Mechanisms and Mitigation Strategies for the Occurrence of Continuous Cropping Obstacles of Legumes in China
Abstract
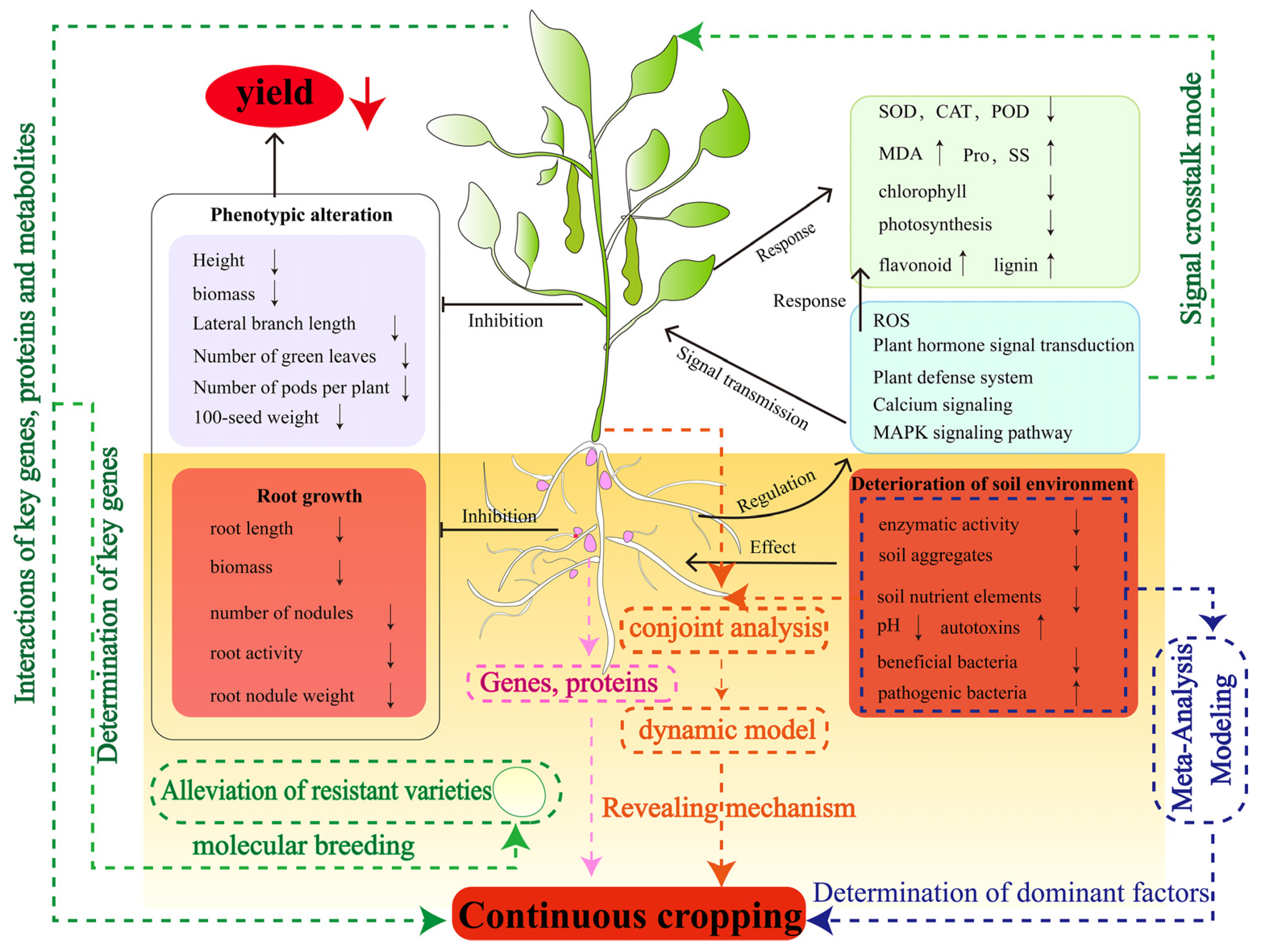
:1. Introduction
2. Effects of Continuous Cropping Obstacles on the Growth of Legume Crops
3. Mechanisms of Legume Crops Responding to Continuous Cropping Obstacles
3.1. Responses of the Physiological and Biochemical Levels of Legume Crops Caused by Continuous Cropping Obstacles
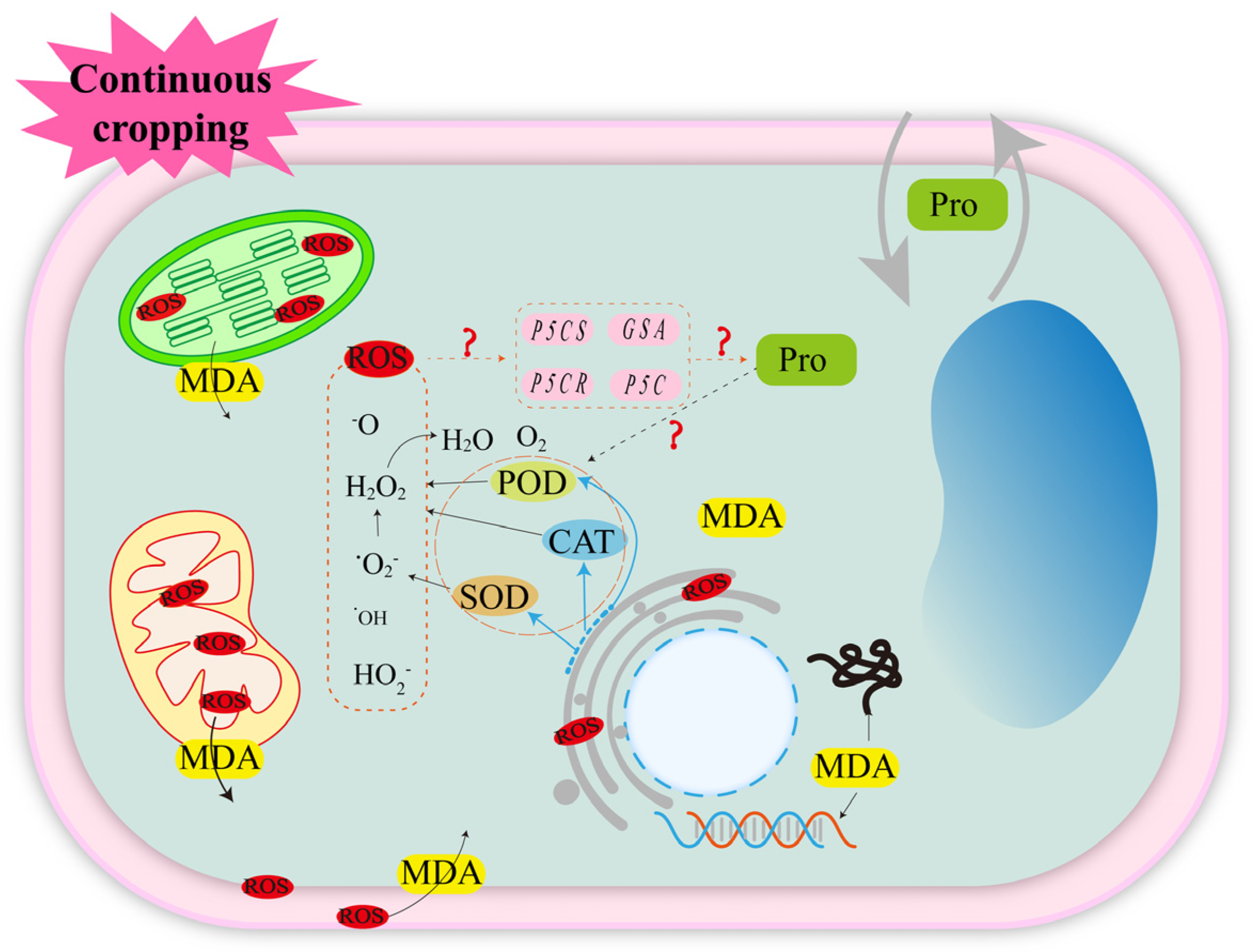
3.1.1. Oxidative Stress Induced by Reactive Oxygen Species (ROS)
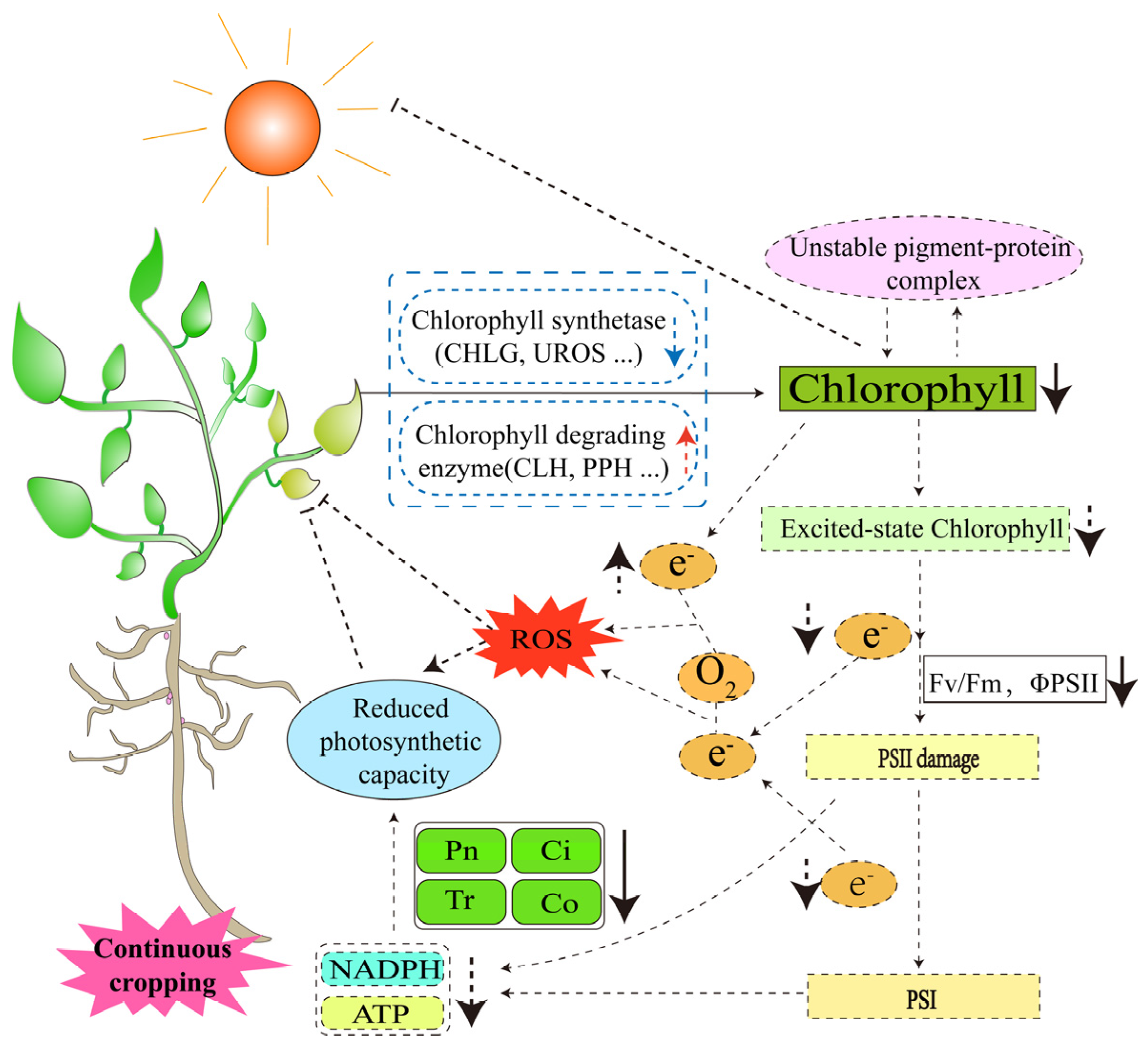
3.1.2. Changes in the Capacity of Photosynthesis
3.2. Molecular Responses of Legume Crops to Continuous Cropping Obstacles
3.2.1. Effects of Continuous Cropping Obstacle on Hormone Signaling Pathways in Legume Crops
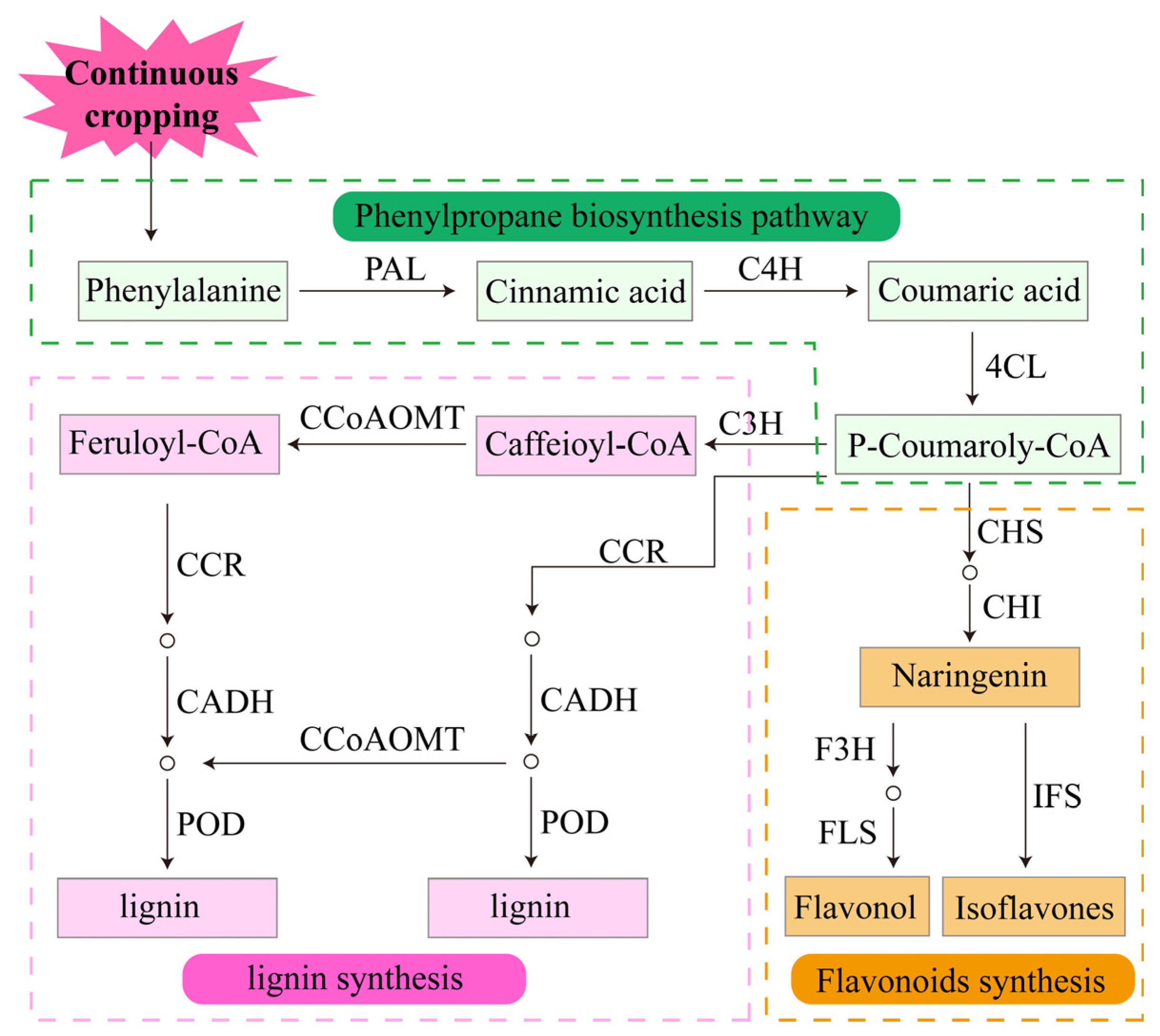
3.2.2. Chemical Defense Response of Legume Crops to Continuous Cropping Obstacles
4. Deterioration of the Soil Environment Leads to the Occurrence of Continuous Cropping Obstacles of Legume Crops
4.1. Response of Soil Physicochemical Properties of Legume Crops to Continuous Cropping
4.2. The Role of Autotoxic Substances in the Initiation of the Continuous Cropping Obstacles of Legume Crops
4.3. The Continuous Cropping System of Legume Crops Causes a Change in Soil Enzyme Activity
4.4. Response of Legume Soil Nematodes to Continuous Cropping
4.5. The Change in Microbial Community Structure in Legume Crop Soil in Continuous Cropping System Is the Key Factor Causing Continuous Cropping Obstacles
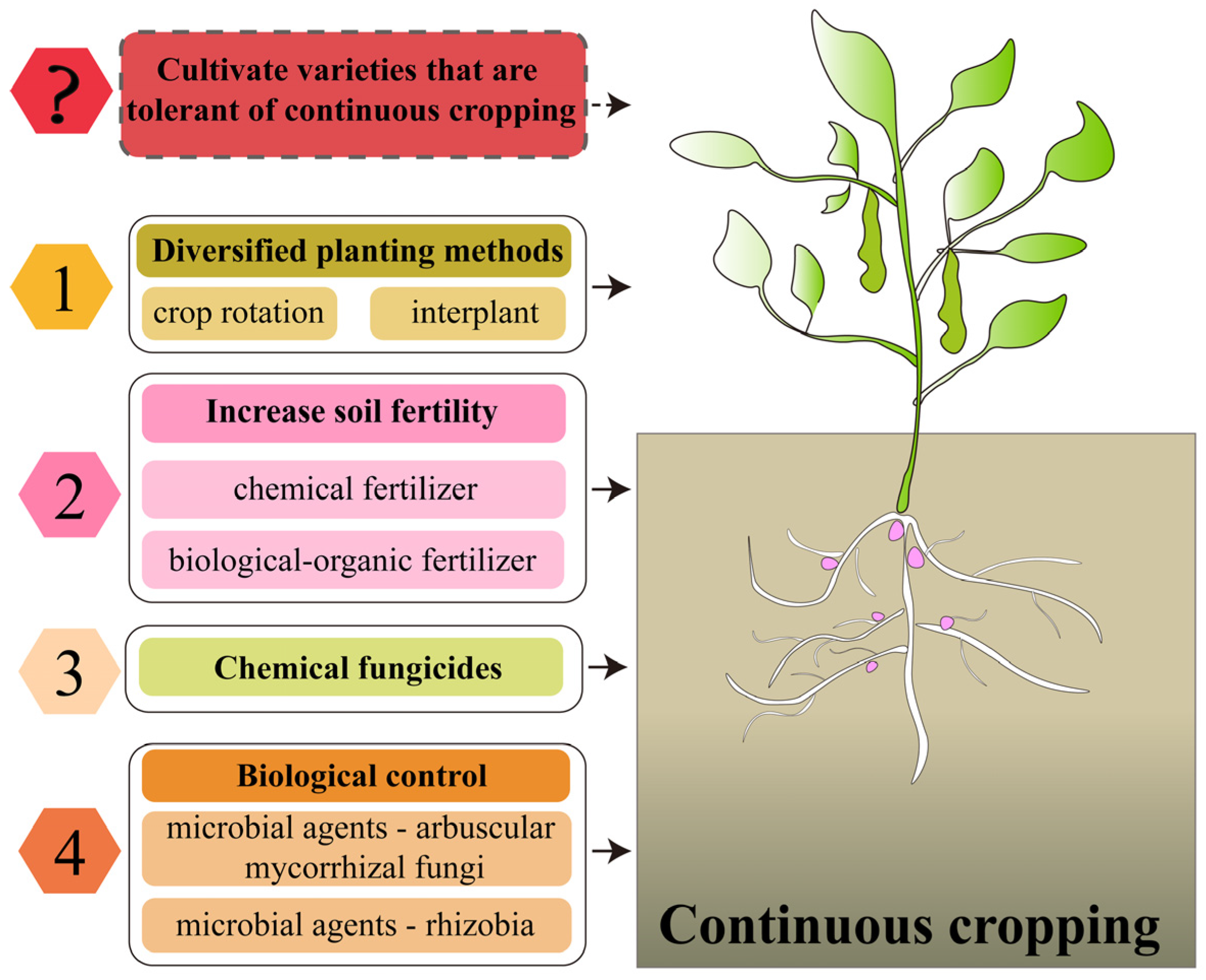
5. The Main Measures to Alleviate the Continuous Cropping Obstacles of Legume Crops and the Change of Ideas
5.1. Diversification of Planting Patterns
5.2. Increase Soil Fertility
5.3. Application of Chemical Fungicides
