Copper- or Zinc-Fortified Nutrient Solution in Vertical Farming System Enriches Copper or Zinc and Elevates Phenolic Acid and Flavonoid Contents in Artemisia annua L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Growth Parameters and Yield
2.3. Preparation of Dried Samples and Their Extracts
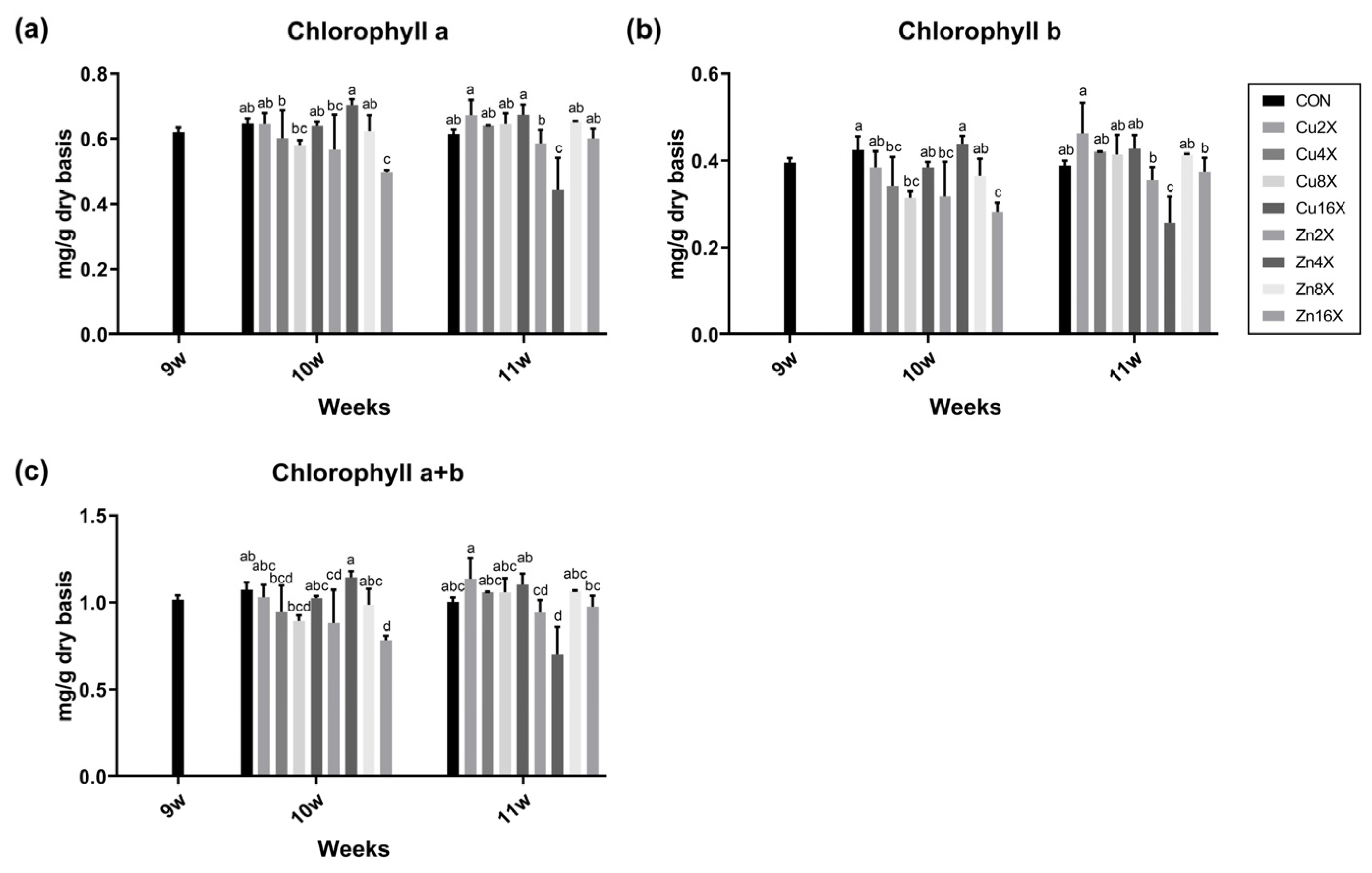
2.4. Chlorophyll Contents of A. annua
2.5. Mineral Analysis
2.6. Determination of Artemisinin Content
2.7. DPPH Radical Scavenging Activity, Total Phenolic Contents, and Total Flavonoid Contents
2.8. Determination of Caffeoylquinic Acid Family Compounds
2.9. Statistical Analysis
3. Results
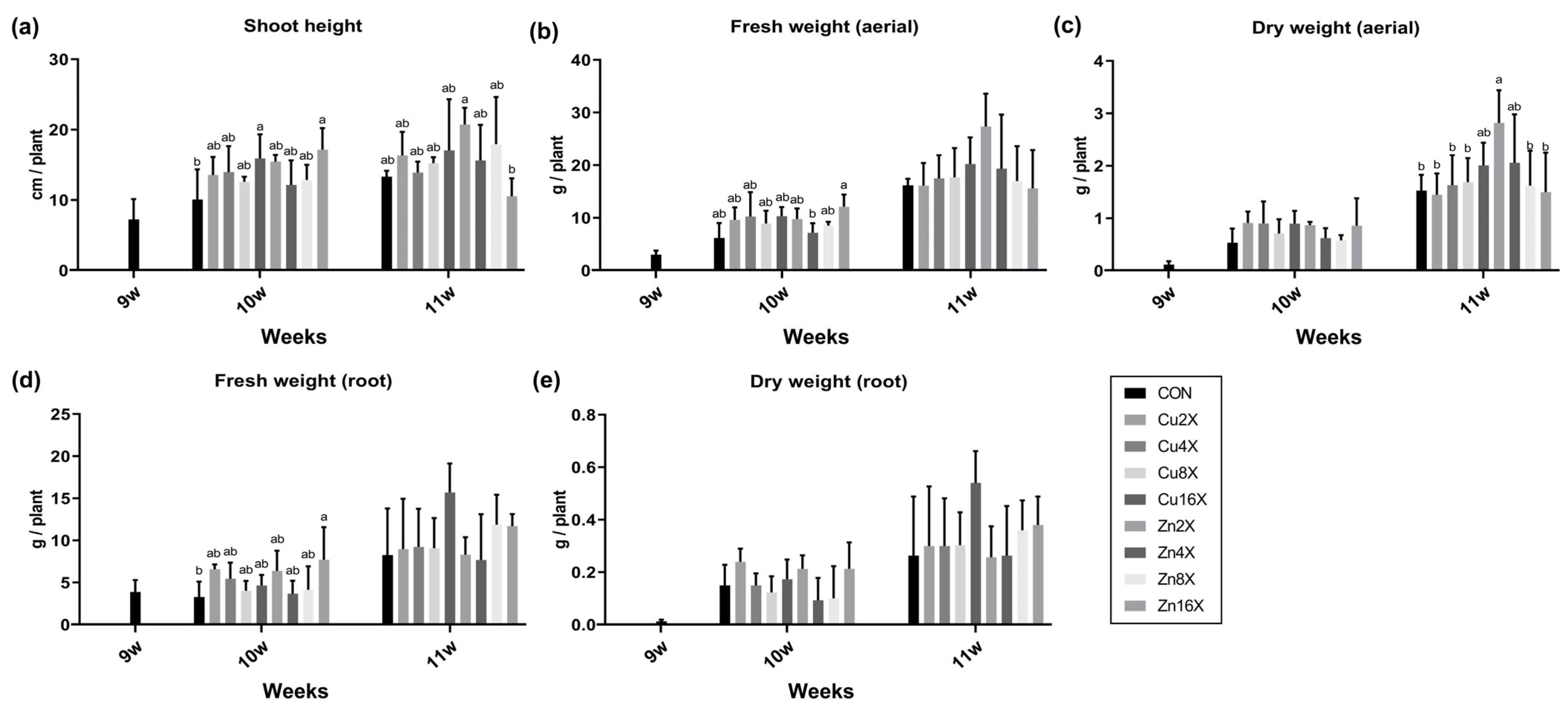
3.1. Effect of EC Condition on Growth Parameters and A. annua Yield
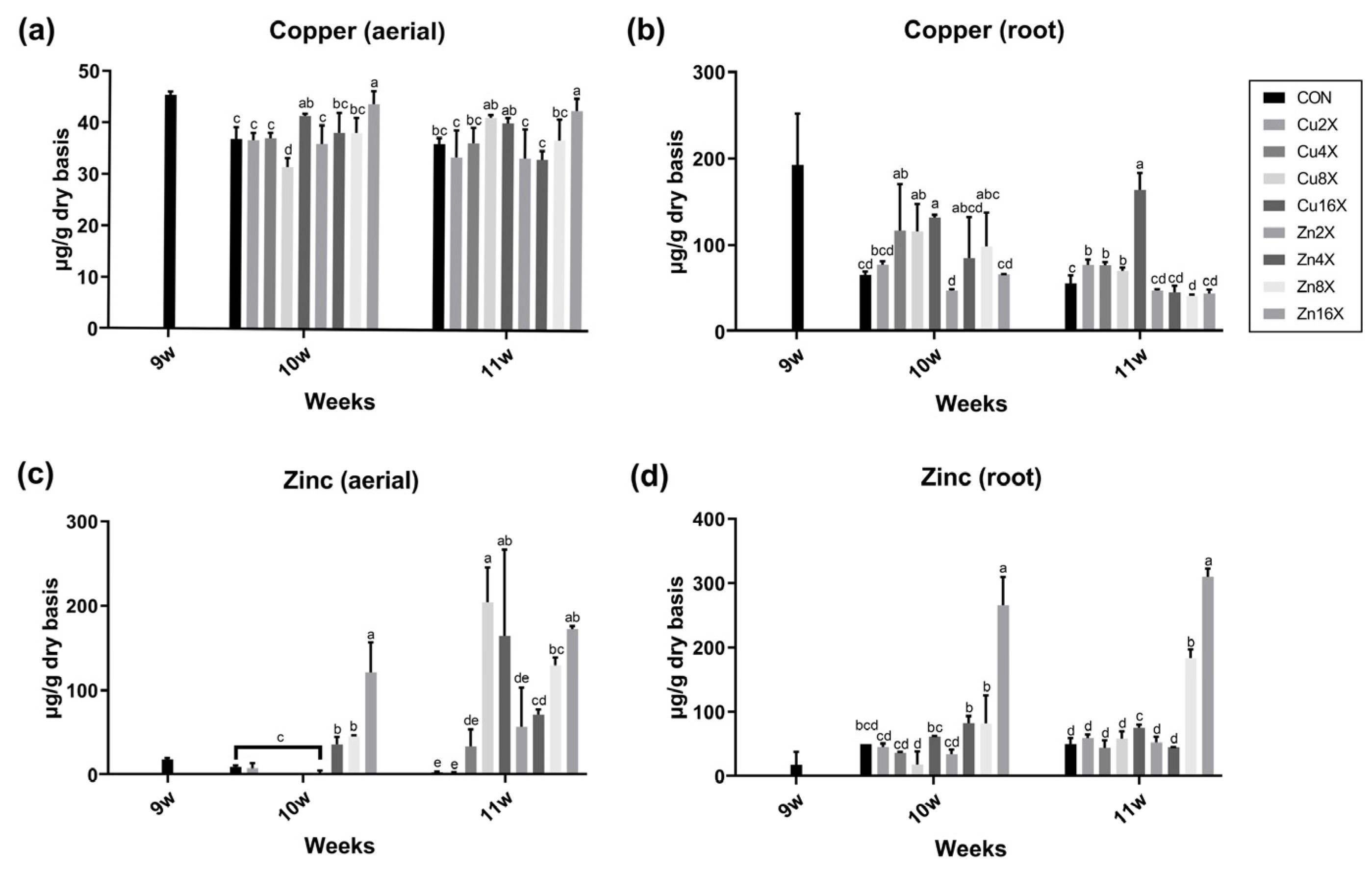
3.2. High Concentration Treatments of Cu or Zn to A. annua Immensely Elevated the Zn Content in Aerial Parts
3.3. Cu or Zn Treatment Decreased the Artemisinin Amount in A. annua
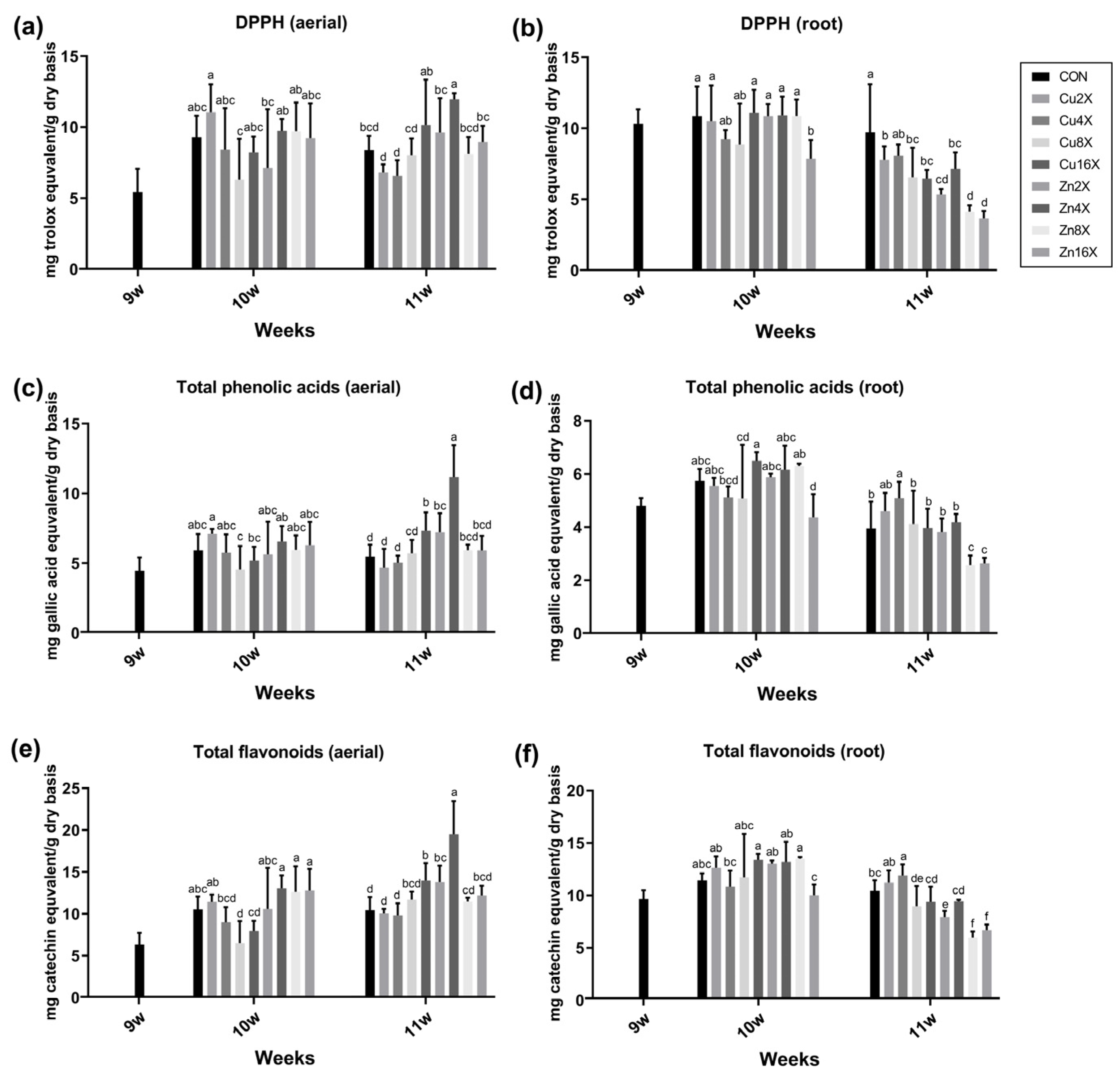
3.4. Cu or Zn Treatments Elevated the Contents of Phenolic Acids and Flavonoids in A. annua
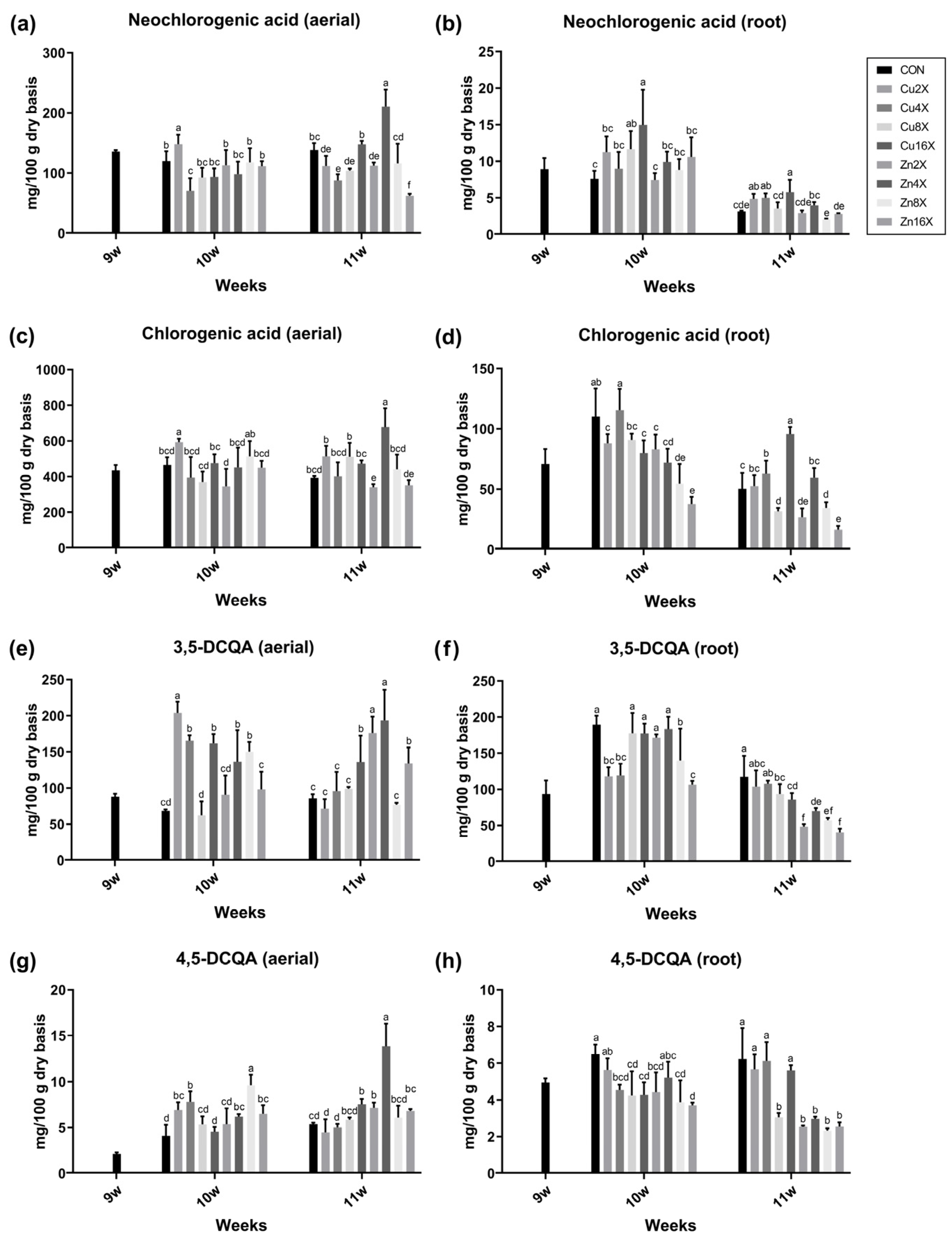
3.5. Contents of Each CQA Compound in A. annua Were Changed by Treatment with Cu or Zn
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feng, X.; Cao, S.; Qiu, F.; Zhang, B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol. Ther. 2020, 216, 107650. [Google Scholar] [CrossRef]
- Sadiq, A.; Hayat, M.Q.; Ashraf, M. Ethnopharmacology of Artemisia annua L.: A review. Artemisia Annu. Pharmacol. Biotechnol. 2013, 9–25. [Google Scholar] [CrossRef]
- Ekiert, H.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Szopa, A. Artemisia annua—Importance in traditional medicine and current state of knowledge on the chemistry, biological activity and possible applications. Planta Med. 2021, 87, 584–599. [Google Scholar] [CrossRef]
