Contribution of Arbuscular Mycorrhizal Fungi (AMF) in Improving the Growth and Yield Performances of Flax (Linum usitatissimum L.) to Salinity Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup, Plant Materials, and Growth Conditions
2.2. Growth Parameters and AMF Root Colonization
2.3. Physiological and Biochemical Parameters
2.4. Nitrogen and Phosphorus Distribution
2.5. Yield Parameters
2.6. Statistical Analysis
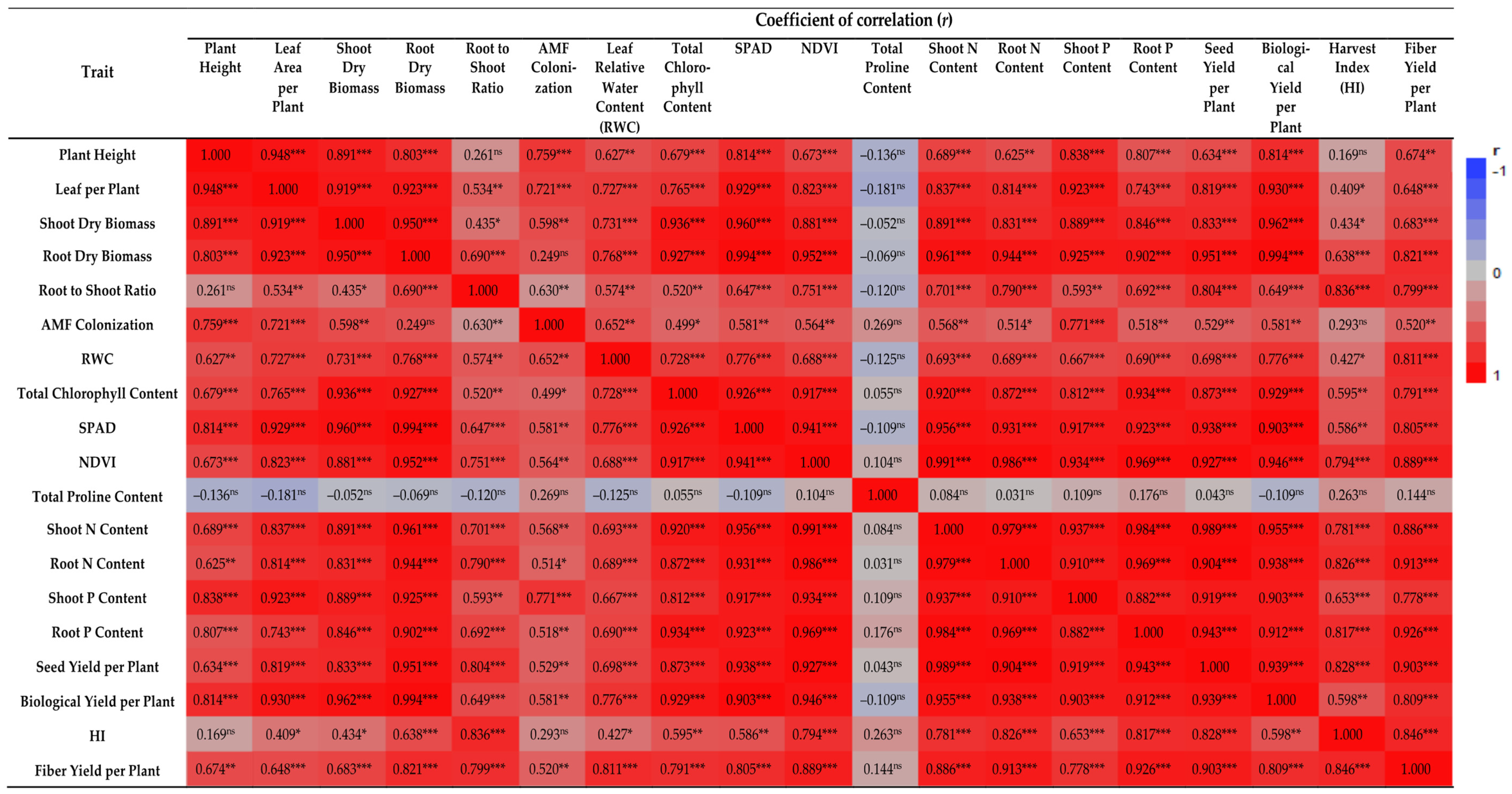
3. Results
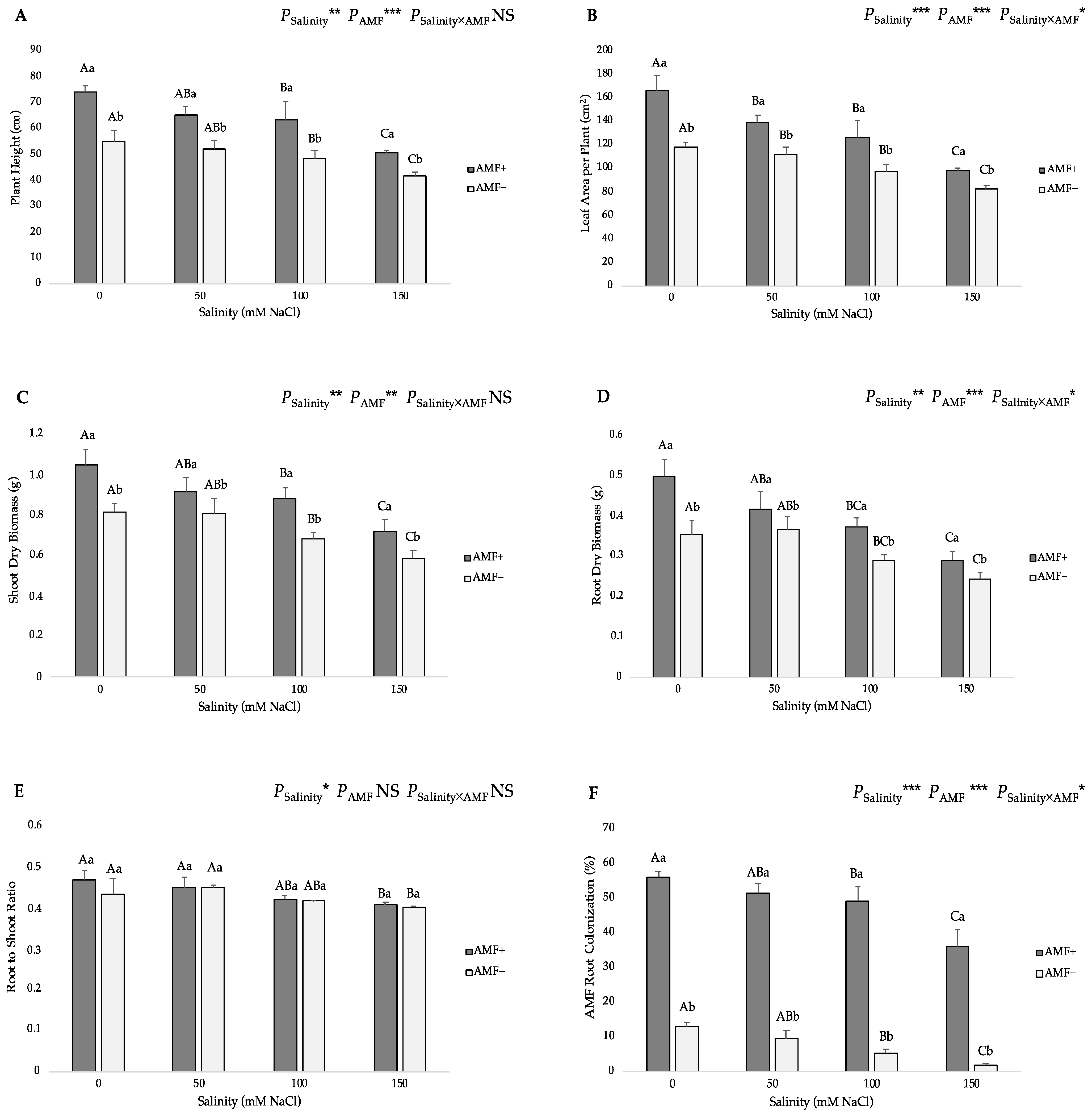
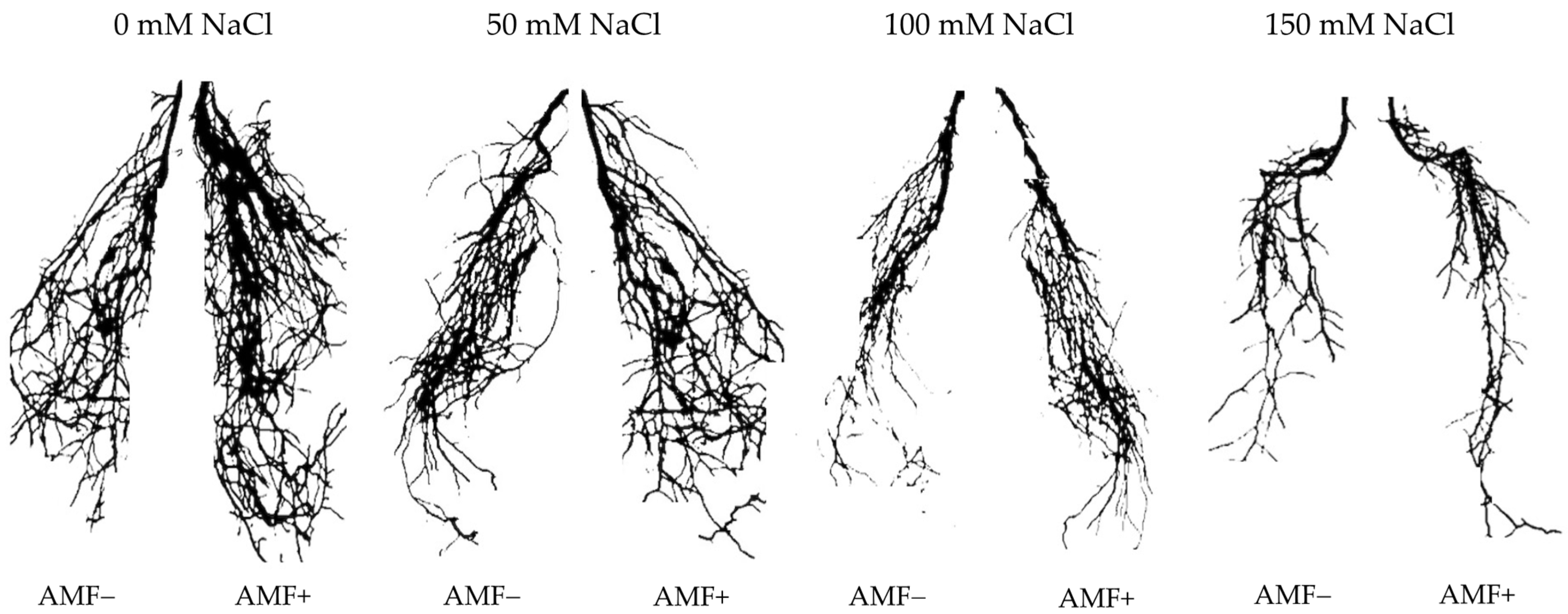
3.1. Growth Parameters and AMF Root Colonization
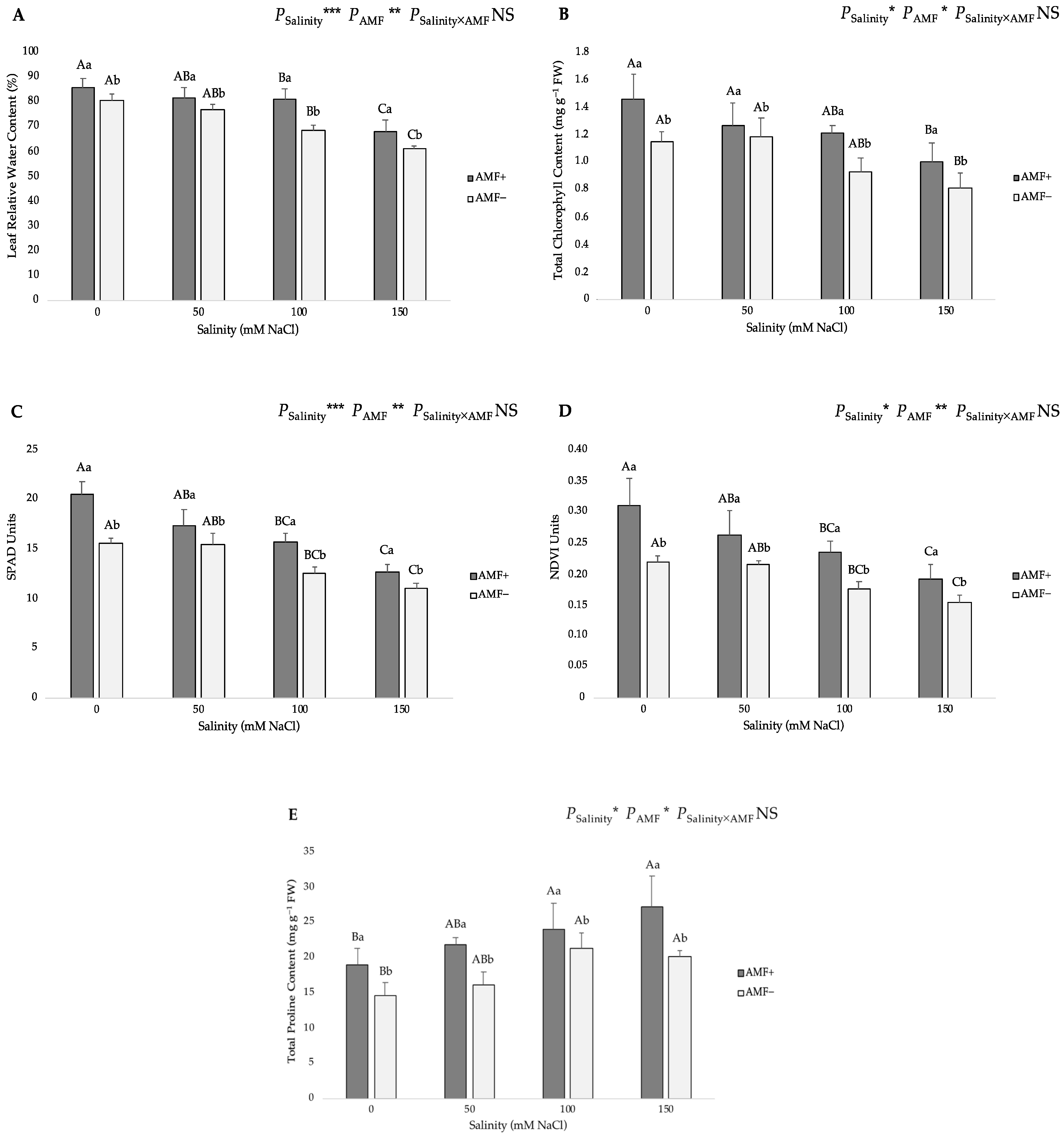
3.2. Physiological and Biochemical Parameters
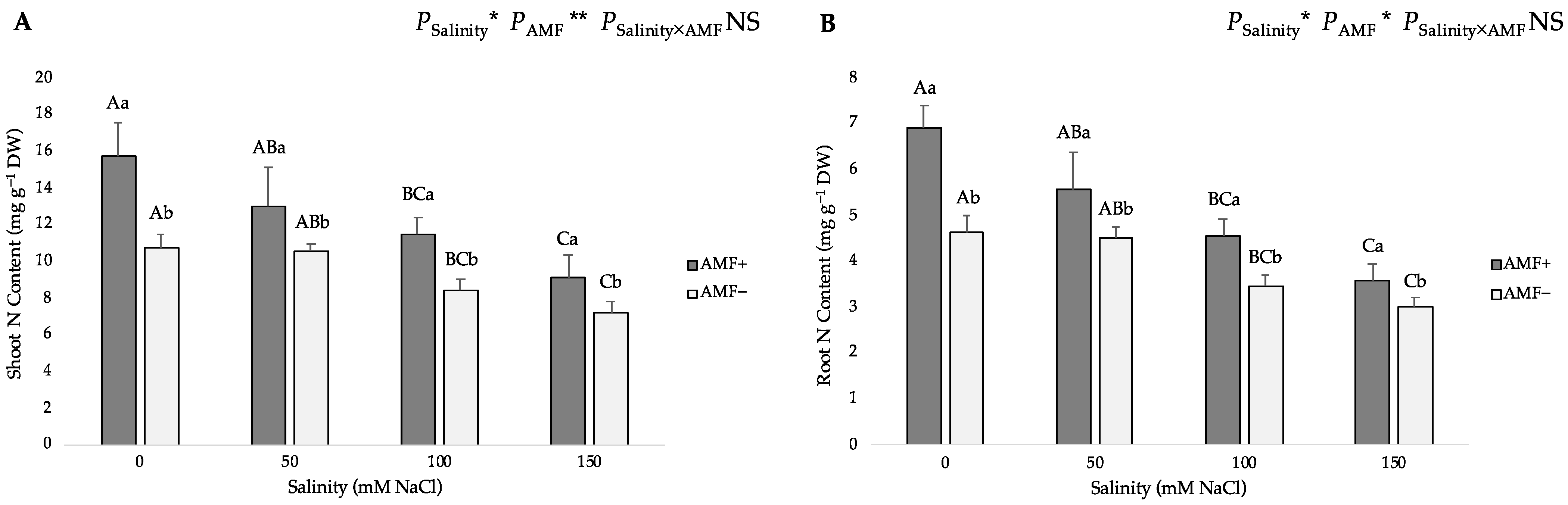
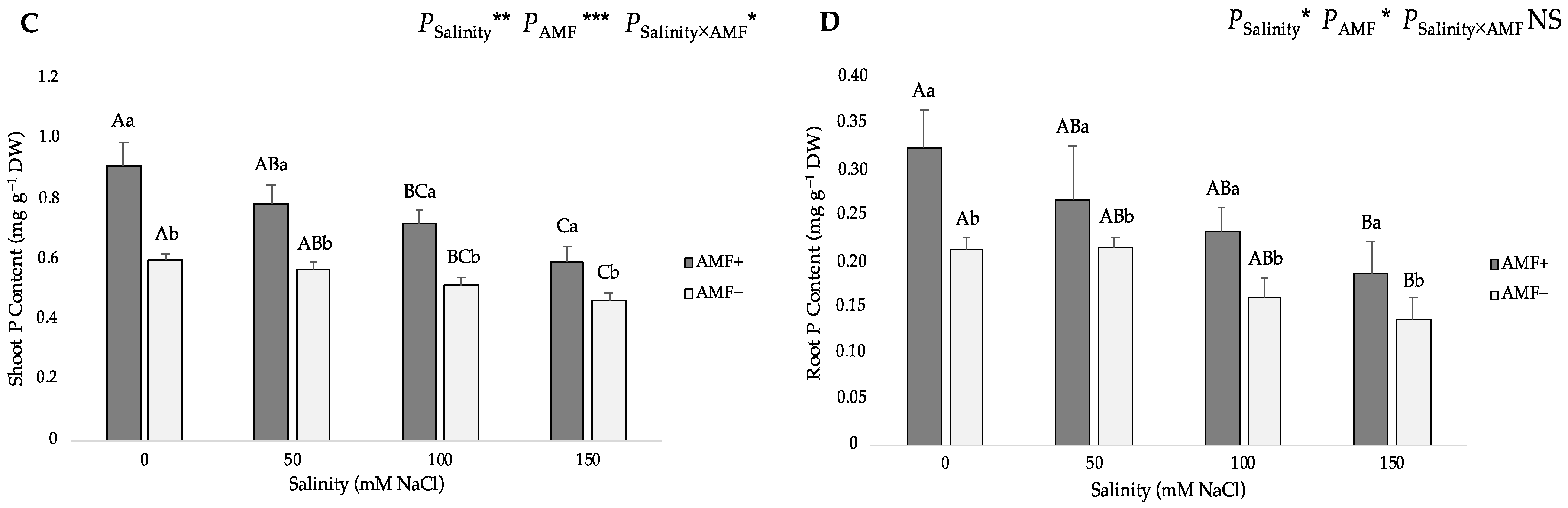
3.3. Nitrogen and Phosphorus Distribution
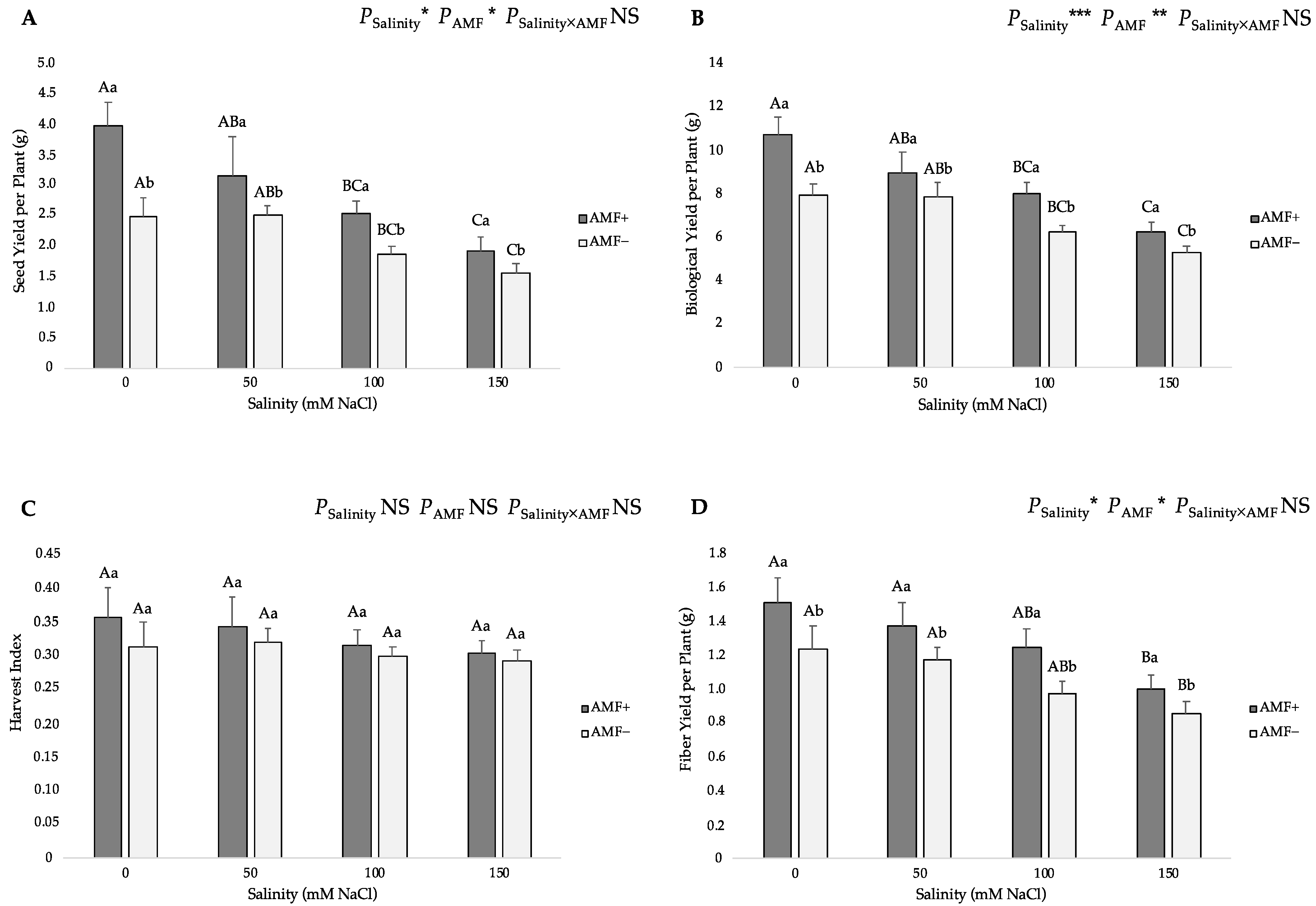
3.4. Yield Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jhala, A.J.; Hall, L.M. Flax (Linum usitatissimum L.): Current uses and future applications. AJBAS 2010, 4, 4304–4312. [Google Scholar]
- Kiryluk, A.; Kostecka, J. Pro-environmental and health-promoting grounds for restitution of flax (Linum usitatissimum L.) cultivation. J. Ecol. Eng. 2020, 21, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Bilalis, D.; Roussis, I.; Cheimona, N.; Kakabouki, I.; Travlos, I. Organic agriculture and innovative feed crops. In Agricultural Research Updates; Gorawala, P., Mandhatri, S., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2018; Volume 23, pp. 55–100. [Google Scholar]
- Debnath, S. Flax fiber extraction to textiles and sustainability: A holistic approach. In Sustainable Fashion and Textiles in Latin America, 1st ed.; Gardetti, M.A., Larios-Francia, R.P., Eds.; Springer: Singapore, 2021; pp. 73–85. [Google Scholar]
- Stavropoulos, P.; Mavroeidis, A.; Papadopoulos, G.; Roussis, I.; Bilalis, D.; Kakabouki, I. On the path towards a “greener” EU: A mini review on flax (Linum usitatissimum L.) as a case study. Plants 2023, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Vaisey-Genser, M.; Morris, D.H. Introduction: History of the cultivation and uses of flaxseed. In Flax, 1st ed.; Muir, A.D., Westcott, N.D., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 13–33. [Google Scholar]
- Cloutier, S. Linseed: Overview. In Encyclopedia of Food Grains, 1st ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 1, pp. 259–264. [Google Scholar]