5.4. Biological Control
6. Future Perspectives and Research to Advance Continuous Cropping of Legumes
- At present, research on the mechanism of continuous cropping obstacles in legume crops mainly focuses on the characteristics of changes in their phenotypes and physiological and biochemical levels, which need to be analyzed in depth from the molecular level by combining with advanced biological techniques. For example, “omics” technology plays an important role in understanding the crop response to continuous cropping and can guide people to find new methods to influence the phenotypic, physiological and biochemical changes. Future studies can use the combination of multi-omics technology and bioinformatics to find key genes, proteins, metabolic pathways and products involved in legumes that significantly respond to continuous cropping obstacles. It will be helpful to further understand the molecular mechanism of legume responses to continuous cropping. Nodulation and nitrogen fixation are the characteristics of legume crops, but how the continuous cropping system affects the genetic pathway of legume crop nodule formation and the mechanism of their symbiotic behavior is still unknown. Clarifying the ecological adaptability and physiological and molecular mechanisms of nodules in continuous cropping is helpful for understanding the relationship between rhizobia and continuous cropping obstacles, and provides a scientific basis for solving the problem of continuous cropping obstacles. In addition, through large-scale genomic data and continuous cropping test data, the mining of candidate genes and functional sites related to continuous cropping obstacles of legume crops is the key task for the cultivation of continuous-cropping-resistant germplasm resources of legume crops in the future.