- Brisibe, E.A.; Umoren, U.E.; Brisibe, F.; Magalhäes, P.M.; Ferreira, J.F.S.; Luthria, D.; Wu, X.; Prior, R.L. Nutritional characterisation and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 2009, 115, 1240–1246. [Google Scholar] [CrossRef]
- White, N.J. Qinghaosu (artemisinin): The price of success. Science 2008, 320, 330–334. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef]
- Ćavar, S.; Maksimović, M.; Vidic, D.; Parić, A. Chemical composition and antioxidant and antimicrobial activity of essential oil of Artemisia annua L. from Bosnia. Ind. Crops Prod. 2012, 37, 479–485. [Google Scholar] [CrossRef]
- Ding, F.; Ma, T.; Hao, M.; Wang, Q.; Chen, S.; Wang, D.; Huang, L.; Zhang, X.; Jiang, D. Mapping Worldwide Environmental Suitability for Artemisia annua L. Sustainability 2020, 12, 1309. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Guo, L.; Qiu, Z.; Huang, L.; Qu, X. Differences in chemical constituents of Artemisia annua L from different geographical regions in China. PLoS ONE 2017, 12, e0183047. [Google Scholar] [CrossRef]
- Ferreira, J.F.; Laughlin, J.; Delabays, N.; de Magalhães, P.M. Cultivation and genetics of Artemisia annua L. for increased production of the antimalarial artemisinin. Plant Genet. Resour. 2005, 3, 206–229. [Google Scholar] [CrossRef]
- Stern, B.R.; Solioz, M.; Krewski, D.; Aggett, P.; Aw, T.-C.; Baker, S.; Crump, K.; Dourson, M.; Haber, L.; Hertzberg, R.; et al. Copper and Human Health: Biochemistry, Genetics, and Strategies for Modeling Dose-response Relationships. J. Toxicol. Environ. Health Part B 2007, 10, 157–222. [Google Scholar] [CrossRef]
- Morrell, A.; Tallino, S.; Yu, L.; Burkhead, J.L. The role of insufficient copper in lipid synthesis and fatty-liver disease. IUBMB Life 2017, 69, 263–270. [Google Scholar] [CrossRef]
- Lazarchick, J. Update on anemia and neutropenia in copper deficiency. Curr. Opin. Hematol. 2012, 19, 58–60. [Google Scholar] [CrossRef]
- Klevay, L.M. Cardiovascular disease from copper deficiency—A history. J. Nutr. 2000, 130, 489S–492S. [Google Scholar] [CrossRef]