- Adorian, T.J.; Pianesso, D.; Bender, A.B.B.; Speroni, C.S.; Mombach, P.I.; Kowalski, É.A.; da Silva, L.P. Fractionation of linseed and obtaining ingredients rich in protein and fibers: Alternatives for animal feed. J. Sci. Food Agric. 2021, 102, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.X.; Shi, L.E.; Wang, X.M.; Dai, G.W.; Cheng, L.A.; Wan, Z.X.; He, H.; Wu, Q.; Wang, Y.B.; Jin, X.Y.; et al. Whole flaxseed-based products and their health benefits. Food Sci. Technol. Res. 2020, 26, 561–578. [Google Scholar] [CrossRef]
- Kolodziejczyk, P.; Ozimek, L.; Kozłowska, J. The application of flax and hemp seeds in food, animal feed and cosmetics production. In Handbook of Natural Fibers, 1st ed.; Kozłowski, R.M., Ed.; Woodhead Publishing: Cambridge, UK, 2012; Volume 2, pp. 329–366. [Google Scholar]
- Begum, H.; Alam, A.K.M.M.; Chowdhury, M.J.A.; Hossain, M.I. Genetic divergence in linseed (Linum usitatissimum L.). Int. J. Sustain. Crop. Prod. 2007, 2, 4–6. [Google Scholar]
- Topnikova, E.V.; Pirogova, E.N.; Danilova, E.S. Quality assessment of linseed oil. IOP Conf. Ser. Earth Environ. Sci. 2022, 1, 012096. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Zahan, M.I.; Tahjib-Ul-Arif, M.; Akter, M.A.; Okazaki, S. Plant salinity tolerance conferred by arbuscular mycorrhizal fungi and associated mechanisms: A meta-analysis. Front. Plant Sci. 2020, 11, 588550. [Google Scholar] [CrossRef]
- Niu, X.; Bressan, R.A.; Hasegawa, P.M.; Pardo, J.M. Ion homeostasis in NaCl stress environments. Plant Physiol. 1995, 109, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Evelin, H.; Giri, B.; Kapoor, R. Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 2012, 22, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Li, Q.S.; Ding, W.Y.; Dong, L.W.; Deng, M.; Chen, J.H.; Tian, X.; Hashem, A.; Al-Arjani, A.F.; Alenazi, M.M.; et al. Arbuscular mycorrhizal fungi inoculation impacts expression of aquaporins and salt overly sensitive genes and enhances tolerance of salt stress in tomato. Chem. Biol. Technol. Agric. 2023, 10, 5. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: New York, NY, USA, 2010. [Google Scholar]
