Green Waste from Cucumber (Cucumis sativus L.) Cultivation as a Source of Bioactive Flavonoids with Hypolipidemic Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals
2.3. Total Flavonoid Spectrophotometric Assay
2.4. Flavonoid Extract Isolation
2.5. Liquid Chromatography–Mass Spectrometry Profiling and Quantification
2.6. Hypolipidemic Activity
2.7. Statistical Analysis
3. Results
3.1. Selection of Cucumber Cultivars Based on the Total Flavonoid Content
3.2. Variations in the Total Flavonoid Content in Cucumber Greens Based on Harvest Time and Organic Fertilization
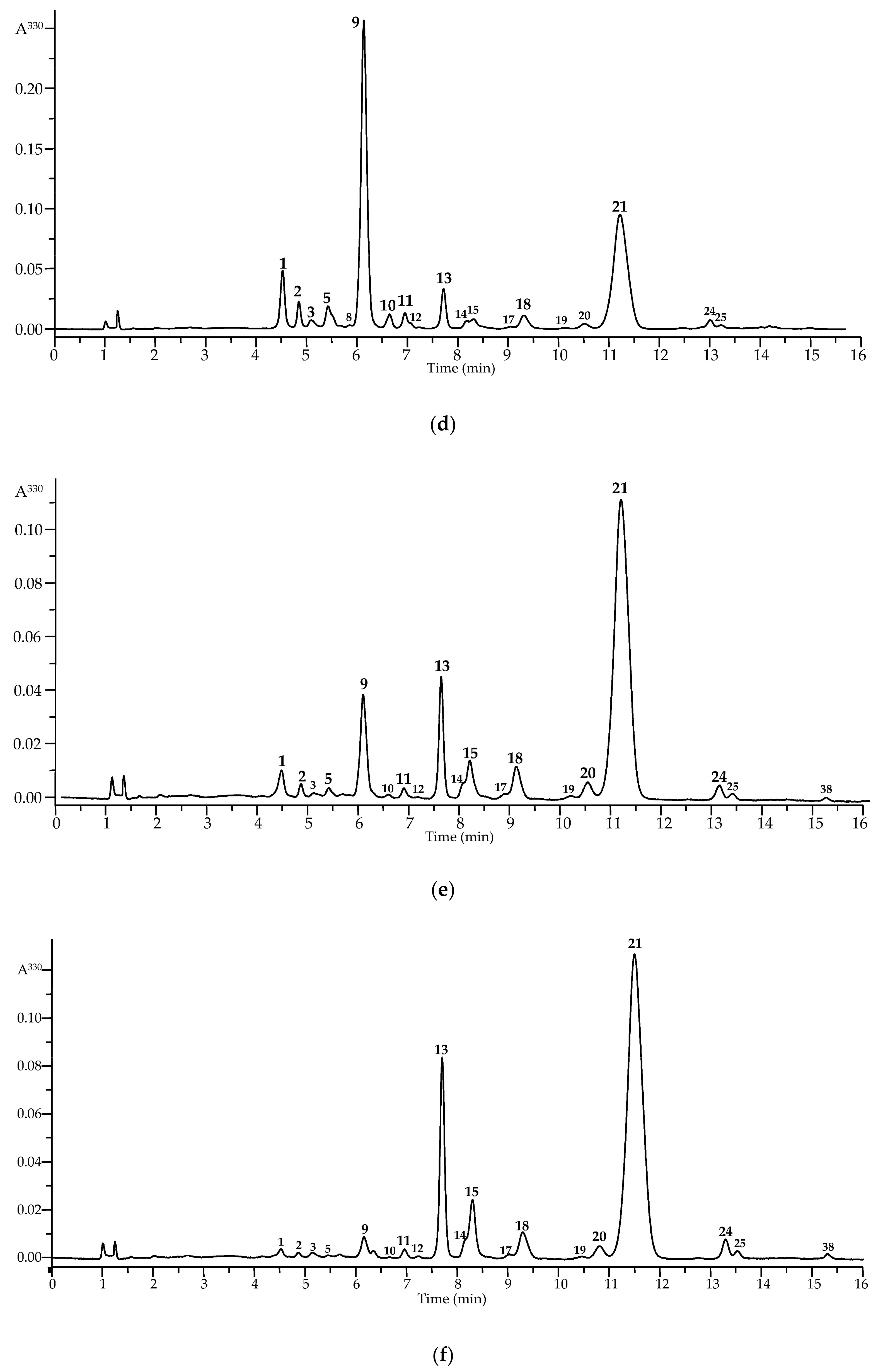
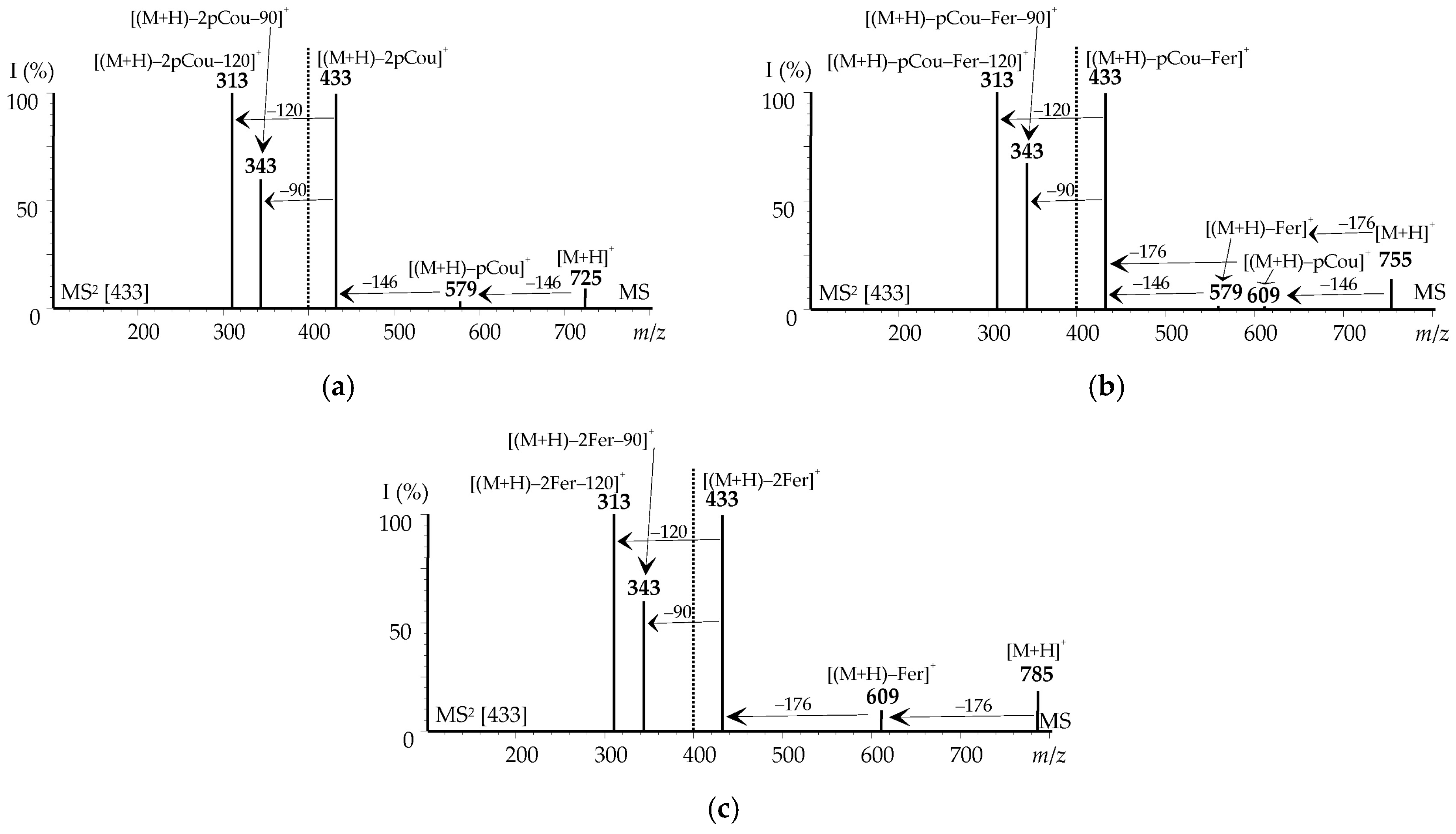
3.3. LC–MS Profiling of Flavonoids in Cucumber Waste Green Biomass Extracts
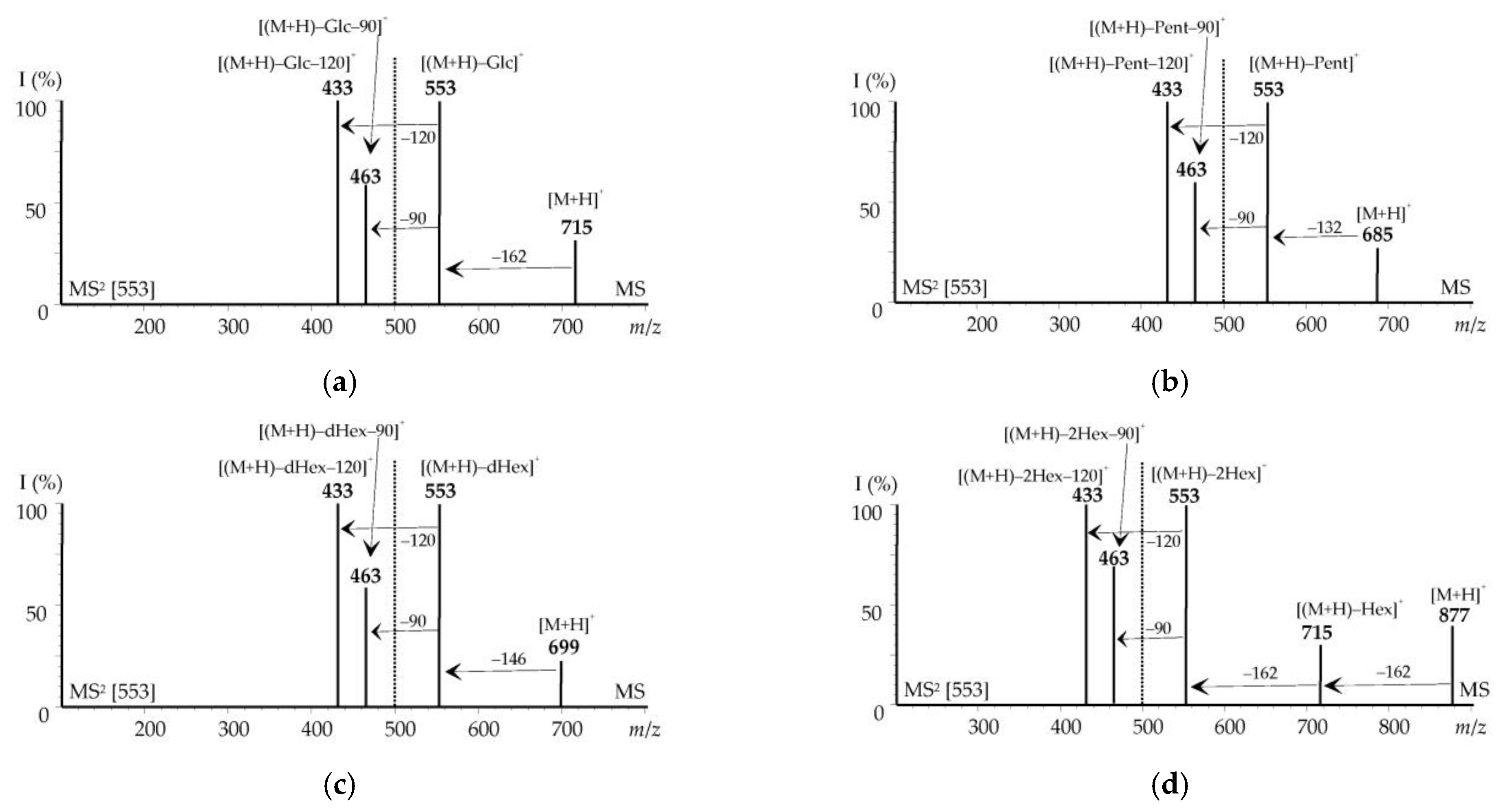
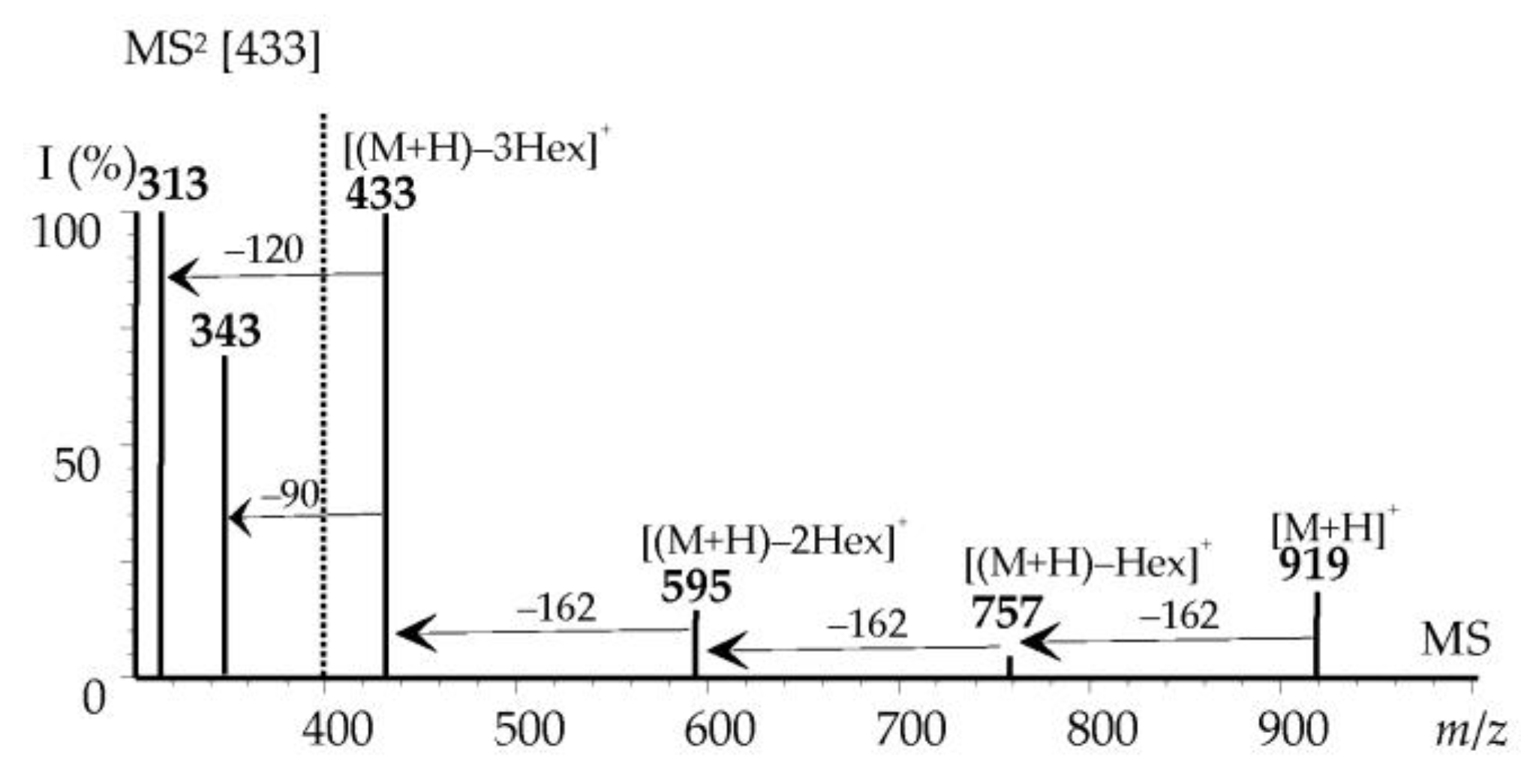
3.3.1. Non-Acylated Flavonoids
3.3.2. Acylated Flavonoids
3.4. Selected Compounds in the Flavonoid Extracts of Cucumber Waste Green Biomass
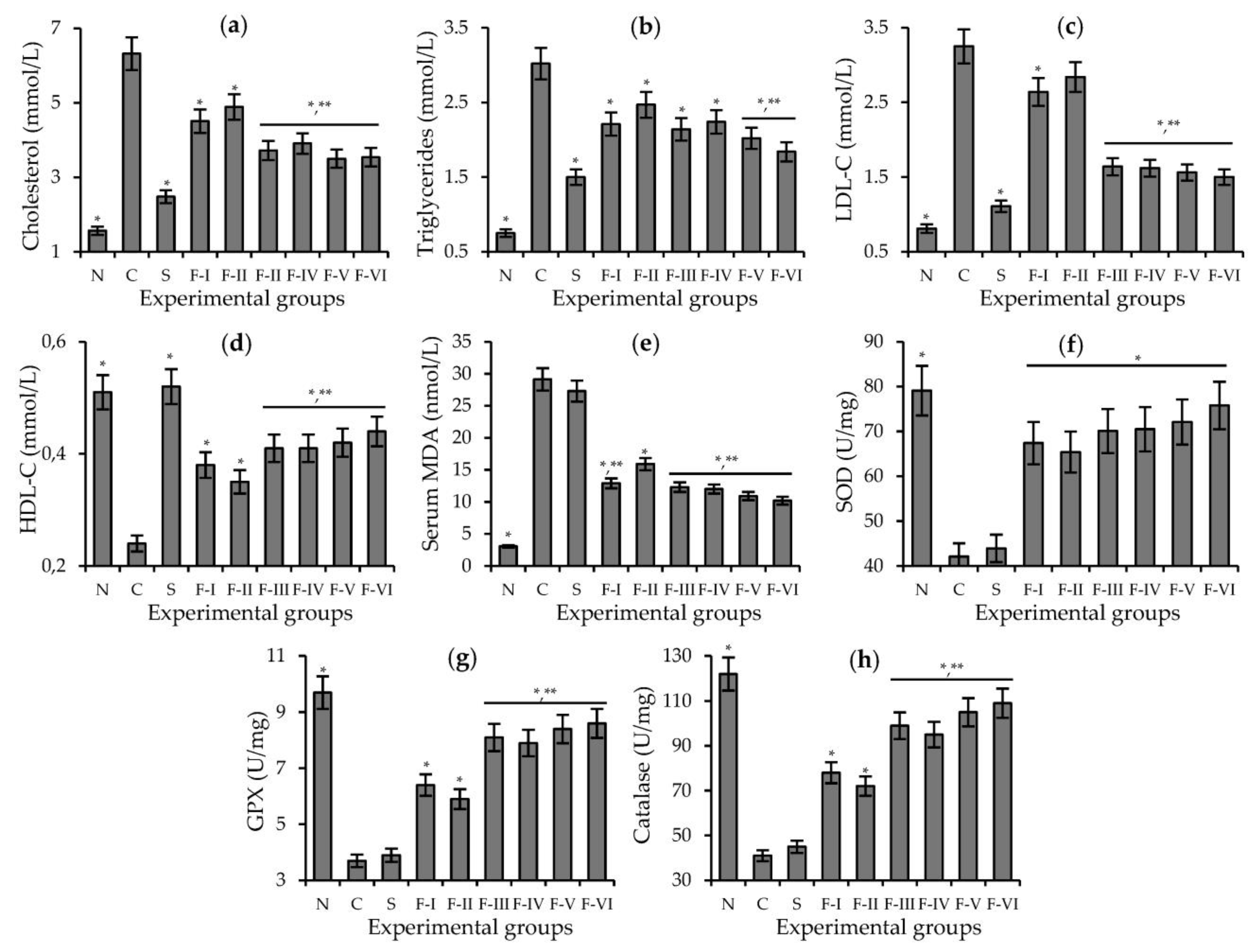
3.5. Hypolipidemic and Antioxidant Potential of the Flavonoid Extract and Selected Compounds in Cucumber Waste Green Biomass on the Hyperlipidemia of Hamsters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, J.; Wu, X.; Wang, Y.; Meyerson, L.A.; Gu, B.; Min, Y.; Xue, H.; Peng, C.; Ge, Y. Does growing vegetables in plastic greenhouses enhance regional ecosystem services beyond the food supply? Front. Ecol. Environ. 2013, 11, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.M.; Rahman, T.T.; Pei, Z.; Ufodike, C.O.; Lee, J.; Elwany, A. Additive manufacturing using agriculturally derived biowastes: A systematic literature review. Bioengineering 2023, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Blasi, A.; Verardi, A.; Lopresto, C.G.; Siciliano, S.; Sangiorgio, P. Lignocellulosic agricultural waste valorization to obtain valuable products: An overview. Recycling 2023, 8, 61. [Google Scholar] [CrossRef]
- Rex, P.; Mohammed Ismail, K.R.; Meenakshisundaram, N.; Barmavatu, P.; Sai Bharadwaj, A.V.S.L. Agricultural biomass waste to biochar: A review on biochar applications using machine learning approach and circular economy. ChemEngineering 2023, 7, 50. [Google Scholar] [CrossRef]