- Changes in the physical and chemical properties and microbial communities in soil ecosystems trigger legume continuous cropping obstacles. The interactions between these factors determine the structure, function and complexity of soil ecosystems. At the same time, it also increases the difficulty of researching the causes of continuous cropping obstacles. In the future, it is necessary to strengthen the detection of the soil environment in the continuous cropping system of legume crops and further analyze the mechanism of soil environment degradation in the continuous cropping system through a meta-analysis, model building and other methods to clarify the leading factors. In addition, soil microorganisms are currently a hot topic in the research of continuous cropping obstacles, but the research of continuous cropping obstacles in legume crops only revealed a decrease in beneficial bacteria and an increase in pathogenic bacteria in the soil. In the future, it is necessary to explore the beneficial bacteria of rhizosphere resistance to continuous cropping according to the existing research results and design specific compound microorganisms for legume crops to effectively alleviate the continuous cropping obstacles of legume crops.
- After long-term (>5–6 years) continuous cropping of soybean, the degree of growth inhibition was weakened, the soil environment was improved, soil nutrients and enzyme activities were increased, and soil pests and diseases were reduced. However, it is not clear why soybean soil self-repaired after long-term continuous cropping. Therefore, in-depth research on the mechanism of soil environment improvement after the long-term continuous cropping of soybean may be an important breakthrough to alleviate the continuous cropping obstacle of legume crops in the future.
- The research on continuous cropping obstacles of legume crops is more focused on the changes in the soil environment, but the research on crop–soil interaction is scarce. The occurrence of continuous cropping obstacles is closely related to the rhizosphere signal exchange of legume crops. The induction effect of root exudates on microorganisms in the continuous cropping system of legume crops and how roots perceive microbial signals are still uncertain. In the future, a systematic dynamic model should be established by linking the changing characteristics of soil environmental factors with the physiological and biochemical characteristics, as well as genes and metabolites of legume crops, to comprehensively and deeply reveal the mechanism of continuous cropping obstacles of legume crops.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fouad, A.W.; Fathy, E.E.; Helmy, H.R. Challenges and opportunities for the global cultivation and adaption of legumes. Biotechnol. Bloeng. 2021, 8, 160–172. [Google Scholar]
- Nguyen, V.; Riley, S.; Nagel, S.; Fisk, I.; Searle, I.R. Common vetch: A drought tolerant, high protein neglected leguminous crop with potential as a sustainable food source. Front Plant Sci. 2020, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, H.; Khan, N.; Tian, J.; Wang, L.; Wu, J.; Cheng, X.; Chen, X.; Liu, Y.; He, Y.J.A. Economic assessment of food legumes breeding in China: Evidence using a provincial level dataset. Agronomy 2022, 12, 2297. [Google Scholar] [CrossRef]
- Bhat, K.A.; Mahajan, R.; Pakhtoon, M.M.; Urwat, U.; Bashir, Z.; Shah, A.A.; Agrawal, A.; Bhat, B.; Sofi, P.A.; Masi, A.; et al. Low temperature stress tolerance: An insight into the omics approaches for legume crops. Front Plant Sci. 2022, 13, 888710. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, P.; Konkin, D.; Polowick, P.; Hodgins, C.L.; Subedi, M.; Xiang, D.; Yu, B.; Patterson, N.; Rajagopalan, N.; Babic, V. CRISPR/Cas9 gene editing in legume crops: Opportunities and challenges. Legume Sci. 2021, 3, e96. [Google Scholar] [CrossRef]
- Tan, G.; Liu, Y.; Peng, S.; Yin, H.; Meng, D.; Tao, J.; Gu, Y.; Li, J.; Yang, S.; Xiao, N.; et al. Soil potentials to resist continuous cropping obstacle: Three field cases. Environ. Res. 2021, 200, 111319. [Google Scholar] [CrossRef] [PubMed]
- Geng, G.; Yang, R.; Yu, L.; Lv, C.; LI, R.R.; Wang, Y. Crop continuous cropping obstacles: Research progress. Chin. Agric. Sci. Bull. 2019, 35, 36–42. [Google Scholar]
- Li, Y. Characteristics of Soil Nematode Community and Its Influencing Factors in Soybean Continuous Cropping in Black Soil Region; Northeast Agricultural University: Harbin, China, 2021. [Google Scholar]
- Wang, H.W.; Tang, M.J.; Su, C.L.; Zhang, W.; Xu, R.S.; Guan, Y.X.; Dai, C.C. The alleopathic compound luteolin from peanut litter affects peanut nodule formation and the rhizosphere microbial community. Agron. J. 2018, 110, 2587–2595. [Google Scholar] [CrossRef]
- Li, Y.; Fang, F.; Wei, J.L.; Cui, R.Z.; Li, G.S.; Zheng, F.L.; Tan, D.S. Physiological effects of humic acid in peanut growing in continuous cropping soil. Agron. J. 2021, 113, 550–559. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, J.; Li, R.Q.; Tan, S.L.; Wang, S.Q. Effects of continuous cropping obstacle on the characteristics of Ejiangdou No.5 and Ejiangdou No.8. Hubei Agric. Sci. 2019, 58, 72–73,78. [Google Scholar]
- Wang, D.; Yang, X.; Fan, Q.; Liang, H.; Tian, F. Effects of continuous cropping on growth development and quality of protected cowpea. North. Hortic. 2023, 3, 51–55. [Google Scholar]
- Ma, Z.; Guan, Z.; Liu, Q.; Hu, Y.; Liu, L.; Wang, B.; Huang, L.; Li, H.; Yang, Y.; Han, M. Obstacles in continuous cropping: Mechanisms and control measures. Adv. Agron. 2023, 179, 205–256. [Google Scholar]
- Zeeshan Ul Haq, M.; Yu, J.; Yao, G.; Yang, H.; Iqbal, H.A.; Tahir, H.; Cui, H.; Liu, Y.; Wu, Y. A systematic review on the continuous cropping obstacles and control strategies in medicinal plants. Int. J. Mol. Sci. 2023, 24, 12470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Elcin, E.; He, L.; Vithanage, M.; Zhang, X.; Wang, J.; Wang, S.; Deng, Y.; Niazi, N.K.; Shaheen, S.M. Using biochar for the treatment of continuous cropping obstacle of herbal remedies: A review. Appl. Soil Ecol. 2023, 2023, 105127. [Google Scholar] [CrossRef]
- Ku, Y.; Li, W.; Mei, X.; Yang, X.; Cao, C.; Zhang, H.; Cao, L.; Li, M. Biological control of melon continuous cropping obstacles: Weakening the negative effects of the vicious cycle in continuous cropping soil. Microbiol. Spectr. 2022, 10, e01776-22. [Google Scholar] [CrossRef]
- Wang, G.Y.; Guo, W.L.; Chen, B.H.; Fan, F.F.; Huang, X.Q.; Cai, Z.C.; Zhou, J.H.; Gu, G.L. Research progress on mechanism and application of reductive soil disinfection (RSD) in prevention and control of continuous cropping obstacles of facility soil and vegetable. J. Henan Agric. Sci. 2023, 52, 1–10. [Google Scholar]
- Li, Y.H.; Zhang, F.; Yang, X.K.; Yang, C.; Lei, Z.J.; Gao, F.; Wang, Y.Y.; Li, X.D. Effects of continuous cropping on agronomic traits and physiological characteristics of peanut and its regulation under plastic mulching. J. Peanut Sci. 2012, 41, 16–20. [Google Scholar]
- Yan, W.; Wan, T.; Wang, Z. Effects of continuous cropping on seed germination and seedling growth, physiological characters of alfalfa. Legume Res. Int. J. 2022, 45, 1434–1439. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, B.; Wang, C.; Zheng, Y.; Liu, J.; Cheng, D.; Gao, X. Effect of continuous cropping on peanut seedling physiological characteristics and pod yield. J. Peanut Sci. 2006, 35, 29–33. [Google Scholar]
- Su, Y.; Yang, Z.; Cai, J.; Li, G.; Qu, S. The effect of continuous cropping on the germination and seedling formation of alfalfa. Heilongjiang Anim. Sci. Vet. 2018, 21, 167–171. [Google Scholar]
- Ruan, W.B.; Li, X.M.; Wang, Y.F.; Wang, J.G.; Zhang, F.S. Study of soybean (Glycine max L.) root growth in monocultural conditions with root-splitting equipment. Soybean Sci. 2001, 20, 183–186. [Google Scholar]
- Zhao, Q.; Chen, L.; Dong, K.; Dong, Y.; Xiao, J.X. Cinnamic acid inhibited growth of faba bean and promoted the incidence of fusarium wilt. Plants 2018, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.Q.; Wang, S.B.; Jiang, S.Q.; Cheng, B.; Li, H.; Chu, C.J.; Wang, C.B. Dffert of continuous cropping on photosynthesis and accumulation of dry matter in peanut. J. Peanut Sci. 2007, 36, 35–37. [Google Scholar]