- Wu, N.; Li, Z.; Wu, F.; Tang, M. Comparative photochemistry activity and antioxidant responses in male and female Populus cathayana cuttings inoculated with arbuscular mycorrhizal fungi under salt. Sci. Rep. 2016, 6, 37663. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Maucieri, C.; Berruti, A.; Borin, M.; Barbera, A. Responses of different Panicum miliaceum L. genotypes to saline and water stress in a Marginal Mediterranean environment. Agronomy 2018, 8, 8. [Google Scholar] [CrossRef]
- Xiao, X.; Liao, X.; Yan, Q.; Xie, Y.; Chen, J.; Liang, G.; Chen, M.; Xiao, S.; Chen, Y.; Liu, J. Arbuscular mycorrhizal fungi improve the growth, water status, and nutrient uptake of Cinnamomum migao and the soil nutrient stoichiometry under drought stress and recovery. J. Fungi 2023, 9, 321. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, P.; Li, F.; Duan, T. Effects of AM fungi and grass endophytes on perennial ryegrass Bipolaris sorokiniana leaf spot disease under limited soil nutrients. Eur. J. Plant Pathol. 2019, 154, 659–671. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Martin, F.M.; Selosse, M.-A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Smith, S.E.; Facelli, E.; Pope, S.; Smith, F.A. Plant performance in stressful environments: Interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 2009, 326, 3–20. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Alguacil, M.; Lozano, Z.; Campoy, M.J.; Roldán, A. Phosphorus fertilization management modifies the biodiversity of am fungi in a tropical savanna forage system. Soil Biol. Biochem. 2010, 42, 1114–1122. [Google Scholar] [CrossRef]
- Kaur, S.; Suseela, V. Unraveling arbuscular mycorrhiza-induced changes in plant primary and secondary metabolome. Metabolites 2020, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Püschel, D.; Bitterlich, M.; Rydlová, J.; Jansa, J. Drought accentuates the role of mycorrhiza in phosphorus uptake. Soil Biol. Biochem. 2021, 157, 108243. [Google Scholar] [CrossRef]
- El-Sawah, A.M.; Abdel-Fattah, G.G.; Holford, P.; Korany, S.M.; Alsherif, E.A.; AbdElgawad, H.; Ulhassan, Z.; Jośko, I.; Ali, B.; Sheteiwy, M.S. Funneliformis constrictum modulates polyamine metabolism to enhance tolerance of Zea mays L. to salinity. Microbiol. Res. 2023, 266, 127254. [Google Scholar] [CrossRef] [PubMed]
- Bowles, T.M.; Jackson, L.E.; Cavagnaro, T.R. Mycorrhizal fungi enhance plant nutrient acquisition and modulate nitrogen loss with variable water regimes. Glob. Change Biol. 2017, 24, e171–e182. [Google Scholar] [CrossRef] [PubMed]