- Atlas Big. World Cucumber Production by Country. Available online: https://www.atlasbig.com/en-us/countries-cucumber-production (accessed on 20 August 2023).
- Gao, Y.; Briers, H.K.; Matharu, A.S.; Fan, J. Exploration of cucumber waste as a potential biorefinery feedstock. Processes 2022, 10, 2694. [Google Scholar] [CrossRef]
- Khan, A.; Mishra, A.; Hasan, S.M.; Usmani, A.; Ubaid, M.; Khan, N.; Saidurrahman, M. Biological and medicinal application of Cucumis sativus Linn.—Review of current status with future possibilities. J. Complement. Integr. Med. 2022, 19, 843–854. [Google Scholar] [CrossRef]
- Rice, C.A.; Rymal, K.S.; Chambliss, O.L.; Johnson, F.A. Chromatographic and mass spectral analysis of cucurbitacins of three Cucumis sativus cultivars. J. Agric. Food Chem. 1981, 29, 194–196. [Google Scholar] [CrossRef]
- Abou-Zaid, M.M.; Lombardo, D.A.; Kite, G.C.; Grayer, R.J.; Veitch, N.C. Acylated flavone C-glycosides from Cucumis sativus. Phytochemistry 2001, 58, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Krauze-Baranowska, M.; Cisowski, W. Flavonoids from some species of the genus Cucumis. Biochem. Syst. Ecol. 2001, 29, 321–324. [Google Scholar] [CrossRef]
- McNally, D.J.; Wurms, K.V.; Labbé, C.; Quideau, S.; Bélanger, R.R. Complex C-glycosyl flavonoid phytoalexins from Cucumis sativus. J. Nat. Prod. 2003, 66, 1280–1283. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. New flavonoids from Cucumis sativus. Chem. Nat. Compd. 2023, 58, 651–654. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Acylated flavonoids from Cucumis sativus inhibit the activity of human pancreatic lipase. Appl. Biochem. Microbiol. 2023, 59, 530–538. [Google Scholar] [CrossRef]
- Olennikov, D.N. Separation, characterization and mammal pancreatic lipase inhibitory potential of cucumber flower flavonoids. Separations 2023, 10, 255. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Ruan, J.; Wang, W.; Chen, J.; Zhang, Q. Structure-activity relationship of dietary flavonoids on pancreatic lipase. Curr. Res. Food Sci. 2022, 6, 100424. [Google Scholar] [CrossRef] [PubMed]