- Brown, K.H.; Wuehler, S.E.; Peerson, J.M. The Importance of Zinc in Human Nutrition and Estimation of the Global Prevalence of Zinc Deficiency. Food Nutr. Bull. 2001, 22, 113–125. [Google Scholar] [CrossRef]
- Gupta, S.; Brazier, A.K.M.; Lowe, N.M. Zinc deficiency in low- and middle-income countries: Prevalence and approaches for mitigation. J. Hum. Nutr. Diet. 2020, 33, 624–643. [Google Scholar] [CrossRef]
- Jothimani, D.; Kailasam, E.; Danielraj, S.; Nallathambi, B.; Ramachandran, H.; Sekar, P.; Manoharan, S.; Ramani, V.; Narasimhan, G.; Kaliamoorthy, I.; et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020, 100, 343–349. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.-F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Bailey, R.L.; Fulgoni, V.L.; Keast, D.R.; Dwyer, J.T. Dietary supplement use is associated with higher intakes of minerals from food sources1234. Am. J. Clin. Nutr. 2011, 94, 1376–1381. [Google Scholar] [CrossRef]
- Di Gioia, F.; Petropoulos, S.A.; Ozores-Hampton, M.; Morgan, K.; Rosskopf, E.N. Zinc and Iron Agronomic Biofortification of Brassicaceae Microgreens. Agronomy 2019, 9, 677. [Google Scholar] [CrossRef]
- Zou, T.; Xu, N.; Hu, G.; Pang, J.; Xu, H. Biofortification of soybean sprouts with zinc and bioaccessibility of zinc in the sprouts. J. Sci. Food Agric. 2014, 94, 3053–3060. [Google Scholar] [CrossRef]
- Mishra, A.K.; Sharma, K.; Misra, R.S. Elicitor recognition, signal transduction and induced resistance in plants. J. Plant Interact. 2012, 7, 95–120. [Google Scholar] [CrossRef]
- Naik, P.M.; Al-Khayri, J.M. Abiotic and biotic elicitors-role in secondary metabolites production through in vitro culture of medicinal plants. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; InTech: Rijeka, Croatia, 2016; p. 247. [Google Scholar]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Ali, B. Salicylic acid: An efficient elicitor of secondary metabolite production in plants. Biocatal. Agric. Biotechnol. 2021, 31, 101884. [Google Scholar] [CrossRef]