- Konvalinková, T.; Püschel, D.; Rezácová, V.; Gryndlerová, H.; Jansa, J. Carbon flow from plant to arbuscular mycorrhizal fungi is reduced under phosphorus fertilization. Plant Soil 2017, 419, 319–333. [Google Scholar] [CrossRef]
- Liu, H.; Chen, W.; Wu, M.; Wu, R.; Zhou, Y.; Gao, Y.; Ren, A. Arbuscular mycorrhizal fungus inoculation reduces the drought-resistance advantage of endophyte-infected versus endophyte-free Leymus chinensis. Mycorrhiza 2017, 27, 791–799. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Yamasaki, S.; Dillenburg, L.R. Measurements of leaf relative water content in Araucaria angustifolia. Rev. Bras. Fisiol. Veg. 1999, 11, 69–75. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Khan, M.M.A.; Naeem, M. Salinity induced changes in growth, enzyme activities, photosynthesis, proline accumulation and yield in linseed genotypes. World J. Agric. Sci. 2007, 3, 685–695. [Google Scholar]
- Wu, N.; Li, Z.; Wu, F.; Tang, M. Microenvironment and microbial community in the rhizosphere of dioecious Populus cathayana at Chaka Salt Lake. J. Soils Sediments 2019, 19, 2740–2751. [Google Scholar] [CrossRef]
- Li, Z.; Wu, N.; Meng, S.; Wu, F.; Liu, T. Arbuscular mycorrhizal fungi (AMF) enhance the tolerance of Euonymus maackii Rupr. at a moderate level of salinity. PLoS ONE 2020, 15, e0231497. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Aroca, R.; Azcón, R.; Ruiz-Lozano, J.M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 2016, 26, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, V.; Shamshiri, M.H.; Alaei, H.; Salehi, H. The role of inoculum identity for growth, photosynthesis, and chlorophyll fluorescence of zinnia plants by arbuscular mycorrhizal fungi under varying water regimes. Photosynthetica 2019, 57, 409–419. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Mathur, N.; Singh, J.; Bohra, S.; Bohra, A.; Vyas, A. Biomass production, productivity and physiological changes in moth bean genotypes at different salinity levels. Am. J. Plant Physiol. 2006, 1, 210–213. [Google Scholar]
- Jamil, M.; Rehman, S.U.; Lee, K.J.; Kim, J.M.; Rha, H.K. Salinity reduced growth PS2 photochemistry and chlorophyll content in radish. Sci. Agric. 2007, 64, 111–118. [Google Scholar] [CrossRef]