- Kostov, O.; Tzvetkov, Y.; Kaloianova, N.; Van Cleemput, O. Cucumber cultivation on some wastes during their aerobic composting. Biores. Technol. 1995, 53, 237–242. [Google Scholar] [CrossRef]
- Lowe, T.B.; Hatch, B.T.; Antle, C.; Nartker, S.; Ammerman, M.L. One and two-stage anaerobic co-digestion of cucumber waste and sewage sludge. Environ. Technol. 2020, 41, 3157–3165. [Google Scholar] [CrossRef] [PubMed]
- Jaouhari, Y.; Travaglia, F.; Giovannelli, L.; Picco, A.; Oz, E.; Oz, F.; Bordiga, M. From industrial food waste to bioactive ingredients: A review on the sustainable management and transformation of plant-derived food waste. Foods 2023, 12, 2183. [Google Scholar] [CrossRef]
- Ries, J.; Chen, Z.; Park, Y. Potential applications of food-waste-based anaerobic digestate for sustainable crop production practice. Sustainability 2023, 15, 8520. [Google Scholar] [CrossRef]
- Solano Porras, R.C.; Artola, A.; Barrena, R.; Ghoreishi, G.; Ballardo Matos, C.; Sánchez, A. Breaking new ground: Exploring the pomising role of solid-state fermentation in harnessing natural biostimulants for sustainable agriculture. Processes 2023, 11, 2300. [Google Scholar] [CrossRef]
- Khan, Z.S.; Amir, S.; Sokač Cvetnić, T.; Jurinjak Tušek, A.; Benković, M.; Jurina, T.; Valinger, D.; Gajdoš Kljusurić, J. Sustainable isolation of bioactive compounds and proteins from plant-based food (and byproducts). Plants 2023, 12, 2904. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K. New C,O-glycosylflavones from Melandrium divaricatum. Chem. Nat. Compd. 2019, 55, 1032–1038. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K. New compounds from Siberian Gentiana species. II. Xanthone and C,O-glycosylflavone. Chem. Nat. Compd. 2021, 57, 681–684. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chemposov, V.V.; Chirikova, N.K. Polymeric compounds of lingonberry waste: Characterization of antioxidant and hypolipidemic polysaccharides and polyphenol-polysaccharide conjugates from Vaccinium vitis-idaea press cake. Foods 2022, 11, 2801. [Google Scholar] [CrossRef] [PubMed]
- State Commission of the Russian Federation for Testing and Protection of Breeding Achievements (FGBU “Gossortkomissia”). Available online: https://reestr.gossortrf.ru (accessed on 20 August 2023).
