Finding Phenotypic Biomarkers for Drought Tolerance in Solanum tuberosum
Abstract
1. Introduction
2. Materials and Methods
2.1. Potato Drought Trials
2.2. Evaluation of Yield Data
2.3. Phenotyping
2.3.1. Measurements and Quality Control
2.3.2. Nonlinear Regression
2.3.3. Correlation Analysis
2.3.4. Multiple Regression Analysis
3. Results
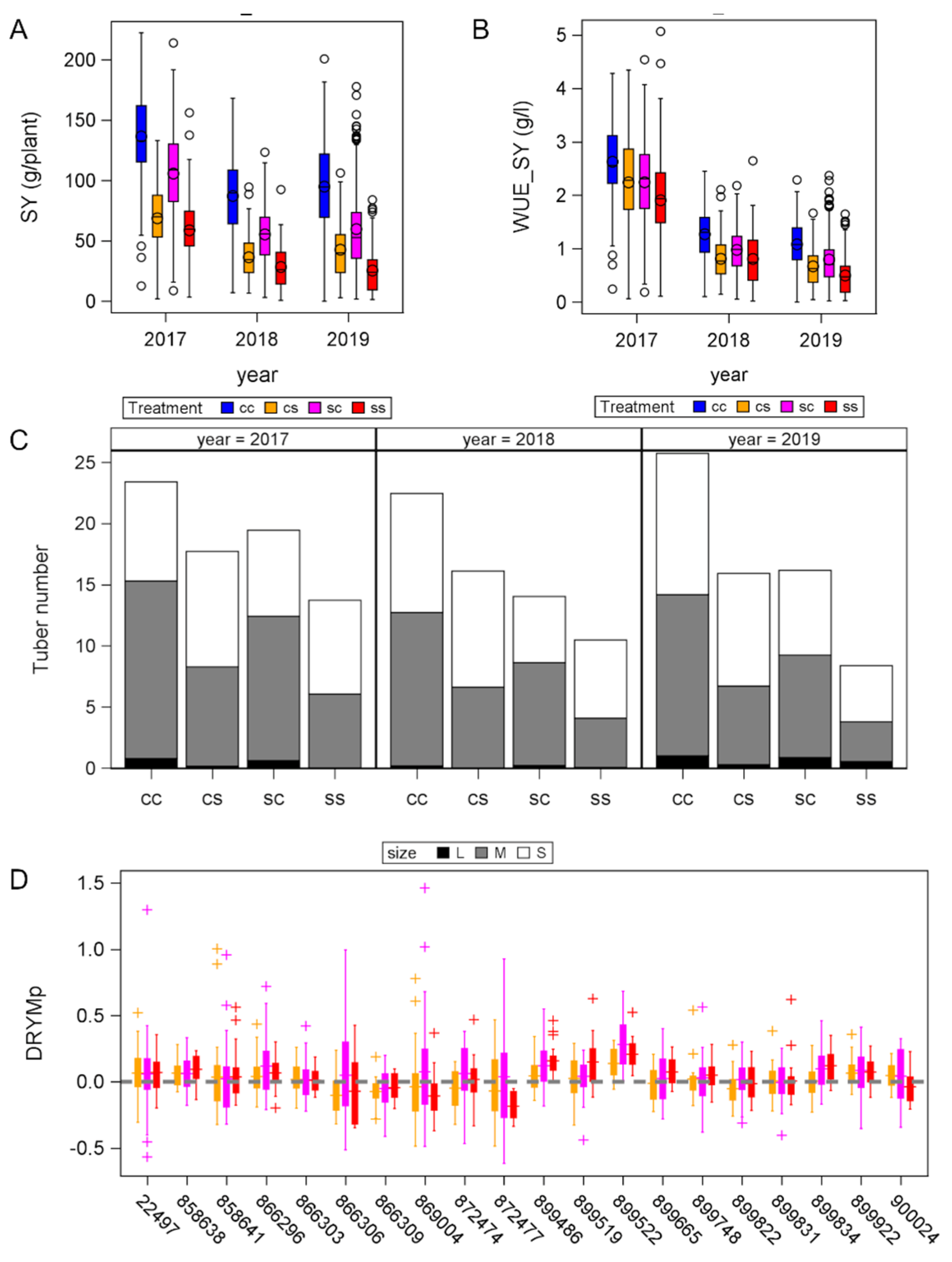
3.1. Tolerance to Different Drought Scenarios
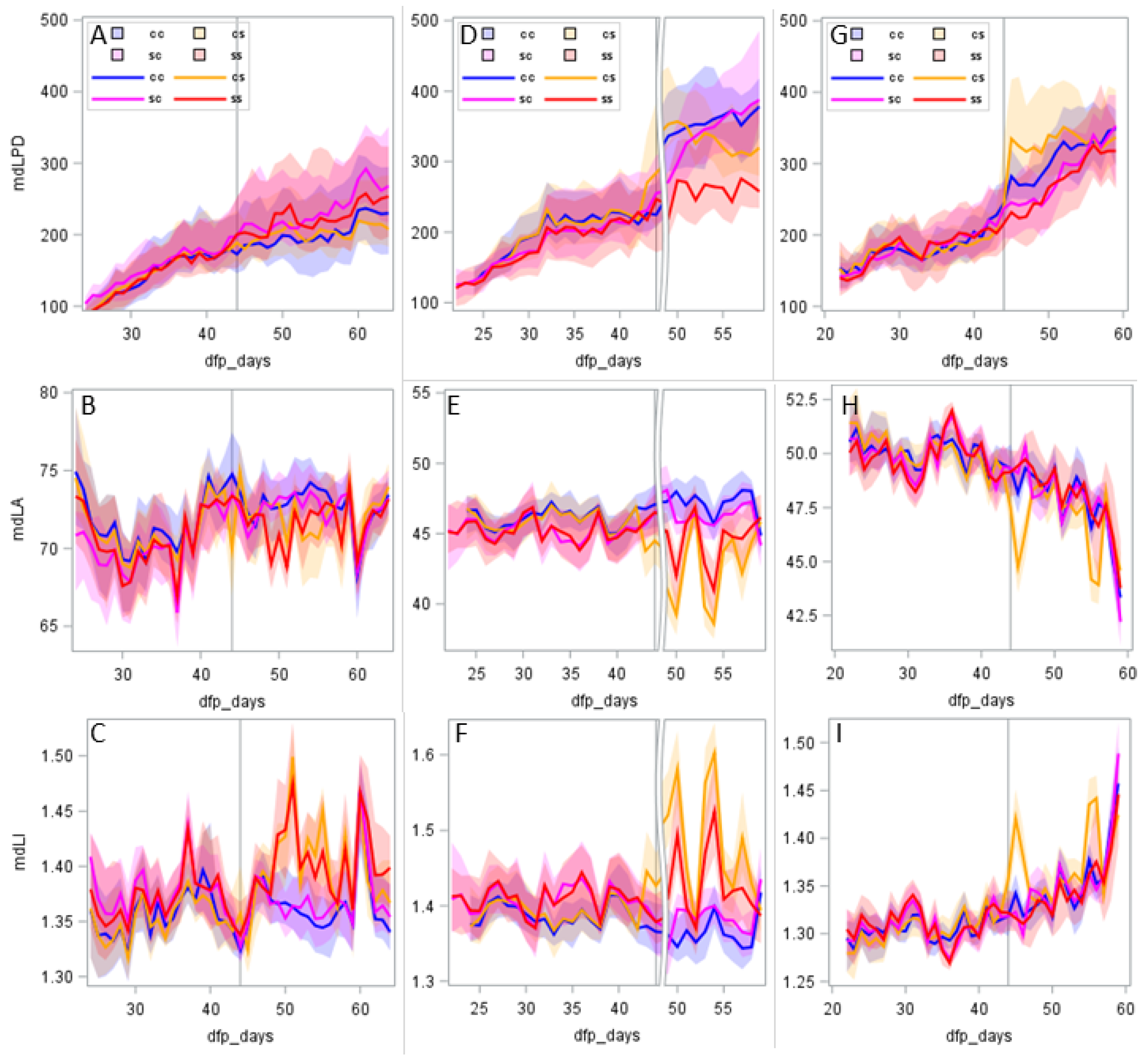
3.2. Phenotyping
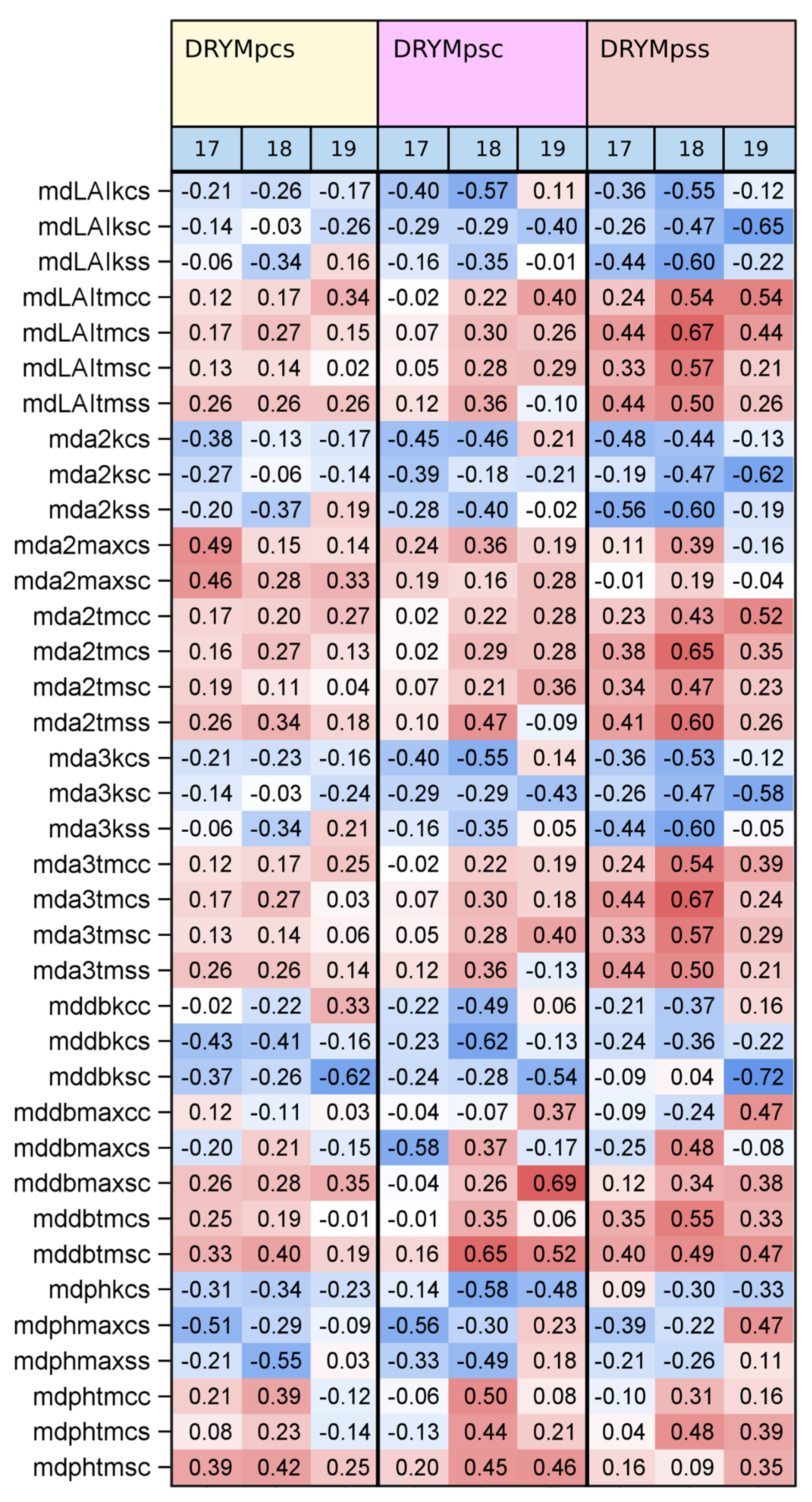
3.3. Relationship between Phenotype and Tolerance
3.4. Multiple Regression Analysis
4. Discussion
4.1. Short-Term Stress Versus Long-Term Stress
4.2. Drought Tolerance Prediction from Phenotypic Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowerman, A.F.; Byrt, C.S.; Roy, S.J.; Whitney, S.M.; Mortimer, J.C.; Ankeny, R.A.; Gilliham, M.; Zhang, D.; Millar, A.A.; Rebetzke, G.J.; et al. Potential abiotic stress targets for modern genetic manipulation. Plant Cell 2022, 35, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, J.; Wood, E.F. Projected changes in drought occurrence under future global warming from multi-model, multi-scenario, IPCC AR4 simulations. Clim. Dyn. 2008, 31, 79–105. [Google Scholar] [CrossRef]
- Lobell, D.B.; Field, C.B. Global scale climate–crop yield relationships and the impacts of recent warming. Environ. Res. Lett. 2007, 2, 014002. [Google Scholar] [CrossRef]
