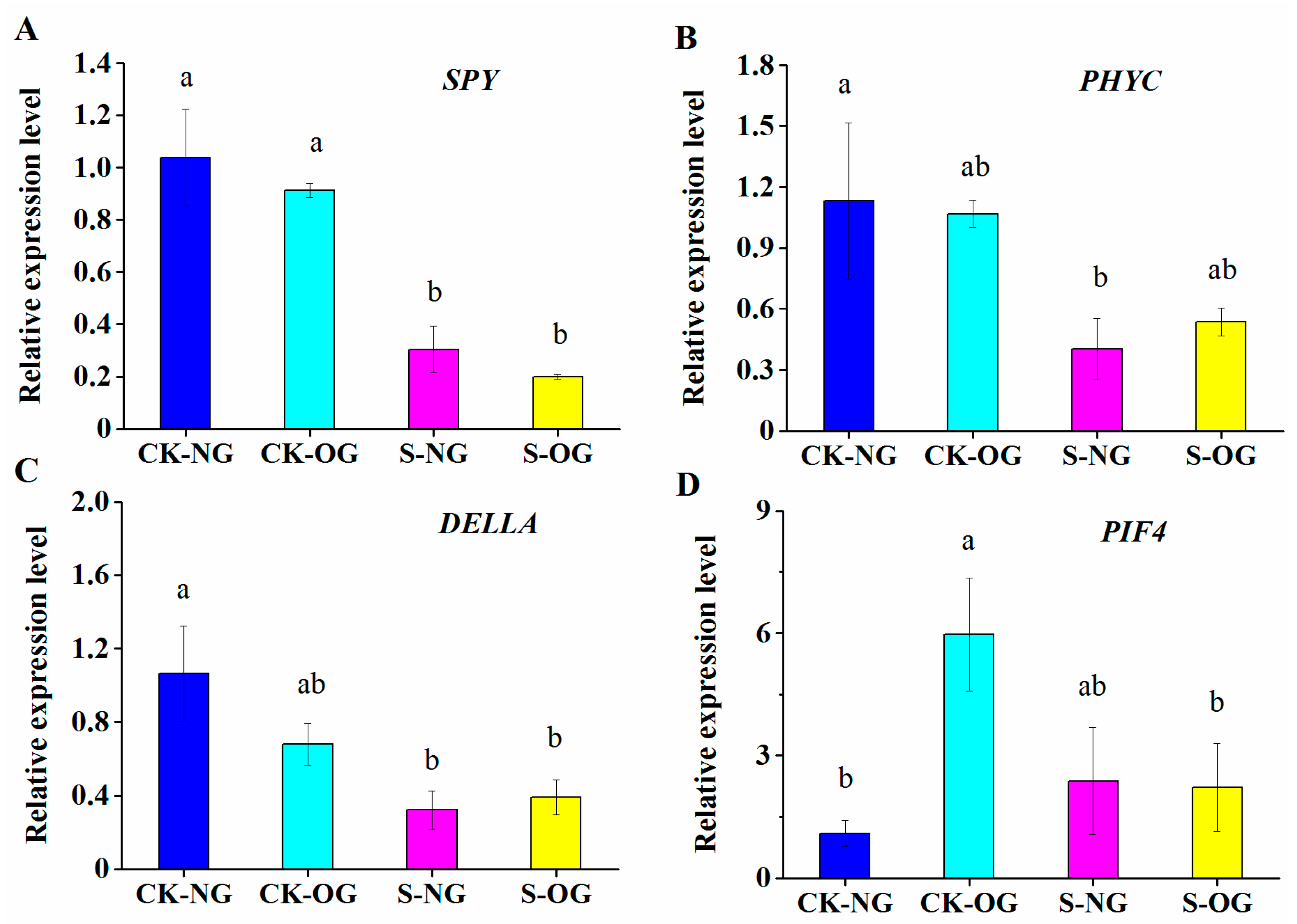
3.4. The Expression Levels of GA Signaling Pathway Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buisson, E.; Archibald, S.; Fidelis, A.; Suding, K.N. Ancient grasslands guide ambitious goals in grassland restoration. Science 2022, 377, 594–598. [Google Scholar] [CrossRef] [PubMed]
- O’Mara, F.P. The role of grasslands in food security and climate change. Ann. Bot. 2012, 110, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Wilsey, B.J. The Biology of Grasslands; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Gang, C.; Zhou, W.; Chen, Y.; Wang, Z.; Sun, Z.; Li, J.; Qi, J.; Odeh, I. Quantitative assessment of the contributions of climate change and human activities on global grassland degradation. Environ. Earth Sci. 2014, 72, 4273–4282. [Google Scholar] [CrossRef]
- Gibbs, H.K.; Salmon, J.M. Mapping the world’s degraded lands. Appl. Geogr. 2015, 57, 12–21. [Google Scholar] [CrossRef]
- Tiscornia, G.; Jaurena, M.; Baethgen, W. Drivers, process, and consequences of native grassland degradation: Insights from a literature review and a survey in río de la plata grasslands. Agronomy 2019, 9, 239. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Bullock, J.M.; Lavorel, S.; Manning, P.; Schaffner, U.; Ostle, N.; Chomel, M.; Durigan, G.; Fry, E.L.; Johnson, D.; et al. Combatting global grassland degradation. Nat. Rev. Earth Env. 2021, 2, 720–735. [Google Scholar] [CrossRef]
- Sun, B.; Li, Z.; Gao, Z.; Guo, Z.; Wang, B.; Hu, X.; Bai, L. Grassland degradation and restoration monitoring and driving forces analysis based on long time-series remote sensing data in Xilin Gol League. Acta Ecol. Sin. 2017, 37, 219–228. [Google Scholar] [CrossRef]
- Schönbach, P.; Wan, H.; Gierus, M.; Bai, Y.; Müller, K.; Lin, L.; Susenbeth, A.; Taube, F. Grassland responses to grazing: Effects of grazing intensity and management system in an Inner Mongolian steppe ecosystem. Plant Soil 2011, 340, 103–115. [Google Scholar] [CrossRef]
- Byrnes, R.C.; Eastburn, D.J.; Tate, K.W.; Roche, L.M. A global meta-analysis of grazing impacts on soil health indicators. J. Environ. Qual. 2018, 47, 758–765. [Google Scholar] [CrossRef]
- Manuel Pulido, S.S.J.F. The impact of heavy grazing on soil quality and pasture production in rangelands of SW Spain. Land Degrad. Dev. 2016, 29, 219–230. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, C. Quantifying grass coverage trends to identify the hot plots of grassland degradation in the Tibetan plateau during 2000–2019. Int. J. Environ. Res. Public Health 2021, 18, 416. [Google Scholar] [CrossRef]
- Stotz, G.C.; Salgado Luarte, C.; Escobedo, V.M.; Valladares, F.; Gianoli, E. Global trends in phenotypic plasticity of plants. Ecol. Lett. 2021, 24, 2267–2281. [Google Scholar] [CrossRef]
- Fahnestock, J.T.; Detling, J.K. Morphological and physiological responses of perennial grasses to long-term grazing in the Pryor Mountains, Montana. Am. Midl. Nat. 2000, 143, 312–320. [Google Scholar] [CrossRef]