- An, L.; Wang, J.; Liu, Y.; Chen, T.; Xu, S.; Feng, H.; Wang, X. The effects of enhanced UV-B radiation on growth, stomata, flavonoid, and ABA content in cucumber leaves. Proc. SPIE 2003, 4896, 223–231. [Google Scholar] [CrossRef]
- Insanu, M.; Zahra, A.A.; Sabila, N.; Silviani, V.; Haniffadli, A.; Rizaldy, D.; Fidrianny, I. Phytochemical and antioxidant profile: Cucumber pulp and leaves extracts. Maced. J. Med. Sci. 2022, 10, 616–622. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Paglia, K.; Vaniya, A.; Wancewicz, B.; Keller, A.A. Metabolomics reveals the molecular mechanisms of copper induced cucumber leaf (Cucumis sativus) senescence. Environ. Sci. Technol. 2018, 52, 7092–7100. [Google Scholar] [CrossRef] [PubMed]
- Dannehl, D.; Becker, C.; Suhl, J.; Josuttis, M.; Schmid, U. Reuse of organomineral substrate waste from hydroponic systems as fertilizer in open-field production of red oak leaf lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2016, 64, 7068–7075. [Google Scholar] [CrossRef]
- Ozdemir, G.; Kitir, N.; Turan, M.; Ozlu, E. Impacts of organic and organo-mineral fertilizers on total phenolic, flavonoid, anthocyanin and antiradical activity of okuzgozu (Vitis vinifera L.) grapes. Acta Sci. Polon. Hort. Cultus 2018, 17, 91–100. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N.; Mladenović, J. The influence of organic, organo-mineral and mineral fertilizers on tree growth, yielding, fruit quality and leaf nutrient composition of apple cv. ‘Golden Delicious Reinders’. Sci. Hort. 2022, 297, 110978. [Google Scholar] [CrossRef]
- El-Mageed, A.T.A.; Semida, W.M. Organo mineral fertilizer can mitigate water stress for cucumber production (Cucumis sativus L.). Agric. Water Manag. 2015, 159, 1–10. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Gadimli, A.I.; Isaev, J.I.; Kashchenko, N.I.; Prokopyev, A.S.; Kataeva, T.N.; Chirikova, N.K.; Vennos, C. Caucasian Gentiana species: Untargeted LC-MS metabolic profiling, antioxidant and digestive enzyme inhibiting activity of six plants. Metabolites 2019, 9, 271. [Google Scholar] [CrossRef]
- Goetz, M.; Jacot-Guillarmod, A. Phytochemistry of genus Gentiana. XX. Identification of new di-O-glucosides of C-glucosylflavones in Gentiana asclepiadea. Helv. Chim. Acta 1977, 60, 1322–1324. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Olennikov, D.N.; Chirikova, N.K. Phytohormones and elicitors enhanced the ecdysteroid and glycosylflavone content and antioxidant activity of Silene repens. Appl. Sci. 2021, 11, 11099. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. New C,O-glycosylflavones from the genus Silene. Chem. Nat. Comp. 2020, 56, 1026–1034. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Olennikov, D.N.; Chirikova, N.K. Metabolites of Geum aleppicum and Sibbaldianthe bifurca: Diversity and α-glucosidase inhibitory potential. Metabolites 2023, 13, 689. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Nikolaev, V.M.; Chirikova, N.K. Sagan Dalya tea, a new “old” probable adaptogenic drug: Metabolic characterization and bioactivity potentials of Rhododendron adamsii leaves. Antioxidants 2021, 10, 863. [Google Scholar] [CrossRef]
- Kachlicki, P.; Piasecka, A.; Stobiecki, M.; Marczak, Ł. Structural characterization of flavonoid glycoconjugates and their derivatives with mass spectrometric techniques. Molecules 2016, 21, 1494. [Google Scholar] [CrossRef]
- Schmidt, J.; Kuck, D.; Franke, K.; Sultani, H.; Laub, A.; Wessjohann, L.A. The unusual fragmentation of long-chain feruloyl esters under negative ion electrospray conditions. J. Mass Spectrom. 2019, 54, 549–556. [Google Scholar] [CrossRef]
- Brown, G.B.; Deakin, J.R.; Wood, M.B. Identification of Cucumis species by paper chromatography of flavonoids. J. Amer. Soc. Hort. Sci. 1969, 94, 231–234. [Google Scholar] [CrossRef]
- Assefa, A.D.; Choi, S.; Lee, J.E.; Sung, J.S.; Hur, O.S.; Ro, N.Y.; Lee, H.S.; Jang, S.W.; Rhee, J.H. Identification and quantification of selected metabolites in differently pigmented leaves of lettuce (Lactuca sativa L.) cultivars harvested at mature and bolting stages. BMC Chem. 2019, 13, 56. [Google Scholar] [CrossRef]
- Beier, R.C.; Ivie, G.W.; Oertli, E.H.; Holt, D.L. HPLC analysis of linear furocoumarins (psoralens) in healthy celery (Apium graveolens). Food Chem. Toxicol. 1983, 21, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N. Coumarins of lovage roots (Levisticum officinale W.D.J.Koch): LC-MS profile, quantification, and stability during postharvest storage. Metabolites 2023, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Khandy, M.T.; Chirikova, N.K. Oriental strawberry metabolites: LC–MS profiling, antioxidant potential, and postharvest changes of Fragaria orientalis fruits. Horticulturae 2022, 8, 975. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Kou, X.; Wang, Y.; Yu, Y.; Zhen, N.; Jiang, J.; Zhaxi, P.; Xue, Z. Synergistic hypolipidemic effects and mechanisms of phytochemicals: A review. Foods 2022, 11, 2774. [Google Scholar] [CrossRef]