- Ramadan, K.M.A.; El-Beltagi, H.S.; Shanab, S.M.M.; El-fayoumy, E.A.; Shalaby, E.A.; Bendary, E.S.A. Potential Antioxidant and Anticancer Activities of Secondary Metabolites of Nostoc linckia Cultivated under Zn and Cu Stress Conditions. Processes 2021, 9, 1972. [Google Scholar] [CrossRef]
- Anjitha, K.S.; Sameena, P.P.; Puthur, J.T. Functional aspects of plant secondary metabolites in metal stress tolerance and their importance in pharmacology. Plant Stress 2021, 2, 100038. [Google Scholar] [CrossRef]
- Saghirzadeh Darki, B.; Shabani, L.; Pourvaez, R.; Ghannadian, M. Effects of CuSO4 and AgNO3 on artemisinin and phenolic compound in shoot cultures of Artemisia annua L. J. Plant Process Funct. Iran. Soc. Plant Physiol. 2019, 8, 1–8. [Google Scholar]
- Golubkina, N.; Logvinenko, L.; Konovalov, D.; Garsiya, E.; Fedotov, M.; Alpatov, A.; Shevchuk, O.; Skrypnik, L.; Sekara, A.; Caruso, G. Foliar application of selenium under nano silicon on Artemisia annua: Effects on yield, antioxidant status, essential oil, artemisinin content and mineral composition. Horticulturae 2022, 8, 597. [Google Scholar] [CrossRef]
- Zarad, M.; Elateeq, A.A.; Toaima, N.; KA Refaey, K.; Atta, R. Copper sulfate and Cobalt chloride effect on total phenolics accumulation and antioxidant activity of Artemisia annua L. callus cultures. Al-Azhar J. Agric. Res. 2021, 46, 26–40. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Son, Y.-J.; Park, J.-E.; Kim, J.; Yoo, G.; Nho, C.W. The changes in growth parameters, qualities, and chemical constituents of lemon balm (Melissa officinalis L.) cultivated in three different hydroponic systems. Ind. Crops Prod. 2021, 163, 113313. [Google Scholar] [CrossRef]
- Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 2008, 69, 1732–1738. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Chen, W.; Eisenberg, R.; Mowrey, W.B.; Wylie-Rosett, J.; Abramowitz, M.K.; Bushinsky, D.A.; Melamed, M.L. Association between dietary zinc intake and abdominal aortic calcification in US adults. Nephrol. Dial. Transplant. 2020, 35, 1171–1178. [Google Scholar] [CrossRef]
- Hussain, A.; Jiang, W.; Wang, X.; Shahid, S.; Saba, N.; Ahmad, M.; Dar, A.; Masood, S.U.; Imran, M.; Mustafa, A. Mechanistic Impact of Zinc Deficiency in Human Development. Front. Nutr. 2022, 9, 717064. [Google Scholar] [CrossRef]
- Li, G.; Lu, A. Study on the accumulation property of Zn in different artemisia plants. Guizhou Agric. Sci. 2012, 7, 242–244. [Google Scholar]
- Landis, H.E.; Getachew, B.; Tizabi, Y. Therapeutic Potential of Flavonoids and Zinc in COVID-19. Medpress Nutr. Food Sci. 2022, 1, 1. [Google Scholar]
- Wessels, I.; Rolles, B.; Rink, L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front. Immunol. 2020, 11, 1712. [Google Scholar] [CrossRef]
- Pal, A.; Squitti, R.; Picozza, M.; Pawar, A.; Rongioletti, M.; Dutta, A.K.; Sahoo, S.; Goswami, K.; Sharma, P.; Prasad, R. Zinc and COVID-19: Basis of Current Clinical Trials. Biol. Trace Elem. Res. 2021, 199, 2882–2892. [Google Scholar] [CrossRef]
- Owusu-Agyei, S.; Newton, S.; Mahama, E.; Febir, L.G.; Ali, M.; Adjei, K.; Tchum, K.; Alhassan, L.; Moleah, T.; Tanumihardjo, S.A. Impact of vitamin A with zinc supplementation on malaria morbidity in Ghana. Nutr. J. 2013, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.A.; Zavaleta, N.; Caulfield, L.E.; Black, R.E.; Witzig, R.S.; Shankar, A.H. Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2006, 75, 126–132. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Piasentin, S.; Furini, A. Nutrient metal elements in plants. Metallomics 2014, 6, 1770–1788. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Galavi, M.; Rezaei, M. Zinc (Zn) importance for crop production—A review. Int. J. Agron. Plant Prod. 2013, 4, 64–68. [Google Scholar]