- Elliott, J.; Deryng, D.; Müller, C.; Frieler, K.; Konzmann, M.; Gerten, D.; Glotter, M.; Flörke, M.; Wada, Y.; Best, N.; et al. Constraints and potentials of future irrigation water availability on agricultural production under climate change. Proc. Natl. Acad. Sci. USA 2014, 111, 3239–3244. [Google Scholar] [CrossRef]
- Lerner, D.N.; Harris, B. The relationship between land use and groundwater resources and quality. J. Land Use Policy 2009, 26, S265–S273. [Google Scholar] [CrossRef]
- Wu, W.-Y.; Lo, M.-H.; Wada, Y.; Famiglietti, J.S.; Reager, J.T.; Yeh, P.J.F.; Ducharne, A.; Yang, Z.-L. Divergent effects of climate change on future groundwater availability in key mid-latitude aquifers. Nat. Commun. 2020, 11, 3710. [Google Scholar] [CrossRef]
- Tardieu, F. Any trait or trait-related allele can confer drought tolerance: Just design the right drought scenario. J. Exp. Bot. 2012, 63, 25–31. [Google Scholar] [CrossRef]
- Cooper, M.; Messina, C.D. Breeding crops for drought-affected environments and improved climate resilience. Plant Cell 2022, 35, 162–186. [Google Scholar] [CrossRef]
- Richards, R.A.; Hunt, J.R.; Kirkegaard, J.A.; Passioura, J.B. Yield improvement and adaptation of wheat to water-limited environments in Australia—A case study. Crop Pasture Sci. 2014, 65, 676–689. [Google Scholar] [CrossRef]
- Richards, R.A.; Rebetzke, G.J.; Watt, M.; Condon, A.G.; Spielmeyer, W.; Dolferus, R. Breeding for improved water productivity in temperate cereals: Phenotyping, quantitative trait loci, markers and the selection environment. Funct. Plant Biol. 2010, 37, 85–97. [Google Scholar] [CrossRef]