- Sudheesh, S.; Presannakumar, G.; Vijayakumar, S. Hypolipidemic effect of flavonoids from Solanum melongena. Plant Foods Hum. Nutr. 1997, 51, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yu, C.; Ying, K.; Hua, J.; Dai, X. Hypolipidemic and antioxidant effects of total flavonoids of Perilla Frutescens leaves in hyperlipidemia rats induced by high-fat diet. Food Res. Int. 2011, 44, 404–409. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zhou, L. Hypolipidemic and antioxidant effects of flavonoids from Hippophae rhamnoides L. pomace in ICR mice with alloxan induced diabetes. Food Sci. 2010, 31, 297–301. [Google Scholar]
- Brajawikalpa, R.S.; Nidhamuddin, U.; Loebis, I.M. Hypolipidemic effect of avocado peel (Persea americana Mill.) extract in rats with dyslipidemia. J. Phys. Conf. Ser. 2020, 1665, 012016. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Z.; Chen, L.; Sun, G. Hypolipidemic effects and preliminary mechanism of chrysanthemum flavonoids, its main components luteolin and luteoloside in hyperlipidemia rats. Antioxidants 2021, 10, 1309. [Google Scholar] [CrossRef]
- Kurowska, E.M.; Manthey, J.A. Hypolipidemic effects and absorption of citrus polymethoxylated flavones in hamsters with diet-induced hypercholesterolemia. J. Agric. Food Chem. 2004, 52, 2879–2886. [Google Scholar] [CrossRef]
- Hu, H.; Weng, J.; Cui, C.; Tang, F.; Yu, M.; Zhou, Y.; Shao, F.; Zhu, Y. The hypolipidemic effect of hawthorn leaf flavonoids through modulating lipid metabolism and gut microbiota in hyperlipidemic rats. Evid. Based Compl. Altern. Med. 2022, 2022, 3033311. [Google Scholar] [CrossRef] [PubMed]
- Aldayel, T.S. Apigenin attenuates high-fat diet-induced nephropathy in rats by hypoglycemic and hypolipidemic effects, and concomitant activation of the Nrf2/antioxidant axis. J. Funct. Foods 2022, 99, 105295. [Google Scholar] [CrossRef]
- Lu, J.; Meng, Z.; Cheng, B.; Liu, M.; Tao, S.; Guan, S. Apigenin reduces the excessive accumulation of lipids induced by palmitic acid via the AMPK signaling pathway in HepG2 cells. Exp. Ther. Med. 2019, 18, 2965–2971. [Google Scholar] [CrossRef]
- Wu, L.; Guo, T.; Deng, R.; Liu, L.; Yu, Y. Apigenin ameliorates insulin resistance and lipid accumulation by endoplasmic reticulum stress and SREBP-1c/SREBP-2 pathway in palmitate-induced HepG2 cells and high-fat diet-fed mice. J. Pharmacol. Exp. Ther. 2021, 377, 146–156. [Google Scholar] [CrossRef]
- Todd, P.A.; Goa, K.L. Simvastatin. Drugs 1990, 40, 583–607. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Yamamoto, K.; Kato, Y.; Nagatomo, A.; Matsuda, H. Floratheasaponins A−C, acylated oleanane-type triterpene oligoglycosides with anti-hyperlipidemic activities from flowers of the tea plant (Camellia sinensis). J. Nat. Prod. 2005, 68, 1360–1365. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, M.-Y.; Cho, J.Y. Apigenin: A therapeutic agent for treatment of skin inflammatory diseases and cancer. Int. J. Mol. Sci. 2023, 24, 1498. [Google Scholar] [CrossRef]
- Boo, Y.C. p-Coumaric acid as an active ingredient in cosmetics: A review focusing on its antimelanogenic effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, H.; Yu, X.; Sui, D.; Lin, G. Hypolipidemic effects of total flavonoid extracted from the leaves of Actinidia kolomikta in rats fed a high-fat diet. Iran J. Basic Med. Sci. 2017, 20, 1141–1148. [Google Scholar] [CrossRef]
- Ling, Y.; Shi, Z.; Yang, X.; Cai, Z.; Wang, L.; Wu, X.; Ye, A.; Jiang, J. Hypolipidemic effect of pure total flavonoids from peel of Citrus (PTFC) on hamsters of hyperlipidemia and its potential mechanism. Exp. Gerontol. 2020, 130, 110786. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, X. Flavonoid-rich extract of Polygonum capitatum attenuates high-fat diet–induced atherosclerosis development and inflammatory and oxidative stress in hyperlipidemia rats. Eur. J. Inflamm. 2018, 16, 2058739218772710. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Xu, C.; Wang, B.; Yu, J.; Kang, X.; Liu, X.; Zhou, L.; Qin, Y.; Liao, L.; et al. Effects of total flavonoids extracted from Polygonum perfoliatum L. on hypolipidemic and antioxidant in hyperlipidemia rats induced by high-fat diet. Int. J. Clin. Exp. Med. 2018, 11, 6758–6766. Available online: https://e-century.us/files/ijcem/11/7/ijcem0069825.pdf (accessed on 20 August 2023).
- Hou, Z.; Zhu, L.; Meng, R.; Wang, B. Hypolipidemic and antioxidant activities of Trichosanthes kirilowii maxim seed oil and flavonoids in mice fed with a high-fat diet. J. Food Biochem. 2020, 44, e13272. [Google Scholar] [CrossRef]
- Nekohashi, M.; Ogawa, M.; Ogihara, T.; Nakazawa, K.; Kato, H.; Misaka, T.; Abe, K.; Kobayashi, S. Luteolin and quercetin affect the cholesterol absorption mediated by epithelial cholesterol transporter niemann-pick c1-like 1 in caco-2 cells and rats. PLoS ONE 2014, 9, e97901. [Google Scholar] [CrossRef]
- Wong, T.Y.; Tan, Y.Q.; Lin, S.M.; Leung, L.K. Apigenin and luteolin display differential hypocholesterolemic mechanisms in mice fed a high-fat diet. Biomed. Pharm. 2017, 96, 1000–1007. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef]




| Cultivar | Extract Yield, % of Dry Plant Weight | Flavonoid Content, % of Dry Plant Weight |
|---|---|---|
| Altai | 1.2 ± 0.0 | 90.83 ± 1.84 |
| Konkurent | 1.6 ± 0.0 | 90.65 ± 1.86 |
| Masha | 3.4 ± 0.1 | 92.61 ± 2.11 |
| Parizhskii Kornishon | 2.8 ± 0.1 | 90.33 ± 2.01 |