- Kapoor, K.; Srivastana, A. Assessment of salinity tolerance of Vigna mungo var. Pu-19 using ex vitro and in vitro methods. Asian J. Biotechnol. 2010, 2, 73–85. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, L.; Carletti, P.; Badeck, F.W.; Rizza, F.; Morcia, C.; Ghizzoni, R.; Rouphael, Y.; Colla, G.; Terzi, V.; Lucini, L. Metabolomic responses triggered by arbuscular mycorrhiza enhance tolerance to water stress in wheat cultivars. Plant Physiol. Biochem. 2019, 137, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Kumar, A.; Al-Dbass, A.M.; Alqarawi, A.A.; Al-Arjani, B.F.; Singh, G.; Farooq, M.; Abd-Allah, E.F. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Biol. Sci. 2019, 26, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Jerbi, M.; Labidi, S.; Laruelle, F.; Tisserant, B.; Dalpé, Y.; Lounès-Hadj Sahraoui, A.; Ben Jeddi, F. Contribution of native and exotic arbuscular mycorrhizal fungi in improving the physiological and biochemical response of hulless barley (Hordeum vulgare ssp. nudum L.) to drought. J. Soil Sci. Plant Nutr. 2022, 22, 2187–2204. [Google Scholar] [CrossRef]
- Bilalis, D.J.; Roussis, I.; Kakabouki, I.; Karydogianni, S. Effects of salinity and arbuscular mycorrhizal fungi (AMF) on root growth development and productivity of chia (Salvia hispanica L.), a promising salt-tolerant crop, under Mediterranean conditions. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Wu, Q.S.; Zou, Y.N.; Abd Allah, E.F. Mycorrhizal association and ROS in plants. In Oxidative Damage to Plants; Ahmad, P., Ed.; Elsevier: New York, NY, USA, 2014; pp. 453–475. [Google Scholar]
- Borde, M.; Dudhane, M.; Kulkarni, M. Role of arbuscular mycorrhizal fungi (AMF) in salinity tolerance and growth response in plants under salt stress conditions. In Mycorrhiza-Eco-Physiology, Secondary Metabolites, Nanomaterials; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 71–86. [Google Scholar]
- Bheemareddy, V.S.; Lakshman, H.C. Effect of salt and acid stress on Triticum aestivum inoculated with Glomus fasciculatum. J. Anim. Plant Sci. 2011, 7, 945–956. [Google Scholar]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Klinsukon, C.; Lumyong, S.; Kuyper, T.W.; Boonlue, S. Colonization by arbuscular mycorrhizal fungi improves salinity tolerance of eucalyptus (Eucalyptus camaldulensis) seedlings. Sci. Rep. 2021, 11, 4362. [Google Scholar] [CrossRef]
- Da Silva, H.F.O.; Tavares, O.C.H.; da Silva, L.S.; Zonta, E.; da Silva, E.M.R.; Júnior, O.J.S.; Nobre, C.P.; Berbara, R.L.L.; García, A.C. Arbuscular mycorrhizal fungi and humic substances increased the salinity tolerance of rice plants. Biocatal. Agric. Biotechnol. 2022, 44, 102472. [Google Scholar] [CrossRef]