- Furbank, R.T.; Tester, M. Phenomics—Technologies to relieve the phenotyping bottleneck. Trend Plant Sci. 2011, 16, 635–644. [Google Scholar] [CrossRef]
- Stockem, J.E.; Korontzis, G.; Wilson, S.E.; de Vries, M.E.; van Eeuwijk, F.A.; Struik, P.C. Optimal Plot Dimensions for Performance Testing of Hybrid Potato in the Field. Potato Res. 2022, 65, 417–434. [Google Scholar] [CrossRef]
- Köhl, K.I.; Mulugeta Aneley, G.; Haas, M.; Peters, R. Confounding Factors in Container-Based Drought Tolerance Assessments in Solanum tuberosum. Agronomy 2021, 11, 865. [Google Scholar] [CrossRef]
- Simmons, C.R.; Lafitte, H.R.; Reimann, K.S.; Brugière, N.; Roesler, K.; Albertsen, M.C.; Greene, T.W.; Habben, J.E. Successes and insights of an industry biotech program to enhance maize agronomic traits. Plant Sci. Int. J. Exp. Plant Biol. 2021, 307, 110899. [Google Scholar] [CrossRef]
- Langstroff, A.; Heuermann, M.C.; Stahl, A.; Junker, A. Opportunities and limits of controlled-environment plant phenotyping for climate response traits. Theor. Appl. Genet. 2022, 135, 1–16. [Google Scholar] [CrossRef]
- Bänziger, M.; Setimela, P.S.; Hodson, D.; Vivek, B. Breeding for improved abiotic stress tolerance in maize adapted to southern Africa. Agric. Water Manag. 2006, 80, 212–224. [Google Scholar] [CrossRef]
- Lafitte, H.R.; Blum, A.; Atlin, G. Using secondary traits to help identify drought-tolerant genotypes. In Breeding Rice for Drought-Prone Environments; Fisher, K., Lafitte, R., Fukai, S., Atlin, G., Hardy, B., Eds.; IRRI: Los Banos, Philippines, 2003; pp. 37–48. [Google Scholar]