| Zasolochnii | 1.2 ± 0.0 | 91.51 ± 1.93 |
| Zozula | 2.0 ± 0.1 | 91.04 ± 2.08 |
| Cultivar | TFC in Leaves, mg/g DPW | TFC in Stems, mg/g DPW | Cultivar Group |
|---|---|---|---|
| Masha | 52.11 ± 1.04 a | 2.17 ± 0.04 a | Cultivars with high flavonoid content: >40 mg/g in leaves, >1 mg/g in stems |
| Parizhskii Kornishon | 48.63 ± 0.97 b | 2.02 ± 0.04 ab | |
| Zozula | 46.82 ± 0.93 bc | 1.92 ± 0.04 b | |
| Zasolochnii | 45.79 ± 0.91 c | 1.27 ± 0.03 c | |
| Altai | 40.26 ± 0.80 d | 1.63 ± 0.03 b | |
| Konkurent | 40.01 ± 0.81 d | 1.52 ± 0.03 b | |
| Alfavit | 32.67 ± 0.65 e | 0.80 ± 0.02 d | Cultivars with medium flavonoid content: 10–40 mg/g in leaves, 0.1–1 mg/g in stems |
| Nezhinskii | 30.09 ± 0.60 e | 0.83 ± 0.02 d | |
| Arcadia | 25.11 ± 0.50 f | 0.64 ± 0.02 e | |
| Titus | 23.67 ± 0.45 f | 0.30 ± 0.01 g | |
| Dachnyi | 20.82 ± 0.41 g | 0.43 ± 0.02 f | |
| Perseus | 18.63 ± 0.37 h | 0.35 ± 0.01 fg | |
| Sibirskaya Girlyanda | 17.14 ± 0.34 h | 0.15 ± 0.00 h | |
| Madhur | 15.22 ± 0.30 i | 0.27 ± 0.01 g | |
| Amur | 15.08 ± 0.30 i | 0.25 ± 0.00 g | |
| April | 14.29 ± 0.26 i | 0.61 ± 0.02 e | |
| Tulsi | 11.27 ± 0.20 j | 0.14 ± 0.00 h | |
| Sibirskii Express | 8.62 ± 0.17 k | ND | Cultivars with low flavonoid content: <10 mg/g in leaves, <0.1 mg/g in stems |
| Dinamit | 7.29 ± 0.14 kl | 0.08 ± 0.00 i | |
| Strelets | 7.04 ± 0.12 l | ND | |
| Kurazh | 6.33 ± 0.12 lm | ND | |
| Tornado | 6.27 ± 0.11 m | ND | |
| Shruti | 6.02 ± 0.11 mn | 0.05 ± 0.00 j | |
| Parisian Gherkin | 5.71 ± 0.14 n | ND | |
| Secret | 3.75 ± 0.07 o | 0.06 ± 0.00 ij | |
| Holland Yellow | 3.56 ± 0.06 o | ND | |
| Kartoshka | 2.83 ± 0.05 p | ND | |
| Sikkim | 1.69 ± 0.03 q | ND | |
| Lemon | 1.54 ± 0.03 q | ND | |
| Crystall Apple | 1.14 ± 0.02 r | ND |
| Fertilizer Concentration | Total Flavonoid Content, mg/g Dry Plant Weight (±S.D.) | |||
|---|---|---|---|---|
| 1st Month | 2nd Month | 3rd Month | 4th Month | |
| cv. Masha | ||||
| 0.00% | 28.11 ± 2.52 | 29.52 ± 2.30 | 31.28 ± 2.53 | 30.63 ± 2.48 |
| 0.01% | 31.27 ± 2.84 | 31.67 ± 2.51 | 32.84 ± 2.69 | 31.62 ± 2.94 |
| 0.05% | 35.69 ± 3.21 * | 36.84 ± 3.31 * | 41.81 ± 3.26 *,** | 40.83 ± 3.14 *,** |
| 0.10% | 36.07 ± 3.54 * | 36.96 ± 3.39 * | 42.67 ± 3.49 *,** | 41.85 ± 3.30 *,** |
| cv. Parizhskii Kornishon | ||||
| 0.00% | 21.67 ± 1.73 | 22.12 ± 1.83 | 22.54 ± 1.78 | 20.81 ± 1.65 |
| 0.01% | 22.16 ± 1.77 | 23.93 ± 1.63 | 24.08 ± 1.92 | 22.08 ± 1.67 |
| 0.05% | 25.18 ± 2.01 * | 27.04 ± 2.14 * | 32.69 ± 2.61 *,** | 30.82 ± 2.40 *,** |
| 0.10% | 25.96 ± 2.11 * | 27.96 ± 2.21 * | 32.93 ± 2.69 *,** | 31.07 ± 2.35 *,** |
| cv. Zozula | ||||
| 0.00% | 17.80 ± 1.42 | 19.63 ± 1.50 | 20.08 ± 1.60 | 20.31 ± 1.60 |
| 0.01% | 18.22 ± 1.47 | 20.11 ± 1.59 | 21.86 ± 1.66 | 21.59 ± 1.75 |
| 0.05% | 22.09 ± 1.79 * | 24.83 ± 1.89 * | 25.69 ± 2.05 *,** | 25.06 ± 2.01 *,** |
| 0.10% | 22.53 ± 1.83 * | 25.69 ± 1.93 * | 26.37 ± 2.12 *,** | 25.93 ± 2.11 *,** |
| cv. Konkurent | ||||
| 0.00% | 14.02 ± 1.12 | 14.02 ± 1.14 | 14.27 ± 1.16 | 14.63 ± 1.20 |
| 0.01% | 14.93 ± 1.22 | 14.69 ± 1.21 | 15.89 ± 1.29 | 15.92 ± 1.29 |
| 0.05% | 17.03 ± 1.40 * | 17.27 ± 1.42 * | 20.93 ± 1.52 *,** | 20.53 ± 1.51 *,** |
| 0.10% | 17.43 ± 1.48 * | 17.53 ± 1.40 * | 21.22 ± 1.60 *,** | 20.99 ± 1.53 *,** |
| cv. Zasolochnii | ||||
| 0.00% | 10.42 ± 0.83 | 10.83 ± 0.80 | 11.93 ± 0.97 | 11.47 ± 0.95 |
| 0.01% | 10.93 ± 0.89 | 11.27 ± 0.92 | 12.89 ± 1.05 | 12.93 ± 1.01 |
| 0.05% | 14.22 ± 1.14 * | 14.97 ± 1.25 * | 16.83 ± 1.45 *,** | 16.29 ± 1.42 *,** |
| 0.10% | 14.59 ± 1.21 * | 15.18 ± 1.26 * | 17.22 ± 1.49 *,** | 16.97 ± 1.53 *,** |
| cv. Altai | ||||
| 0.00% | 8.97 ± 0.75 | 9.04 ± 0.70 | 9.18 ± 0.75 | 9.02 ± 0.72 |
| 0.01% | 10.22 ± 0.85 | 10.54 ± 0.82 | 10.69 ± 0.85 | 10.75 ± 0.82 |
| 0.05% | 11.83 ± 0.95 * | 11.93 ± 0.99 * | 15.22 ± 1.22 *,** | 15.02 ± 1.20 *,** |
| 0.10% | 12.69 ± 1.04 * | 12.90 ± 1.07 * | 15.89 ± 0.27 *,** | 15.93 ± 1.22 *,** |
| No. | t, min | UV Pattern a | ESI–MS, [M + H]−, m/z | Compound [Ref.] | IL b | Found in Cultivar c | Early Data d | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | |||||||
| 1 | 4.45 | A | 715 | Cucumerin C [12] | 1 | + | + | + | + | + | + | L [12], F [14] |
| 2 | 4.83 | A | 715 | Cucumerin D [12] | 1 | + | + | + | + | + | + | L [12], F [14] |
| 3 | 5.11 | A | 685 | Cucumerin A/B O-pentoside [11,12] | 2 | + | − | + | + | + | + | |
| 4 | 5.18 | A | 919 | Apigenin-C-hexoside-tri-O-hexoside [9,13,33] | 2 | + | + | − | − | − | − | |
| 5 | 5.42 | A | 757 | Isovitexin-7,4′-O-glucoside (saponarin-4′-O-glucoside) [33,34] | 1 | + | + | + | + | + | + | L [12], L* [9] |
| 6 | 5.47 | A | 699 | Cucumerin A/B O-desoxy hexoside [11,12] | 2 | + | − | − | − | − | − | |
| 7 | 5.63 | A | 757 | Isovitexin-4′,2′′-O-glucoside [33,34] | 1 | + | + | − | − | − | − | L [12] |
| 8 | 5.80 | A | 699 | Cucumerin A/B O-desoxyhexoside [11,12] | 2 | + | − | + | + | − | − | |
| 9 | 6.02 | B | 903 | Apigenin-C-hexoside-di-O-hexoside-O-p-coumarate [9,13,33] | 2 | + | + | + | + | + | + | |
| 10 | 6.53 | A | 757 | Isovitexin-7-O-(6′′-O-glucosyl)-glucoside [35] | 1 | + | + | + | + | + | + | |
| 11 | 6.90 | B | 903 | Isovitexin-4′-O-glucoside-2′′-O-(6′′′′- O-p-coumaroyl)-glucoside [9,13] | 1 | + | + | + | + | + | + | L [13], L* [9] |