- Liu, M.; Gong, J.; Yang, B.; Ding, Y.; Zhang, Z.; Wang, B.; Zhu, C.; Hou, X. Differences in the photosynthetic and physiological responses of Leymus chinensis to different levels of grazing intensity. BMC Plant Biol. 2019, 19, 558. [Google Scholar] [CrossRef]
- Zheng, S.X.; Lan, Z.C.; Li, W.H.; Shao, R.X.; Shan, Y.M.; Wan, H.W.; Taube, F.; Bai, Y. Differential responses of plant functional trait to grazing between two contrasting dominant C3 and C4 species in a typical steppe of Inner Mongolia, China. Plant Soil 2011, 340, 141–155. [Google Scholar] [CrossRef]
- Qu, K.; Cheng, Y.; Gao, K.; Ren, W.; Fry, E.L.; Yin, J.; Liu, Y. Growth-defense trade-offs induced by long-term overgrazing could act as a stress memory. Front. Plant Sci. 2022, 13, 917354. [Google Scholar] [CrossRef]
- Yin, J.; Li, X.; Guo, H.; Zhang, J.; Kong, L.; Ren, W. Legacy effects of historical grazing alter leaf stomatal characteristics in progeny plants. PeerJ 2020, 8, e9266. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci. 2007, 173, 603–608. [Google Scholar] [CrossRef]
- Galloway, L.F. Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytol. 2005, 166, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Waterman, R.; Sultan, S.E. Transgenerational effects of parent plant competition on offspring development in contrasting conditions. Ecology 2021, 102, e3531. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, I.; Hussain, S.; Raza, M.A.; Iqbal, N.; Asghar, M.A.; Raza, A.; Yuan-Fang, Y.-F.F.; Mumtaz, M.; Shoaib, M.; Ansar, M.; et al. Crop photosynthetic response to light quality and light intensity. J. Integr. Agric. 2021, 20, 4–23. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Bell, D.L.; Galloway, L.F. Plasticity to neighbour shade: Fitness consequences and allometry. Funct. Ecol. 2007, 21, 1146–1153. [Google Scholar] [CrossRef]
- Fu, L.; Bo, T.; Du, G.; Zheng, X. Modeling the responses of grassland vegetation coverage to grazing disturbance in an alpine meadow. Ecol. Model. 2012, 247, 221–232. [Google Scholar] [CrossRef]
- Karimipoor, Z.; Rashtian, A.; Amirkhani, M.; Ghasemi, S. The effect of grazing intensity on vegetation coverage and nitrogen mineralization kinetics of steppe rangelands of Iran (Case study: Nodoushan Rangelands, Yazd, Iran). Sustainability 2021, 13, 8392. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Wang, Z.; Zhao, M.; Han, G. Overgrazing leads to decoupling of precipitation patterns and ecosystem carbon exchange in the desert steppe through changing community composition. Plant Soil 2023, 486, 607–620. [Google Scholar] [CrossRef]
- Borer, E.T.; Seabloom, E.W.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Lind, E.M.; Adler, P.B.; Alberti, J.; Anderson, T.M.; Bakker, J.D.; et al. Herbivores and nutrients control grassland plant diversity via light limitation. Nature 2014, 508, 517–520. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Pierik, R. The shade-avoidance syndrome: Multiple signals and ecological consequences. Plant Cell Environ. 2017, 40, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Hersch, M.; Lorrain, S.; de Wit, M.; Trevisan, M.; Ljung, K.; Bergmann, S.; Fankhauser, C. Light intensity modulates the regulatory network of the shade avoidance response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 6515–6520. [Google Scholar] [CrossRef]