- Zhao, H.; Wu, L.; Chai, T.; Zhang, Y.; Tan, J.; Ma, S. The effects of copper, manganese and zinc on plant growth and elemental accumulation in the manganese-hyperaccumulator Phytolacca americana. J. Plant Physiol. 2012, 169, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Luis Abreu, J. Artemisia annua+ Zinc for the Treatment of COVID-19: A Potential Successful Combination Therapy with Ivermectin. Rev. Daena (Int. J. Good Conscienc.) 2021, 16, 1–41. [Google Scholar]
- Singh, C.K.; Chhabra, G.; Patel, A.; Chang, H.; Ahmad, N. Dietary Phytochemicals in Zinc Homeostasis: A Strategy for Prostate Cancer Management. Nutrients 2021, 13, 1867. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, K.; Raghu, P.; Nair, K.M. Polyphenol-rich beverages enhance zinc uptake and metallothionein expression in Caco-2 cells. J. Food Sci. 2010, 75, H123–H128. [Google Scholar] [CrossRef]
- Yang, J.-G.; Yu, H.-N.; Sun, S.-L.; Zhang, L.-C.; He, G.-Q.; Das, U.N.; Ruan, H.; Shen, S.-R. Epigallocatechin-3-gallate affects the growth of LNCaP cells via membrane fluidity and distribution of cellular zinc. J. Zhejiang Univ. Sci. B 2009, 10, 411–421. [Google Scholar] [CrossRef]
- Dabbagh-Bazarbachi, H.; Clergeaud, G.; Quesada, I.M.; Ortiz, M.; O’Sullivan, C.K.; Fernández-Larrea, J.B. Zinc Ionophore Activity of Quercetin and Epigallocatechin-gallate: From Hepa 1-6 Cells to a Liposome Model. J. Agric. Food Chem. 2014, 62, 8085–8093. [Google Scholar] [CrossRef]
- Chen, X.; Yu, H.; Shen, S.; Yin, J. Role of Zn2+ in epigallocatechin gallate affecting the growth of PC-3 cells. J. Trace Elem. Med. Biol. 2007, 21, 125–131. [Google Scholar] [CrossRef]
- Yu, H.-N.; Shen, S.-R.; Xiong, Y.-K. Cytotoxicity of epigallocatechin-3-gallate to LNCaP cells in the presence of Cu2+. J. Zhejiang Univ. Sci. B 2005, 6, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ayadi Hassan, S.; Soleimani, T. Improvement of artemisinin production by different biotic elicitors in Artemisia annua by elicitation–infiltration method. Banat. J. Biotechnol. 2016, 82–94. [Google Scholar] [CrossRef]
