Abstract
Phenological shifts in peaches have been observed over the last few years due to the fluctuation of the seasonal climate conditions experienced during dormancy, affecting orchard management practices and influencing production and harvest dates. This study aimed to model the vegetative and floral budbreak of selected peach cultivars. Three peach cultivars, including “Rubyprince”, “Harvester”, and “Red Globe”, were considered in this study based on the representation of the early, early-mid, and mid-seasons. The prediction of the budbreak in peaches was assessed using different models that integrate the combination of chill and heat requirements. Models used include the Weinberger model, the modified Weinberger model, Utah, the dynamic model, and the growing degree model. The accumulation of chill varies according to the season evaluated. A model that considers both chill and heat accumulation is presented for each cultivar. Budbreak as an indicator of dormancy completion was established for each cultivar. The outcome of this study is to determine the amount of chilling accumulation and thermal time required to mark the beginning of the budbreak in selected cultivars with a model that predicts the duration of the dormancy. These results are valuable information that can be used for crop management practices and support the mitigation of cold damage during this critical period of crop development.
1. Introduction
Weather and changes in climate influence crop phenology and, consequently, may affect fruit quality and yield. The interrelation of these factors and unanticipated shifts in the climate and weather patterns can influence the phenological process, leading to potential implications in the timing of dormancy release and growth resumption affecting fruit quality [1]. The phenological characterization permits us to relate variations in climate and their impacts on crops [2,3,4]. A series of phenological events that occur during an annual cycle are essential to ensuring appropriate crop management practices [5]. Deciduous fruit tree orchards can experience unpredictable effects by advancing or delaying phenological stages due to warmer winters [6,7,8].
A period of rest known as “dormancy” with low-temperature conditions (chilling) is required for peach trees prior to resuming growth under warm temperatures after winter [2,3,9,10].
The Southeastern peach industry faces multiple challenges every year regarding production and demand; climate variability tops the list of challenges, with increased incidences of warm winters in recent years, and one of the major growers’ concerns during this stage is the completion of the chill hours requirement for each cultivar.
Three peach cultivars considered in this study were selected according to chilling hours (CH) requirements “Rubyprince” (850 CH), “Red Globe” (850 CH), and “Harvester” (750 CH) [11,12]. These cultivars have also been popular during peak growing season in the Southeast US [13], and they have been studied in quality aspects such as variability in sugars, acids, firmness, color, fruit size, and peach skin properties [14,15,16].
Some fruit trees survive low temperatures during the winter with dormancy as a physiological response to those challenging conditions [7,17,18,19]. Temperate tree species use the dormancy process to delay or inhibit floral and vegetative bud growth as a part of their physiological response to low temperatures and short photoperiods [7,20,21]. Chilling refers to the number of low temperatures required by vegetative and floral buds during winter to break dormancy and initiate normal growth and development each growing season. Insufficient chilling symptoms vary with species, and one effect is the delay in anthesis and vegetative budbreak [8,17,18].
The consecutive completion of chilling and heat requirements are decisive in defining the moment of the budbreak in peach [Prunus persica (L.) Batsch] [22]. Before the growing season, cultivars need to complete specific chilling requirements as a condition to obtain the heat for floral development [23]. If the required chill is not satisfied, potential consequences in bloom delay, fruit growth, and asynchronous growth could happen, affecting maturity stages and reducing yield [7,24,25]. Although chill accumulation is still happening, heat accumulation can occur at the same time, especially when the plant is in the dormant stage. In peaches, chilling and heat accumulation interact to control the time of bloom [26,27]. Nevertheless, the completion of chilling and heat requirements can be affected by variations in temperatures year by year due to temperatures not being sufficiently low even between nearby places [24,28,29].
The knowledge of the likelihood of chill accumulation reduction could lead to cultivar selection in perennial crops like peaches and orchard management practices [30].
Knowing the moment when the floral bud fulfills the chilling requirement and subsequently begins to accumulate heat is critical to predicting the floral budbreak. Controlled-condition experiments using empirical and statistical methods are one of the most common approaches to determining chilling and heat requirements [24]. The empirical approach focuses on the forced single method, which involves the sequential evaluation of shoots under controlled conditions using growth chambers during the winter season [6]. The same procedure has been used to evaluate the dormancy release in peaches, grapes, apples, almonds, and cherries [22,31,32].
Statistical methods have been applied in the estimation of chilling requirements for different species and varieties using shoots or young potted trees. Phenological records of forcing chill experiments in several climatic conditions have been used, and differences have been found among the same species and varieties in different climatic areas [10,24,33,34,35,36,37,38,39].
Chilling can be quantified as chill hours [40] and chill units (that allow for partial chill-hour accumulation and chill negation) [41]. Chill hours refer to the number of hours of low temperature within a specific range that regulates growth in processes such as dormancy. The latter model was adapted according to varying climatic conditions for different locations [42,43,44,45].
As an adaptation for cultivars with low chilling requirements, the modified Weinberger model considers temperatures below 11 °C for the accumulation of CH [46,47].
The total accumulation of the difference between the daily mean temperature and the base temperature (Tb) is known as Growing Degree Days (GDD). Tb is defined as the minimum temperature below which significant crop development is not expected. Consecutively, the development of flowering depends on the fulfillment of those thermal requirements as part of plant phenology, which has been defined as a seasonal calendar of biological events [5,48].
Several tools and approaches have been developed to guarantee the feasibility of temperate fruit production. Mathematical models are the most common methods in the quantification of chilling and heat requirements and are broadly applied to numerous species for bloom prediction [6,17,49,50].
The variation in temperature between places and several cultivars makes it difficult to generate a unique model that explains the moment when a floral bud completes the requirements [42]. Peach phenology models used to predict the development stages of typical peach cultivars help growers evaluate the potential response of a peach cultivar in a specific location [2]. This can explain how crops are closely linked to their geographical origin and their adaptability to climatic conditions [3,51].
Numerous models have been developed for the simulation of phenological stages; however, few of them simulate budbreak using an integration of chill and heat requirements. This study aims to model the budbreak in peaches using a basic, simple approach of integrating chill and heat accumulation in the prediction of dormancy release of three commercial peach cultivars in Alabama, USA.
2. Materials and Methods
2.1. Plant Material
Five stem segment samples per cultivar with growing and dormant flower and vegetative buds were collected randomly every week for 23 weeks for two different seasons starting from September 2021 to March 2022 (season 1) and September 2022 to March 2023 (season 2) from the ten-year-old peach orchard of three commercial cultivars, including “Rubyprince”, “Harvester”, and “Red Globe”. Management practices were followed according to commercial recommendations for the area [52]. The orchard was located at the Chilton Regional Research and Extension Center in Clanton, Alabama (32°55′14″ N; 86°40′20″ W).