- Albacete, A.; Ghanem, M.E.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Martinez, V.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Glaría, A.; Eichler-Löbermann, B.; Loiret, F.G.; Ortega, E.; Kavka, M. Root-system architectures of two Cuban rice cultivars with salt stress at early development stages. Plants 2021, 10, 1194. [Google Scholar] [CrossRef] [PubMed]
- Juniper, S.; Abbott, L.K. Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 2006, 16, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Nacoon, S.; Ekprasert, J.; Riddech, N.; Mongkolthanaruk, W.; Jogloy, S.; Vorasoot, N.; Cooper, J.; Boonlue, S. Growth enhancement of sunchoke by arbuscular mycorrhizal fungi under drought condition. Rhizosphere 2021, 17, 100308. [Google Scholar] [CrossRef]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Kapoor, R.; Sharma, D.; Bhatnagar, A.K. Arbuscular mycorrhizae in micropropagation systems and their potential applications. Sci. Hortic. 2008, 116, 227–239. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass, and mineral nutrition of Acacia auriculiformis. Biol. Fertil. Soils 2003, 38, 170–175. [Google Scholar] [CrossRef]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.A.; Hungria, M.; Giller, K.E. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Bechtaoui, N.; Rabiu, M.K.; Raklami, A.; Oufdou, K.; Hafidi, M.; Jemo, M. Phosphate-dependent regulation of growth and stresses management in plants. Front. Plant Sci. 2021, 12, 679916. [Google Scholar] [CrossRef]
- Chang, W.; Sui, X.; Fan, X.X.; Jia, T.T.; Song, F.Q. Arbuscular mycorrhizal symbiosis modulates antioxidant response and ion distribution in salt-stressed Elaeagnus angustifolia seedlings. Front. Microbiol. 2018, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Frosi, G.; Barros, V.A.; Oliveira, M.T.; Santos, M.; Ramos, D.G.; Maia, L.C.; Santos, M.G.J.T.P. Arbuscular mycorrhizal fungi and foliar phosphorus inorganic supply alleviate salt stress effects in physiological attributes, but only arbuscular mycorrhizal fungi increase biomass in woody species of a semiarid environment. Tree Physiol. 2018, 38, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Ferrarezi, R.; Geiger, T.C.; Greenidge, J.; Dennery, S.; Weiss, S.A.; Vieira, G.S. Microirrigation equipment for okra cultivation in the U.S. Virgin Islands. HortScience 2020, 55, 1045–1052. [Google Scholar] [CrossRef]