- Aliche, E.B.; Oortwijn, M.; Theeuwen, T.P.J.M.; Bachem, C.W.B.; Visser, R.G.F.; van der Linden, C.G. Drought response in field grown potatoes and the interactions between canopy growth and yield. Agric. Water Manag. 2018, 206, 20–30. [Google Scholar] [CrossRef]
- Bojaca, C.R.; Garcia, S.J.; Schrevens, E. Analysis of Potato Canopy Coverage as Assessed Through Digital Imagery by Nonlinear Mixed Effects Models. Potato Res. 2011, 54, 237–252. [Google Scholar] [CrossRef]
- Jensen, C.R.; Battilani, A.; Plauborg, F.; Psarras, G.; Chartzoulakis, K.; Janowiak, F.; Stikic, R.; Jovanovic, Z.; Li, G.; Qi, X.; et al. Deficit irrigation based on drought tolerance and root signalling in potatoes and tomatoes. Agric. Water Manag. 2010, 98, 403–413. [Google Scholar] [CrossRef]
- Mulugeta Aneley, G.; Haas, M.; Köhl, K. LIDAR-Based Phenotyping for Drought Response and Drought Tolerance in Potato. Potato Res. 2022. [Google Scholar] [CrossRef]
- Reynolds, M.; Skovmand, B.; Trethowan, R.; Pfeiffer, W. Evaluating a conceptual model for drought tolerance. In Molecular Approaches for the Genetic Improvement of Cereals for Stable Production in Water-Limited Environments; Ribaut, J.M., Poland, S.D., Eds.; CIMMYT: Texcoco, Mexico, 2000; pp. 49–53. [Google Scholar]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trend Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Berger, B.; de Regt, B.; Tester, M. High-throughput phenotyping of plant shoots. Methods Mol. Biol. 2012, 918, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Jindo, K.; Kozan, O.; Iseki, K.; Maestrini, B.; van Evert, F.K.; Wubengeda, Y.; Arai, E.; Shimabukuro, Y.E.; Sawada, Y.; Kempenaar, C. Potential utilization of satellite remote sensing for field-based agricultural studies. Chem. Biol. Technol. Agric. 