- Patra, N.; Srivastava, A.; Sharma, S. Study of various factors for enhancement of artemisinin in Artemisia annua hairy roots. Int. J. Chem. Eng. Appl. 2013, 4, 157. [Google Scholar] [CrossRef][Green Version]
- Keng, C.L.; Singaram, N.; Lim, B.P. Production of artemisinin from cell suspension culture of Artemisia annua L. In Proceedings of the Asia Pacific Conference on Plant Tissue and Agribiotechnology (APaCPA), Kuala Lumpur, Malaysia, 17–21 June 2007; p. 21. [Google Scholar]
- Irfan Qureshi, M.; Israr, M.; Abdin, M.Z.; Iqbal, M. Responses of Artemisia annua L. to lead and salt-induced oxidative stress. Environ. Exp. Bot. 2005, 53, 185–193. [Google Scholar] [CrossRef]
- Chukwurah, P.N.; Brisibe, E.A.; Osuagwu, A.N.; Okoko, T. Protective capacity of Artemisia annua as a potent antioxidant remedy against free radical damage. Asian Pac. J. Trop. Biomed. 2014, 4, S92–S98. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.K.; Sangwan, R.S.; Srivastava, A.K.; Sangwan, N.S. Prolonged exposure to salt stress affects specialized metabolites-artemisinin and essential oil accumulation in Artemisia annua L.: Metabolic acclimation in preferential favour of enhanced terpenoid accumulation accompanying vegetative to reproductive phase transition. Protoplasma 2017, 254, 505–522. [Google Scholar] [CrossRef]
- Nasim, S.A.; Dhir, B. Heavy metals alter the potency of medicinal plants. Rev. Environ. Contam. Toxicol. 2010, 203, 139–149. [Google Scholar] [CrossRef]
- Alirzayeva, E.; Neumann, G.; Horst, W.; Allahverdiyeva, Y.; Specht, A.; Alizade, V. Multiple mechanisms of heavy metal tolerance are differentially expressed in ecotypes of Artemisia fragrans. Environ. Pollut. 2017, 220, 1024–1035. [Google Scholar] [CrossRef]