- Monje, O.A.; Bugbee, B. Inherent limitations of nondestructive chlorophyll meters: A comparison of two types of meters. HortScience 1992, 27, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dunn, B.L.; Arnall, D.B.; Mao, P. Use of an active canopy sensor and SPAD chlorophyll meter to quantify geranium nitrogen status. HortScience 2012, 47, 45–50. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, F.; Zheng, X.; Pan, H.; Wen, Y.; Song, F. Effects of AMF compound inoculants on growth, ion homeostasis, and salt tolerance-related gene expression in Oryza sativa L. under salt treatments. Rice 2023, 16, 18. [Google Scholar] [CrossRef]
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.S. Salinity effects on nitrogen metabolism in plants—Focusing on the activities of nitrogen metabolizing enzymes: A review. J. Plant Nutr. 2018, 41, 1065–1108. [Google Scholar] [CrossRef]
- Hoff, T.; Stummann, B.M.; Henningsen, K.W. Structure, function and regulation of nitrate reductase in higher plants. Physiol. Plant. 1992, 84, 616–624. [Google Scholar] [CrossRef]
- Marschner, H.; Dell, B. Nutrient uptake in mycorrhizal symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Influence of arbuscular mycorrhizae on yield, nutrients, organic solutes, and antioxidant enzymes of two wheat cultivars under salt stress. J. Plant Nutr. Soil Sci. 2011, 174, 283–291. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhiza on organic solutes in maize leaves under salt stress. Mycorrhiza 2011, 21, 423–430. [Google Scholar] [CrossRef]
- Heydari, S.; Pirzad, A. Mycorrhizal fungi and Thiobacillus co-inoculation improve the physiological indices of Lallemantia iberica under salinity stress. Curr. Microbiol. 2020, 77, 2523–2534. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, X.; Zhukova, A.; Tang, Z.; Weng, Y.; Li, Z.; Yang, Y. Arbuscular mycorrhizal fungi (AMF) species and abundance exhibit different effects on saline-alkaline tolerance in Leymus chinensis. J. Plant Interact. 2020, 15, 266–279. [Google Scholar] [CrossRef]
- Poss, J.A.; Pond, E.; Menge, J. Effect of salinity on mycorrhizal onion and tomato in soil with and without additional phosphate. Plant Soil. 1985, 88, 307–309. [Google Scholar] [CrossRef]
- Volkamar, K.M.; Hu, Y.; Steppuhn, H. Physiological responses of plants to salinity: A review. Can. J. Plant Sci. 1998, 78, 19–27. [Google Scholar] [CrossRef]