2021, 8, 58. [Google Scholar] [CrossRef]
- West, H.; Quinn, N.; Horswell, M. Remote sensing for drought monitoring & impact assessment: Progress, past challenges and future opportunities. Remote Sens. Environ. 2019, 232, 111291. [Google Scholar] [CrossRef]
- Kempenaar, C.; Been, T.; Booij, J.; van Evert, F.; Michielsen, J.-M.; Kocks, C. Advances in Variable Rate Technology Application in Potato in The Netherlands. Potato Res. 2017, 60, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Barbedo, J. A Review on the Use of Unmanned Aerial Vehicles and Imaging Sensors for Monitoring and Assessing Plant Stresses. Drones 2019, 3, 40. [Google Scholar] [CrossRef]
- Zhang, J.; Virk, S.; Porter, W.; Kenworthy, K.; Sullivan, D.; Schwartz, B. Applications of Unmanned Aerial Vehicle Based Imagery in Turfgrass Field Trials. Front. Plant Sci. 2019, 10, 279. [Google Scholar] [CrossRef]
- Su, J.; Liu, C.; Coombes, M.; Hu, X.; Wang, C.; Xu, X.; Li, Q.; Guo, L.; Chen, W.-H. Wheat yellow rust monitoring by learning from multispectral UAV aerial imagery. Comput. Electron. Agric. 2018, 155, 157–166. [Google Scholar] [CrossRef]
- YARA. N-SensorTM, How Does It Work? Available online: https://www.yara.de/pflanzenernaehrung/pure-nutrient/info-3-intelligente-stickstoffdungung/funktion-nsensor/ (accessed on 3 March 2023).