- Zehra, A.; Choudhary, S.; Mukarram, M.; Naeem, M.; Khan, M.M.A.; Aftab, T. Impact of long-term copper exposure on growth, photosynthesis, antioxidant defence system and artemisinin biosynthesis in soil-grown Artemisia annua genotypes. Bull. Environ. Contam. Toxicol. 2020, 104, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ye, M.; Qiao, X.; Xu, M.; Wang, B.-r.; Guo, D.-A. Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2008, 47, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, T.; Pascale, R.; Argentieri, M.P.; Papadia, P.; Fanizzi, F.P.; Villanova, L.; Avato, P. Phytochemical analysis of a herbal tea from Artemisia annua L. J. Pharm. Biomed. Anal. 2012, 62, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, Y.-J.; Park, J.-E.; Lee, N.; Ju, Y.-W.; Pyo, S.-H.; Oh, C.; Yoo, G.; Nho, C.W. Copper- or Zinc-Fortified Nutrient Solution in Vertical Farming System Enriches Copper or Zinc and Elevates Phenolic Acid and Flavonoid Contents in Artemisia annua L. Agronomy 2024, 14, 135. https://doi.org/10.3390/agronomy14010135
Son Y-J, Park J-E, Lee N, Ju Y-W, Pyo S-H, Oh C, Yoo G, Nho CW. Copper- or Zinc-Fortified Nutrient Solution in Vertical Farming System Enriches Copper or Zinc and Elevates Phenolic Acid and Flavonoid Contents in Artemisia annua L. Agronomy. 2024; 14(1):135. https://doi.org/10.3390/agronomy14010135
Chicago/Turabian StyleSon, Yang-Ju, Jai-Eok Park, Nakhyun Lee, Young-Woong Ju, Su-Hyeon Pyo, Changmin Oh, Gyhye Yoo, and Chu Won Nho. 2024. "Copper- or Zinc-Fortified Nutrient Solution in Vertical Farming System Enriches Copper or Zinc and Elevates Phenolic Acid and Flavonoid Contents in Artemisia annua L." Agronomy 14, no. 1: 135. https://doi.org/10.3390/agronomy14010135
APA StyleSon, Y.-J., Park, J.-E., Lee, N., Ju, Y.-W., Pyo, S.-H., Oh, C., Yoo, G., & Nho, C. W. (2024). Copper- or Zinc-Fortified Nutrient Solution in Vertical Farming System Enriches Copper or Zinc and Elevates Phenolic Acid and Flavonoid Contents in Artemisia annua L. Agronomy, 14(1), 135. https://doi.org/10.3390/agronomy14010135