The shoots selected were positioned from 1.8 to 2.4 m from the ground and oriented at 45° angles vertically from around the canopy [53]. The average length of the shoots ranged between 20 cm and 50 cm. Samples were taken using pruners from either the north or the south-facing side of the tree to minimize the influence of microclimate and sunlight and have a homogeneous sample. Shoots were then wrapped in moistened paper towels, placed into plastic Ziploc bags to avoid desiccation, and transported in a cooler with ice to Auburn, AL. Once in the laboratory, the shoot’s base was cut diagonally and submerged in water to keep it moist.
2.2. Assessment of Dormancy Break and Data Acquisition
The dynamic of floral budbreaks was estimated using a biological cutting test performed on about 1570 buds, including both floral and vegetative. These buds were evaluated through daily observations for both seasons [54,55,56,57]. Three sections—the apex, midsection, and base—were identified according to the number of nodes and the shoot length. Observations for each section were conducted daily for vegetative and floral budbreaks and recorded in an Excel database. The same method was used for both seasons.
2.3. Controlled Conditions
During season 1 (2021–2022), samples remained in laboratory conditions with a constant temperature of 23 °C. For the 2022–2023 years (season 2), the beakers were placed in growth chambers (Arabidopsis units) to obtain control of relative humidity and photoperiod. Two units (Percival and Conviron), each with two shelves and two light bars, were used for the experiment. Stem segments were evaluated under the same temperature condition (23 °C) [58,59,60] with relative humidity at 60% and a 12 h/24 h photoperiod under artificial fluorescent lighting.
2.4. Weather Data
Daily weather records were obtained from the nearest weather stations of the Chilton Regional Research and Extension Center and Clanton 2 NE weather station (https://wx.medius.re). Hourly temperature data were used to calculate heat and chilling requirements for both seasons.
2.5. Heat Requirements
Growing Degree Days were used for heat requirements (HR) calculation in terms of thermal time (TT) and were determined as the accumulation of the difference between the daily average temperature (Ti) above the base temperature (Tb) [41,61] (Equation (1)).
where TT is thermal time, accumulated from the first day (i) of September (2021) until the day when dormancy release occurred (n), Tb of 4.5 °C was used for the calculation of the thermal time [62,63,64].
2.6. Chill Accumulation
Four different chilling models, the Weinberger model, the modified Weinberger model, the Utah model, and the dynamic model, were used to evaluate the dormancy release (budbreak) in all cultivars assessed for both seasons (2021–2022 and 2022–2023).
2.6.1. Weinberger Model
Is one of the most common and used models due to its simplicity. It determines the accumulation of effective chilling hours at temperatures lower than 7.2 °C. Hours below this temperature account for one chilling hour. In this study, the chilling requirement (CR) was estimated by calculating the sum of chill hours (CHs) using Equation (2) [40,49,65].
where T = temperature; CH = chill hour; °C = degree Celsius. This model has been used widely by several authors and applied to different crops [22,63,66].
2.6.2. Modified Weinberger
This model uses the hourly temperature to calculate the chilling hours. It uses a range of temperatures between 0 °C and 7.2 °C. One hour between those ranges will be equivalent to 1.0 chilling hours (Equation (3)) [67,68].
1 h between 0 °C and 7.2 °C = 1.0 chill hours
2.6.3. Utah Model
This model considers different chilling efficiencies based on a weight function where the permanence of buds on a range of temperatures between 2.5 and 12.5 °C for 1 h effectively accumulates chill units (CU). One chill unit is accumulated at 6 °C. Relative chilling and negative chilling accumulation are counted in this model. Null chill occurs at temperatures below 0 °C, while negative chill accumulation appears at temperatures above 16 °C [41,44].
2.6.4. Dynamic Model
Based on some principles of the Utah model, the dynamic model calculates chill portions and defines the maximum effectiveness of chilling hours at 6 °C and the null effect when the temperature is equal to −2 °C and 14 °C. The model postulates the accumulation of winter chill in two steps, combining the effects of temperature. First, the cold temperatures lead to the formation of intermediate products. Finally, once the intermediate has accumulated in a certain quantity, it will transform into a chilling portion by a process involving the interaction of relatively warm temperatures. However, high temperatures can negatively affect the chilling accumulation in peach buds. Diurnal modification of low temperatures with temperatures above a certain threshold negates the chilling effect. Thus, the effect of high temperatures on the chilling accumulation will depend on the level, duration, and cycle length [44,69,70,71,72]. An Excel format [73] was used for the calculation of chill portions in this study.
In addition, we used the Weinberger model to accumulate the chilling hours of 24 seasons in Chilton, AL, from 1998 to 2023, to demonstrate the variability in the number of chilling hours during that period.
2.7. Data Analysis and Model Integration
The GLIMMIX SAS procedure (SAS version 9.4; SAS Institute, Cary, NC, USA) was used to compare the budbreak duration in days of the bud position (Base, Medium (Mid), Appex), type of bud (Vegetative and Floral), cultivars (“Harvester”, “Red Globe”, and “Rubyprince”), and interaction Cultivar Bud type; to compare the factors and interaction, Tukey–Kramer pairwise comparison (Alpha = 0.05) was applied.
To determine the relationship between chilling models and GDD, a Pearson linear correlation was calculated using the CORR SAS procedure. Nonlinear sigmoid models were adjusted to the logistic curve to describe the progress of the number of budbreaks for each cultivar through the NLIN SAS procedure.
To model the budbreak, a multiple linear regression including GDD and Chilling units was estimated (Equation (4)).
where β0 is the intercept, β1 is the regression coefficient of chilling units and β2 is the regression coefficient of the GDD. Evaluation of the adjusted models was performed using the coefficient of determination (R2), the root means square error (RMSE) [74,75,76,77] in Equation (5), and the regression 1:1 of the predicted and the observed values for each cultivar.
where Pi and Oi are the predicted and observed percentages of the budbreak, I goes from 1 to n dates per year that were measured.
Y = β0 + β1Chill+ β2GDD
3. Results
3.1. Assessment of Dormancy Break
The total floral and vegetative budbreak was determined by cultivars as a function of the days to budbreak and shoot position for both seasons (Figure 1). Differences between the days needed for budbreak among the three cultivars were observed for season 1 (2021–2022) (Figure 1A). A total of 268 budbreaks occurred; 95 of the buds recorded were vegetative, and 173 were floral. For season 2 (2022–2023), a total of 878 budbreaks were recorded (580 were floral and 298 were vegetative) (Figure 1B). The total number of buds for both seasons was distributed in all three positions. The highest number of budbreak events were recorded during February and March for all cultivars and seasons 1 (2021–2022) and 2 (2022–2023).
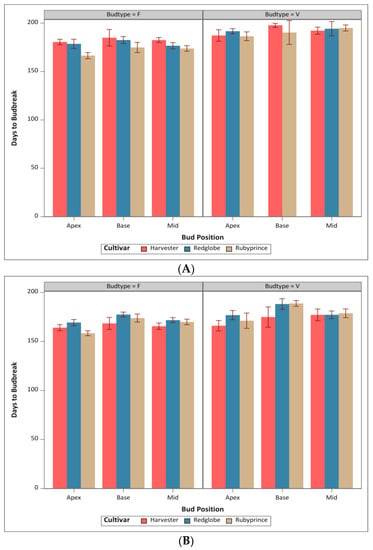
Figure 1.