- Passioura, J.B. Phenotyping for drought tolerance in grain crops: When is it useful to breeders? Funct. Plant Biol. 2012, 39, 851–859. [Google Scholar] [CrossRef]
- Bellvert, J.; Zarco-Tejada, P.J.; Girona, J.; Fereres, E. Mapping crop water stress index in a ‘Pinot-noir’ vineyard: Comparing ground measurements with thermal remote sensing imagery from an unmanned aerial vehicle. Precis. Agric. 2014, 15, 361–376. [Google Scholar] [CrossRef]
- Zhao, T.; Doll, D.; Wang, D.; Chen, Y. A new framework for UAV-based remote sensing data processing and its application in almond water stress quantification. In Proceedings of the 2017 International Conference on Unmanned Aircraft Systems (ICUAS), Miami, FL, USA, 13–16 June 2017; pp. 1794–1799. [Google Scholar]
- Monneveux, P.; Ramirez, D.A.; Pino, M.T. Drought tolerance in potato (S. tuberosum L.) Can we learn from drought tolerance research in cereals? Plant Sci. 2013, 205, 76–86. [Google Scholar] [CrossRef]
- Xu, X.; Pan, S.; Cheng, S.; Zhang, B.; Mu, D.; Ni, P.; Zhang, G.; Yang, S.; Li, R.; Wang, J.; et al. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar] [CrossRef]
- Caruana, B.M.; Pembleton, L.W.; Constable, F.; Rodoni, B.; Slater, A.T.; Cogan, N.O.I. Validation of Genotyping by Sequencing Using Transcriptomics for Diversity and Application of Genomic Selection in Tetraploid Potato. Front. Plant Sci. 2019, 10, 670. [Google Scholar] [CrossRef]
- Slater, A.T.; Cogan, N.O.I.; Forster, J.W.; Hayes, B.J.; Daetwyler, H.D. Improving genetic gain with genomic selection in autotetraploid potato. Plant Genome 2016, 9, 1–15. [Google Scholar] [CrossRef]
- Cabello, R.; Monneveux, P.; Bonierbale, M.; Khan, M.A. Heritability of yield components under irrigated and drought conditions in andigenum potatoes. Am. J. Potato Res. 2014, 91, 492–499. [Google Scholar] [CrossRef]
- Sprenger, H.; Rudack, K.; Schudoma, C.; Neumann, A.; Seddig, S.; Peters, R.; Zuther, E.; Kopka, J.; Hincha, D.K.; Walther, D.; et al. Assessment of drought tolerance and its potential yield penalty in potato. Funct. Plant Biol. 2015, 42, 655–667. [Google Scholar] [CrossRef]
- Wishart, J.; George, T.S.; Brown, L.K.; White, P.J.; Ramsay, G.; Jones, H.; Gregory, P.J. Field phenotyping of potato to assess root and shoot characteristics associated with drought tolerance. Plant Soil 2014, 378, 351–363. [Google Scholar] [CrossRef]
- Khan, M.S.; Struik, P.C.; van der Putten, P.E.L.; Jansen, H.J.; van Eck, H.J.; van Eeuwijk, F.A.; Yin, X.Y. A model-based approach to analyse genetic variation in potato using standard cultivars and a segregating population. I. Canopy cover dynamics. Field Crops Res. 2019, 242, 107581. [Google Scholar] [CrossRef]
- Khan, M.S.; Yin, X.; van der Putten, P.E.L.; Jansen, H.J.; van Eck, H.J.; van Eeuwijk, F.A.; Struik, P.C. A model-based approach to analyse genetic variation in potato using standard cultivars and a segregating population. II. Tuber bulking and resource use efficiency. Field Crops Res. 2019, 242, 107582. [Google Scholar] [CrossRef]
- Haas, M.; Sprenger, H.; Zuther, E.; Peters, R.; Seddig, S.; Walther, D.; Kopka, J.; Hincha, D.K.; Köhl, K.I. Can metabolite- and transcript-based selection for drought tolerance in Solanum tuberosum replace selection on yield in arid environments? Front. Plant Sci. 2020, 11, 1071. [Google Scholar] [CrossRef]
- Shoot Development of Twenty Potato Cultivars under Early, Late and Long-Term Drought Stress 2023. Available online: https://doi.ipk-gatersleben.de/DOI/10.5447/ipk/2023/9 (accessed on 22 May 2023).