- Ballaré, C.L. Illuminated behaviour: Phytochrome as a key regulator of light foraging and plant anti-herbivore defence. Plant Cell Environ. 2009, 32, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, L. Hormonal regulation in shade avoidance. Front. Plant Sci. 2017, 8, 1527. [Google Scholar] [CrossRef]
- Carriedo, L.G.; Maloof, J.N.; Brady, S.M. Molecular control of crop shade avoidance. Curr. Opin. Plant Biol. 2016, 30, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Jiang, H.; Sun, X.; Li, Y.; Liu, Y.; Sun, M.; Fan, Z.; Cao, Q.; Feng, L.; Shang, J.; et al. Auxin and gibberellins are required for the receptor-like kinase erecta regulated hypocotyl elongation in shade avoidance in arabidopsis. Front. Plant Sci. 2018, 9, 124. [Google Scholar] [CrossRef]
- Xiong, H.; Lu, D.; Li, Z.; Wu, J.; Ning, X.; Lin, W.; Bai, Z.; Zheng, C.; Sun, Y.; Chi, W.; et al. The DELLA-ABI4-HY5 module integrates light and gibberellin signals to regulate hypocotyl elongation. Plant Commun. 2023, 4, 100597. [Google Scholar] [CrossRef] [PubMed]
- Djakovic-Petrovic, T.; Wit, M.D.; Voesenek, L.A.C.J.; Pierik, R. DELLA protein function in growth responses to canopy signals. Plant J. 2007, 51, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Fleet, C.M.; Sun, T. A DELLA cate balance: The role of gibberellin in plant morphogenesis. Curr. Opin. Plant Biol. 2005, 8, 77–85. [Google Scholar] [CrossRef]
- Sarwar, R.; Zhu, K.M.; Jiang, T.; Ding, P.; Gao, Y.; Tan, X.L. DELLAs directed gibberellins responses orchestrate crop development: A brief review. Crop Sci. 2023, 63, 1–28. [Google Scholar] [CrossRef]
- Achard, P.; Liao, L.; Jiang, C.; Desnos, T.; Bartlett, J.; Fu, X.; Harberd, N.P. DELLAs contribute to plant photomorphogenesis. Plant Physiol. 2007, 143, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Martinez, C.; Gusmaroli, G.; Wang, Y.; Zhou, J.; Wang, F.; Chen, L.; Yu, L.; Iglesias-Pedraz, J.M.; Kircher, S.; et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 2008, 451, 475–479. [Google Scholar] [CrossRef]
- Dong, B.C.; Meng, J.; Yu, F.H.; Adams, W.; Adams, W. Effects of parental light environment on growth and morphological responses of clonal offspring. Plant Biol. 2019, 21, 1083–1089. [Google Scholar] [CrossRef]
- Chen, L.; Baoyin, T.; Minggagud, H. Effects of mowing regimes on above- and belowground biota in semi-arid grassland of northern China. J. Environ. Manag. 2021, 277, 111441. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, X.; Bajgain, R.; Starks, P.; Steiner, J.; Doughty, R.B.; Chang, Q. Estimating leaf area index and aboveground biomass of grazing pastures using sentinel-1, sentinel-2 and Landsat images. ISPRS J. Photogramm. Remote Sens. 2019, 154, 189–201. [Google Scholar] [CrossRef]