| 12 | 7.09 | B | 933 | Apigenin-C-hexoside-di-O-hexoside-O-ferulate [9,13,33] | 2 | + | − | − | + | + | + | |
| 13 | 7.53 | C | 933 | Isovitexin-4′-O-glucoside-2′′-O-(6′′′′- O-feruloyl)-glucoside [9,13] | 1 | + | + | + | + | + | + | L [13], L* [9] |
| 14 | 8.03 | B | 903 | Apigenin-C-hexoside-di-O-hexoside-O-p-coumarate [9,13,33] | 2 | + | − | − | + | + | + | |
| 15 | 8.17 | A | 595 | Isovitexin-2′′-O-glucoside (meloside A) [34,36] | 1 | + | − | + | + | + | + | L [10,12] |
| 16 | 8.46 | A | 595 | Isovitexin-4′-O-glucoside [34,36] | 1 | + | + | − | − | − | − | |
| 17 | 8.82 | C | 933 | Apigenin-C-hexoside-di-O-hexoside-O-ferulate [3,9,13] | 2 | + | − | − | + | + | + | |
| 18 | 9.20 | B | 903 | Apigenin-C-hexoside-di-O-hexoside-O-p-coumarate [9,13,33] | 2 | + | + | + | + | + | + | |
| 19 | 10.24 | C | 609 | Apigenin-7-O-(6′′-O-feruloyl)-glucoside (saponarin-6′′-O-ferulate) [35] | 1 | + | − | + | + | + | + | F [14] |
| 20 | 10.63 | B | 741 | Isovitexin-2′′-O-(6′′′′-O-p-coumaroyl)-glucoside [9,13] | 1 | + | + | + | + | + | + | L [13], L* [9] |
| 21 | 11.27 | B | 741 | Isovitexin-2′′-O-glucoside-6′′-O-p-coumarate [13] | 1 | + | − | + | + | + | + | L [13] |
| 22 | 12.61 | C | 771 | Isovitexin-2′′-O-glucoside-6′′-O-ferulate [13] | 1 | + | + | − | − | − | − | L [13] |
| 23 | 12.94 | B | 741 | Apigenin-C-hexoside-O-hexoside-O-p-coumarate [9,13,33] | 2 | + | − | − | − | − | − | |
| 24 | 13.18 | C | 771 | Isovitexin-2′′-O-(6′′′′-O-feruloyl)-glucoside [9,13] | 1 | + | + | + | + | + | + | L [13], L* [9] |
| 25 | 13.47 | B | 579 | Isovitexin-2′′-O-p-coumarate [33] | 1 | + | − | + | + | + | + | |
| 26 | 3.62 | A | 877 | Cucumerin C/D O-hexoside [11,12] | 2 | − | + | − | − | − | − | |
| 27 | 3.71 | A | 877 | Cucumerin C/D O-hexoside [11,12] | 2 | − | + | − | − | − | − | |
| 28 | 6.98 | A | 595 | Isovitexin-7-O-glucoside (saponarin) [35] | 1 | − | + | − | − | − | − | L [12], L* [9], F [14] |
| 29 | 7.24 | B | 741 | Apigenin-C-hexoside-O-hexoside-O-p-coumarate [9,13,33] | 2 | − | + | − | − | − | − | |
| 30 | 7.71 | B | 741 | Apigenin-C-hexoside-O-hexoside-O-p-coumarate [9,13,33] | 2 | − | + | − | − | − | − | |
| 31 | 7.92 | B | 741 | Apigenin-C-hexoside-O-hexoside-O-p-coumarate [9,13,33] | 2 | − | + | − | − | − | − | |
| 32 | 9.31 | A | 579 | Isovitexin-2′′-O-rhamnoside [34,36] | 1 | − | + | − | − | − | − | L [12] |
| 33 | 9.94 | A | 433 | Isovitexin [36] | 1 | − | + | − | − | − | − | L [10,12], L* [9,11], F [14] |
| 34 | 13.54 | C | 947 | Isovitexin-2′′-O-(6′′′′-O-feruloyl)-glucoside-6′′-O-ferulate [13] | 1 | − | + | − | − | − | − | L [13] |
| 35 | 13.81 | B | 579 | Isovitexin-6′′-O-p-coumarate [34,36] | 1 | − | + | − | − | − | − | |
| 36 | 14.27 | B | 725 | Apigenin-C-hexoside-di-O-p-coumarate [9,13,33] | 2 | − | + | − | − | − | − | |
| 37 | 14.81 | C | 755 | Apigenin-C-hexoside-O-p-coumarate-O-ferulate [9,13,33] | 2 | − | + | − | − | − | − | |
| 38 | 15.21 | C | 785 | Apigenin-C-hexoside-di-O-ferulate [9,13,33] | 2 | − | − | − | − | + | + | |
| Compound | Content in Flavonoid Extract from the Cultivar a, mg/g ± S.D. | |||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | |
| Non-acylated flavone C- and C,O-glycosides | ||||||
| Isovitexin | - | <1.00 | - | - | - | - |
| Isovitexin-7-O-glucoside | - | 6.03 ± 0.14 b | - | - | - | 4.71 ± 0.09 d |
| Isovitexin-4′-O-glucoside | <1.00 | 59.73 ± 1.14 i | - | - | - | - |
| Isovitexin-2′′-O-rhamnoside | - | 21.44 ± 0.24 e | - | - | - | - |
| Isovitexin-2′′-O-glucoside | 22.08 ± 0.41 e | - | 5.72 ± 0.12 c | 15.62 ± 0.32 g | 38.06 ± 0.77 j | 54.73 ± 1.02 k |
| Isovitexin-7,4′-O-glucoside | 20.06 ± 0.40 e | 47.21 ± 0.95 g | 15.83 ± 0.32 g | 33.41 ± 0.69 i | 6.14 ± 0.12 e | 1.42 ± 0.03 a |
| Isovitexin-4′,2′′-O-glucoside | 4.21 ± 0.08 b | 33.18 ± 0.69 f | - | - | - | - |
| Isovitexin-7-O-(6′′-O-glucosyl)-glucoside | 10.32 ± 0.22 d | 2.86 ± 0.06 a | 18.30 ± 0.37 h | 15.04 ± 0.32 g | 2.18 ± 0.05 b | <1.00 |
| Apigenin-C-hexoside-tri-O-hexoside 4 | <1.00 | 11.08 ± 0.23 c | - | - | - | - |
| Acylated flavone C- and C,O-glycosides | ||||||
| Isovitexin-2′′-O-p-coumarate | <1.00 | - | 2.63 ± 0.04 a | 4.37 ± 0.09 c | 6.02 ± 0.11 f | 6.73 ± 0.12 e |
| Isovitexin-6′′-O-p-coumarate | - | <1.00 | - | - | - | - |
| Isovitexin-2′′-O-(6′′′′-O-p-coumaroyl)-glucoside | 11.02 ± 0.23 d | 51.26 ± 1.01 h | 15.64 ± 0.34 g | 9.43 ± 0.19 e | 22.18 ± 0.45 i | 17.41 ± 0.35 i |
| Isovitexin-2′′-O-(6′′′′-O-feruloyl)-glucoside | 4.04 ± 0.09 b | 115.03 ± 2.34 k | 9.97 ± 0.18 e | 11.86 ± 0.23 f | 14.35 ± 0.29 h | 15.24 ± 0.33 h |
| Isovitexin-2′′-O-glucoside-6′′-O-p-coumarate | 183.39 ± 3.69 h | - | 309.27 ± 6.22 k | 329.02 ± 6.71 l | 613.09 ± 12.26 m | 643.34 ± 12.89 m |
| Isovitexin-2′′-O-glucoside-6′′-O-ferulate | <1.00 | <1.00 | - | - | - | - |
| Isovitexin-4′-O-glucoside-2′′-O-(6′′′′- O-p-coumaroyl)-glucoside | 22.07 ± 0.45 e | 104.82 ± 2.14 j | 15.24 ± 0.29 g | 12.93 ± 0.14 f | 7.25 ± 0.15 f | 6.08 ± 0.12 e |
| Isovitexin-4′-O-glucoside-2′′-O-(6′′′′- O-feruloyl)-glucoside | 81.50 ± 1.63 | <1.00 | 20.35 ± 0.42 i | 39.29 ± 0.78 j | 81.83 ± 1.65 k | 137.10 ± 2.75 l |
| Isovitexin-2′′-O-(6′′′′-O-feruloyl)-glucoside-6′′-O-ferulate | - | <1.00 | - | - | - | - |
| Apigenin-C-hexoside-O-hexoside-O-p-coumarate 23 | <1.00 | - | - | - | - | - |