- Qiao, Y.F.; Han, X.Z. Effects of different continuous cropping patterns on biological nitrogen fixation of soybean. J. Anhui Agric. Sci. 2008, 36, 13588–13589. [Google Scholar]
- Bekuzarova, S.A.; Kozyrev, A.K.; Shabanova, I.A.; Lushenko, G.V.; Weissfeld, L.I. Enhancing of nitrogen fixation by legumes. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2020; p. 02006. [Google Scholar]
- O’Hara, G. Nutritional constraints on root nodule bacteria affecting symbiotic nitrogen fixation: A review. Aust. J. Exp. Agric. 2001, 41, 417–433. [Google Scholar] [CrossRef]
- Zhang, X.H.; Ma, S.Y.; Ma, L.; Yang, N.; Wang, N.; Zhang, W.X.; Lian, R.F.; Li, S.; Chai, Q. Effects of rhizobium inculation on the growth and photosynthetic characteristics of pea plants. Acta Agrestia Sin. 2021, 29, 1234–1241. [Google Scholar]
- Jani, A.D.; Motis, T.N.; Longfellow, J.M.; Lingbeek, B.J.; Aiuto, C.J. Continuous cropping legumes in semi-arid southern africa: Legume productivity and soil health implications. Exp. Agric. 2022, 58, e15. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Yang, N.; Ma, S.; Ma, L.; Zhang, X.; Wang, N.; Li, S.; Chai, Q. Physiological response of continuously cropped pea seedlings to inoculation with compound rhizobia preparations. Acta Agrestia Sin. 2020, 29, 144–152. [Google Scholar]
- Wang, N.; Ma, S.Y.; Ma, L.; Yang, N.; Zhang, X.H.; Zhang, W.X. Allelopathy effects of cinnamic acid and palmitic acid on seed germination and seedling growth of pea. Plant Physiol. J. 2021, 57, 1657–1667. [Google Scholar]
- Hang, C.M. Effect of vanillic acid on vigna sesquipedatis wight seed germination and anti-oxidant enzyme activity. Seed 2013, 32, 65–66. [Google Scholar]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, C.; Chen, H.Y.H.; Ruan, H. Response of plants to water stress: A Meta-analysis. Front. Plant Sci. 2020, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Minkina, T.; Yaning, C.; Sushkova, S.; Chapligin, V.A.; Mandzhieva, S. A review on salinity adaptation mechanism and characteristics of populus euphratica, a boon for arid ecosystems. Acta Ecologica Sin. 2016, 36, 497–503. [Google Scholar] [CrossRef]
- Herbette, S.; de Labrouhe, D.T.; Drevet, J.R.; Roeckel, P. Transgenic tomatoes showing higher glutathione peroxydase antioxidant activity are more resistant to an abiotic stress but more susceptible to biotic stresses. Plant Sci. 2011, 180, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bloch. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Dumanovic, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2020, 11, 552969. [Google Scholar] [CrossRef]
- Zhou, X.; Xiang, Y.; Li, C.; Yu, G. Modulatory role of reactive oxygen species in root development in model plant of arabidopsis thaliana. Front. Plant Sci. 2020, 11, 485932. [Google Scholar] [CrossRef] [PubMed]
- Somal, M.K.; Sachan, R.S.K.; Bhagat, D.; Khusbhoo; Bala, R.; Kumar, M. An Update on Reactive Oxygen Species Synthesis and Its Potential Application; Springer Nature Singapore: Singapore, 2023; pp. 1–15. [Google Scholar]
- Janku, M.; Luhova, L.; Petrivalsky, M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef]
- Morales, M.; Munne, B.S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savoure, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, L.; Lin, X.; Sun, C. Hydrogen peroxide alleviates salinity-induced damage through enhancing proline accumulation in wheat seedlings. Plant Cell Rep. 2020, 39, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Ben Rejeb, K.; Abdelly, C.; Savoure, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; Villamor, J.G.; Lin, W.; Sharma, S.; Verslues, P.E. Proline Coordination with fatty acid synthesis and redox metabolism of chloroplast and mitochondria. Plant Physiol. 2016, 172, 1074–1088. [Google Scholar] [CrossRef]
- Ozden, M.; Demirel, U.; Kahraman, A. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 2009, 119, 163–168. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hasanuzzaman, M.; Fujita, M. Coordinate induction of antioxidant defense and glyoxalase system by exogenous proline and glycinebetaine is correlated with salt tolerance in mung bean. Front. Agric. China 2011, 5, 1–14. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Y.G. Effect of exogenous proline on SOD and POD activity for soybean callus under salt stress. Acta Agric. Boreali-Sin. 2002, 17, 37–40. [Google Scholar]
- Banu, N.A.; Hoque, A.; Watanabe-Sugimoto, M.; Matsuoka, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol. 2009, 166, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.H.; Ahmad, H.; Li, F.B. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front. Plant Sci. 2020, 11, 615942. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Stewart, J.J.; Baker, C.R.; Adams, W.W. Optimization of photosynthetic productivity in contrasting environments by regulons controlling plant form and function. Int. J. Mol. Sci. 2018, 19, 872. [Google Scholar] [CrossRef] [PubMed]
- Paunov, M.; Koleva, L.; Vassilev, A.; Vangronsveld, J.; Goltsev, V. Effects of different metals on photosynthesis: Cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int. J. Mol. Sci. 2018, 19, 787. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Singh, M.P.; Kaur, S.; Bhardwaj, R.; Zheng, B.; Sharma, A. Modulation of the functional components of growth, photosynthesis, and anti-oxidant stress markers in cadmium exposed Brassica juncea L. Plants 2019, 8, 260. [Google Scholar] [CrossRef]
- Najeeb, U.; Jilani, G.; Ali, S.; Sarwar, M.; Xu, L.; Zhou, W. Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J. Hazard. Mater. 2011, 186, 565–574. [Google Scholar] [CrossRef]
- Gratão, P.L.; Monteiro, C.C.; Rossi, M.L.; Martinelli, A.P.; Peres, L.E.P.; Medici, L.O.; Lea, P.J.; Azevedo, R.A. Differential ultrastructural changes in tomato hormonal mutants exposed to cadmium. Environ. Exp. Bot. 2009, 67, 387–394. [Google Scholar] [CrossRef]
- Patterson, D.T. Effects of allelopathic chemicals on growth and physiological responses of soybean (Glycine max). Weed Sci. 2017, 29, 53–59. [Google Scholar] [CrossRef]
- Zheng, X.X.; Zhu, Y.; Liang, H.B.; Zhuan, J.P.; Shi, J.Q.; Wang, X.F. Cloning and functional analysis of uroporphyrinogen Ⅲ synthase gene bnHemd from brassica napus. Chin. J. Oil Crop Sci. 2020, 42, 380–389. [Google Scholar]
- Rudiger, W.; Benz, J.; Guthoff, C. Detection and partial characterization of activity of chlorophyll synthetase in etioplast membranes. Eur. J. Biochem. 1980, 109, 193–200. [Google Scholar] [CrossRef]
- Schelbert, S.; Aubry, S.; Burla, B.; Agne, B.; Kessler, F.; Krupinska, K.; Hortensteiner, S. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell. 2009, 21, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, S.S.; Azoulay, T.; Arazi, T.; Ben-Yaakov, E.; Mett, A.; Shiboleth, Y.M.; Hortensteiner, S.; Gidoni, D.; Gal-On, A.; Goldschmidt, E.E.; et al. Chlorophyllase is a rate-limiting enzyme in chlorophyll catabolism and is posttranslationally regulated. Plant Cell. 2007, 19, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Grimm, B. Connecting chlorophyll metabolism with accumulation of the photosynthetic apparatus. Trends Plant Sci. 2021, 26, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Figueiredo, P.; Duarte, I.F.; Gil, A.M.; Santos, C. Different responses of young and expanded lettuce leaves to fungicide mancozeb: Chlorophyll fluorescence, lipid peroxidation, pigments and proline content. Photosynthetica 2014, 52, 148–151. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2019, 39, 509–531. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, G.W.; Hu, W.Z.; Xu, S.C.; Gun, Y.M. Effects of cinnamic acid on growth and chlorophyll fluorescence parameters of Pisum sativum L. seedlings. China Veg. 2013, 2013, 44–49. [Google Scholar]
- Du, C.Y.; Li, D.M.; Pang, Q.G. Study on the effect of successive planting soybean to nutrient chlorophyll photosynthetic efficiency and photosynthesis of soybean plants. Soybean Sci. 2003, 22, 146–150. [Google Scholar]