- Hameed, A.; Egamberdieva, D.; Abd Allah, E.F.; Hashem, A.; Kumar, A.; Ahmad, P. Salinity stress and arbuscular mycorrhizal symbiosis in plants. In Use of Microbes for the Alleviation of Soil Stresses; Miransari, M., Ed.; Springer: New York, NY, USA, 2014; Volume 1, pp. 139–159. [Google Scholar]
- Dubey, S.; Bhargava, A.; Fuentes, F.; Shukla, S.; Srivastava, S. Effect of salinity stress on yield and quality parameters in flax (Linum usitatissimum L.). Not. Bot. Horti Agrobot. 2020, 48, 954–966. [Google Scholar] [CrossRef]
- Abdullah; Mahmood, A.; Bibi, S.; Naqve, M.; Javaid, M.M.; Zia, M.A.; Jabbar, A.; Ud-Din, W.; Attia, K.A.; Khan, N.; et al. Physiological, biochemical, and yield responses of linseed (Linum usitatissimum L.) in α-tocopherol-mediated alleviation of salinity stress. Front. Plant Sci. 2022, 13, 867172. [Google Scholar] [CrossRef]
| Substrate Properties | Soil, Peat, and Perlite (2:1:1) |
|---|---|
| Soil type | Clay loam (28.4% clay, 33.7% silt, 37.9% sand) |
| pH (1:2 H2O) | 7.42 |
| Organic matter (%) | 0.41 |
| CaCO3 | 15.48 |
| P (Olsen) (mg kg−1) | 9.87 |
| NO3− | 114.8 |
| Na+ | 107.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakabouki, I.; Stavropoulos, P.; Roussis, I.; Mavroeidis, A.; Bilalis, D. Contribution of Arbuscular Mycorrhizal Fungi (AMF) in Improving the Growth and Yield Performances of Flax (Linum usitatissimum L.) to Salinity Stress. Agronomy 2023, 13, 2416. https://doi.org/10.3390/agronomy13092416
Kakabouki I, Stavropoulos P, Roussis I, Mavroeidis A, Bilalis D. Contribution of Arbuscular Mycorrhizal Fungi (AMF) in Improving the Growth and Yield Performances of Flax (Linum usitatissimum L.) to Salinity Stress. Agronomy. 2023; 13(9):2416. https://doi.org/10.3390/agronomy13092416
Chicago/Turabian StyleKakabouki, Ioanna, Panteleimon Stavropoulos, Ioannis Roussis, Antonios Mavroeidis, and Dimitrios Bilalis. 2023. "Contribution of Arbuscular Mycorrhizal Fungi (AMF) in Improving the Growth and Yield Performances of Flax (Linum usitatissimum L.) to Salinity Stress" Agronomy 13, no. 9: 2416. https://doi.org/10.3390/agronomy13092416
APA StyleKakabouki, I., Stavropoulos, P., Roussis, I., Mavroeidis, A., & Bilalis, D. (2023). Contribution of Arbuscular Mycorrhizal Fungi (AMF) in Improving the Growth and Yield Performances of Flax (Linum usitatissimum L.) to Salinity Stress. Agronomy, 13(9), 2416. https://doi.org/10.3390/agronomy13092416