| Apigenin-C-hexoside-O-hexoside-O-p-coumarate 29 | - | 7.11 ± 0.15 b | - | - | - | - |
| Apigenin-C-hexoside-O-hexoside-O-p-coumarate 30 | - | <1.00 | - | - | - | - |
| Apigenin-C-hexoside-O-hexoside-O-p-coumarate 31 | - | <1.00 | - | - | - | - |
| Apigenin-C-hexoside-di-O-hexoside-O-p-coumarate 9 | 222.61 ± 4.48 i | 105.39 ± 2.16 j | 453.18 ± 9.11 l | 371.53 ± 7.49 l | 91.03 ± 1.85 l | 17.24 ± 0.33 i |
| Apigenin-C-hexoside-di-O-hexoside-O-p-coumarate 14 | <1.00 | - | - | 7.26 ± 0.15 e | 9.43 ± 0.19 g | 11.18 ± 0.21 g |
| Apigenin-C-hexoside-di-O-hexoside-O-p-coumarate 18 | 2.34 ± 0.05 a | <1.00 | 6.08 ± 0.12 d | 22.46 ± 0.43 h | 34.62 ± 0.69 j | 32.92 ± 0.66 j |
| Apigenin-C-hexoside-di-O-hexoside-O-ferulate 12 | <1.00 | - | - | 5.62 ± 0.12 d | 1.01 ± 0.02 a | 1.52 ± 0.03 a |
| Apigenin-C-hexoside-di-O-hexoside-O-ferulate 17 | <1.00 | - | 5.14 ± 0.10 c | 2.31 ± 0.05 b | 4.14 ± 0.08 d | 3.41 ± 0.07 c |
| Apigenin-C-hexoside-di-O-p-coumarate 36 | - | 18.12 ± 0.34 d | - | - | - | - |
| Apigenin-C-hexoside-O-p-coumarate-O-ferulate 37 | - | <1.00 | - | - | - | - |
| Apigenin-C-hexoside-di-O-ferulate 38 | - | - | - | - | - | 3.84 ± 0.07 c |
| Flavone O-glycosides | ||||||
| Apigenin-7-O-(6′′-O-feruloyl)-glucoside | <1.00 | - | <1.00 | 1.52 ± 0.03 a | 4.03 ± 0.08 d | 2.81 ± 0.05 b |
| Cucumerins | ||||||
| Cucumerin C | 154.12 ± 3.08 h | <1.00 | 60.83 ± 1.22 j | 53.92 ± 1.09 k | 22.71 ± 0.46 i | 7.43 ± 0.15 f |
| Cucumerin D | 86.71 ± 1.73 g | 210.44 ± 4.22 l | 14.06 ± 0.29 f | 21.18 ± 0.42 h | 7.16 ± 0.15 f | 3.02 ± 0.06 b |
| Cucumerin C/D O-hexoside 26 | - | <1.00 | - | - | - | - |
| Cucumerin C/D O-hexoside 27 | - | <1.00 | - | - | - | - |
| Cucumerin A/B O-pentoside 3 | 46.21 ± 0.92 f | - | 10.63 ± 0.21 e | 11.73 ± 0.23 f | 3.42 ± 0.06 c | 4.22 ± 0.09 c |
| Cucumerin A/B O-desoxyhexosides 6 | 6.27 ± 0.12 c | - | 4.24 ± 0.08 b | - | - | - |
| Cucumerin A/B O-desoxyhexosides 8 | <1.00 | - | <1.00 | 3.25 ± 0.07 c | - | - |
| Subtotal non-acylated C- and C,O-glycosides | 56.67 | 181.53 | 39.85 | 64.07 | 46.38 | 60.86 |
| Subtotal acylated C- and C,O-glycosides | 526.97 | 401.73 | 837.50 | 816.08 | 884.95 | 892.17 |
| Subtotal O-glycosides | <1.00 | - | <1.00 | 1.52 | 4.03 | 2.81 |
| Subtotal cucumerins | 293.31 | 210.44 | 89.76 | 90.08 | 33.29 | 14.67 |
| Total flavonoids | 876.95 | 793.70 | 967.11 | 971.75 | 968.65 | 970.51 |
| Experimental Group | Dose, mg/kg/day | TC, mmol/L | TTG, mmol/L | LDLC, mmol/L | HDLC, mmol/L |
|---|---|---|---|---|---|
| Normal Diet | - | 1.57 ± 0.08 * | 0.75 ± 0.06 * | 0.81 ± 0.06 * | 0.51 ± 0.04 * |
| 1% Cholesterol Diet | - | 6.32 ± 0.53 | 3.02 ± 0.21 | 3.25 ± 0.26 | 0.24 ± 0.02 |
| 1% Cholesterol Diet + Simvastatin | 10 | 2.48 ± 0.18 * | 1.50 ± 0.11 * | 1.11 ± 0.09 * | 0.54 ± 0.05 * |
| 1% Cholesterol Diet + IVitGC | 20 | 3.69 ± 0.32 * | 1.96 ± 0.14 * | 1.83 ± 0.14 * | 0.40 ± 0.04 * |
| 50 | 2.95 ± 0.23 *,** | 1.71 ± 0.12 *,** | 1.40 ± 0.11 *,** | 0.49 ± 0.04 *,** | |
| 1% Cholesterol Diet + IVitG | 20 | 6.20 ± 0.57 | 3.05 ± 0.24 | 3.20 ± 0.25 | 0.25 ± 0.25 |
| 50 | 5.72 ± 0.45 | 2.91 ± 0.22 | 3.06 ± 0.21 | 0.30 ± 0.02 | |
| 1% Cholesterol Diet + IVit | 20 | 6.27 ± 0.52 | 2.96 ± 0.21 | 3.18 ± 0.26 | 0.22 ± 0.02 |
| 50 | 5.68 ± 0.47 | 2.78 ± 0.20 | 2.93 ± 0.23 | 0.32 ± 0.02 |
| Experimental Group | Dose, mg/kg/day | MDA, nmol/L | SOD, U/mg | GPx, U/mg | CAT, U/mg |
|---|---|---|---|---|---|
| Normal Diet | - | 3.0 ± 0.18 * | 79.1 ± 5.2 * | 9.7 ± 0.6 * | 122.7 ± 9.8 * |
| 1% Cholesterol Diet | - | 29.1 ± 1.8 | 42.1 ± 2.8 | 3.7 ± 0.2 | 41.6 ± 3.3 |
| 1% Cholesterol Diet + Simvastatin | 10 | 27.3 ± 1.7 | 43.9 ± 3.5 | 3.9 ± 0.2 | 45.9 ± 3.4 |
| 1% Cholesterol Diet + IViGC | 20 | 12.9 ± 0.8 * | 60.7 ± 4.1 * | 7.8 ± 0.5 * | 86.1 ± 6.9 * |
| 50 | 9.5 ± 5.6 *,** | 75.9 ± 5.0 *,** | 9.2 ± 0.7 *,** | 115.3 ± 9.2 *,** | |
| 1% Cholesterol Diet + IVitG | 20 | 27.1 ± 1.6 | 54.7 ± 3.5 * | 4.5 ± 0.3 * | 62.7 ± 5.0 * |
| 50 | 17.8 ± 1.1 *,** | 61.8 ± 4.1 *,** | 5.8 ± 0.3 *,** | 73.7 ± 5.9 *,** | |
| 1% Cholesterol Diet + IVit | 20 | 26.4 ± 1.6 | 57.8 ± 4.6 * | 5.0 ± 0.3 * | 69.3 ± 5.6 * |
| 50 | 15.9 ± 0.9 *,** | 69.7 ± 5.6 *,** | 6.1 ± 0.4 *,** | 81.2 ± 6.9 *,** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olennikov, D.N.; Kashchenko, N.I. Green Waste from Cucumber (Cucumis sativus L.) Cultivation as a Source of Bioactive Flavonoids with Hypolipidemic Potential. Agronomy 2023, 13, 2410. https://doi.org/10.3390/agronomy13092410
Olennikov DN, Kashchenko NI. Green Waste from Cucumber (Cucumis sativus L.) Cultivation as a Source of Bioactive Flavonoids with Hypolipidemic Potential. Agronomy. 2023; 13(9):2410. https://doi.org/10.3390/agronomy13092410
Chicago/Turabian StyleOlennikov, Daniil N., and Nina I. Kashchenko. 2023. "Green Waste from Cucumber (Cucumis sativus L.) Cultivation as a Source of Bioactive Flavonoids with Hypolipidemic Potential" Agronomy 13, no. 9: 2410. https://doi.org/10.3390/agronomy13092410
APA StyleOlennikov, D. N., & Kashchenko, N. I. (2023). Green Waste from Cucumber (Cucumis sativus L.) Cultivation as a Source of Bioactive Flavonoids with Hypolipidemic Potential. Agronomy, 13(9), 2410. https://doi.org/10.3390/agronomy13092410