- Köhl, K.I.; Basler, G.; Luedemann, A.; Selbig, J.; Walther, D. A plant resource and experiment management system based on the Golm Plant Database as a basic tool for omics research. Plant Methods 2008, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- van Eeuwijk, F.A.; Bustos-Korts, D.; Millet, E.J.; Boer, M.P.; Kruijer, W.; Thompson, A.; Malosetti, M.; Iwata, H.; Quiroz, R.; Kuppe, C.; et al. Modelling strategies for assessing and increasing the effectiveness of new phenotyping techniques in plant breeding. Plant Sci. 2019, 282, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Archontoulis, S.V.; Miguez, F.E. Nonlinear regression models and applications in agricultural research. Agron. J. 2014, 107, 786–798. [Google Scholar] [CrossRef]
- O’Brien, P.J.; Allen, E.J.; Firman, D.M. REVIEW A review of some studies into tuber initiation in potato (Solanum tuberosum) crops. J. Agric. Sci. 1998, 130, 251–270. [Google Scholar] [CrossRef]
- Puangbut, D.; Jogloy, S.; Toomsan, B.; Vorasoot, N.; Akkasaeng, C.; Kesmala, T.; Rachaputi, R.C.N.; Wright, G.C.; Patanothai, A. DROUGHT STRESS: Physiological Basis for Genotypic Variation in Tolerance to and Recovery from Pre-flowering Drought in Peanut. J. Agron. Crop Sci. 2010, 196, 358–367. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic Variation in Growth and Physiological Response to Drought Stress and Re-Watering Reveals the Critical Role of Recovery in Drought Adaptation in Maize Seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. 2015, 91, 1118–1133. [Google Scholar] [CrossRef]
- Jacques, C.; Salon, C.; Barnard, R.L.; Vernoud, V.; Prudent, M. Drought Stress Memory at the Plant Cycle Level: A Review. Plants 2021, 10, 1873. [Google Scholar] [CrossRef]
- Cavagnaro, J.B.; De Lis, B.R.; Tizio, R.M. Drought hardening of the potato plant as an aftereffect of soil drought conditions at planting. Potato Res. 1971, 14, 181–192. [Google Scholar] [CrossRef]
- Zhang, S.-H.; Xu, X.-F.; Sun, Y.-M.; Zhang, J.-L.; Li, C.-Z. Influence of drought hardening on the resistance physiology of potato seedlings under drought stress. J. Integr. Agric. 2018, 17, 336–347. [Google Scholar] [CrossRef]
- Schumacher, C.; Krannich, C.T.; Maletzki, L.; Köhl, K.; Kopka, J.; Sprenger, H.; Hincha, D.; Seddig, S.; Peters, R.; Hamera, S.; et al. Unravelling differences in candidate genes for drought tolerance in potato (Solanum tuberosum L.) by use of new functional microsatellite markers. Genes 2021, 12, 494. [Google Scholar] [CrossRef]
- John, M.; Haselbeck, F.; Dass, R.; Malisi, C.; Ricca, P.; Dreischer, C.; Schultheiss, S.J.; Grimm, D.G. A comparison of classical and machine learning-based phenotype prediction methods on simulated data and three plant species. Front. Plant Sci. 2022, 13, 932512. [Google Scholar] [CrossRef]
- Montesinos-López, O.A.; Montesinos-López, A.; Crossa, J.; de los Campos, G.; Alvarado, G.; Suchismita, M.; Rutkoski, J.; González-Pérez, L.; Burgueño, J. Predicting grain yield using canopy hyperspectral reflectance in wheat breeding data. Plant Methods 2017, 13, 4. [Google Scholar] [CrossRef]