- Lü, X.; Dijkstra, F.A.; Kong, D.; Wang, Z.; Han, X. Plant nitrogen uptake drives responses of productivity to nitrogen and water addition in a grassland. Sci. Rep. 2014, 4, 4817. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Z.; Liu, Z.; Hou, X.; Badgery, W.; Guo, H.; Zhao, Q.; Hu, N.; Duan, J.; Ren, W. Contrasting effects of long-term grazing and clipping on plant morphological plasticity: Evidence from a rhizomatous grass. PLoS ONE 2015, 10, e141055. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ren, W.; Fry, E.L.; Sun, S.; Han, H.; Guo, F. DNA methylation mediates overgrazing-induced clonal transgenerational plasticity. Sci. Total Environ. 2023, 897, 165338. [Google Scholar] [CrossRef] [PubMed]
- Kvastad, L.; Carlberg, K.; Larsson, L.; Villacampa, E.G.; Stuckey, A.; Stenbeck, L.; Mollbrink, A.; Zamboni, M.; Magnusson, J.P.; Basmaci, E.; et al. The spatial RNA integrity number assay for in situ evaluation of transcriptome quality. Commun. Biol. 2021, 4, 57. [Google Scholar] [CrossRef]
- Pandey, A.; Khan, M.K.; Hamurcu, M.; Brestic, M.; Topal, A.; Gezgin, S. Insight into the root transcriptome of a boron-tolerant triticum zhukovskyi genotype grown under boron toxicity. Agronomy 2022, 12, 2421. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.M.; Ball, C.; Blake, J.; Botstein, D.; Sherlock, G. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25. [Google Scholar] [CrossRef]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2Go: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Götz, S.; Garcia-Gomez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the blast2Go suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Fang, L.; Zheng, H.; Zhang, Y.; Chen, J.; Zhang, Z.; Wang, J.; Li, S.; Li, R.; Bolund, L.; et al. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2006, 34, 293–297. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Sanchez-Gomez, D.; Zavala, M.A. Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]
- Guo, F.; Li, X.; Jimoh, S.O.; Ding, Y.; Zhang, Y.; Shi, S.; Hou, X. Overgrazing-induced legacy effects may permit Leymus chinensis to cope with herbivory. PeerJ 2020, 8, e10116. [Google Scholar] [CrossRef]
- Ren, W.; Hu, N.; Hou, X.; Zhang, J.; Guo, H.; Liu, Z.; Kong, L.; Wu, Z.; Wang, H.; Li, X. Long-term overgrazing-induced memory decreases photosynthesis of clonal offspring in a perennial grassland plant. Front. Plant Sci. 2017, 8, 419. [Google Scholar] [CrossRef]
- Dong, B.; Fu, T.; Luo, F.; Yu, F. Herbivory-induced maternal effects on growth and defense traits in the clonal species alternanthera philoxeroides. Sci. Total Environ. 2017, 605–606, 114–123. [Google Scholar] [CrossRef]
- Champagne, F.A. Epigenetic mechanisms and the transgenerational effects of maternal care. Front. Neuroendocrin. 2008, 29, 386–397. [Google Scholar] [CrossRef]