Number of days to reach budbreak for floral and vegetative buds by position (Apex, Base, and Mid) for “Harvester”, “Red Globe”, and “Rubyprince” cultivars. (A) Season 1 and (B) season 2.
During season 1, significant differences were found for cultivars, bud positions, bud types, and the interaction between cultivar and bud type p-value (<0.0001). For Season 2, there were significant differences among cultivars, bud positions, and bud type p-values (<0.0001), but not for the interaction of cultivar × bud type (Table 1).

Table 1.
Tukey–Kramer Grouping Least Squares Means (Alpha = 0.05). By cultivars, cultivar × bud type, and bud type for both seasons. LS-means (LS-m) with the same letter are not significantly different.
Cultivars “Harvester” and “Red Globe” did not show a significant difference between them regarding the number of days to reach dormancy release for season 1. Both cultivars “Harvester” and “Red Globe” were different compared to “Rubyprince” for the number of days of breaking the floral stage; nevertheless, “Harvester” and “Rubyprince” were different from “Red Globe” for season 2. The interaction of cultivar × by bud type was significant for the season 1 p-value (<0.0001) but not for the season 2 p-value (<0.0846) in the number of days for budbreak. For bud type, both seasons showed that vegetative buds took significantly longer than floral buds to break (Table 1).
Regarding the position of the floral buds in the shoot (apex, mid, and base), there were no significant differences between the apex and mid positions, but they were different from the base for season 1. However, all positions showed significant differences for season 2.
3.2. Onset and Late Release of Dormancy (Budbreak)
The dates and number of days necessary for the earliest and latest floral and vegetative budbreaks for each cultivar were obtained using the chilling hours model as well as the growing degree days model (Table 2). In general, “Rubyprince” was the earliest cultivar to release dormancy for season 1, and “Harvester” was the earliest for season 2. “Rubyprince” was the earliest cultivar in floral budbreak, followed by Red Globe and “Harvester” for season 1, while “Harvester” was the earliest, followed by “Rubyprince” and “Red Globe” for floral budbreak during season 2.

Table 2.
Dates for onset (E. Date) and latest release of dormancy (budbreaks L. Date), a total of chilling hours (chill), and thermal requirements (GDD) accumulated for “Harvester”, “Red Globe”, and “Rubyprince” for both seasons in Chilton, AL, USA.
January, February, and March were the months when all the floral and vegetative buds were released from dormancy during both seasons. In general, cultivars needed between 137 and 207 days to reach budbreaks for season 1 and from 123 to 200 for season 2, with September as an initial sampling date. The range of chilling hours accumulated in the earliest and latest budbreaks was between 200 and 985 for season 1 and 232 and 816 for season 2.
Floral and vegetative buds accumulated, in general, a total of 1785.8 to 2577.2 GDD for season 1 and 1744.4 to 2298.5 GDD for season 2 to complete the dormancy release process. The highest value of chill accumulation (802) for floral early budbreak was reported only for “Harvester” during the first season. This might be because samples collected on 2/4/2022. already had accumulated significant amounts of chilling in the field compared to samples that were collected early and fell into the lab. For “Red Globe” and “Ruby Prince”, less chill accumulation of 200 and 264 were presented, perhaps due to those buds that burst early accumulating less chilling in the field since they were collected early. In this study, additional chill was not provided to the samples after arriving at the laboratory (Table 2).
3.3. Modeling the Progression of the Budbreak
A logistic adjustment was made to fit the sigmoidal trend for the budbreak distribution over time for both floral and vegetative buds. The floral budbreak for season 1 was, in general, delayed compared to season 2. Equations fit statistics, and the curves of the adjustments are presented (Figure 2). Season 1 presented in general less budbreak than Season 2. The environmental factors before the arrival of the samples to the laboratory for both seasons could affect the accumulation of chill in the field since this was a progressive sampling over the season and each year was different. Another possible aspect that could contribute to the lower budbreak was the laboratory conditions. During the first season, mortality of the buds was observed due to the location of the experiment, compared to the second season, where we have more control over the growing chambers.
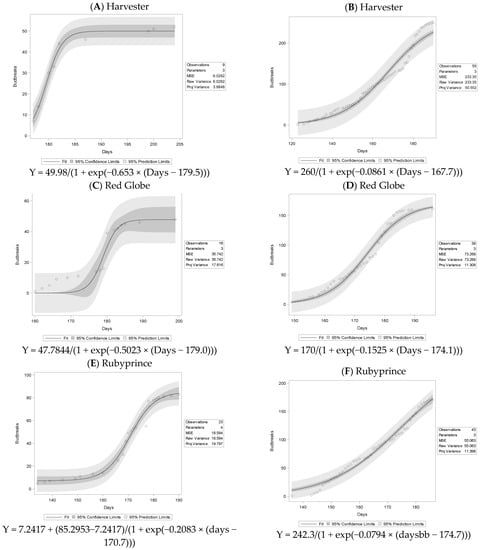
Figure 2.
Logistic adjustment for floral budbreaks for all cultivars and seasons evaluated. Season 1 left panel Season 2 right panel.
3.4. Heat and Chilling Requirements
A significant high correlation among all the models for chill accumulation was found for both seasons; GDD was negatively correlated with each of the chill accumulation models in season 1 (Table 3). The high correlation among the chill accumulation models suggests that any of the models can be used for chill accumulation combined with GDD. Similar results for season 2 were obtained (Table 3).

Table 3.
Pearson correlation coefficients among chilling and GDD models for seasons 1 and 2.
Chilling accumulation for 24 seasons was quantified starting from September to April from 1998 to 2023 to indicate the effect of climate variability using the chill requirements referenced [12] ranging from 750 to 850 (horizontal lines) for the cultivars evaluated (Figure 3). We observed that every year the chill requirements were fulfilled, even though there were some early (January) or late (February) completions. In the same way, the GDD accumulation was conducted for 24 seasons from 1998 to 2023, starting from September to April (Figure 4), to display the effect of warm temperatures accumulated during the same period.
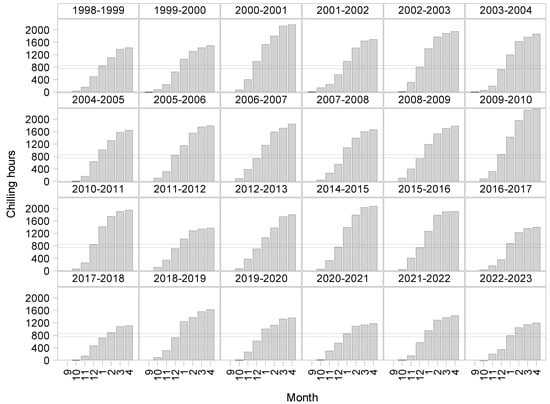
Figure 3.