- Li, R.; He, Y.; Chen, J.; Zheng, S.; Zhuang, C. Research progress in improving photosynthetic efficiency. Int. J. Mol. Sci. 2023, 24, 9286. [Google Scholar] [CrossRef]
- Dietz, K.J.; Turkan, I.; Liszkay, A.K. Redox and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Dolferus, R. To grow or not to grow: A stressful decision for plants. Plant Sci. 2014, 229, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Tang, T.; Zhang, D.P.; Cheng, Y.; Lu, B. Recent advances in auxin–cytokinin interactions involved in shaping architecture of rice root system. Plant Physiol. J. 2020, 56, 2495–2509. [Google Scholar]
- Zaynaba, M.; Fatimab, M.; Abbasc, S.; Sharifd, Y.; Umairc, M.; Zafare, M.H.; Bahadarf, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Jogawat, A. Crosstalk among Phytohormone Signaling Pathways during Abiotic Stress; National Institute ofPlant Genome Research: NewDelhi, India, 2019. [Google Scholar]
- Guilfoyle, T.J.; Hagen, G. Auxin response factors. Curr. Opin. Plant Biol. 2007, 10, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Dreher, K.A.; Brown, J.; Saw, R.E.; Callisa, J. The Arabidopsis Aux/IAA protein family has diversified in degradation and auxin responsiveness. Plant Cell. 2006, 18, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Lavy, M.; Estelle, M. Mechanisms of auxin signaling. Development 2016, 143, 3226–3229. [Google Scholar] [CrossRef]
- Li, X.; Jousset, A.; Boer, W.; Carrion, V.J.; Zhang, T.; Wang, X.; Kuramae, E.E. Legacy of land use history determines reprogramming of plant physiology by soil microbiome. ISME J. 2019, 13, 738–751. [Google Scholar] [CrossRef]
- Yang1, W.; Cortijo, S.; Korsbo, N.; Roszak, P.; Schiessl, K.; Gurzadyan, A.; Wightman, R.; Jönsson, H.; Meyerowitz, E. Molecular mechanism of cytokinin-activated cell division in Arabidopsis. Science 2021, 371, 1350–1355. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ohnishi, A.; Sasaki, D.; Fujii, S.; Iwase, A.; Sugimoto, K.; Masuda, T.; Wada, H. Shoot removal induces chloroplast development in roots via cytokinin signaling. Plant Physiol. 2017, 173, 2340–2355. [Google Scholar] [CrossRef]
- Xie, M.; Chen, H.; Huang, L.; O’Neil, R.C.; Shokhirev, M.N.; Ecker, J.R. A B-ARR-mediated cytokinin transcriptional network directs hormone cross-regulation and shoot development. Nat. Commun. 2018, 9, 1604. [Google Scholar] [CrossRef]
- Yan, Z.W.; Liu, X.; Ljung, K.; Li, S.; Zhao, W.; Yang, F.; Wang, M.; Tao, Y. Type B response regulators act as central integrators in transcriptional control of the auxin biosynthesis enzyme TAA1. Plant Physiol. 2017, 175, 1438–1454. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.J.; Cheng, Z.J.; Sang, Y.L.; Zhang, M.M.; Rong, X.F.; Wang, Z.W.; Tang, Y.Y.; Zhang, X.S. Type-b Arabidopsis response regulators specify the shoot stem cell niche by dual regulation of WUSCHEL. Plant Cell. 2017, 29, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.F.; Zhang, S.W.; Cheng, J.J.; Wang, X.C. Functional analysis of the ARR21 gene in Arabidopsis thaliana during growth and development and in vitro regeneration. J. Shanxi Agric. Sci. 2021, 49, 689–693. [Google Scholar]
- Sakai, H.; Honma, T.; Aoyama, T.; Sato, S.; Kato, T.; Tabata, S.; Oka, A. ARR1, a transcription factor for genes immediately responsive to cytokinins. Science 2001, 294, 1519–1521. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.L.; Feng, L.Z.; Dong, Y.B.; Li, Y.L. Progress on ethylene signal transduction pathway and related genes in plants. Curr. Biotechnol. 2019, 9, 449–454. [Google Scholar]
- Ma, L.; Ma, S.; Chen, G.; Lu, X.; Wei, R.; Xu, L.; Feng, X.; Yang, X.; Chai, Q.; Zhang, X.; et al. New insights into the occurrence of continuous cropping obstacles in pea (Pisum sativum L.) from soil bacterial communities, root metabolism and gene transcription. BMC Plant Biol. 2023, 23, 226. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Tian, H.; Wang, S.; Chen, J.G. The small ethylene response factor ERF96 is involved in the regulation of the abscisic acid response in Arabidopsis. Front. Plant Sci. 2015, 6, 1064. [Google Scholar] [CrossRef]
- Ahmad, P.; Rasoo, S.; Gul, A.; Sheikh, S.A.; Akram, N.A.; Ashraf, M.; Kazi, A.M.; Gucel, S. Jasmonates: Multifunctional roles in stress tolerance. Front. Plant Sci. 2016, 7, 813. [Google Scholar] [CrossRef]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Wang, D.; Amornsiripanitch, N.; Dong, X. A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog. 2006, 2, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Scott, A.; BowLing, A.; Gordon, S.; Dong, X.N. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 1994, 6, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Withers, J.; Dong, X.N. Posttranslational modifications of NPR1: A single protein playing multiple roles in plant immunity and physiology. PLoS Pathog. 2016, 12, e1005707. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Withers, J.; Mohan, R.; Marques, J.; Gu, Y.; Yan, S.; Zavaliev, R.; Nomoto, M.; Tada, Y.; Dong, X. Posttranslational modifications of the master transcriptional regulator NPR1 enable dynamic but tight control of plant immune responses. Cell Host Microbe 2015, 18, 169–182. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Fan, W.H.; Kinkema, M.; Li, X.; Dong, X.N. Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene. Proc. Natl. Acad. Sci. USA 1999, 96, 6523–6528. [Google Scholar] [CrossRef]
- Zhou, J.M.; Trifa, Y.; Klessig, D.F.; Silva, H.; Pontier, D.; Lam, E.; Shah, J.; Klessig, D.F. NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 gene required for induction by salicylic acid. Mol. Plant Microbe Interact. 2000, 33, 191–202. [Google Scholar] [CrossRef]
- Kesarwani, M.; Yoo, J.; Dong, X. Genetic interactions of TGA transcription factors in the regulation of pathogenesis-related genes and disease resistance in Arabidopsis. Plant Physiol. 2007, 144, 336–346. [Google Scholar] [CrossRef]
- Yadav, V.; Mallappa, C.; Gangappa, S.N.; Bhatia, S.; Chattopadhyay, S. A basic helix-loop-helix transcription factor in Arabidopsis, MYC2, acts as a repressor of blue light-mediated photomorphogenic growth. Plant Cell 2005, 17, 1953–1966. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Francini, A.; Khan, M.I.R.; Ferrante, A. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Ludwig, A.A.; Saitoh, H.; Felix, G.; Freymark, G.; Miersch, O.; Wasternack, C.; Boller, T.; Jones, J.D.G.; Romeis, T. Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc. Acad. Natl. Sci. USA 2005, 102, 10736–10741. [Google Scholar] [CrossRef] [PubMed]
- Mockaitis, K.; Howell, S.H. Auxin induces mitogenic activated protein kinase (MAPK) activation in roots of Arabidopsis seedlings. Plant J. 2000, 24, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Kroymann, J.; Ballhorn, D.J.; Kautz, S.; Heil, M.; Hegeman, A.D. Cyanogenesis of wild lima bean (Phaseolus lunatus L.) is an efficient direct defence in nature. PLoS ONE 2009, 4, e5450. [Google Scholar]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Hidalgo, S.C.; Bonawitz, N.D.; Franke, R.B.; Chapple, C. Spatio-temporal control of phenylpropanoid biosynthesis by inducible complementation of a cinnamate 4-hydroxylase mutant. J. Exp. Bot. 2021, 72, 3061–3073. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, K.K.; Higashi, Y.; Nakabayashi, R. The qrigin and evolution of plant flavonoid metabolism. Front. Plant Sci. 2019, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.S.; Ng, M.S.; Cheng, S.S.; Lo, A.W.; Xiao, Z.; Shin, T.S.; Chung, G.; Lam, H.M. Understanding the composition, biosynthesis, accumulation and transport of flavonoids in crops for the promotion of crops as healthy sources of flavonoids for human consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef]
- Scherbakov, A.V.; Ivanov, V.B.; Ivanova, A.V.; Usmanov, I.Y. The equifinal achievement of the total antioxidant activity of flavonoids by plants in various habitats. IOP Conf. Ser. Earth Environ. Sci. 2021, 670, 012018. [Google Scholar] [CrossRef]
- Dong, W.; Song, Y. The significance of flavonoids in the process of biological nitrogen fixation. Int. J. Mol. Sci. 2020, 21, 5926. [Google Scholar] [CrossRef]