- Fallet, M.; Blanc, M.; Di Criscio, M.; Antczak, P.; Engwall, M.; Guerrero Bosagna, C.; Rüegg, J.; Keiter, S.H. Present and future challenges for the investigation of transgenerational epigenetic inheritance. Environ. Int. 2023, 172, 107776. [Google Scholar] [CrossRef] [PubMed]
- González, A.P.R.; Chrtek, J.; Dobrev, P.I.; Dumalasova, V.; Fehrer, J.; Mraz, P.; Latzel, V. Stress-induced memory alters growth of clonal offspring of white clover (Trifolium repens). Am. J. Bot. 2016, 103, 1567–1574. [Google Scholar] [CrossRef]
- Herman, J.J.; Sultan, S.E. Adaptive transgenerational plasticity in plants: Case studies, mechanisms, and implications for natural populations. Front. Plant Sci. 2011, 2, 102. [Google Scholar] [CrossRef] [PubMed]
- Baduel, P.; Sasaki, E. The genetic basis of epigenetic variation and its consequences for adaptation. Curr. Opin. Plant Biol. 2023, 75, 102409. [Google Scholar] [CrossRef]
- Herman, J.J.; Sultan, S.E. DNA methylation mediates genetic variation for adaptive transgenerational plasticity. Proc. R. Soc. B. Biol. Sci. 2016, 283, 20160988. [Google Scholar] [CrossRef]
- Liu, K.; Wang, T.; Xiao, D.; Liu, B.; Yang, Y.; Xu, K.; Qi, Z.; Wang, Y.; Li, J.; Xiang, X.; et al. The role of DNA methylation in the maintenance of phenotypic variation induced by grafting chimerism in Brassica. Hortic. Res. 2023, 10, uhad008. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.H.; Berg, L.J.; Sultan, S.E. Context-dependent developmental effects of parental shade versus sun are mediated by DNA methylation. Front. Plant Sci. 2018, 9, 1251. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, H.; Liu, C.; Dong, B.; Yu, F.; Fischer, M. Adaptive plasticity in response to light and nutrient availability in the clonal plant Duchesnea indica. J. Plant Ecol. 2022, 15, 795–807. [Google Scholar] [CrossRef]
- Nicotra, A.; Atkin, O.; Bonser, S.; Davidson, A.; Finnegan, E.; Mathesius, U.; Poot, P.; Purugganan; Richards, C.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Dlugos, D.M.; Collins, H.; Bartelme, E.M.; Drenovsky, R.E. The non-native plantrosa multiflora expresses shade avoidance traits under low light availability. Am. J. Bot. 2015, 102, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Seidlova, L.; Verlinden, M.; Gloser, J.; Milbau, A.; Nijs, I. Which plant traits promote growth in the low-light regimes of vegetation gaps? Plant Ecol. 2009, 200, 303–318. [Google Scholar] [CrossRef]
- Jaillais, Y.; Chory, J. Unraveling the paradoxes of plant hormone signaling integration. Nat. Struct. Mol. Biol. 2010, 17, 642–645. [Google Scholar] [CrossRef] [PubMed]
- González, A.P.R.; Dumalasová, V.; Rosenthal, J.; Skuhrovec, J.; Latzel, V. The role of transgenerational effects in adaptation of clonal offspring of white clover (Trifolium repens) to drought and herbivory. Evol. Ecol. 2017, 31, 345–361. [Google Scholar] [CrossRef]
- Latzel, V.; Klimešová, J. Transgenerational plasticity in clonal plants. Evol. Ecol. 2010, 24, 1537–1543. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, Y.; Xue, W.; Gao, J.; Lei, N.; Chen, J.; Yu, F. Clonal transgenerational effects transmit for multiple generations in a floating plant. Phyton 2023, 92, 1589–1601. [Google Scholar] [CrossRef]