Chill accumulation using the Weinberger model for 24 seasons starting in 1998–2023. The line indicates the range of chill hours accumulated from 750 to 850.
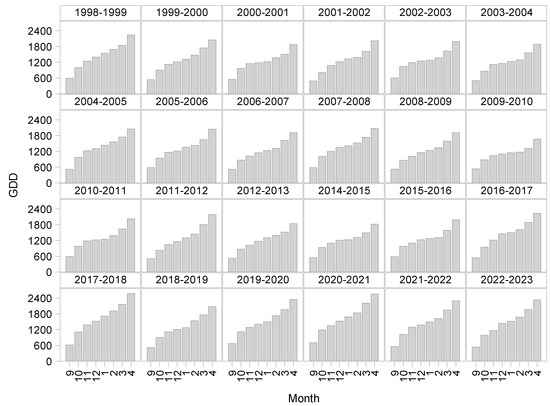
Figure 4.
GDD accumulation for 24 seasons starting in 1998–2023.
3.5. Model Integration for Chill and Heat Requirements
The integration of chill and heat requirements and the distribution of budbreaks were obtained for both vegetative and floral buds. All models for the accumulation of chill requirements (chilling hours, M45, Utah, and dynamic model) were used individually in combination with the GDD using 3D graphs to analyze the distribution of chilling hours (x-axis), the GDD (y-axis), and the percentage of the budbreaks (z-axis). An example of the Weinberger model application in a 3D graph displays the relationship between chill (x-axes), heat requirements (y-axes), and the percentage of budbreak (z-axes), indicating that as chill requirements are fulfilled, the GDD requirements would be less. The 3D graphs support the negative correlation between chilling and heat models described before in the correlation matrix. High values of budbreak percentage were obtained with high chill accumulation and low heat accumulation. This tendency was observed for both seasons and bud types (Figure 5).
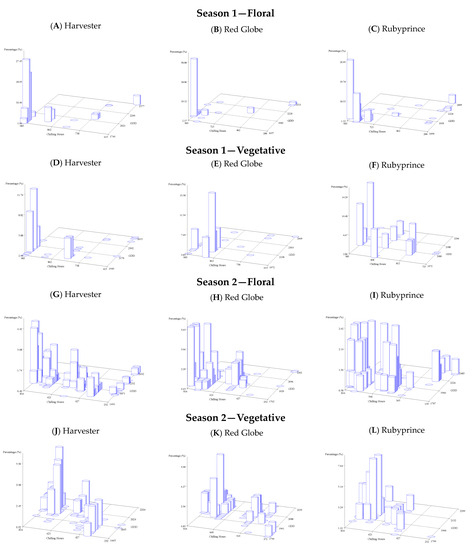
Figure 5.
Floral and vegetative budbreak 3D graphs for seasons 1 and 2 using the Weinberger model and its integration with the GDD model for the percentage of budbreak.
3.6. Prediction Model
The adjusted models were evaluated by the coefficient of determination (R2), the root mean square error (RSME), and the line 1:1 for observed vs. predicted values for each cultivar and both seasons for floral and vegetative budbreak. As an example, the Weinberger model adequately fitted the prediction; R-square was above 98% for all the seasons, types of budbreak, and cultivars evaluated. The root means square error (RMSE) varied between 0.34 and 1.02 days for floral budbreak for season 1 and 0.91- and 1.51 days for season 2. For vegetative, the RMSR varied from 0.25 to 0.49 days for season 1 and 0.76 to 1.01 days for season 2.
The statistics applied Indicated a good fit between the models obtained and confirmed that the simulated values are within an acceptable range of the observed data (Figure 6). These results were similar for all the chilling accumulation models in combination with the GDD model for all seasons, cultivars, and types of buds because of the high correlation among chilling models.
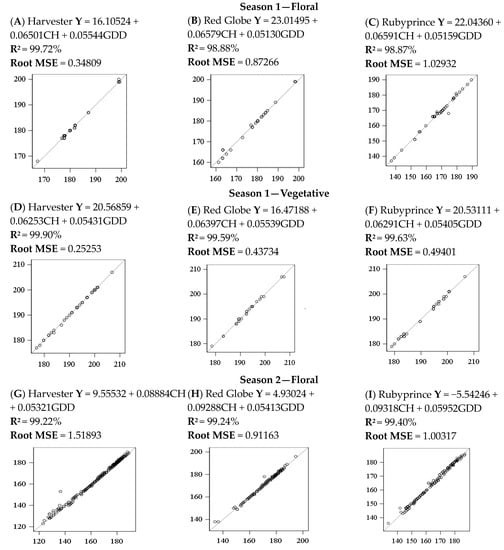
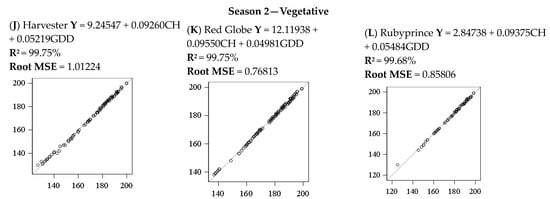
Figure 6.
Regression one-to-one for the predicted days for floral and vegetative budbreaks in peach cultivars for both seasons using the Weinberger model.
4. Discussion
Studies have indicated that extreme weather events around the world are happening more often. Variations in temperature from extreme low to extreme high are occurring, affecting the development of any crop. Integration of many aspects, such as proper fertilization with mineral nutrients like iron, nitrogen, potassium, magnesium, and phosphorus, has a relevant impact on fruit load and quality [25,78], soil quality, water availability, pest management, viruses, and bacteria are determined with economic importance on the reduction of physical peach characteristics and production, crop genetics using potential genes in peaches to enhance the fruit characteristics and production [79], among others, play vital roles in fruit quality and yield [25,78,79,80].
The phenology response to climate change on peaches has been evaluated to show the effect of warming conditions on early blossom dates and late fall events extending the growing season for the past decades [81]. It is important to analyze the temperature variation under laboratory conditions due to the actual temperature fluctuation in the field and the ongoing climate variability. Our results show that given the possibility of warm winters when the number of required chilling hours is not completed, the accumulation of growing degree days would be relevant for the floral and vegetative budbreak. The difference in the number of days to release dormancy in both seasons can be explained since the amount of chill accumulated in both years affected the GGD accumulation differently and consequently the budbreak. Furthermore, the previously presented results would be explained by the effects of the controlled conditions. Although this is one of the most common and used methodologies, shoots are susceptible to drying out; thus, they have to be constantly trimmed to reach greater contact with the water and reduce this possibility. Hypothetically, it is mentioned that controlled conditions may encourage more rapid development [82].