- Bosse, M.A.; Silva, M.B.D.; Oliveira, N.; Araujo, M.A.; Rodrigues, C.; Azevedo, J.P.; Reis, A.R.D. Physiological impact of flavonoids on nodulation and ureide metabolism in legume plants. Plant Physiol. Biochem. 2021, 166, 512–521. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, D.I.L.; Lima, R.B.; Ferrarese, M.d.L.L.; Bubna, G.A.; Ferrarese-Filho, O. Soybean root growth inhibition and lignification induced by p-coumaric acid. Environ. Exp. Bot. 2009, 66, 25–30. [Google Scholar] [CrossRef]
- Lima, R.; Salvador, V.; Santos, W.; Bubna, G.; Finger-Teixeira, A.; Soares, A.; Marchiosi, R.; Ferrarese, M.L.; Ferrarese, F.O. Enhanced lignin monomer production caused by cinnamic acid and its hydroxylated derivatives inhibits soybean root growth. PLoS ONE 2013, 8, e80542. [Google Scholar] [CrossRef] [PubMed]
- Bubna, G.A.; Lima, R.B.; Zanardo, D.Y.L.; Santos, W.D.; Ferrarese, M.d.L.L.; Ferrarese-Filho, O. Exogenous caffeic acid inhibits the growth and enhances the lignification of the roots of soybean (Glycine max). J. Plant Physiol. 2011, 168, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Santos, W.D.; Ferrarese, M.L.; Finger, A.; Teixeira, A.C.N.; Ferrarese-Filho, O. Lignification and related enzymes in glycine max root growth-inhibition by ferulic acid. J. Chem. Ecol. 2014, 30, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Bohm, P.A.F.; Zanardo, F.M.L.; Ferrarese, M.L.L.; Ferrarese-Filho, O. Peroxidase activity and lignification in soybean root growth-inhibition by juglone. Biol. Plant. 2006, 50, 315–317. [Google Scholar] [CrossRef]
- Finger, T.A.; Ferrarese, M.d.L.L.; Soares, A.R.; Da Silva, D.; Ferrarese-Filho, O. Cadmium-induced lignification restricts soybean root growth. Ecotoxicol. Environ. Saf. 2010, 73, 1959–1964. [Google Scholar] [CrossRef]
- Rui, H.; Chen, C.; Zhang, X.; Shen, Z.; Zhang, F. Cd-induced oxidative stress and lignification in the roots of two Vicia sativa L. varieties with different Cd tolerances. J. Hazard. Mater. 2016, 301, 304–313. [Google Scholar] [CrossRef]
- Huang, L.F.; Song, L.X.; Xia, X.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Plant-soil feedbacks and soil sickness: From mechanisms to application in agriculture. J. Chem. Ecol. 2013, 39, 232–242. [Google Scholar] [CrossRef]
- She, S.; Niu, J.; Zhang, C.; Xiao, Y.; Chen, W.; Dai, L.; Liu, X.; Yin, H. Significant relationship between soil bacterial community structure and incidence of bacterial wilt disease under continuous cropping system. Arch. Microbiol. 2017, 199, 267–275. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, Q.; Zhao, J.; Xun, W.; Li, R.; Zhang, R.; Wu, H.; Shen, Q. Different continuous cropping spans significantly affect microbial community membership and structure in a vanilla-grown soil as revealed by deep pyrosequencing. Microb. Ecol. 2015, 70, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.D.; Du, J.F.; Li, Y.; Tang, H.; Yin, Z.Y.; Yang, L.; Ding, X.H. Evolutions and managements of soil microbial community structure drove by continuous cropping. Front. Microbiol. 2022, 13, 839494. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.D.; Yang, L.; Zheng, Y.L.; Yang, W.W.; Zhang, L.M.; Deng, L.L.; Gao, Q.; Li, J.R.; Mi, Q.; Li, X.M.; et al. Autotoxins in continuous tobacco cropping soils and their management. Front. Plant Sci. 2023, 14, 1106033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Cheng, Y.; Lei, Y.F.; Li, J.S.; Shi, F.; Dou, M.M.; Ma, L.H.; Cheng, X.F. Advances in research on allelopathic autotoxicity effects of medicinal plants. Chin. Tradit. Herb. Drugs 2018, 49, 1946–1956. [Google Scholar]
- Zhang, Z.Z.; Wu, J.H.; Xi, Y.P.; Zhang, L.Z.; Gao, Q.; Wang-Pruski, G. Effects of autotoxicity on seed germination, gas exchange attributes and chlorophyll fluorescence in melon seedlings. J. Plant Growth Regul. 2021, 41, 993–1003. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Liu, T.; Wang, H.; Yang, Y.; Chen, X.; Zhu, S. Analysis of bacterial and fungal communities in continuous-cropping ramie (Boehmeria nivea L. Gaud) fields in different areas in China. Sci. Rep. UK 2020, 10, 3264. [Google Scholar] [CrossRef]
- Yang, J.I.; Ruegger, P.M.; McKenry, M.V.; Becker, J.O.; Borneman, J. Correlations between root-associated microorganisms and peach replant disease symptoms in a California soil. PLoS ONE 2012, 7, e46420. [Google Scholar] [CrossRef]
- Pervaiz, Z.H.; Iqbal, J.; Zhang, Q.; Chen, D.; Wei, H.; Saleem, M. Continuous cropping alters multiple biotic and abiotic indicators of soil health. Soil Syst. 2020, 4, 59. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Li, T.; Zhao, D.; Liao, Y. Tillage practices with different soil disturbance shape the rhizosphere bacterial community throughout crop growth. Soil Tillage Res. 2020, 197, 104501. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.F.; Zhang, Y.F.; Chen, T.Y.; Wu, P.; Yang, L.; Jian, L.Q.; Zhang, J.S.; Guo, W.; Xue, Y.W.; et al. Effects of tillage methods on soil structure and yield of maize in Sanjiang Plain. Soil Fertolizer Sci. China 2021, 6, 95–103. [Google Scholar]
- Almajmaiea, A.; Hardiea, M.; Acunaa, T.; Birchb, C. Evaluation of methods for determining soil aggregate stability. Soil Tillage Res. 2017, 167, 39–45. [Google Scholar] [CrossRef]
- Amézketa, E. Soil aggregate stability: A review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Dong, X.L.; Wang, Y.T.; Tian, L.; Lou, B.Y.; Zhang, X.J.; Liu, T.; Liu, X.J.; Sun, Y.Y. Review of relationships between soil aggregates, microorganisms and soil organic matter in salt-affected soil. Chin. J. Eco-Agric. 2023, 31, 364–372. [Google Scholar]
- Wang, L.; Wu, J.G.; Zhang, Z.Y.; Yang, T.Y. Efferts of soybean continuous cropping on the aggregation and combines forms of humus in black soil. J. Soil Water Conserv. 2014, 28, 238–242. [Google Scholar]
- Karlen, D.L.; Hurley, E.G.; Andrews, S.S.; Cambardella, C.A.; Meek, D.W.; Duffy, M.D.; Mallarino, A.P. Crop rotation effects on soil quality at three northern corn/soybean belt locations. Agron. J. 2006, 98, 484–495. [Google Scholar] [CrossRef]
- Li, C.H.; Wang, Q.; Hao, S.P. Advances of studies on the effect of soil physical properties on soil biological activity and crop growth. J. Henan Agric. Univ. 2002, 36, 33–37. [Google Scholar]
- Yang, J.Y.; Gu, S.Y.; Li, Y.H.; He, W.Y.; Tang, Y.; Zhai, C.; Du, L. Effects of deep ploughing-rotary tillage combined with organic fertilizer on black soil physical properties. Chin. J. Soil Sci. 2021, 52, 1290–1298. [Google Scholar]
- Zhang, X.H.; Ma, S.Y.; Li, S.; Zeng, R.F.; Ma, L.; Yang, N.; Pu, Z.H. Effects of rhizobium inoculation on soil nutrients and enzyme activities of continuous crop pea. Chin. J. Soil Sci. 2022, 53, 1360–1367. [Google Scholar]
- Shi, C.R.; Yu, S.L.; Du, B.H.; Yang, Z.; Chen, N.; Pan, L.J.; Wang, T.; Wang, M.; Chi, X.Y.; Chen, M.N. The characteristics variation of soil physical and chemical properties and its correlation with soil microorganisms under continuous peanut croppiing. J. Peanut Sci. 2018, 4, 1–6. [Google Scholar]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). S. Afr. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- Ma, S.Y.; Chen, G.P.; Wang, N.; Ma, L.; Lian, R.F.; Li, S.; Zhang, X.C. Identification of potential autotoxic substances in pea soil and analysis of their autotoxic effects. Acta Pratacul. Turae Sin. 2023, 32, 134–145. [Google Scholar]
- Fernandez, C.; Monnier, Y.; Ormeño, E.; Baldy, V.; Greff, S.; Pasqualini, V.; Mévy, J.-P.; Bousquet-Mélou, A. Variations in allelochemical composition of leachates of different organs and maturity stages of pinus halepensis. J. Chem. Ecol. 2009, 35, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, S.Y.; Lu, X.; Chai, Q.; Zhang, X.C.; Li, S. Isolation, identification and biological effects of allelochemicals from the root exudates of pea. Plant Physiol. J. 2018, 54, 500–508. [Google Scholar]
- Yu, J.Q.; Yoshihisa, M. Autointoxication of root exudates in Pisum sativus. Acta Hortic. Sin. 1999, 26, 175–179. [Google Scholar]