| Parameter. | DF (Error) | Genotype | Treatment | G × E | Year |
|---|---|---|---|---|---|
| SY | 1619 | 66.3 | 818.6 | 1.86 | 703.6 |
| WUE(SY) | 1619 | 69.9 | 143.2 | 1.38 | 2030.3 |
| TY | 1619 | 74.7 | 675.4 | 1.59 | 333.5 |
| WUE (TY) | 1619 | 78.5 | 96.7 | 1.35 | 1372.0 |
| Starch content | 1619 | 53.0 | 61.5 | 1.26 | 280.9 |
| Tuber number (S) | 1587 | 27.8 | 53.2 | 2.21 | 3.0 |
| Tuber number (M) | 1585 | 40.1 | 435.8 | 1.61 | 92.1 |
| Tuber number (L) | 1585 | 24.3 | 31.0 | 3.27 | 52.1 |
| DRYMp | 1201 | 6.0 | 7.1 | 1.02 | 7.5 |
| Parameter | Y | G | Y×G | E | Y × E | G × E | Y × E × G |
|---|---|---|---|---|---|---|---|
| a2k | 349.26 | 8.64 | 2.88 | 167.69 | 27.74 | 1.54 | 1.30 |
| a2max | 924.69 | 27.13 | 4.85 | 75.50 | 13.21 | 1.10 | 0.94 |
| a2tm | 2285.4 | 20.63 | 7.08 | 5.76 | 1.16 | 1.01 | 0.75 |
| a3k | 362.7 | 10.04 | 2.81 | 182.86 | 22.59 | 1.53 | 1.21 |
| a3max | 1027.4 | 27.27 | 4.28 | 52.00 | 9.19 | 1.09 | 0.97 |
| a3tm | 2257.98 | 22.67 | 6.63 | 4.27 | 1.02 | 1.04 | 0.79 |
| dbk | 291.82 | 8.22 | 3.39 | 691.40 | 94.25 | 1.49 | 0.98 |
| dbmax | 90.52 | 5.05 | 1.71 | 305.74 | 11.35 | 1.28 | 0.88 |
| dbtm | 2003.5 | 19.58 | 6.06 | 203.44 | 11.21 | 1.78 | 0.84 |
| phk | 267.25 | 12.42 | 2.93 | 726.15 | 133.89 | 1.56 | 1.20 |
| phmax | 122.67 | 5.55 | 2.24 | 462.98 | 34.76 | 1.86 | 1.31 |
| phtm | 861.25 | 17.11 | 6.77 | 354.99 | 51.82 | 2.32 | 1.41 |
| LAIk | 356.81 | 9.99 | 2.96 | 172.61 | 22.97 | 1.45 | 1.32 |
| LAImax | 523.95 | 25.78 | 4.12 | 50.20 | 9.38 | 1.17 | 0.98 |
| LAItm | 2288.79 | 22.37 | 6.25 | 4.02 | 1.07 | 0.97 | 0.77 |
| Tolerance | Phenotype | Year | R2 (Full Model) | N (Full Model) | R2 (n ≤ 4) |
|---|---|---|---|---|---|
| DRYMp(ss) | cc | 2017 | 0.98 | 6 | 0.95 |
| 2018 | 0.87 | 6 | 0.79 | ||
| 2019 | 0.75 | 4 | 0.75 | ||
| ss | 2017 | 0.88 | 3 | 0.88 | |
| 2018 | 0.96 | 6 | 0.88 | ||
| 2019 | 0.82 | 5 | 0.78 | ||
| DRYMP(sc) | cc | 2017 | 0.98 | 7 | 0.88 |
| 2018 | 0.89 | 4 | 0.89 | ||
| 2019 | 0.89 | 6 | 0.78 | ||
| sc | 2017 | 0.79 | 4 | 0.79 | |
| 2018 | 0.39 | 2 | 0.39 | ||
| 2019 | 0.997 | 10 | 0.86 | ||
| DRYMP(cs) | cc | 2017 | 0.78 | 5 | 0.75 |
| 2018 | 0.94 | 7 | 0.85 | ||
| 2019 | 0.98 | 8 | 0.83 | ||
| cs | 2017 | 0.999 | 12 | 0.89 | |
| 2018 | 0.999 | 14 | 0.73 | ||
| 2019 | 0.44 | 2 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köhl, K.I.; Aneley, G.M.; Haas, M. Finding Phenotypic Biomarkers for Drought Tolerance in Solanum tuberosum. Agronomy 2023, 13, 1457. https://doi.org/10.3390/agronomy13061457
Köhl KI, Aneley GM, Haas M. Finding Phenotypic Biomarkers for Drought Tolerance in Solanum tuberosum. Agronomy. 2023; 13(6):1457. https://doi.org/10.3390/agronomy13061457
Chicago/Turabian StyleKöhl, Karin I., Gedif Mulugeta Aneley, and Manuela Haas. 2023. "Finding Phenotypic Biomarkers for Drought Tolerance in Solanum tuberosum" Agronomy 13, no. 6: 1457. https://doi.org/10.3390/agronomy13061457
APA StyleKöhl, K. I., Aneley, G. M., & Haas, M. (2023). Finding Phenotypic Biomarkers for Drought Tolerance in Solanum tuberosum. Agronomy, 13(6), 1457. https://doi.org/10.3390/agronomy13061457