The impact of climate change on the probability of low chill accumulation during winter has increased, showing a lack of chill accumulation for commonly grown peach cultivars in the southeastern United States [30]. Uneven or delayed budbreak occurs in the absence of chill exposure during the fall and early winter. Likewise, the evaluation of floral bud chilling requirements related to the dormancy release process in different fruits has been studied, i.e., in grapevines, to obtain a better understanding of temperature variation. [83,84]. In species like cherries (Prunus avium L.), the increase in chilling hours using trees under controlled conditions showed a relationship with the intensification of budbreaks influencing the flower size and fruit set [85].
Chilling is needed to induce the floral and vegetative budbreak under controlled conditions for peaches and nectarines were similar [86]. However, the duration of budbreak for dormant buds for peaches ranged from 20 to 40 days [32,86]. In our case, buds came from the field with a different accumulation of chilling in a progressive sampling from fall until late winter. The average duration for early budbreak varied according to the cultivar from 137 to 197 days in season 1 and from 123 to 138 days to budbreak for season 2.
In this study, we found that peach floral and vegetative buds differ in heat requirements for budbreak. Similar results were obtained in an experiment with different peach cultivars using artificially chilled excised shoots and potted trees, where flower and vegetative buds have different heat requirements during ecodormancy [62].
We applied existing well-referenced models for chilling accumulation ([40,41,65,70,71], among other authors) using a combination of those models with the GDD concept in a very simple approach to developing and evaluating a model for the prediction of floral and vegetative budbreaks in three different peach cultivars, characterized by the different requirements on chill accumulation using a multiple regression model.
This approach is unique and has not been reported before for the peach cultivars evaluated. The data collection was progressive over time, obtaining shoots with different accumulation values for heat and chill requirements. We found a high correlation among all chilling accumulation models for both seasons, a highly negatively correlated GDD with each of the models for season 1, and a low negative correlation for season 2. This agrees with previous results obtained for ornamental peaches, where a significant negative correlation is demonstrated between the models to calculate the chilling and heat requirements in the dormancy release process [87]. In apricots, a negative correlation was found in the interaction between chilling requirements and heat requirements in the transition between budbreak and full bloom stage [39].
The model efficiently estimates the number of budbreaks for two types of buds: floral and vegetative. In this study, the highest correlations were found among the chill accumulation models integrated with Growing Degree Days. Several authors have made applications of models for chilling accumulation and heat accumulation to predict peach phenology ([2,22,71,88], among others), but an integration of both models for the prediction of floral and vegetative budbreak has not been extensively reported.
Our model presents a very simple and flexible approach to be used for predicting the budbreak of floral and vegetative buds across cultivars in different locations and for different years since the conditions of the experiment were controlled in a laboratory. Other models are complex and include a great number of parameters. However, validation with a different set of data is desirable to extend the robustness of the model.
5. Conclusions
This study provides a consistent statistical model built for the estimation of budbreak for three peach cultivars from the beginning of fall until the end of spring. Considering the simplicity of the model, it can be a useful tool to assess the budbreak during the critical months for planning crop management practices. The results of this study contribute to an understanding of the chill accumulation and heat requirements for the floral and vegetative budbreak of three peach cultivars with different requirements.
Author Contributions
Conceptualization, M.R.S.-G.; Methodology, B.C.-C., E.V. and M.R.S.-G.; Formal analysis, A.C.-C., B.C.-C. and M.R.S.-G.; Investigation, A.C.-C. and M.R.S.-G.; Resources, E.V., E.D.C. and M.R.S.-G.; Writing—original draft, A.C.-C. and M.R.S.-G.; Writing—review and editing, B.C.-C., E.V., E.D.C., D.C. and M.R.S.-G.; Visualization, B.C.-C.; Funding acquisition, M.R.S.-G. All authors have read and agreed to the published version of the manuscript.
Funding
AAES Awards for Production Agriculture Research (PAR), Seed grant Auburn University Grants # 3702263039272055; 1036263039272055. Southern Sustainable Agriculture Research and Education Program (SARE) Grant # 2457061214012002.
Data Availability Statement
Data sharing is not applicable.
Acknowledgments
Salazar’s Lab graduate research students for assistance in data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Graves, W.R.; Gimondo, A. Phenology of annual dormancy release and its association with fruit set of Dirca occidentalis (Thymelaeaceae). Madrono 2021, 68, 416–424. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Carbone, G.J.; Reighard, G.L.; Okie, W.R. A Model to Predict Peach Phenology and Maturity Using Meteorological Variables. HortScience 1997, 32, 213–216. [Google Scholar] [CrossRef]
- Tadeu, M.H.; Pio, R.; Nunes, S.G.; Olmstead, M.; Damião, C.C.; Bittencourt, F.M.S.; Barcelos, B.R.; Locatelli, G. Duration of the Phenological Stages of Peach Trees At tropics duration of the phenological stages of peach trees at tropics. Sci. Hortic. 2020, 261, 108976. [Google Scholar] [CrossRef]
- Schwartz, M.D. Advancing to full bloom: Planning phenological research for the 21st century. Int. J. Biometeorol. 1999, 42, 113–118. [Google Scholar] [CrossRef]
- Mounzer, O.H.; Conejero, W.; Nicolás, E.; Abrisqueta, I.; García-Orellana, Y.V.; Tapia, L.M.; Vera, J.; Abrisqueta, J.M.; Ruiz-Sánchez, M.C. Growth Pattern and Phenological Stages of Early-maturing Peach Trees under a Mediterranean Climate. Hortscience 2008, 43, 1813–1818. [Google Scholar] [CrossRef]
- Fadón, E.; Herrera, S.; Guerrero, B.I.; Guerra, M.E.; Rodrigo, J. Review Chilling and Heat Requirements of Temperate Stone Fruit Trees (Prunus sp.). Agronomy 2020, 10, 409. [Google Scholar] [CrossRef]
- Campoy, J.A.; Ruiz, D.; Egea, J. Dormancy in temperate fruit trees in a global warming context: A review. Sci. Hortic. 2011, 130, 357–372. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Brennan, R.M.; Jones, H.G. Declining chilling and its impact on temperate perennial crops. Environ. Exp. Bot. 2013, 91, 48–62. [Google Scholar] [CrossRef]
- Weinberger, J.H. Some temperature relations in natural breaking of the rest of peach flower buds in the San Joaquin Valley, California. Proc. Am. Soc. Hort. Sci. 1967, 56, 122–128. [Google Scholar]
- Campoy, J.A.; Ruiz, D.; Allderman, L.; Cook, N.; Egea, J. The fulfillment of chilling requirements and the adaptation of apricot (Prunus armeniaca L.) in warm winter climates: An approach in Murcia (Spain) and the Western Cape (South Africa). Eur. J. Agron. 2012, 37, 43–55. [Google Scholar] [CrossRef]
- Alabama Cooperative Extension A&M & Auburn University. Report. Estimated Chill Portion and Chill Hour Requirements by Variety. 2019. Available online: https://www.aces.edu/blog/topics/fruits-nuts-crops/chill-hour-and-chill-portion-comparison-in-peaches/ (accessed on 8 October 2022).