- Ghimire, B.K.; Ghimire, B.; Yu, C.Y.; Chung, M. Allelopathic and autotoxic effects of medicago sativa-derived allelochemicals. Plants 2019, 8, 233. [Google Scholar] [CrossRef]
- Zhao, R.L.; Cai, L.Q. The determination of root exudates of Medicago sativa and the study on allelopathic effect of typical exudation 3,5-di-tert-butyl-4-hydroxybenzaldehyde. Chin. Agric. Sci. Bull. 2013, 29, 34–41. [Google Scholar]
- Chai, Q.; Huang, G.B.; HUang, P.; Zhang, E.H.; Fen, F.X. Identification of cicer arietinum root exudation and allelopathy of benzaldhyde. Acta Pratacul. Turae Sin. 2005, 14, 106–111. [Google Scholar]
- Ma, F.M.; Wang, A.; Wu, L.; Wang, C.C.; Li, Y.C.; Liu, C.; Ma, G.W.; Shi, X.Y. Identification of soybean root exudates and cloning of the PAL1, PAL2, C4H Genes. Crops 2011, 2, 65–70. [Google Scholar]
- Carlsen, S.C.K.; Kudsk, P.; Laursen, B.; Mathiassen, S.K.; Mortensen, A.G.; Fomsgaarda, I.S. Allelochemicals in rye (Secale cereale L.): Cultivar and tissue differences in the production of benzoxazinoids and phenolic acids. Nat. Prod. Commun. 2009, 4, 199–208. [Google Scholar]
- Gao, L.L.; Guo, P.Y. Research progress on the inhibitory effects of phenolic acid allelochemicals on algae. Technol. Water Treat. 2012, 38, 1–4. [Google Scholar]
- Huang, Y.Q.; Han, X.R.; Yang, J.F.; Liang, C.H.; Zhan, X.M. Autotoxicity of peanut and identification of phytotoxic substances in rhizosphere soil. Allelopath. J. 2013, 31, 297–308. [Google Scholar]
- Huang, X.X.; Bie, Z.L.; Huang, Y. Identification of autotoxins in rhizosphere soils under the continuous cropping of cowpea. Allelopath. J. 2010, 25, 383–392. [Google Scholar]
- Chen, L.; Zhang, M.L.; Xin, M.Y.; Li, J.D. Effects of exogenous phenolic acids on allelopathy of potted soybean seedlings. ACS Agric. Sci. Technol. 2015, 15, 1151–1154. [Google Scholar]
- Li, Q.K.; Liu, P.; Tang, C.H.; Zhao, H.J.; Wang, J.T.; Song, X.Z.; Yang, L.; Wang, S.B. Effects of two phenolic acids on root zone soil nutrients, soil enzyme activities and pod yield of peanut. Chin. J. Appl. Ecol. 2016, 27, 1189–1195. [Google Scholar]
- Cheng, L.; Zhao, Q.; Dong, K.; Zhi, X.Y.; Dong, Y. Physiological mechanism of faba bean Fusarium wilt promoted by benzoic acid and cinnamic acid. J. Plant Prot. 2019, 46, 298–304. [Google Scholar]
- Yuan, T.T.; Dong, K.; Guo, Z.P.; Dong, Y. Allelopathic effects of ferulic acid inducing fusarium wilt occurrence and abnormal root tissue structure of faba bean. J. Plant Nutr. Fertil. 2020, 26, 914–923. [Google Scholar]
- Blum, U. Effects of microbial utilization of phenolic acids and their phenolic acid breakdown products on allelopathic interactions. J. Chem. Ecol. 1998, 24, 685–708. [Google Scholar] [CrossRef]
- Blair, A.C.; Nissen, S.J.; Brunk, G.R.; Hufbauer, R.A. A lack of evidence for an ecological role of the putative allelochemical (+/−)-catechin in spotted knapweed invasion success. J. Chem. Ecol. 2006, 32, 2327–2331. [Google Scholar] [CrossRef]
- Perry, L.G.; Thelen, G.C.; Ridenour, W.M.; Callaway, R.M.; Paschke, M.W.; Vivanco, J.M. Concentrations of the allelochemical (+/−)-catechin in Centaurea maculosa soils. J. Chem. Ecol. 2007, 33, 2337–2344. [Google Scholar] [CrossRef]
- Wan, Z.M.; Song, C.C. Advance on response of soil enzyme activity to ecological environment. Chin. J. Soil Sci. 2009, 40, 951–956. [Google Scholar]
- Liu, G.M.; Zhang, X.C.; Wang, X.P.; Shao, H.B.; Yang, J.S.; Wang, X.P. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar]
- Schloter, M.; Dilly, O.; Munch, J.C. Indicators for evaluating soil quality. Agric. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Z.; Zou, Y.; Wang, S.; Han, L. Effect of soybean continuous cropping on soil enzyme activity. J. Plant Nutr. Fertil. 1996, 2, 4. [Google Scholar]
- Li, Z.; Jiang, L.G.; Tang, R.H.; Guo, W.F. Effects of long-term continuous peanut cropping on dry matter weight of different peanut varieties, soil nutrient contents and enzyme activities. Soils 2018, 50, 491–497. [Google Scholar]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Pulavarty, A.; Horgan, K.; Kakouli-Duarte, T. Effect of an Alltech soil health product on entomopathogenic nematodes, root-knot nematodes and on the growth of tomato plants in the greenhouse. J. Nematol. 2020, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Han, X.; Zou, W. Effect of spring soybean long-term monoculture on soil nematode community structure and food web. Soybean Sci. 2017, 36, 8. [Google Scholar]
- Cheng, H. Microbial Communities in the Rhizoplane and Rhizosphere as Affected by Soybean Cultivar and Cropping System; Agricultural University: Beijing, China, 2005. [Google Scholar]
- Li, X.G.; Ding, C.F.; Liu, J.G.; Zhang, T.L.; Wang, X.X. Evident response of the soil nematode community to consecutive peanut monoculturing. Agron. J. 2015, 107, 195–203. [Google Scholar] [CrossRef]
- Masamune, T.; Anetai, M.; Takasugi, M.; Katsui, N. Isolation of a natural hatching stimulus, glycinoeclepin A, for the soybean cyst nematode. Nature 1982, 297, 495–496. [Google Scholar] [CrossRef]
- Halbrendt, J. Allelopathy in the management of plant-parasitic nematodes. J. Nematol. 1996, 28, 8. [Google Scholar]
- Bulgarelli, D.; Garrido-Oter, R.; Munch, P.C.; Weiman, A.; Droge, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.; Heijden, M.G.; Roussely, V.P.; Walser, J.C.; Schlaeppi, K. Deciphering composition and function of the root microbiome of a legume plant. Microbiome 2017, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pan, F.; Han, X.; Song, F.; Zhang, Z.; Yan, J.; Xu, Y. Response of soil fungal community structure to long-term continuous soybean cropping. Front. Microbiol. 2018, 9, 3316. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, Z.; Jia, H.; Li, L.; Wu, F. Continuously monocropped Jerusalem artichoke changed soil bacterial community composition and ammonia-oxidizing and denitrifying bacteria abundances. Front. Microbiol. 2018, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, N. Research progress on formation mechanism of plant continuous cropping disorder. Sci. J. Technol. 2022, 4, 8–13. [Google Scholar]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fert. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Tang, H.; Xiao, C.; Ma, J.; Yu, M.; Li, Y.; Wang, G.; Zhang, L. Prokaryotic diversity in continuous cropping and rotational cropping soybean soil. FEMS Microbiol. Lett. 2009, 298, 267–273. [Google Scholar] [CrossRef]
- Pan, F.; Han, X.; Zou, W.; Wang, C.; Zhang, Z.; Liu, H.; Xu, Y. Shifts of bacterial community structure and function in long-term soybean monoculture. Arch. Agron. Soil Sci. 2020, 67, 793–808. [Google Scholar] [CrossRef]
- Alves, R.J.E.; Minh, B.Q.; Urich, T.; Haeseler, A.; Schleper, C. Unifying the global phylogeny and environmental distribution of ammonia-oxidising archaea based on amoA genes. Nat. Commun. 2018, 9, 1517. [Google Scholar] [CrossRef]
- Gong, G.L.; Zhang, T.; Shi, Z.H.; Wei, X.M.; Wang, L. To clone the ammonia monooxygenase gene of autotrophic bacteria ammonium oxidation capacity. J. Shanxi Univ. Sci. Technol. 2015, 33, 125–129. [Google Scholar]
- Liu, Z.X.; Liu, J.J.; Yu, Z.H.; Yao, Q.; Li, Y.S.; Liang, A.Z.; Zhang, W.; Mi, G.; Jin, J.; Liu, X.B.; et al. Long-term continuous cropping of soybean is comparable to crop rotation in mediating microbial abundance, diversity and community composition. Soil Tillage Res. 2020, 197, 104503. [Google Scholar] [CrossRef]
- Zhou, Y.J.; JIa, X.; Zhao, Y.H.; Lu, Y.; Tian, G.; Liu, L. A review on soil fungal community and its affecting factors in forest ecosystem. Ecol. Environ. Sci. 2020, 29, 1703–1712. [Google Scholar]
- Liu, J.; Yao, Q.; Li, Y.; Zhang, W.; Mi, G.; Chen, X.; Yu, Z.; Wang, G. Continuous cropping of soybean alters the bulk and rhizospheric soil fungal communities in a mollisol of northeast PR China. Land Degrad. Dev. 2019, 30, 1725–1738. [Google Scholar] [CrossRef]
- Ma, L.J.; van der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Fang, X.L. Study of spatial distribution of alpine meadow in hezuo cit. Chin. J. Grassl. 2021, 43, 106–114. [Google Scholar]