- Baker, B.H.; Sultan, S.E.; Lopez-Ichikawa, M.; Waterman, R. Transgenerational effects of parental light environment on progeny competitive performance and lifetime fitness. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2019, 374, 20180182. [Google Scholar] [CrossRef]
- Heger, T. Light availability experienced in the field affects ability of following generations to respond to shading in an annual grassland plant. J. Ecol. 2016, 104, 1432–1440. [Google Scholar] [CrossRef]
- Pfeiffer, A.; Shi, H.; Tepperman, J.M.; Zhang, Y.; Quail, P.H. Combinatorial complexity in a transcriptionally centered signaling hub in arabidopsis. Mol. Plant 2014, 7, 1598–1618. [Google Scholar] [CrossRef]
- Ponnu, J. What plants do in the shadows: Gene transcription precedes histone methylation during shade responses. Plant Physiol. 2022, 190, 1552–1553. [Google Scholar] [CrossRef]
- Ranade, S.S.; Delhomme, N.; García-Gil, M.R. Transcriptome analysis of shade avoidance and shade tolerance in Conifers. Planta 2019, 250, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.C.; Kathare, P.K.; Paik, I.; Huq, E. Phytochrome signaling networks. Annu. Rev. Plant Biol. 2021, 72, 217–244. [Google Scholar] [CrossRef] [PubMed]
- Lohman, G.J.S.; Zhang, Y.; Zhelkovsky, A.M.; Cantor, E.J.; Evans, T.C. Efficient DNA ligation in DNA–RNA hybrid helices by chlorella virus DNA ligase. Nucleic Acids Res. 2014, 42, 1831–1844. [Google Scholar] [CrossRef] [PubMed]
- Pascal, J.M. DNA and RNA ligases: Structural variations and shared mechanisms. Curr. Opin. Struct. Biol. 2008, 18, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.; Harada, B.T.; Behm, M.; He, C. RNA modifications modulate gene expression during development. Science 2018, 361, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Karijolich, J.; Kantartzis, A.; Yu, Y. RNA Modifications: A Mechanism that Modulates Gene Expression; Humana Press: Totowa, NJ, USA, 2010; pp. 1–19. [Google Scholar]
- Roundtree, I.A.; Evans, M.E.; Pan, T.; He, C. Dynamic RNA modifications in gene expression regulation. Cell 2017, 169, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Licht, K.; Jantsch, M.F. Rapid and dynamic transcriptome regulation by RNA editing and RNA modifications. J. Cell Biol. 2016, 213, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, B.S.; Roundtree, I.A.; Lu, Z.; Han, D.; Ma, H.; Weng, X.; Chen, K.; Shi, H.; He, C. N6-methyladenosine modulates messenger RNA translation efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef]
- Nan, F.; Feng, J.; Lv, J.; Liu, Q.; Xie, S. Transcriptome analysis of the typical freshwater rhodophytes Sheathia arcuata grown under different light intensities. PLoS ONE 2018, 13, e197729. [Google Scholar] [CrossRef]