- Chen, C. Three New Peach Cultivars from the USDA. 1. University of Georgia Peach Blog. Popular Publication. 2020. Available online: https://www.ars.usda.gov/research/publications/publication/?seqNo115=381001 (accessed on 11 August 2023).
- Zhang, Y.; Han, I.; Dawson, P. Antioxidant activity assessment and color analysis of skin from different peach varieties grown in South Carolina. Food Nutr. Sci. 2015, 6, 18. [Google Scholar] [CrossRef]
- Byrne, D.H.; Nikolic, A.N.; Burns, E.E. Variability in sugars, acids, firmness, and color characteristics of 12 peach genotypes. J. Am. Soc. Hortic. Sci. 1991, 116, 1004–1006. [Google Scholar] [CrossRef]
- Budde, C.O.; Blanco, M.P.; Altube, H.A. Fruit firmness, ground color and ethylene evolution in two cultivars of peach (Prunus persica L. Batsch). Agriscientia 2000, 17, 69–72. [Google Scholar]
- Reighard, G.L.; Rauh, B. Predicting peach fruit size potential from GDD 30 days post-bloom. Acta Hortic. 2015, 1084, 753–758. [Google Scholar] [CrossRef]
- Salama, A.M.; Ezzat, A.; El-Ramady, H.; Alam-Eldein, S.M.; Okba, S.K.; Elmenofy, H.M.; Hassan, I.F.; Illés, A.; Holb, I.J. Temperate fruit trees under climate change: Challenges for dormancy and chilling requirements in warm winter regions. Horticulturae 2021, 7, 86. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Díaz-Vivancos, P.; Martínez-Sanchez, G.; Alburquerque, N.; Martínez, D.; Barba-Espín, G.; Acosta-Motos, J.R.; Carrera, E.; García-Brunton, J. Physiological and biochemical characterization of bud dormancy: Evolution of carbohydrate and antioxidant metabolisms and hormonal profile in a low chill peach variety. Sci. Hortic. 2021, 281, 109957. [Google Scholar] [CrossRef]
- Faust, M.; Erez, A.; Rowland, L.J.; Wang, S.Y.; Norman, H.A. Bud dormancy in perennial fruit trees: Physiological basis for dormancy induction, maintenance, and release. HortScience 1997, 32, 623–629. [Google Scholar] [CrossRef]
- Zhao, X.; Han, X.; Wang, Q.; Wang, X.; Chen, X.; Li, L.; Ful, X.; Gao, D. Early bud break 1 triggers bud break in peach trees by regulating hormone metabolism, the cell cycle, and cell wall modifications. J. Exp. Bot. 2020, 71, 3512–3523. [Google Scholar] [CrossRef]
- Viol, R.E.; Peche, P.M.; Farias, D.H.; Vilas Boas, L.V.; Curi, P.N.; Schiassi, M.C.E.; Pio, R. Dormancy breaking of ‘Kampai’ peach trees with alternative products in subtropical regions. J. Agric. Sci. 2021, 159, 688–695. [Google Scholar] [CrossRef]
- Bielenberg, D.G.; Gasic, K. Peach [Prunus persica (L.) Batsch] Cultivars Differ in Apparent Base Temperature and Growing Degree Hour Requirement for Floral Budbreak. Front. Plant Sci. 2022, 13, 801606. [Google Scholar] [CrossRef]
- Drogoudi, P.; Cantín, C.M.; Brandi, F.; Butcaru, A.; Cos-Terrer, J.; Cutuli, M.; Giovannini, D. Impact of Chill and Heat Exposures under Diverse Climatic Conditions on Peach and Nectarine Flowering Phenology. Plants 2023, 12, 584. [Google Scholar] [CrossRef] [PubMed]
- Luedeling, E.; Kunz, A.; Blanke, M.M. Identification of chilling and heat requirements of cherry trees—A statistical approach. Int. J. Biometeorol. 2013, 57, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, T.; Zhou, P.; Huang, X.; Liu, D.; Jin, W.; Zhang, H.; Zhou, J.; Wang, Z.; Gao, Z. Prediction and optimization of fruit quality of peach based on artificial neural network. J. Food Compos. Anal. 2022, 111, 104604. [Google Scholar] [CrossRef]
- Richardson, E.A.; Seeley, S.D.; Walker, D.R.; Anderson, J.L.; Ascroft, G.L. Phenoclimatography of spring peach bud development. HortScience 1975, 10, 236–237. [Google Scholar] [CrossRef]
- Gibson, P.G.; Reighard, G.L. Chilling Requirement and Postrest Heat Accumulation in Peach Trees Inoculated with Peach Latent Mosaic Viroid. J. Am. Soc. Hort. Sci. 2022, 127, 333–336. [Google Scholar] [CrossRef]
- Noorazar, H.; Kalcsits, L.; Jones, V.P.; Jones, M.S.; Rajagopalan, K. Climate change and chill accumulation: Implications for tree fruit production in cold--winter regions. Clim. Chang. 2022, 171, 34. [Google Scholar] [CrossRef]
- Pertille, R.F.; Citadin, I.; De Souza de Oliveira, L.; De Camargo Broch, J.; Kvitschal, M.V.; Araujo, L. The influence of temperature on the phenology of apple trees grown in mild winter regions of Brazil, based on long-term records. Sci. Hortic. 2022, 305, 111354. [Google Scholar] [CrossRef]
- Parker, L.E.; Abatzoglou, J.T. Warming winters reduce chill accumulation for peach production in the Southeastern United States. Climate 2019, 7, 94. [Google Scholar] [CrossRef]
- Pérez, F.J.; Rubio, S. Relationship Between Bud Cold Hardiness and Budbreak in Two Vitis vinifera L Cultivars, Chardonnay and Thompson Seedless. J. Plant Growth Regul. 2022, 41, 840–847. [Google Scholar] [CrossRef]
- Camargo Alvarez, H.; Salazar-Gutiérrez, M.; Zapata, D.; Keller, M.; Hoogenboom, G. Time-to-event analysis to evaluate dormancy status of single-bud cuttings: An example for grapevines. Plant Methods 2018, 14, 94. [Google Scholar] [CrossRef]
- Alburquerque, N.; García-Montiel, F.; Carrillo, A.; Burgos, L. Chilling and heat requirements of sweet cherry cultivars and the relationship between altitude and the probability of satisfying the chill requirements. Environ. Exp. Bot. 2008, 64, 162–170. [Google Scholar] [CrossRef]
- Benmoussa, H.; Ghrab, M.; Ben Mimoun, M.; Luedeling, E. Chilling and heat requirements for local and foreign almond (Prunus dulcis Mill.) cultivars in a warm Mediterranean location based on 30 years of phenology records. Agric. For. Meteorol. 2017, 239, 34–46. [Google Scholar] [CrossRef]
- Egea, J.; Ortega, E.; Martinez-Gomez, P.; Dicenta, F. Chilling and heat requirements of almond cultivars for flowering. Environ. Exp. Bot. 2003, 50, 79–85. [Google Scholar] [CrossRef]
- Fernandez, E.; Krefting, P.; Kunz, A.; Do, H.; Fadón, E.; Luedeling, E. Boosting statistical delineation of chill and heat periods in temperate fruit trees through multi-environment observations. Agric. For. Meteorol. 2021, 310, 108652. [Google Scholar] [CrossRef]
- Guo, L.; Xu, J.C.; Dai, J.H.; Cheng, J.M.; Luedeling, E. Statistical identification of chilling and heat requirements for apricot flower buds in Beijing. China. Sci. Hortic. 2015, 195, 138–144. [Google Scholar] [CrossRef]
- Pope, K.S.; Da Silva, D.; Brown, P.H.; DeJong, T.M. A biologically based approach to modeling spring phenology in temperate deciduous trees. Agric. For. Meteorol. 2014, 198, 15–23. [Google Scholar] [CrossRef]