- Chen, M.N.; Zhang, J.C.; Liu, H.; Wang, M.; Pan, L.J.; Chen, N.; Wang, T.; Jing, Y.; Chi, X.; Du, B. Long-term continuously monocropped peanut significantly disturbed the balance of soil fungal communities. J. Microbiol. 2020, 58, 563–573. [Google Scholar] [CrossRef]
- Li, C.G.; Li, X.M.; Kong, W.D.; Wu, Y.; Wang, J.G. Effect of monoculture soybean on soil microbial community in the northeast China. Plant Soil. 2009, 330, 423–433. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, C.H.; Song, X.; Liu, Y.; Gao, Q.X.; Zheng, R.; Li, J.T.; Zhang, P.C.; Liu, X.L. Impacts of continuous and rotational cropping practices on soil chemical properties and microbial communities during peanut cultivation. Sci. Rep. UK 2022, 12, 2758. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J. Effects of continuous soybean monoculture on soil nematode community. J. Plant Nutr. Fertil. 2015, 21, 1022–1031. [Google Scholar]
- Yao, Q.; Xu, Y.; Liu, X.; Liu, J.; Huang, X.; Yang, W.; Yang, Z.; Lan, L.; Zhou, J.; Wang, G. Dynamics of soil properties and fungal community structure in continuous-cropped alfalfa fields in northeast China. PeerJ 2019, 7, e7127. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; Heijden, M.G.A.; et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef] [PubMed]
- Wille, L.; Messmer, M.M.; Studer, B.; Hohmann, P. Insights to plant-microbe interactions provide opportunities to improve resistance breeding against root diseases in grain legumes. Plant Cell Environ. 2019, 42, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Broeckling, C.D.; Broz, A.K.; Bergelson, J.; Manter, D.K.; Vivanco, J.M. Root exudates regulate soil fungal community composition and diversity. Appl. Environ. Microbiol. 2008, 74, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Ding, C.; Hua, K.; Zhang, T.F.; Zhang, Y.N.; Zhao, L.; Yang, Y.R.; Liu, J.G.; Wang, X.X. Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy. Soil Biol. Biochem. 2014, 78, 149–159. [Google Scholar] [CrossRef]
- Zou, X.; Liu, Y.; Huang, M.; Li, F.; Si, T.; Wang, Y.; Yu, X.; Zhang, X.; Wang, H.; Shi, P. Rotational strip intercropping of maize and peanut enhances productivity by improving crop photosynthetic production and optimizing soil nutrients and bacterial communities. Field Crops Res. 2023, 291, 108770. [Google Scholar] [CrossRef]
- Knight, J.D. Frequency of field pea in rotations impacts biological nitrogen fixation. Can. J. Plant Sci. 2012, 92, 1005–1011. [Google Scholar] [CrossRef]
- Han, Y.; Dong, Q.Q.; Zhang, K.Z.; Sha, D.J.; Jiang, C.J.; Yang, X.; Liu, X.B.; Zhang, H.; Wang, X.G.; Guo, F.; et al. Maize-peanut rotational strip intercropping improves peanut growth and soil properties by optimizing microbial community diversity. PeerJ 2022, 10, e13777. [Google Scholar] [CrossRef]
- Moussart, A.; Even, M.N.; Lesné, A.; Tivoli, B. Successive legumes tested in a greenhouse crop rotation experiment modify the inoculum potential of soils naturally infested by Aphanomyces euteiches. Plant Pathol. 2013, 62, 545–551. [Google Scholar] [CrossRef]
- Jie, G.S.; Wei, G.D.; Chao, M.M.; Wei, Z.; Jun, L.; Long, S.D. Effects of fertilization on bacterial community under the condition of continuous soybean monoculture in black soil in northeast China. Sci. Agric. Sin. 2017, 50, 1271–1281. [Google Scholar]
- Xue, Q.X. Effects of crop stubbles and fertilization on the percentage of seeds damaged by pest of continuous cropping soybean. Chin. Agric. Sci. Bull. 2012, 28, 131–137. [Google Scholar]
- Bhatt, M.K.; Labanya, R.; Joshi, H.C. Influence of long-term chemical fertilizers and organic manures on soil fertility-A review. Univers. J. Agric. Res. 2019, 7, 177–188. [Google Scholar]
- Tang, B.; Xu, H.; Song, F.; Ge, H.; Yue, S. Effects of heavy metals on microorganisms and enzymes in soils of lead–zinc tailing ponds. Environ. Res. 2022, 207, 112174. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Ma, Y.; An, X.; Kan, L.; Xie, C.; Mei, X.; Wang, Z.; Xu, Y.; Dong, C. Effects on the root morphology and mircostructure of young pear (Pyrus pyrifolia) tree by split-root supply of bioorganic and chemical fertilizer. Rhizosphere 2022, 22, 100504. [Google Scholar] [CrossRef]
- Liu, S.X.; Li, X.R.; Ding, F.H.; Wu, Q.Q. Effects of organic fertilizerson yield and quality of cowpeas and avilable nutrients in soil under continuous cropping cultication. Fujian J. Agric. Sci. 2016, 31, 728–732. [Google Scholar]
- Liu, J.G.; Li, X.G.; Wang, X.X. Effects of successive application of organic fertilizers on rhizosphere microbial populations and enzyme activities of monoculture peanut. Soils 2018, 50, 305–311. [Google Scholar]
- Wang, D.C.; WU, J.G.; Li, J.M. Effect of organic manure on nematodes in rhizosphere soil of soybean under continuous cropping. Acta Pedol. Sin. 2018, 55, 490–502. [Google Scholar]
- Chen, D.L.; Wang, X.X.; Zhang, Y.; Yang, Z.; Yao, X.D.; Li, X.G. Effect of persistent application of bioorganic fertilizer on peanut yield and rhizosphere bacterial community. Soils 2021, 53, 537–544. [Google Scholar]
- Wang, X.B.; Liu, W.X.; Li, Z.G.; Teng, Y.; Christle, P.; Luo, Y.M. Effects of long-term fertilizer applications on peanut yield and quality and on plant and soil heavy metal accumulation. Pedosphere 2017, 17, 60457. [Google Scholar] [CrossRef]
- Ge, H.B.; Liu, Z.F.; Ma, Z.W.; Xu, B.Q.; Zeng, X.H.; Huang, H.Y.; Xiong, Q.Y.; Zou, X.; Hu, Y.; Hu, J.H. Effects of different fungicides on leaf spot disease control and yield of continuous cropping peanut. J. Peanut Sci. 2014, 43, 52–55. [Google Scholar]
- Chen, Y.; Yang, D.Q.; Tang, C.H.; Zhang, J.L.; Wang, J.G. Effects of Different fungicides and spraying times on leaf spot disease and yield of continuous cropping peanut in dryland. Shandong Agric. Sci. 2021, 53, 94–97. [Google Scholar]
- Liu, X.M.; Cheng, X.K.; Wang, H.Y.; Wang, K.Y.; Qiao, K. Effect of fumigation with 1, 3-dichloropropene on soil bacterial communities. Chemosphere 2015, 139, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Goldman, G.H.; Hayes, C.; Harman, G.E. Molecular and cellular biology of biocontrol trichoderma spp. Trends Biotechnol. 1994, 12, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Asante, M.; Ahiabor, B.D.K.; Atakora, W.K. Growth, nodulation, and yield responses of groundnut (Arachis hypogaea L.) as influenced by combined application of rhizobium inoculant and phosphorus in the guinea savanna zone of ghana. Int. J. Agron. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Jie, W.G.; Yao, Y.X.; Guo, N.; Zhang, Y.Z.; Qiao, W. Effects of Rhizophagus intraradices on plant growth and the composition of microbial communities in the roots of continuous cropping soybean at maturity. Sustainability 2021, 13, 6623. [Google Scholar] [CrossRef]
- Wu, J.R.; Xu, F.J.; Cao, W.; Zhang, W.; Guan, Y.X.; Dai, C.C. Fungal endophyte Phomopsis Liquidambari B3 enriches the diversity of nodular culturable endophytic bacteria associated with continuous cropping of peanut. Arch. Agron. Soil Sci. 2018, 65, 240–252. [Google Scholar] [CrossRef]
- Bai, L.; Sun, H.B.; Liang, R.T.; Cai, B.Y. iTRAQ proteomic analysis of continuously cropped soybean root inoculated with Funneliformis mosseae. Front. Microbiol. 2019, 10, 1–13. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Ma, S.; Chen, G.; Lu, X.; Chai, Q.; Li, S. Mechanisms and Mitigation Strategies for the Occurrence of Continuous Cropping Obstacles of Legumes in China. Agronomy 2024, 14, 104. https://doi.org/10.3390/agronomy14010104
Ma L, Ma S, Chen G, Lu X, Chai Q, Li S. Mechanisms and Mitigation Strategies for the Occurrence of Continuous Cropping Obstacles of Legumes in China. Agronomy. 2024; 14(1):104. https://doi.org/10.3390/agronomy14010104
Chicago/Turabian StyleMa, Lei, Shaoying Ma, Guiping Chen, Xu Lu, Qiang Chai, and Sheng Li. 2024. "Mechanisms and Mitigation Strategies for the Occurrence of Continuous Cropping Obstacles of Legumes in China" Agronomy 14, no. 1: 104. https://doi.org/10.3390/agronomy14010104
APA StyleMa, L., Ma, S., Chen, G., Lu, X., Chai, Q., & Li, S. (2024). Mechanisms and Mitigation Strategies for the Occurrence of Continuous Cropping Obstacles of Legumes in China. Agronomy, 14(1), 104. https://doi.org/10.3390/agronomy14010104