- Lu, D.; Liu, B.; Ren, M.; Wu, C.; Ma, J.; Shen, Y. Light deficiency inhibits growth by affecting photosynthesis efficiency as well as JA and Ethylene signaling in endangered plant Magnolia sinostellata. Plants 2021, 10, 2261. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, H.; Zeng, R.; Wang, X.; Huang, L.; Wang, L.; Wang, X.; Zhang, L. Shade effects on peanut yield associate with physiological and expressional regulation on photosynthesis and sucrose metabolism. Int. J. Mol. Sci. 2020, 21, 5284. [Google Scholar] [CrossRef] [PubMed]
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I. Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 2013, 35, 62–74. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, W.; Yang, W. Shade inhibits leaf size by controlling cell proliferation and enlargement in soybean. Sci. Rep. 2017, 7, 9259. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, L.; Wang, S.; Yu, N.; Shan, H.; Shi, Z.; Li, F.; Zhong, X. Effect of gibberellic acid on photosynthesis and oxidative stress response in maize under weak light conditions. Front Plant Sci 2023, 14, 1128780. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, Q.; Zhu, G.; Hussien Ibrahim, M.E.; Zhou, G. Gibberellin increased yield of Sesbania pea grown under saline soils by improving antioxidant enzyme activities and photosynthesis. Agronomy 2022, 12, 1855. [Google Scholar] [CrossRef]
- Lorrain, S.; Allen, T.; Duek, P.D.; Whitelam, G.C.; Fankhauser, C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting BHLH transcription factors. Plant J. 2008, 53, 312–323. [Google Scholar] [CrossRef]
- Kim, N.; Jeong, J.; Kim, J.; Oh, J.; Choi, G. Shade represses photosynthetic genes by disrupting the DNA binding of GOLDEN2-LIKE1. Plant Physiol. 2023, 191, 2334–2352. [Google Scholar] [CrossRef] [PubMed]
- Leivar, P.; Quail, P.H. PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 2011, 16, 19–28. [Google Scholar] [CrossRef]
- Hornitschek, P.; Lorrain, S.; Zoete, V.; Michielin, O.; Fankhauser, C. Inhibition of the shade avoidance response by formation of non-DNA binding BHLH heterodimers. Embo J. 2009, 28, 3893–3902. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Ren, W.; Fry, E.L.; Xu, K.; Qu, K.; Gao, K.; Bao, H.; Guo, F. Clonal Transgenerational Effects of Parental Grazing Environment on Offspring Shade Avoidance. Agronomy 2024, 14, 1085. https://doi.org/10.3390/agronomy14051085
Yin J, Ren W, Fry EL, Xu K, Qu K, Gao K, Bao H, Guo F. Clonal Transgenerational Effects of Parental Grazing Environment on Offspring Shade Avoidance. Agronomy. 2024; 14(5):1085. https://doi.org/10.3390/agronomy14051085
Chicago/Turabian StyleYin, Jingjing, Weibo Ren, Ellen L. Fry, Ke Xu, Kairi Qu, Kairu Gao, Hailong Bao, and Fenghui Guo. 2024. "Clonal Transgenerational Effects of Parental Grazing Environment on Offspring Shade Avoidance" Agronomy 14, no. 5: 1085. https://doi.org/10.3390/agronomy14051085
APA StyleYin, J., Ren, W., Fry, E. L., Xu, K., Qu, K., Gao, K., Bao, H., & Guo, F. (2024). Clonal Transgenerational Effects of Parental Grazing Environment on Offspring Shade Avoidance. Agronomy, 14(5), 1085. https://doi.org/10.3390/agronomy14051085