- Ruiz, D.; Campoy, J.A.; Egea, J. Chilling and heat requirements of apricot cultivars for flflowering. Environ. Exp. Bot. 2007, 61, 254–263. [Google Scholar] [CrossRef]
- Weinberger, J.H. Chilling requirements of peach varieties. Proc. Am. Soc. Hortic. Sci. 1950, 56, 122–128. [Google Scholar]
- Richardson, E.A.; Seeley, S.D.; Walker, D.R. A model for estimating the completion of rest for ‘redhaven’ and ‘elberta’peach trees1. HortScience 1974, 9, 331–332. [Google Scholar] [CrossRef]
- Zhang, J.; Taylor, C. The Dynamic Model Provides the Best Description of the Chill Process on ‘Sirora’ Pistachio Trees in Australia. HortScience 2011, 46, 420–425. [Google Scholar] [CrossRef]
- Melke, A. The Physiology of Chilling Temperature Requirements for Dormancy Release and Bud-break in Temperate Fruit Trees Grown at Mild Winter Tropical Climate. J. Plant Stud. 2015, 4, 2. [Google Scholar] [CrossRef]
- Chhetri, A.; Ramjan, M.; Dolley, N. Various models to calculate chill units in fruit crops. Indian Farmer 2018, 5, 439–442. [Google Scholar]
- Rodríguez, A.; Pérez-López, D.; Sánchez, E.; Centeno, A.; Gómara, I.; Dosio, A.; Ruiz-Ramos, M. Chilling accumulation in fruit trees in Spain under climate change. Nat. Hazards Earth Syst. Sci. 2019, 19, 1087–1103. [Google Scholar] [CrossRef]
- Milech, C.G.; Dini, M.; Scariotto, S.; Santos, J.; Herter, F.G.; Raseira, M.C.B. Chilling Requirement of Ten Peach Cultivars Estimated by Different Models. J. Exp. Agric. Int. 2018, 20, 1–9. [Google Scholar] [CrossRef]
- Chavarria, G.; Raseira, M.C.B.; Zanandrea, A. Chilling requirement in peach. In Prunus Breeders Meeting; Embrapa CPACT: Pelotas, Brazil, 2000; Volume 75. [Google Scholar]
- Jochner, S.; Menzel, A. Does flower phenology mirror the slowdown of global warming? Ecol. Evol. 2015, 5, 2284–2295. [Google Scholar] [CrossRef]
- Miranda, C.; Santesteban, L.G.; Royo, J.B. Evaluation and fitting of models for determining peach phenological stages at a regional scale. Agric. For. Meteorol. 2013, 178, 129–139. [Google Scholar] [CrossRef]
- Fernandez, E.; Schiffers, K.; Urbach, C.; Luedeling, E. Unusually warm winter seasons may compromise the performance of current phenology models–Predicting bloom dates in young apple trees with PhenoFlex. Agric. For. Meteorol. 2022, 322, 109020. [Google Scholar] [CrossRef]
- Andreini, L.; García de Cortázar-Atauri, I.; Chuine, I.; Viti, R.; Bartolini, S.; Ruiz, D.; Campoy, J.A.; Legave, J.M.; Audergon, J.M.; Bertuzzi, P. Understanding dormancy release in apricot flower buds (Prunus armeniaca L.) using several process-based phenological models. Agric. For. Meteorol. 2014, 184, 210–219. [Google Scholar] [CrossRef]
- Blaauw, B.; Brannen, P.; Lockwood, D.; Schnabel, G.; Ritchie, D. Southeastern Peach, Nectarine, and Plum Pest Management and Culture Guide. UGA Cooperative Extension Bulletin 1171. 2023. Available online: https://ssl.acesag.auburn.edu/dept/peaches/peachipm/images/2023_SE_peach_pest_guide.pdf (accessed on 8 October 2022).
- Okie, W.R.; Werner, D.J. Genetic Influence on Flower Bud Density in Peach and Nectarine Exceeds That of Environment. Hortscience 1996, 31, 1010–1012. [Google Scholar] [CrossRef]
- Balandier, P.; Gendraud, M.; Rageau, R.; Bonhomme, M.; Richard, J.P.; Parisot, E. Bud break delay on single node cuttings and bud capacity for nucleotide accumulation as parameters for endo-and paradormancy in peach trees in a tropical climate. Sci. Hortic. 1993, 55, 249–261. [Google Scholar] [CrossRef]
- Bonhomme, M.; Rageau, R.; Richard, J.P.; Erez, A.; Gendraud, M. Influence of three contrasted climatic conditions on endodormant vegetative and floral peach buds: Analyses of their intrinsic growth capacity and their potential sink strength compared with adjacent tissues. Sci. Hortic. 1999, 80, 157–171. [Google Scholar] [CrossRef]
- Maurel, K.; Leite, G.B.; Bonhomme, M.; Guilliot, A.; Rageau, R.; Pétel, G.; Sakr, S. Trophic control of bud break in peach (Prunus persica) trees: A possible role of hexoses. Tree Physiol. 2004, 24, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, M.; Rageau, R.; Lacointe, A.; Gendraud, M. Influences of cold deprivation during dormancy on carbohydrate contents of vegetative and floral primordia and nearby structures of peach buds (Prunus persica L. Batch). Sci. Hortic. 2005, 105, 223–240. [Google Scholar] [CrossRef]
- Erez, A.; Lavee, S. The Effect of Climatic Conditions on Dormancy Development of Peach Buds. I. Temperature1. J. Am. Soc. Hortic. Sci. 1971, 96, 711–714. [Google Scholar] [CrossRef]
- Horsáková, J.; Krška, B. Evaluation of dormancy break in some selected peach (Prunus persica) cultivars. Hort. Sci. 2016, 43, 181–187. [Google Scholar] [CrossRef]
- Prudencio, A.S.; Martínez-Gómez, P.; Dicenta, F. Evaluation of breaking dormancy, flowering and productivity of extra-late and ultra-late flowering almond cultivars during cold and warm seasons in South-East of Spain. Sci. Hortic. 2018, 235, 39–46. [Google Scholar] [CrossRef]
- Pulido, S.; Bojaca, C.R.; Salazar, M.; Chaves, B. Node appearance model for Lulo (Solanum quitoense Lam.) in the high altitude tropics. Biosyst. Eng. 2008, 101, 383–387. [Google Scholar] [CrossRef]
- Citadin, I.; Raseira, M.D.C.; Herter, F.G.; Da Silva, J.B. Heat requirement for blooming and leafing in peach. HortScience 2001, 36, 305–307. [Google Scholar] [CrossRef]
- Atagul, O.; Calle, A.; Demirel, G.; Lawton, J.M.; Bridges, W.C.; Gasic, K. Estimating Heat Requirement for Flowering in Peach Germplasm. Agronomy 2022, 12, 1002. [Google Scholar] [CrossRef]
- Łysiak, G.P.; Szot, I. The Use of Temperature Based Indices for Estimation of Fruit Production Conditions and Risks in Temperate Climates. Agriculture 2023, 13, 960. [Google Scholar] [CrossRef]
- Chandler, W.H. Deciduous Orchards; Lea & Febiger: Philadelphia, PA, USA, 1942; p. 438. [Google Scholar]
- Luedeling, E. Climate change impacts on winter chill for temperate fruit and nut production: A review. Sci. Hortic. 2012, 144, 218–229. [Google Scholar] [CrossRef]
- Razavi, F.; Hajilou, J.; Tabatabaei, S.J.; Dadpour, M.R. Comparison of chilling and heat requirement in some peach and apricot cultivars. Res. Plant Biol. 2011, 1. Available online: https://www.updatepublishing.com/journal/index.php/ripb/article/view/2571 (accessed on 5 July 2023).
- Eggert, F.P. A study of rest in several varieties of apple and in other fruit species grown in New York State. Proc. Am. Soc. Hort. Sci. 1951, 51, 169–178. [Google Scholar]
- Erez, A.; Couvillon, G.A.; Hendershott, C.H. Quantitative Chilling Enhancement and Negation in Peach Buds by High Temperatures in a Daily Cycle1. J. Am. Soc. Hortic. Sci. 1979, 104, 536–540. [Google Scholar] [CrossRef]
- Fishman, S.; Erez, A.; Couvillon, G.A. The temperature dependence of dormancy breaking in plants: Computer simulation of processes studied under controlled temperatures. J. Theor. Biol. 1987, 126, 309–321. [Google Scholar] [CrossRef]
- Erez, A.; Fishman, S.; Linsley-Noakes, G.C.; Allan, P. The dynamic model for rest completion in peach buds. In Proceedings of the II International Symposium on Computer Modelling in Fruit Research and Orchard Management. Acta Hortic. 1990, 276, 165–174. [Google Scholar] [CrossRef]
- Luedeling, E.; Zhang, M.; Luedeling, V.; Girvetz, E.H. Sensitivity of winter chill models for fruit and nut trees to climatic changes expected in California’s Central Valley. Agric. Ecosyst. Environ. 2009, 133, 23–31. [Google Scholar] [CrossRef]
- Glozer, K. The Dynamic Model and Chill Accumulation. 2016. Retrieved June 16 h. Available online: https://ucanr.edu/sites/fruittree/files/49320.pdf (accessed on 16 June 2023).
- Willmott, C.J. On the validation of models. Phys. Geogr. 1981, 2, 184–194. [Google Scholar] [CrossRef]
- Willmott, C.J. Some comments on the evaluation of model performance. Bull. Am. Meteorol. Soc. 1982, 63, 1309–1313. [Google Scholar] [CrossRef]
- Willmott, C.J.; Ackleson, S.G.; Davis, R.E.; Feddema, J.J.; Klink, K.M.; Legates, D.R.; O’Donnell, J.; Rowe, C.M. Statistics for the evaluation and comparison of models. J. Geophys. Res. Ocean. 1985, 90, 8995–9005. [Google Scholar] [CrossRef]
- Janssen, P.H.; Heuberger, P.S. Calibration of process-oriented models. Ecol. Model. 1995, 83, 55–66. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, B.; Guo, S.; Guo, L.; Chen, X.; He, X.; Ma, R.; Yu, M. Effects of fruit load on photosynthetic characteristics of peach leaves and fruit quality. Sci. Hortic. 2022, 299, 110977. [Google Scholar] [CrossRef]
- Gradziel, T.M. Exotic genes for solving emerging peach production challenges. Sci. Hortic. 2022, 295, 110801. [Google Scholar] [CrossRef]
- Luo, C.X.; Schnabel, G.; Hu, M.; De Cal, A. Global distribution and management of peach diseases. Phytopathol. Res. 2022, 4, 1–15. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhu, G.; Fang, W.; Cao, K.; Chen, C.; Wang, X.; Wang, X. Phenological response of peach to climate change exhibits a relatively dramatic trend in China, 1983–2012. Sci. Hortic. 2016, 209, 192–200. [Google Scholar] [CrossRef]
- Basconsuelo, S.; Reinoso, H.; Lorenzo, E.; Bottini, R. Dormancy in peach (Prunus persica L.) flower buds: IV. Morphogenesis of excised buds as influenced by chilling and gibberellin A 3. Plant Growth Regul. 1995, 16, 113–119. [Google Scholar] [CrossRef]
- Camargo-Alvarez, H.; Salazar-Gutiérrez, M.; Keller, M.; Hoogenboom, G. Modeling the effect of temperature on bud dormancy of grapevines. Agric. For. Meteorol. 2020, 280, 107782. [Google Scholar] [CrossRef]
- Andreini, L.; Viti, R.; Scalabrelli, G. Study on the morphological evolution of bud break in Vitis vinifera L. Vitis 2009, 48, 153–158. [Google Scholar]
- Mahmood, K.; Carew, J.G.; Hadley, P.; Battey, N.H. The effect of chilling and post-chilling temperatures on growth and flowering of sweet cherry (Prunus avium L.). J. Hortic. Sci. Biotechnol. 2000, 75, 598–601. [Google Scholar] [CrossRef]
- Gariglio, N.; González Rossia, D.E.; Mendow, M.; Reig, C.; Agusti, M. Effect of artificial chilling on the depth of endodormancy and vegetative and Flower Budbreak of peach and nectarine cultivars using excised shoots. Sci. Hortic. 2006, 108, 371–377. [Google Scholar] [CrossRef]
- Pawasut, A.; Fujishige, N.; Yamane, K.; Yamaki, Y.; Honjo, H. Relationships between chilling and heat requirement for flowering in ornamental peaches. J. Jpn. Soc. Hortic. Sci. 2004, 73, 519–523. [Google Scholar] [CrossRef][Green Version]
- Martinez, J.D.; Hernández, I.C.; Reyes, J.N.G.; Nájera, J.B.P.; Güereca, M.C.G.; Pérez, E.C. Growth models of peach fruit Prunus persica (L.) in three handling systems. Interciencia 2017, 42, 597–602. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
